Abstract
Fabry disease, a lysosomal storage disorder, is a rare inborn error of metabolism caused by deficiency of the enzyme alpha galactosidase A and resulting accumulation of globotriaosylceramide. The symptoms of Fabry disease are heterogeneous including renal failure, cardiac hypertrophy, and stroke and may not be well recognized by non-specialist physicians. Patients with milder, later onset of disease often have a delay in diagnosis.
Fabry patients may suffer significant neuropathic pain in the extremities (acroparasthesia) but the degree to which musculoskeletal symptoms contribute to total pain and disability is unknown. Here, we present a questionnaire study focusing on joint manifestations and their impact on daily life of patients with Fabry disease.
Seventy-seven patients with Fabry disease and age-matched healthy controls (49 female and 28 male) took part in a survey focused on joint problems, pain, disability, and quality of life. Joint pain and swelling were reported by 43% of male and 39% of female Fabry patients. Analysis by age group showed higher prevalence of joint problems and decreased quality of life, in terms of mobility, activity, pain, and anxiety, in Fabry patients younger than 50 years compared to healthy controls. Female Fabry patients had higher fatigue scores compared to control subjects. Fabry patients reported problems with vigorous daily activities and gripping.
Musculoskeletal symptoms are common in Fabry patients and contribute to overall pain and decreased quality of life. Awareness of Fabry disease by physicians may be raised to ensure timely diagnosis of this rare disease.
Electronic supplementary material
The online version of this article (10.1007/8904_2017_84) contains supplementary material, which is available to authorized users.
Keywords: Fabry disease, Joints manifestations, Lysosomal storage disorder, Multidisciplinary team-care, Musculoskeletal symptoms, Patient-reported outcomes
Introduction
Fabry disease is a lysosomal storage disorder caused by a genetic defect resulting in a deficiency of a lysosomal protein alpha galactosidase A (α-GAL) (Brady et al. 1967).
A defect in the GLA gene results in partial or complete deficiency of α-GAL and inability to catabolize lipids with terminal α-galactosyl residues, mainly globotriaosylceramide (Desnick et al. 2001). This leads to its accumulation in a variety of cell types including capillary endothelial cells, renal, cardiac, and nerve cells (Germain et al. 2007).
Fabry disease often has its onset in childhood with pain in the hands and feet, fever, hypohidrosis, fatigue, and exercise intolerance (Shelley et al. 1995; Morgan and Crawfurd 1988; Hopkin et al. 2008).
Despite X-linked inheritance clinical manifestations are frequent in female patients and are more variable than among male patients, with later onset and slower progression (Gupta et al. 2005; Deegan et al. 2006; Wang et al. 2007). A number of atypical variants (non-classical disease) exist with males having residual plasma α-GAL levels (1–30% of normal) and few or none of the early symptoms of classical Fabry disease (von Scheidt et al. 1991; Nakao et al. 1995; Yoshitama et al. 2001).
Despite the early onset of Fabry symptoms, the patients often remain undiagnosed with a mean delay of approximately 12 years between onset of symptoms and diagnosis reported for male and female patients enrolled in the Fabry Registry (Wilcox et al. 2008). By the third to fifth decade, renal, cardiac, and cerebrovascular complications typically occur and may become life-threatening (Shelley et al. 1995; Colombi et al. 1967; Sims et al. 2009). Given that enzyme replacement therapy (ERT) is generally more effective in less advanced stages of the disease (Banikazemi et al. 2007; Schiffmann et al. 2007; Weidemann et al. 2009), timely diagnosis of Fabry patients is important.
