Abstract
Background: One of the major metabolic consequences of using nitisinone to treat patients with alkaptonuria is that circulating tyrosine concentrations increase. As tyrosine is required for the biosynthesis of catecholamine neurotransmitters, it is possible that their metabolism is altered as a consequence. Herein we report the 24-h urinary excretion of normetadrenaline (NMA), metadrenaline (MA), 3-methoxytyramine (3-MT) (catecholamine metabolites) and 5-hydroxyindole acetic acid (5-HIAA, metabolite of serotonin) in a cohort of AKU patients before and after a 4-week treatment trial with nitisinone.
Materials and Methods: 24 h urinary excretions of NMA, MA, 3-MT and 5-HIAA were determined by liquid chromatography tandem mass spectrometry. Interassay coefficient of variation was <10% for all analytes measured, at all concentrations tested.
Results: Urine samples were assayed at baseline (pre-nitisinone, n = 36) and 4 weeks later; 7 received no nitisinone (4 male, mean age (±SD) 46.3 (16.4) years), and 29 received a daily dose of nitisinone [1 mg (n = 7, 6 male, mean age 45.9 (10.9) years), 2 mg (n = 8, 5 male, mean age 43.9 (13.7) years), 4 mg (n = 8, 5 male, mean age 47.3 (10.7) years) and 8 mg (n = 6, 4 male, mean age 53.8 (8.3) years)]. 3-MT concentrations increase significantly (p < 0.01, at all doses) following nitisinone therapy but not in a dose-dependent manner. NMA concentrations decreased (p < 0.05, at all doses) following nitisinone therapy at all doses. 5-HIAA concentrations decreased following nitisinone therapy and were significantly lower at a daily dose of 8 mg only (p < 0.05).
Conclusions: This study shows that catecholamine and serotonin metabolism is altered by treatment with nitisinone.
Keywords: 5-Hydroxyindole acetic acid, Alkaptonuria, Metadrenalines
Introduction
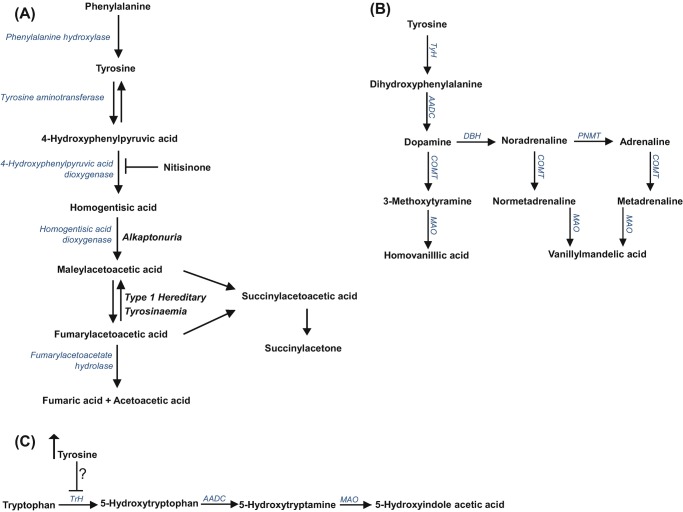
Alkaptonuria (AKU, OMIM: 203500) is a rare autosomal recessive disorder of tyrosine metabolism (Fig. 1a) resulting from a congenital lack in the enzyme homogentisate-1,2-dioxygenase (HGD, E.C.1.12.11.5). It occurs in 1 in 250,000 of the general population (Phomphutkul et al. 2002); however in certain countries, it is observed more commonly; for instance, in Slovakia it is estimated to occur in 1 in 19,000 of the population (Zatkova 2011; Milch 1960). One of the major biochemical consequences of AKU is that the circulating concentration of homogentisic acid (HGA) significantly increases. HGA is central to the pathophysiology of the disease and is thought to be responsible for a number of abnormalities including spondyloarthropathy, characterised by progressive kyphoscoliosis and impaired spinal and thoracic mobility, as well as renal and prostate stones, aortic valve stenosis, osteoporosis, fractures and ruptures of tendons, ligaments and muscle (Ranganath et al. 2013).
Fig. 1.
(a) Tyrosine metabolic pathway – highlighting the site of the enzyme defect observed in alkaptonuria and type 1 hereditary tyrosinaemia and the site of action of nitisinone. (b) Catecholamine metabolic pathway showing the formation of metadrenalines and (c) tryptophan metabolic pathway – highlighting the proposed site tyrosine inhibits tryptophan hydroxylase activity. TyH tyrosine hydroxylase, AADC aromatic acid decarboxylase, COMT catechol-O-methyl transferase, MAO monoamine oxidase, TrH tryptophan hydroxylase
Supportive medical management of AKU includes a low protein diet, analgesia and arthroplasty (Ranganath et al. 2013). An additional HGA lowering therapy is nitisinone (Fig. 1a), a competitive reversible inhibitor of the enzyme hydroxyphenylpyruvic acid dioxygenase (HPPD, E.C. 1.13.11.27). Its action reduces the accumulation of HGA and thus has the potential to prevent or slow the complications observed in patients with AKU. Currently nitisinone is not licenced for the treatment of patients with AKU.
In contrast nitisinone is already licensed for the treatment of hereditary tyrosinaemia type 1 (HT1) (HT I, OMIM 276700) and has proved to be a very efficacious mode of treatment (McKiernan 2013).