Pain is common in patients with Fabry disease; however, the degree to which musculoskeletal symptoms contribute to total body pain is unknown. No systematic review of patients aimed to reveal association of Fabry disease with joints manifestations has previously been completed. Single instances of osteonecrosis (Lien and Lai 2005), burning pain in hands and feet (Paira et al. 1992), femoral head avascular necrosis (Ross et al. 1993), arthropathy of hand joints (MacDermot et al. 2001), and polyarthralgia (Manger et al. 2007) have been reported. Moreover, a survey among 360 clinical rheumatologists and paediatricians has shown that Fabry manifestations are poorly recognized and awareness of relevant diagnostic steps is low (Cimaz et al. 2011). Musculoskeletal manifestations in Fabry disease may constitute the presenting symptoms of which the most common are past or present pain in the extremities and osteoporosis. Their misinterpretation may delay diagnosis and, therefore, initiation of specific therapy (Lidove et al. 2016).
This study aimed to evaluate the prevalence of joint manifestations, limitations in daily life experienced due to pain and joint problems and general quality of life reported by Fabry patients compared to age-matching control healthy subjects.
Methodology
Patients
Patients with Fabry disease known to the Lysosomal Storage Disease Unit (LSDU) at the Royal Free Hospital, London were invited to participate in a questionnaire-based study approved by the Royal Free Hospital Research Ethics Committee. A control group of healthy volunteers matched by age and sex was also enrolled. Period of recruitment and data collection comprised between 2010 and 2012.
For Fabry patients, clinical data was obtained by retrospective case notes review performed by the Royal Free LSDU care team, to maintain patients’ confidentiality. Clinical data comprised:
Confirmation of Fabry diagnosis based on enzyme activity and mutation
Multi-organ manifestations of Fabry disease based on Mainz Severity Score Index (MSSI) (Whybra et al. 2004)
ERT administration
Study Design
The “Joint Pain Questionnaire” included ten general questions on presence of musculoskeletal problems, as well as parts of established and validated questionnaires, i.e. Euro Quality of Life 5D (EQ5D), Brief Pain Inventory (BPI), Patient’s assessment of physical function (HAQ-DI), SF-36 Health Survey and McGregor’s questionnaire. It was designed to investigate and determine whether there is a prevalence of joints involvement in patients with Fabry as well as to evaluate the impact of joint problems on quality of life, pain and ability to look after themselves for patients with Fabry in comparison to the control group.
Statistical Analysis
Data were analysed using SPSS statistical software, version 20 (IBM Company, Armonk, NY). Descriptive statistical analysis was used to evaluate the prevalence of joint disease in Fabry patients. Socio-demographic characteristics were tabulated (age, sex). Distribution of joint disease scores were tabulated or shown graphically. Mann Whitney tests were used for comparisons of continuous variables and two-tailed Fisher’s exact tests for categorical variables. A P-value of 0.05 or less was considered statistically significant. The relationship between the fatigue score values and joint scores was analysed with Spearman rank correlation analysis.
Results
Demographics and Disease Characteristics
Seventy-seven of 135 Fabry patients known to the LSDU at the Royal Free Hospital who had been invited to participate returned the surveys, of which 11 patients (14%) did not complete the questionnaire but reported that they were not experiencing any joint problems. These 11 patients were included in the evaluation, with exception of quality of life part, assuming they have had no musculoskeletal problems. Additionally, age- and sex-matching control group of 77 subjects was enrolled.
Fabry patients had median age of 54 years with min-max of 18 and 83 years, 49 (64%) of them were female and 28 (36%) were male. Control subjects (the same number of male and female subjects) had median age of 52 with min-max of 25 and 79 years. Female patients with Fabry disease had a median MSSI score of 16 (min-max range: 1–41) with 67% receiving the ERT. Male patients had a median MSSI score of 28 (min-max range: 14–45); and the majority (96%) received ERT. Eleven Fabry patients who said they did not have joint problems and did not complete the survey presented with similar disease characteristics and demographic data as the remaining patients with median MSSI score of 23 (range: 6–41) for female and 33 (range: 30–34) for male patients. Demographic characteristics of the evaluated Fabry patients and control subjects are summarized in Table S1.
Joints Manifestations History
Both male and female Fabry patients reported joint problems with similar patterns of responses to single questions (see Table 1). Male and female Fabry patients reported current joint pain and swelling experienced at the time of questionnaire completion (43% and 39%, respectively). However, female patients reported slightly more joint pain in the past lasting more than 4 continuous weeks (45% vs. 29%) and morning stiffness (31% vs. 18%). 20% of female and 7% of male Fabry patients had already been given a diagnosis of arthritis. Although the overall prevalence of joint problems and pain reported in questionnaires appears higher in patients with Fabry disease than in control subjects, the differences reached statistical significance only for joint pain in the past and ongoing morning stiffness in female Fabry patients compared to control subjects (p < 0.05).
Table 1.
Joint manifestations questionnaire
| Question | Answers with “Yes”, n (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Male < 50 years | Male ≥ 50 years | Female | Female < 50 years | Female ≥ 50 years | |||||||||||||
| F | C | F | C | F | C | F | C | F | C | F | C | |||||||
| Total number of subjects | 28 | 28 | pa | 10 | 12 | pa | 18 | 16 | pa | 49 | 49 | pa | 24 | 24 | pa | 25 | 25 | pa |
| Have you ever experienced any joint swelling lasting more than four continuous weeks? | 6 21% |
4 14% |
– | 2 20% |
1 8.3% |
– | 4 22% |
3 19% |
– | 11 23% |
8 16% |
– | 3 13% |
0 | – | 8 32% |
8 32% |
– |
| Have you ever experienced any joint pain lasting more than four continuous weeks? | 8 29% |
4 14% |
– | 2 20% |
0 | 0.20 | 6 33% |
4 25% |
0.72 | 22 45% |
7 14% |
0.02 | 9 36% |
2 8.3% |
0.04 | 13 52% |
5 20% |
0.04 |
| Do you currently have any joint pain or swelling? | 12 43% |
7 25% |
– | 4 40% |
0 | 0.03 | 8 44% |
7 44% |
– | 19 39% |
16 33% |
– | 6 25% |
2 8.3% |
– | 13 52% |
14 56% |
– |
| Do you currently have any morning stiffness lasting more than 30 min? | 5 18% |
3 11% |
– | 0 | 0 | – | 5 28% |
3 19% |
0.69 | 15 31% |
5 10% |
0.02 | 5 21% |
0 | 0.05 | 10 40% |
5 20% |
– |
| Have you been told in the past that you had arthritis? | 2 7.1% |
5 18% |
– | 0 | 0 | 2 11% |
5 31% |
0.21 | 10 20% |
9 18% |
– | 3 13% |
0 | – | 7 28% |
9 36% |
– | |
| Have you been seen by a doctor in the past for any joint problem? | 8 29% |
9 32% |
– | 2 20% |
1 8.3% |
– | 6 33% |
8 50% |
0.49 | 18 37% |
24 49% |
– | 8 33% |
7 29% |
– | 10 40% |
17 68% |
0.09 |
| Have you ever had a bone fracture or joint injury? | 11 39% |
12 43% |
– | 3 30% |
3 25% |
– | 8 44% |
9 56% |
0.73 | 14 29% |
16 33% |
– | 6 25% |
6 25% |
– | 8 32% |
10 40% |
– |
| Have you ever been told that you have osteoporosis? | 0 | 0 | – | 0 | – | 0 | 0 | – | 1 2.0% |
4 8.2% |
– | 1 4.2% |
0 | – | 0 | 4 16% |
– | |
| Do you have immobility of any joint? | 3 11% |
1 3.6% |
– | 0 | 0 | – | 3 17% |
1 6.3% |
0.61 | 6 12% |
3 6.1% |
– | 1 4.2% |
0 | – | 5 20% |
3 12% |
– |
| Do your symptoms get worse with exercise? | 8 29% |
7 25% |
– | 3 30% |
0 | 0.08 | 5 28% |
7 44% |
0.48 | 18 37% |
12 25% |
– | 8 33% |
2 8.3% |
0.07 | 10 40% |
10 40% |
– |
Note: C control subjects, F Fabry patients
Bold indicates statistical significance
a2-Tailed Fisher’s exact test
When divided into two age groups (with a cut-off of 50 years) female Fabry patients who were younger than the median age of 50 years (n = 24) showed higher prevalence of joint problems in Fabry patients than age-matched healthy controls. Pain in the past (p < 0.05) and morning stiffness (p = 0.05) were higher than in age-matched control group and a trend toward higher prevalence of symptoms worsening with exercise was observed. Male Fabry patients younger than 50 (n = 10) had significantly higher prevalence of current joint pain or swelling and showed a trend toward higher prevalence of symptoms worsening with exercise. Fabry patients of both genders aged 50 years and older reported similar patterns of joint problems to control subjects with the exception of higher pain in the past in female Fabry patients (Table 1).