One of the major metabolic consequences of treating patients with nitisinone in AKU and HT1 is that circulating tyrosine concentrations increase significantly (Lindstedt et al. 1992; Suwannarat et al. 2005; Introne et al. 2011; Ranganath et al. 2016; Olsson et al. 2015; McKiernan et al. 2015; Milan et al. 2017). As tyrosine is the metabolic substrate required for the biosynthesis of catecholamine neurotransmitters (adrenaline (Ad), noradrenaline (NA) and dopamine (DP)) (Fig. 1b), it is possible that they may be altered during the 4-week treatment trial with nitisinone.
Hypertyrosinaemia is observed in patients with AKU that attend the National AKU Centre in Liverpool (patients are given 2 mg of nitisinone daily, off-licence) and during the Suitability Of Nitisinone In Alkaptonuria 1 (SONIA-1) clinical trial (Ranganath et al. 2016), which evaluated the efficacy of different daily doses of nitisinone over a 4-week period.
Significant elevations in serum and cerebral spinal fluid tyrosine concentrations have also been a documented consequence of nitisinone treatment in HT1 (Thimm et al. 2011). In patients with HT1, it is believed that the supraphysiological concentrations of tyrosine may be responsible for the reduced intelligence quotient (IQ) and cognitive function observed (Masurel-Paulet et al. 2008; De Laet et al. 2011; Thimm et al. 2012; Bendadi et al. 2014; Mckiernan et al. 2015).
The impact of hypertyrosinaemia on neurotransmitter metabolism (Fig. 1b, c) in patients with AKU following nitisinone therapy has not been previously reported. Herein we report the 24-h urinary excretion of normetadrenaline (NMA), metadrenaline (MA) and 3-methoxytyramine (3-MT) (catecholamine metabolites) and 5-hydroxyindole acetic acid (5-HIAA) (metabolite of serotonin) in patients before and during the 4-week treatment trial with nitisinone.
Materials and Methods
Subjects
All urine samples were from subjects included in the SONIA-1 clinical trial (trial registration number EudraCT number: 2012-005340-24. Registered at ClinicalTrials.gov: NCTO1828463). Twenty-four hour urine samples were collected into 2.5 L bottles containing 30 mL of 5N H2SO4; aliquots were stored away from bright light at −80°C. Urine samples analysed in this study were from baseline (pre-nitisinone, n = 36) and 4 weeks following no treatment (n = 7) or a daily dose of nitisinone [1 mg (n = 7), 2 mg (n = 8), 4 mg (n = 8) and 8 mg (n = 6)]. No patients included in this study had renal impairment (eGFR > 60 mL/min/1.73 m2 in all cases). Completeness of 24-h urine collection was assessed by measurement of urine creatinine (Roche Diagnostics, Germany); all patients had urine creatinine concentrations within the normal reference range (9.0–18.0 μmol/24 h, in-house reference range).
Analytical Methods
Urine Metadrenalines
The concentrations of urinary NMA, MA and 3-MT were determined by liquid chromatography tandem mass spectrometry (Banks et al. 2014). 1 mL of urine underwent acid hydrolysis (5M-HCl) at 100°C for 30 min. 50 μL of hydrolysate was diluted in 2 mL of deionised water containing deuterated internal standards (D3-NMA, 0.11 μmol/L, D3-MA 0.10 μmol/L and D4-3-MT 0.12 μmol/L. CDN Isotopes, Essex) and loaded onto the solid phase extraction plate (30 mg Evolute-SCX, Biotage, Hengoed). 20 μL of extract was injected onto a C18 phenyl-hexyl column (2.6 μ, 4.6 × 100 mm, Phenomenex, Cheshire) using a Waters Acquity UPLC separations module coupled to a Xevo TQS tandem mass spectrometer. Initial conditions of 90:10 water/methanol with 0.1% formic acid (v/v) increased linearly to 40:60 over 2 min, returning to starting conditions by 4 min. In-house calibration standards and commercial quality controls materials (Recipe ClinChek, Germany) were used. Calibrator concentrations were 0–46.8 μmol/L for NMA, 0–24.8 μmol/L for MA and 0–27.7 μmol/L for 3-MT. Interassay coefficients of variation for NMA, MA and 3-MT were 4.6, 4.7 and 7.2% at 1.7, 0.8 and 1.6 μmol/L, respectively.
Urine 5-Hydroxyindole Acetic Acid
The concentration of urinary 5-HIAA was determined by liquid chromatography tandem mass spectrometry. Samples were diluted (20 μL urine in 2 mL) in deionised water containing a deuterated internal standard (D5-5-hydroxyindole acetic acid, 0.34 μmol/L, CDN Isotopes, Essex). 20 μL of the diluted urine sample was injected onto an Atlantis dC18 column (2.6 μ, 4.6 × 100 mm, Waters, Milford) using a Waters Alliance 2795 separations module coupled to a Waters Quattro Premier XE tandem mass spectrometer. Initial conditions of 95:5 water/methanol with 0.1% formic acid (v/v) and 2 mmol/L ammonium acetate increased linearly to 5:95 over 2 min, returning to starting conditions by 4 min. Commercial calibration standards (3.6–356 μmol/L, Chromsystems, Germany) and quality controls materials (Recipe ClinChek, Germany) were used. Interassay coefficient of variation was 7.2% for 5-HIAA at 26.2 μmol/L.
Serum Tyrosine and Phenylalanine
The concentrations of serum tyrosine and phenylalanine were determined by liquid chromatography tandem mass spectrometry; for details see Hughes et al. (2015). Tyrosine concentration was previously reported in the SONIA-1 clinical trial (Ranganath et al. 2016).
In this study data from only 36 of 40 subjects from the SONIA-1 clinical were included. Serum tyrosine and phenylalanine concentrations presented herein are from subjects that had urine samples analysed for urinary NMA, MA, 3-MT and 5-HIAA. All serum samples were collected from patients after an overnight fast (at least 8 h). Patients’ dietary intake of protein was not restricted during this study, nor was it monitored.
Statistical Analysis
All statistical analysis was performed using GraphPad Instat (version 3.10, 2009). Kolmogorov-Smirnov testing was performed to assess if urinary NMA, MA, 3-MT and 5-HIAA concentrations and serum tyrosine and phenylalanine concentrations were normally distributed. Unpaired two-tailed student t-test was used to assess significant differences in urinary metabolites pre- and post-treatment with nitisinone; a p value <0.05 was deemed significant.
Results
Forty patients were included in the SONIA-1 study (Ranganath et al. 2016). Urine samples from 36 of these patients were available for inclusion in this study. Of the 36 patients, 7 patients received no treatment with nitisinone (4 male, mean age (±SD) 46.3 (16.4) years), and 29 patients received a daily dose of nitisinone [1 mg (n = 7, 6 male, mean age 45.9 (10.9) years), 2 mg (n = 8, 5 male, mean age 43.9 (13.7) years), 4 mg (n = 8, 5 male, mean age 47.3 (10.7) years) and 8 mg (n = 6, 4 male, mean age 53.8 (8.3) years)].
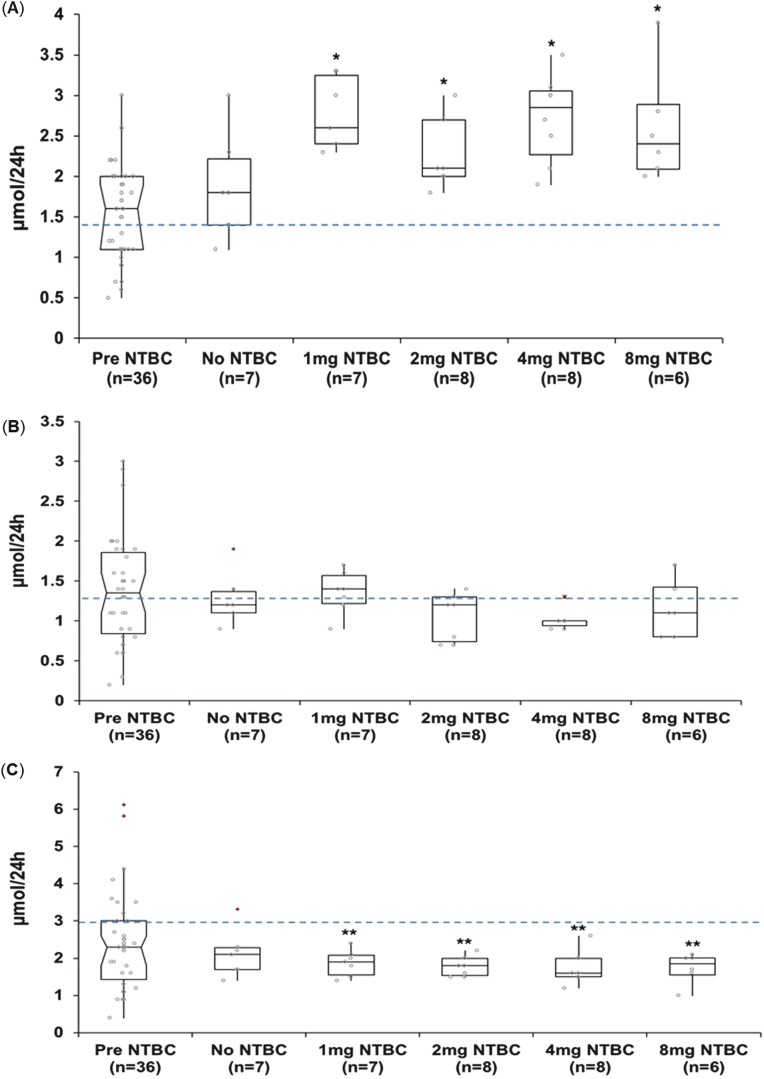
Urinary 3-MT concentrations increased significantly (p ≤ 0.01, at all doses) following daily nitisinone therapy. This occurred at all doses but not in a dose-dependent manner. In contrast urinary NMA concentrations decreased (p ≤ 0.03, at all doses) following nitisinone therapy. Urinary MA concentrations were not significantly different post-nitisinone therapy (Fig. 2).
Fig. 2.