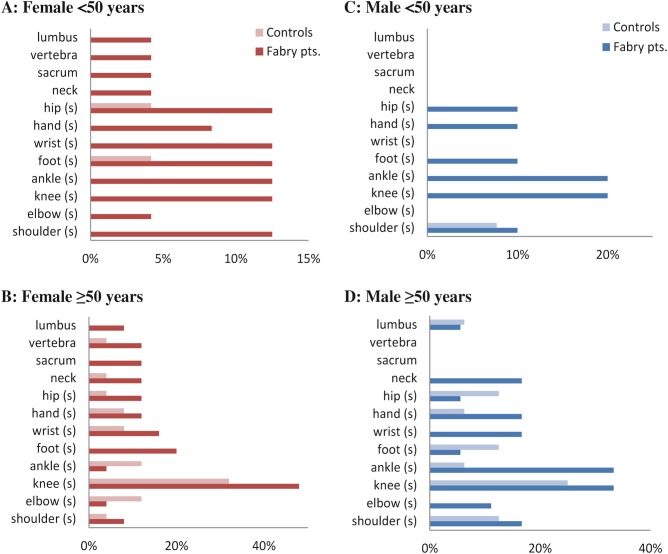
Study participants were asked to mark their current joint swelling or pain location, if any, on a body diagram. Male and female Fabry patients under 50 years reported more areas of pain or swelling on the diagram compared with healthy controls. Younger than 50 male patients experienced pain or swelling in extremities (ankle, foot, hand, and hip), whereas female patients reported varied sites including shoulder, elbow, knee, and vertebra (see Fig. 1a, c). For subjects of 50 years and older, no obvious differences in proportion reporting pain or swelling between control and Fabry group were observed (see Fig. 1b, d).
Fig. 1.
Joint swelling or pain per area in percent of subjects. (a) Female <50 years, (b) Female ≥50 years, (c) Male <50 years, (d) Male ≥50 years
A joint score consisting of a sum of the positive answers plus number of sites of pain was calculated for Fabry patients who completed the survey on joint problems. The median joint score was 3 in female and male Fabry patients. In the group younger than 50 years, the median joint score was 2 (range 0–7) and 1 (range 0–13) for male and female Fabry patients, respectively. In Fabry patients of 50 years and older, the median joint score was 4 (range 0–13) and 7 (range 0–17) for male and female, respectively.
Quality of Life
Quality of life was evaluated in 66 Fabry patients who completed the survey on joint problems and 77 healthy control subjects.
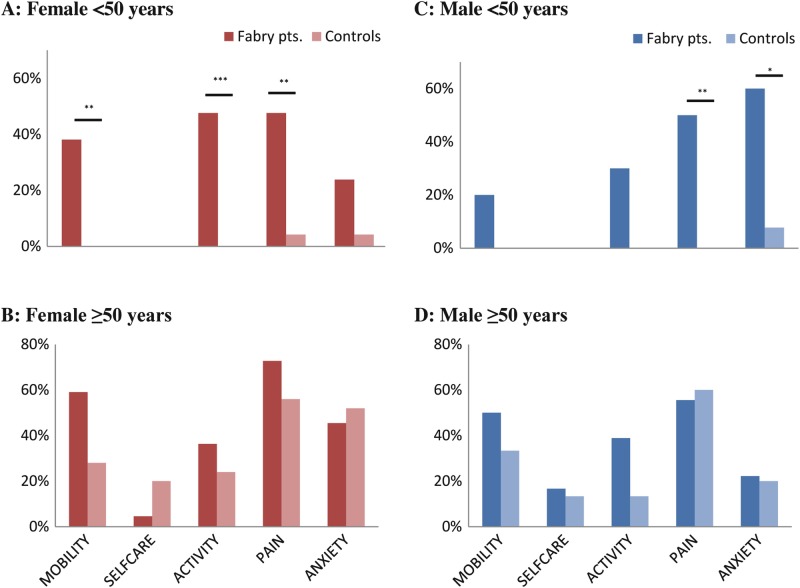
More difficulties in mobility, activity, pain, and anxiety were reported in male and female Fabry patients younger than 50 years compared to age-matched healthy controls (see Fig. 2a, c). No apparent differences in quality of life were observed in the patients of 50 years and older (see Fig. 2b, d) compared to age-matched healthy controls. Self-care problems were reported only by a small percent of patients and control subjects.
Fig. 2.
Quality of life: percent of Fabry patients and control subjects reporting problems. *p < 0.05, **p < 0.01, ***p < 0.001 Fisher’s exact test. (a) Female <50 years, (b) Female ≥50 years, (c) Male <50 years, (d) Male ≥50 years
The female Fabry patients had significantly higher mean fatigue scores compared to control female subjects (4.9 vs. 2.8, p < 0.001). In male Fabry patients, the mean fatigue value was slightly higher (4.2 vs. 2.8, p = 0.056) than in controls. The scatterplots of fatigue scores versus age are shown in Fig. S1. Fatigue scores marginally increased with age in female Fabry patients and remaining almost the same in male Fabry patients. In contrast, the fatigue scores increased continuously with age in male and female control subjects.
Statistical analysis showed greater correlation of fatigue scores with joint scores in Fabry patients (r = 0.471, p < 0.001) than in control subjects (r = 0.266, p = 0.019).
Assessment of Physical Function
Fabry patients and healthy controls were asked how their pain affects their activities of daily life. Sixty percent (60%) of male Fabry patients younger than 50 years reported problems in vigorous activities compared to 8% of age-matching healthy subjects. Limitations in other activities were reported by 10–30% of younger male patients, which was higher than in healthy controls. No differences were observed between patients of 50 years and older and their healthy controls (Table 2).
Table 2.