Urinary metadrenaline concentrations in patients with alkaptonuria before treatment with nitisinone and after no treatment (n = 7); treatment with 1 mg nitisinone daily (n = 7); treatment with 2 mg nitisinone daily (n = 8); treatment with 4 mg nitisinone daily (n = 8) and treatment with 8 mg nitisinone daily (n = 6). (a) 3-Methoxytyramine, (b) metadrenaline and (c) normetadrenaline. Dashed line = indicates upper limit of normal urine reference range for 3-methoxytyramine <1.4 μmol/24 h; metadrenaline <1.3 μmol/24 h; normetadrenaline <3.0 μmol/24 h; − = 95% confidence notched outlier boxplot; + = outlier; circle = individual patient concentrations. Significance testing compared metadrenaline concentrations before and after a 4-week trial of nitisinone. * = p ≤ 0.01; ** = p ≤ 0.03
Of interest a large proportion of patients had NMA (reference range < 3.0 μmol/24 h), MA (reference range < 1.3 μmol/24 h) and 3-MT (reference range < 1.4 μmol/24 h) concentrations outside of the normal reference range pre-nitisinone therapy. Following nitisinone therapy, NMA concentrations were within the normal reference range in all but one patient; however 3-MT concentrations were outside of the reference range in all patients. MA concentrations remained unchanged overall post-nitisinone therapy, although there was less of a spread of concentrations observed.
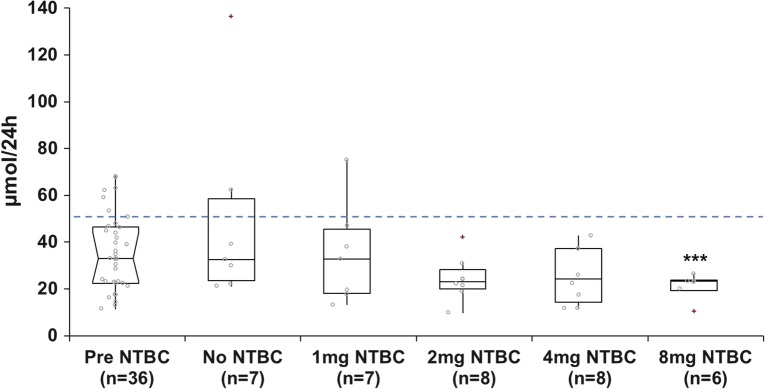
5-HIAA concentrations decreased following nitisinone therapy (Fig. 3) and were significantly lower at a daily dose of 8 mg (p < 0.05) only. 5-HIAA concentrations were within the normal reference range (reference range < 50 μmol/24 h) in the majority of patients pre- (7/36 patients had elevated 5-HIAA) and post- (2/8 patients receiving no nitisinone and 1/7 patients receiving 1 mg nitisinone daily had elevated 5-HIAA) nitisinone therapy.
Fig. 3.
Urinary 5-hydroxyindole acetic acid concentrations in patients with alkaptonuria before treatment with nitisinone and after no treatment (n = 7); treatment with 1 mg nitisinone daily (n = 7); treatment with 2 mg nitisinone daily (n = 8); treatment with 4 mg nitisinone daily (n = 8) and treatment with 8 mg nitisinone daily (n = 6). Dashed line = indicates upper limit of normal urine reference range for 5-hydroxyindole acetic acid, <50 μmol/24 h; − = 95% confidence notched outlier boxplot; + = outlier; circle = individual patient concentrations. Significance testing compared 5-hydroxyindole acetic acid concentrations before and after a 4-week trial of nitisinone. *** = p < 0.05
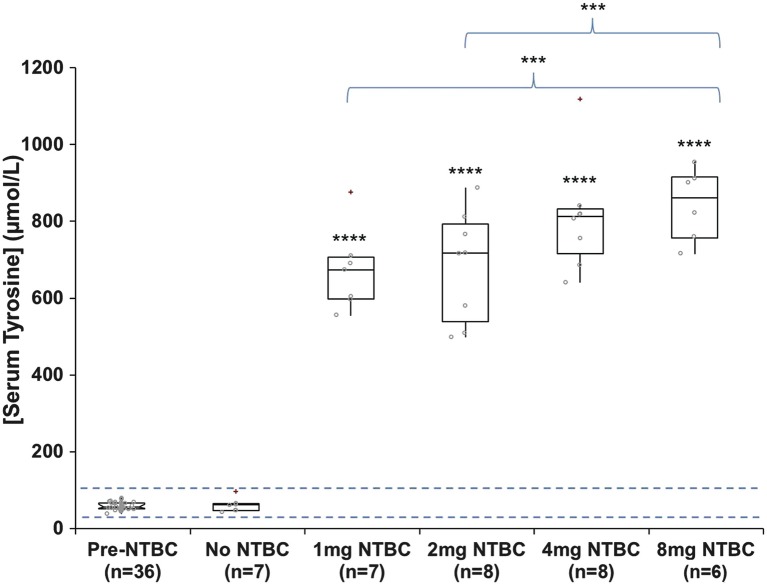
Fasting serum tyrosine concentrations increased significantly following treatment with nitisinone at all doses (p = <0.0001). There were also significantly higher tyrosine concentrations observed between subjects receiving 1 mg nitisinone versus 8 mg nitisinone (p = <0.05) and subjects receiving 2 mg nitisinone versus 8 mg nitisinone (p = <0.05), thus indicating a dose-dependent increase in serum tyrosine concentrations following nitisinone. Mean (± standard deviation) concentrations were 59 (±10) μmol/L and 750 (±140) μmol/L pre- and post-nitisinone therapy (Fig. 4), respectively (reference range 30–87 μmol/L, Davison et al. 2015).
Fig. 4.
Serum tyrosine concentrations in patients with alkaptonuria before treatment with nitisinone and after no treatment (n = 7); treatment with 1 mg nitisinone daily (n = 7); treatment with 2 mg nitisinone daily (n = 8); treatment with 4 mg nitisinone daily (n = 8) and treatment with 8 mg nitisinone daily (n = 6). Dashed line = indicates normal serum reference range for tyrosine, 30–87 μmol/L; − = 95% confidence notched outlier boxplot; + = outlier; circle = individual patient concentrations. Significance testing compared tyrosine concentrations before and after a 4-week trial of nitisinone and tyrosine concentrations in patients that received different doses of nitisinone at 4 weeks of therapy (brackets compare 1 mg vs. 8 mg nitisinone and 2 mg vs. 8 mg nitisinone). **** = p < 0.0001; *** = p < 0.05
Serum phenylalanine concentrations were not significantly different following a 4-week trial of nitisinone (p > 0.05 at all doses) and were within the normal reference range (30–76 μmol/L, in-house reference range). Mean (±standard deviation) phenylalanine concentrations were 57.1 μmol/L (7.9) (pre-nitisinone, n = 36), 55.7 μmol/L (17.8) (no nitisinone, n = 7), 53.0 μmol/L (6.0) (1 mg nitisinone, n = 7), 51.6 μmol/L (13.0) (2 mg nitisinone, n = 8), 53.8 μmol/L (12.2) (4 mg nitisinone, n = 8) and 53.8 μmol/L (13.4) (8 mg nitisinone, n = 6).
Discussion
Significant hypertyrosinaemia following nitisinone therapy has been previously reported in AKU (Suwannarat et al. 2005; Introne et al. 2011; Ranganath et al. 2016; Olsson et al. 2015; Milan et al. 2017). To date there are no reports on the impact this may have on closely related metabolic pathways that require tyrosine as a substrate, including the biosynthesis of the catecholamine neurotransmitters (Fig. 1b).
Much of the literature focuses on hypertyrosinaemia following nitisinone therapy in patients with HT1. It is believed that the supraphysiological concentrations observed may contribute to neurodevelopmental delay (Masurel-Paulet et al. 2008; De Laet et al. 2011; Thimm et al. 2012; Bendadi et al. 2014; Mckiernan et al. 2015). It is estimated that up to 35% of children treated with nitisinone have learning difficulties (McKiernan et al. 2015). It is not known whether this may also be related to nitisinone treatment itself or low phenylalanine concentrations, presenting with acute liver disease or an intrinsic effect of HT1.
Several mechanisms for neurodevelopmental delay have been postulated. These include increased transport of tyrosine into the brain, decreased transport of other neutral amino acids into the brain (specifically tryptophan, the precursor of serotonin), increased central nervous system DP, decreased central nervous system serotonin, oxidative damage from δ-aminolevulinic acid and succinylacetone modification of neuronal proteins (Thimm et al. 2011; Harding et al. 2014; Hillgartner et al. 2016).
It has also been suggested that altered serotonin metabolism may be due to direct inhibition of tryptophan hydroxylase (TPH; EC 1.14.16.4) activity by tyrosine (Fig. 1c), which leads to a reduced biosynthesis of serotonin (Thimm et al. 2011). TPH is the rate-limiting step in the biosynthesis of serotonin.
As it has been suggested that hypertyrosinaemia may have an impact on neurodevelopmental delay in HT1, concern exists around the use of nitisinone in AKU, which is currently not licenced for treatment of this disorder. While the dose prescribed to patients with AKU is much lower than those with HT1 (2 mg daily at the National Centre for AKU in Liverpool versus 0.5–2.5 mg/kg per day in HT1), the concentration of serum and urine tyrosine observed following nitisinone therapy is similar.
Herein for the first time, we report the impact of nitisinone on the urinary excretion of the NMA, MA and 3-MT and 5-HIAA, which serve as surrogate markers for catecholamine and serotonin neurotransmitter biosynthesis, respectively (Fig. 1b, c). Urinary metabolites were measured as collecting urine is non-invasive and is the primary route for neurotransmitter elimination.
The marked increase in 3-MT and decreased NMA excretion post-nitisinone therapy indicate that nitisinone therapy alters the metabolism of catecholamines as the concentration of their respective O-methylated metabolites is altered when compared to pretreatment concentrations.
The metabolism of catecholamine neurotransmitters is complex (Fig. 1b), and they have multiple origins (for detailed overview, see Eisenhofer et al. (2004)), including the sympathetic nerves, adrenal medulla, brain and mesenteric organs (Eisenhofer et al. 2004). It has been demonstrated that the concentration of monoamine neurotransmitters in urine is dependent on plasma concentration and uptake via organic cation transporters (OCT) in the kidney (Eisenhofer et al. 1996; Graefe et al. 1997). However it is also essential to consider the degree to which renal neurotransmitter synthesis can contribute to the urinary concentrations. In the kidneys DP is produced via the uptake and decarboxylation of circulating DOPA and not just from the filtration of circulating DP (Eisenhofer et al. 2004).
In this study 3-MT was shown to increase significantly post-nitisinone therapy. 3-MT is a direct metabolite of DP and thus may reflect increased circulating DP concentrations and or synthesis in the kidney. It is proposed that the latter is highly likely as the tyrosine load delivered to the kidney increased significantly post-nitisinone therapy. This was reported in the SONIA-1 dosing study (Ranganath et al. 2016) and shown in the data presented herein where mean serum tyrosine concentrations increased significantly (p = <0.0001) after a 4-week treatment with nitisinone therapy (reference range 30–87 μmol/L, Davison et al. 2015). The increased tyrosine load delivered to the kidney may provide a substrate for dihydroxyphenylalanine (DOPA) synthesis, and thus the subsequent decarboxylation of DOPA to DP may lead to the consequent increase in 3-MT observed.