Limitations through pain in daily life
| Subjects reporting problems (n, %) | Male < 50 years | Male ≥ 50 years | Female < 50 years | Female ≥ 50 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | C | pa | F | C | pa | F | C | pa | F | C | pa | |
| N | 10 | 12 | 18 | 16 | 24 | 23 | 25 | 25 | ||||
| Vigorous activities | 6 60% |
1 8.3% |
0.02 | 8 44% |
13 75% |
0.04 | 10 42% |
1 4.4% |
0.004 | 16 64% |
10 40% |
– |
| Moderate activities | 2 20% |
0 | – | 6 33% |
6 38% |
– | 8 33% |
0 | 0.004 | 12 48% |
9 36% |
– |
| Lifting or carrying groceries | 2 20% |
0 | – | 5 28% |
5 31% |
– | 7 29% |
0 | 0.009 | 10 40% |
8 32% |
– |
| Climbing several flights of stairs | 2 20% |
0 | – | 8 44% |
8 50% |
– | 9 38% |
0 | 0.002 | 15 60% |
12 48% |
– |
| Climbing one flights of stairs | 1 10% |
0 | – | 7 39% |
5 31% |
– | 3 13% |
0 | – | 10 40% |
8 32% |
– |
| Bending, kneeling, or stooping | 3 30% |
0 | 0.08 | 10 56% |
10 56% |
– | 8 33% |
0 | 0.004 | 14 56% |
11 44% |
– |
| Walking more than a mile | 3 30% |
0 | 0.08 | 9 50% |
9 50% |
– | 9 38% |
0 | 0.002 | 15 60% |
9 36% |
– |
| Walking several blocks | 2 20% |
0 | – | 7 39% |
4 25% |
– | 5 21% |
0 | 0.05 | 14 56% |
7 28% |
0.09 |
| Walking one block | 1 10% |
0 | 7 39% |
3 19% |
– | 3 13% |
0 | – | 11 44% |
4 16% |
0.06 | |
| Bathing or dressing yourself | 0 | 0 | – | 4 22% |
2 13% |
– | 0 | 0 | – | 7 28% |
5 20% |
– |
Note: C control subjects, F Fabry patients
Bold indicates statistical significance
a2-Tailed Fisher’s exact test
In female Fabry patients younger than 50 years, 42% reported problems with vigorous activities and other daily activities such as moderate activities (33%), climbing of several flights of stairs (38%), walking more than a mile (38%) were reported and were apparently higher than in healthy subjects (Table 2). As with males, almost no differences were observed between patients of 50 years and older and their healthy controls.
Despite limitation in physical activities few Fabry patients needed help from other people to perform daily tasks, other than gripping and opening (7.7% male and 23% female patients), and errands and chores (7.7% male and 17% female patients). Female Fabry patients needed help from others more often than male patients. Both female and male Fabry patients showed apparent differences to healthy subjects with regard to help from other people (Table S3).
Patients were questioned about their ability to grip different objects demonstrating difficulties in opening new milk carton (11.5% male and 20.8% female patients compared to 3.9% and 6.4% in male and female healthy subjects, respectively). Few difficulties in other activities such as opening a car door, opening previously opened jars, or turning faucets on and off were reported (Table S3).
Discussion
This study is the first systematic survey focusing on joint manifestations and their impact on daily life in patients with Fabry disease.
Both male and female Fabry patients reported high numbers of joint problems currently and in the past with pain being the most common symptom. In particular, Fabry patients younger than 50 years reported suffering significantly more from joint problems compared to age-matched control subjects.
The number and severity of Fabry crises tend to decrease during adulthood (Paira et al. 1992; Mehta et al. 2004). Patients diagnosed with Fabry disease at a younger age are more likely to be more severe and possibly more symptomatic from joint manifestations whereas those patients surviving beyond 50 years are more likely to have a milder later onset phenotype.
The clinical manifestations of Fabry disease are more variable among female patients than among male patients (Whybra et al. 2004; Mehta et al. 2004). In this study, male Fabry patients reported mostly pain in extremities whereas female patients had pain in extremities and various further sites including neck, sacrum, vertebra, and lumbar region.
In this study, 37% female and 29% male patients had been seen by a doctor for joint problems and 20% and 7% of female and male patients, respectively, had been given a diagnosis of rheumatoid arthritis. However, we do not know in which cases there had been a misinterpretation of Fabry symptoms. This may be because initial symptoms such as burning pain can be accompanied by fever and an elevation of the erythrocyte sedimentation rate (Paira et al. 1992). Additionally, Fabry manifestations are generally poorly recognized and awareness of appropriate diagnostic tests is low, as demonstrated by survey among 360 rheumatologists and paediatricians clinically managing patients with rheumatologic conditions (Cimaz et al. 2011).
Previous studies showed significant decrease in bone mineral density in Fabry patients before the start of ERT (Mersebach et al. 2007). In this study, only one female patient was reported to have osteoporosis; however, dual-energy X-ray absorptiometry (DXA) scans have not been systematically performed.