The elevated serum tyrosine concentrations observed post-nitisinone therapy were accompanied by a significant dose-dependent decrease in urinary excretion of HGA, across the studied dose interval of 1–8 mg. The 8 mg dose resulted in a mean reduction of 24-h urinary HGA excretion of 98.8% compared with baseline (Ranganath et al. 2016). Serum phenylalanine concentrations were not affected by the marked increase in tyrosine following a 4-week trial with nitisinone, indicating that tyrosine has an alternative metabolic fate.
The impact of hypertyrosinaemia on serum tryptophan concentrations was not evaluated in this short-term dose evaluation study as it was not included in the original study design. However it has been shown at the National Centre for AKU in Liverpool that serum tryptophan concentrations do not change following a 2 mg daily dose of nitisinone (unpublished, personal communication with Ranganath L. R.). The authors postulate that serum tryptophan concentrations will not be altered at a higher dose of nitisinone as marked hypertyrosinaemia is observed at all doses of nitisinone evaluated. Further work is required to confirm this.
In this study tissue concentrations of tyrosine were not determined to assess whether they correlate with serum and urine concentrations. Further work into this area is required to help understand the pathophysiology of AKU at a tissue level.
Previously Thimm et al. (2011) reported tyrosine and homovanillic acid (dopamine metabolite) concentrations in the cerebral spinal fluid (CSF) of three patients with HT1 during long-term treatment with nitisinone. In this small study, tyrosine concentrations were markedly increased in the CSF and plasma, as expected. However homovanillic acid concentrations were within the normal reference range, suggesting that there is no alteration in dopamine metabolism in the CNS. This supports the postulate that the increased urinary 3-MT observed in this study is a consequence of increased tyrosine load being delivered to the kidney. It is also important to consider that dietary constituents have been shown to influence urinary concentrations of 3-MT and thus represent one peripheral source of dopamine metabolites (de Jong et al. 2009).
In this study dietary components rich in biogenic amines were not restricted or documented. However as the data presented herein show an increase in 3-MT in all patients following a 4-week treatment with nitisinone, it is believed that diet alone is not solely responsible for the increase in 3-MT observed.
The parallel decrease in NMA suggests that there may be a reduction in the synthesis of NA as a consequence of nitisinone therapy. While there is limited information available on the exact contribution of central and peripheral output to urinary excretion of neurotransmitters, Graefe et al. (1997) demonstrated that a significant proportion of urinary NA and Ad stems from circulation. In this study one may postulate that the observed decrease in NMA may be a consequence of reduced sympathetic nerve excitation as subjects are less depressed or anxious, as they are on treatment for AKU. Previous studies (Hughes et al. 2004; Roy et al. 1986a, b; Grossman and Potter 2009) have evaluated the urinary concentration of NA and Ad in subjects with depression, demonstrating that urinary concentrations of NA and Ad were significantly higher in subjects with depression compared to controls. It is not surprising that MA concentrations were not significantly different post-nitisinone therapy as Ad is produced by the adrenal gland and not the sympathetic nervous system, mesenteric organs or kidney (Eisenhofer et al. 2004). The reduced variability of MA concentrations observed following a 4-week trial of nitisinone is of unknown significance and is unlikely to reflect a change in diet; this requires further investigation.
Another possibility is that there is an alteration in inflammatory signalling following nitisinone therapy. Elenkov et al. (2000) demonstrated that Ad and NA can inhibit the production of pro-inflammatory cytokines (i.e. interleukin-2, tumour necrosis factor-α and interferon-γ) and stimulate the production of anti-inflammatory cytokines (i.e. interleukin-10 and transforming growth factor-β). It is postulated that altering these pathways may cause a selective suppression of T helper-1 cells and cellular immunity and enhancement of T helper-2 cell activity and a shift towards humoral immunity.
While changes in urinary concentrations of NA, Ad and their respective metabolites were not evaluated in this study, one can postulate that they may be altered as a consequence of changes in the inflammatory processes observed following nitisinone therapy.
The decrease in 5-HIAA excretion at a daily dose of 8 mg only suggests that there is a dose-dependent effect of nitisinone therapy. This supports that serotonin metabolism is altered following treatment with nitisinone. Thimm et al. (2011) also reported alterations in serotonin metabolism in patients with HT1 treated with nitisinone. This study showed a decrease in CSF 5-HIAA concentrations following nitisinone therapy.
It is proposed that the decrease in CSF 5-HIAA occurred as increased tyrosine concentrations compete with tryptophan (serotonin precursor) via a neutral amino acid transporter across the blood–brain barrier, thus reducing tryptophan availability for intracerebral serotonin synthesis (Pratt 1982).
In addition it has been hypothesised that elevated tyrosine may inhibit tryptophan hydroxylase (TPH; EC 1.14.16.4) activity (Fig. 2c), the rate-limiting step for serotonin metabolism (Thimm et al. 2011). Although this study was small and analysis was performed in CSF, it has been shown that urinary analysis of serotonin and 5-HIAA reflects parallel changes in immunoreactivity in the dorsal raphe nucleus, demonstrating a positive correlation between CNS serotonergic activity and urinary serotonin concentrations (Lynn-Bullock et al. 2004).
Conclusion
For the first time, alterations in neurotransmitter metabolism have been reported in patients with AKU following nitisinone therapy, specifically increased urinary 3-MT (dopaminergic neurotransmitter metabolite), decreased NMA (noradrenaline neurotransmitter metabolite) and increased 5-HIAA (serotoninergic neurotransmitter metabolite) concentrations. The exact mechanism(s) causing these changes are not known, and further work is required to elucidate this and to establish whether these observations truly reflect changes in CNS monoamine neurotransmitter metabolism or contributions from peripheral or renal metabolism.
Moreover it is recognised that these data are based on a short-term dosing study, and it is necessary to assess whether these changes would be observed in patients on long-term therapy with nitisinone and to see if biochemical changes correlate with changes in behaviour or mood.
Abbreviations
- 3-MT
3-Methoxytyramine
- 5HIAA
5-Hydroxyindole acetic acid
- AADC
Aromatic acid decarboxylase
- Ad
Adrenaline
- AKU
Alkaptonuria
- COMT
Catechol-O-methyl transferase
- CSF
Cerebral spinal fluid
- DP
Dopamine
- HGA
Homogentisic acid
- HGD
Homogentisate-1,2-dioxygenase
- HPPD
Hydroxyphenylpyruvic acid dioxygenase
- HT1
Hereditary tyrosinaemia type 1
- IQ
Intelligence quotient
- MA
Metadrenaline
- MAO
Monoamine oxidase
- NA
Noradrenaline
- NMA
Normetadrenaline
- OCT
Organic cation transporter
- SONIA-1
Suitability Of Nitisinone In Alkaptonuria 1
- TrH
Tryptophan hydroxylase
- TyH
Tyrosine hydroxylase
Compliance with Ethics Guidelines
All procedures reported in this review were in accordance with the ethical standards of the local hospital ethics committee and with the Helsinki Declaration of 1975, as revised in 2000.
All samples used in this study were from a registered clinical trial. Trial registration number EudraCT number: 2012-005340-24. Registered at ClinicalTrials.gov: NCTO1828463.
Conflict of Interest
Davison A. S., Milan A. M., Hughes A. T., Norman B., Khedr M., Rovensky J., Gallagher J. A. and Ranganath L. R. have no conflict of interest.
Davison A. S. was the main author who performed the laboratory analysis and wrote the manuscript. He is funded through a National Institute for Health Research grant (grant code: HCS DRF-2014-05-009).
Milan A. M., Khedr M. and Gallagher J. are senior colleagues in the AKU research group; they reviewed and made corrections to the manuscript. Hughes A. T. and Norman B. contributed to the laboratory analysis and reviewed and made corrections to the manuscript. Rovensky J. is a major collaborator from Slovakia. He recruited patients and supplied samples for analysis. Ranganath L. R. is the clinical director of the AKU society and AKU research group and was responsible for conceiving the ideas behind this article and made corrections to the manuscript.
Contributor Information
A. S. Davison, Email: andrew.davison@rlbuht.nhs.uk
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Banks A, Dutton JJ, Phillipson K, et al. Development of a LC-MS/MS method for the measurement of total fractionated urine metadrenalines and determination of a healthy population reference range. Clin Chem Lab Med. 2014;52(11):eA307. [Google Scholar]
- Bendadi F, de Koning TJ, Visser G, et al. Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J Pediatr. 2014;164:398–401. doi: 10.1016/j.jpeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Davison AS, Milan AM, Hughes AT, et al. Serum concentrations and urinary excretion of tyrosine and homogentisic acid in normal subjects. Clin Chem Lab Med. 2015;53:e81–e83. doi: 10.1515/cclm-2014-0668. [DOI] [PubMed] [Google Scholar]
- de Jong WHA, Eisenhofer G, Post WJ, et al. Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors. J Clin Endocrinol Metab. 2009;94:2841–2849. doi: 10.1210/jc.2009-0303. [DOI] [PubMed] [Google Scholar]
- De Laet C, Munoz VT, Jaeken J, FranÅois B, et al. Neuropsychological outcome of NTBC-treated patients with tyrosinaemia type 1. Dev Med Child Neurol. 2011;53:962–964. doi: 10.1111/j.1469-8749.2011.04048.x. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, McCarty R, Pacak K, et al. Disprocynium24, a novel inhibitor of the extraneuronal monoamine transporter, has potent effects on the inactivation of circulating noradrenaline and adrenaline in conscious rat. Naunyn Schmiedeberg's Arch Pharmacol. 1996;354:287–294. doi: 10.1007/BF00171059. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJU, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, et al. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Graefe KH, Friedgen B, Wolfel R, et al. 1,1-Diisopropyl-2,4-cyanine (disprocynium24), a potent uptake2 blocker, inhibits the renal excretion of catecholamines. Naunyn Schmiedeberg's Arch Pharmacol. 1997;356:115–125. doi: 10.1007/PL00005018. [DOI] [PubMed] [Google Scholar]
- Grossman F, Potter WZ. Catecholamines in depression: a cumulative study of urinary norepinephrine and its major metabolites in unipolar and bipolar depressed patients versus healthy volunteers at the NIMH. Psychiatry Res. 2009;87:21–27. doi: 10.1016/S0165-1781(99)00055-4. [DOI] [PubMed] [Google Scholar]