Recurring pain and exercise intolerance limit the activity of symptomatic patients with AFD to a sedentary lifestyle (Kolodny and Pastores 2002). Having little confidence in medical science some Fabry patients become withdrawn and fatalistic (Kolodny and Pastores 2002). With increasing disability and awareness of the natural history of the condition, the unpredictability and difficulty of controlling the pain, patients are at risk for depression (Cole et al. 2007). The prevalence of depression in Fabry patients is found to be approximately 43%, a rate higher than that of the general population (Cole et al. 2007). There are Fabry cases documenting dependence on narcotics taken for neuropathic pain (Kolodny and Pastores 2002). Major depression has also been reported in female Fabry carriers (Sadek et al. 2004).
Nephropathy is common manifestation of Fabry disease and is characterized by proteinuria and development of structural changes including glomerular sclerosis, tubular atrophy, and interstitial fibrosis (Wilcox et al. 2008). Standard medical management of joint disease with aggressive use of nonsteroidal anti-inflammatory drugs and methotrexate could cause potential renal side effects and lead to additional organ damage and it is, therefore, important that rheumatologists are aware of the potential diagnosis.
A recent multicenter study showed that quality of life in patients with AFD is related to phenotype, age, pain, and disease severity with classically affected patients of older age having more severe disease and thus a decreased quality of life (Arends et al. 2017). However, the study by Arends et al. did not include an age-matched control group and did not specifically comment on joint pain making a direct comparison to results of our study difficult.
In this study, quality of life in terms of mobility, activity, pain, and anxiety was decreased in Fabry patients younger than 50 years, but not in older group, compared to healthy controls. Female Fabry patients had higher fatigue values, compared to healthy controls and male Fabry patients.
Fabry patients younger than 50 years reported problems with vigorous activities as well as other daily activities such as moderate physical activity, climbing of several flights of stairs, and walking more than in healthy subjects. Despite limitation in physical activities not many Fabry patients required help from other people to perform their daily routine, mostly with gripping and opening things.
The relatively small sample of Fabry patients, especially males in this study might be seen as a limitation; however, it should be highlighted that for a rare disorder such as Fabry disease this is a significant number of subjects taking part in a questionnaire study.
Other limitations might be difficulties in distinguishing the origin of musculoskeletal versus neuropathic pain. In our research, we have made efforts to obtain specific information about joint pain. The subjects were asked to indicate location of their joint pain on the body graph. Areas characterized by subjects as main locations of musculoskeletal pain are not usually associated with acroparasthesia (larger joints, i.e. knee, hip, wrist etc., see Fig. 1). Part of a established and validated rheumatologic HAQ-DI questionnaire was used to assess grip.
Although swelling can be a manifestation of lymphedema (Amann-Vesti et al. 2003) and not just a local inflammatory response, only one patient with Fabry disease had lymphedema in our study.
Another limitation was the lack of comparison of weight or body-mass index between Fabry patients and healthy controls. Nevertheless, we can report that the Fabry patients have the mean weight of 83.4 kg for male and 68 kg for female, comparable to statistical data on the weight of the UK population collected between 2010 and 2015 with the mean weight of 83.9–85.0 kg in men and 70.7–71.3 kg in women (NHS Digital 2015).
The strengths include the age and gender matched control population. The results do provide evidence to draw attention of multidisciplinary medical practitioners to the topic of musculoskeletal manifestations in Fabry disease and to encourage further research in this matter.
Additional research is required to investigate the underlying pathophysiology mechanisms of joint symptoms in Fabry disease.
Conclusion
By recognizing the early signs and symptoms the multidisciplinary medical practitioners including rheumatologists could have an opportunity to diagnose Fabry disease on early stages of the condition, resulting in more favourable therapeutic outcomes with ERT. Presentation of musculoskeletal symptoms, in conjunction with a family history of early renal or cardiac disease, should alert to the possibility of Fabry disease. In addition physicians treating patients with Fabry disease should be alerted to the joint manifestation and work with rheumatology and orthopaedic colleagues to improve symptom control.