- Harding CO, Winn SR, Gibson KM, et al. Pharmacologic inhibition of L-tyrosine degradation ameliorates cerebral dopamine deficiency in murine phenylketonuria (PKU) J Inherit Metab Dis. 2014;37(5):735–743. doi: 10.1007/s10545-013-9675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillgartner MA, Coker SB, Koenig AE, et al. Tyrosinemia type I and not treatment with NTBC causes slower learning and altered behavior in mice. J Inherit Metab Dis. 2016;39:673–682. doi: 10.1007/s10545-016-9949-6. [DOI] [PubMed] [Google Scholar]
- Hughes JW, Watkins L, Blumenthal JA, et al. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. 2004;57:353–358. doi: 10.1016/S0022-3999(04)00064-9. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Milan AM, Davison AS, et al. Serum markers in alkaptonuria: simultaneous analysis of homogentisic acid, tyrosine and nitisinone by liquid chromatography tandem mass spectrometry. Ann Clin Biochem. 2015;52(5):597–605. doi: 10.1177/0004563215571969. [DOI] [PubMed] [Google Scholar]
- Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103:307–314. doi: 10.1016/j.ymgme.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt S, Holme E, Lock EA, et al. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340(8823):813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- Lynn-Bullock CP, Welshhans K, Pallas SL, et al. The effect of oral 5-HTP administration on 5-HTP and 5-HT immunoreactivity in monoaminergic brain regions of rats. J Chem Neuroanat. 2004;27:129–138. doi: 10.1016/j.jchemneu.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Masurel-Paulet A, Poggi-Bach J, Rolland MO, et al. NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis. 2008;31:81–87. doi: 10.1007/s10545-008-0793-1. [DOI] [PubMed] [Google Scholar]
- McKiernan PJ. Nitisinone for the treatment of hereditary tyrosinemia type I. Expert Opin Orphan Drugs. 2013;1:491–497. doi: 10.1517/21678707.2013.800807. [DOI] [Google Scholar]
- McKiernan PJ, Preece MA, Chakrapani A. Outcome of children with hereditary tyrosinaemia following newborn screening. Arch Dis Child. 2015;100:738–741. doi: 10.1136/archdischild-2014-306886. [DOI] [PubMed] [Google Scholar]
- Milan AM, Hughes AT, Davison AS, et al. The effect of nitisinone on homogentisic acid and tyrosine: a two-year survey of patients attending the National Alkaptonuria Centre, Liverpool. Ann Clin Biochem. 2017;54:323–330. doi: 10.1177/0004563217691065. [DOI] [PubMed] [Google Scholar]
- Milch RA. Studies of alcaptonuria: inheritance of 47 cases in eight highly inter-related Dominican kindreds. Am J Hum Genet. 1960;12:76–85. [PMC free article] [PubMed] [Google Scholar]
- Olsson B, Cox TF, Psarelli EE, et al. Relationship between serum concentrations of nitisinone and its effect on homogentisic acid and tyrosine in patients with alkaptonuria. J Inherit Metab Dis Rep. 2015;24:21–27. doi: 10.1007/8904_2015_412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phomphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347:2111–2121. doi: 10.1056/NEJMoa021736. [DOI] [PubMed] [Google Scholar]
- Pratt O. Transport inhibition in the pathology of phenylketonuria and other inherited metabolic diseases. J Inherit Metab Dis. 1982;5:S75–S81. doi: 10.1007/BF01805567. [DOI] [Google Scholar]
- Ranganath LR, Jarvis JC, Gallagher JA. Recent advances in management of alkaptonuria. J Clin Pathol. 2013;66:367–373. doi: 10.1136/jclinpath-2012-200877. [DOI] [PubMed] [Google Scholar]
- Ranganath LR, Milan AM, Hughes AT, et al. Suitability Of Nitisinone In Alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis. 2016;75(2):362–367. doi: 10.1136/annrheumdis-2014-206033. [DOI] [PubMed] [Google Scholar]
- Roy A, Linnoila M, Karoum F, et al. Relative activity of metabolic pathways for norepinephrine in endogenous depression. Acta Psychiatr Scand. 1986;73:624–628. doi: 10.1111/j.1600-0447.1986.tb02734.x. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, Douillet P, et al. Urinary monoamines and monoamine metabolites in subtypes of unipolar depressive disorder and normal controls. Psychol Med. 1986;16:541–546. doi: 10.1017/S0033291700010308. [DOI] [PubMed] [Google Scholar]
- Suwannarat P, O’Brien K, Perry MB, et al. Use of nitisinone in patients with alkaptonuria. Metab Clin Exp. 2005;54:719–728. doi: 10.1016/j.metabol.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Thimm E, Herebian D, Assmann B, et al. Increase of CSF tyrosine and impaired serotonin turnover in tyrosinemia type I. Mol Genet Metab. 2011;102:122–125. doi: 10.1016/j.ymgme.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Thimm E, Richter-Werkle R, Kamp G, et al. Neurocognitive outcome in patients with hypertyrosinemia type I after long-term treatment with NTBC. J Inherit Metab Dis. 2012;35:263–268. doi: 10.1007/s10545-011-9394-5. [DOI] [PubMed] [Google Scholar]
- Zatkova A. An update on molecular genetics of alkaptonuria (AKU) J Inherit Metab Dis. 2011;34:1127–1136. doi: 10.1007/s10545-011-9363-z. [DOI] [PubMed] [Google Scholar]