Electronic Supplementary Material
Scatterplots of fatigue scores for Fabry patients and healthy controls. Upper graph displays data on females, lower graph on males (TIFF 114 kb)
Response to Reveiwers Comments R2 (DOCX 17 kb)
Acknowledgments
We are thankful to the patients and control subjects for participation in the survey. The authors acknowledge Royal Free Hospital Research Nurses Capt. Alan Milligan and Mrs. Linda Richfield for their help in patients study enrolment and work with patient records; as well as Mr. Andrew Burgess for critical review on statistical evaluations.
Compliance with Ethics Guidelines
Conflict of Interest
Alexandra Ivleva, Ekaterina Weith, Atul Mehta, and Derralynn A. Hughes declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Royal Free Hospital Research Ethics Committee) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
The following approach to Informed Consent process was reviewed and approved by the Royal Free Hospital Research Ethics Committee and applied in this study.
Research packs were sent via post to each patient on the Fabry Registry of the LSDU at the Royal Free Hospital and also provided to the subjects of the control group. The pack included an invitation letter, a full information sheet, and the “Joint Pain Questionnaire”. The potential participant had the right to refuse to take part in the research without jeopardizing their future treatment. Those who did not wish to participate were encouraged to tick “no” to the consent question on the invitation letter and return it in the prepaid envelope or in person to the investigator.
A fully detailed information sheet, approved by the EC provided to potential participants was aimed to help the individual in deciding whether or not to participate. Potential participants were given the opportunity to discuss any concerns and clarify any queries regarding the study either over the phone or face to face with their site before completing the study documents.
It was anticipated that all eligible subjects would complete the study questionnaire administration (for both AFD and control group).
Explicit written informed consent was not collected for the study. Rather, implied informed consent was collected by the receipt of the completed questionnaire.
Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Details of the Contributions of Individual Authors
Alexandra Ivleva: Planning; Creation of the study documents (protocol, research information sheet, study questionnaire); Ethics Committee submissions and communications; Study conduct & coordination; Data collection and analysis; Creation of the first draft of the manuscript, Revision of the manuscript.
Ekaterina Weith: Data evaluation, Statistical analysis, Writing and revision of the manuscript.
Atul Mehta: Planning and coordination; Writing of the manuscript.
Derralynn A. Hughes: Principal Investigator of the study, Planning and coordination, Review of the study documents; Data analysis; Work with confidential patients’ records; Writing and revision of the manuscript.
Competing Interests
No interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Derralynn A. Hughes, Email: rmgvdah@ucl.ac.uk
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Amann-Vesti BR, Gitzelmann G, Widmer U, et al. Severe lymphatic microangiopathy in Fabry disease. Lymphat Res Biol. 2003;1(3):185–189. doi: 10.1089/153968503768330229. [DOI] [PubMed] [Google Scholar]
- Arends M, Körver S, Hughes DA et al (2017) Phenotype, disease severity and pain are major determinants of quality of life in Fabry disease: results from a large multicenter cohort study. J Inherit Metab Dis. 10.1007/s10545-017-0095-6. Epub ahead of print [DOI] [PMC free article] [PubMed]
- Banikazemi M, Bultas J, Waldek S, et al. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146:77–86. doi: 10.7326/0003-4819-146-2-200701160-00148. [DOI] [PubMed] [Google Scholar]
- Brady RO, Gal AE, Bradley RM, et al. Enzymatic defect in Fabry’s disease: ceramide-trihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- Cimaz R, Guillaume S, Hilz MJ. Awareness of Fabry disease among rheumatologists – current status and perspectives. Clin Rheumatol. 2011;30:467–475. doi: 10.1007/s10067-010-1445-z. [DOI] [PubMed] [Google Scholar]
- Cole AL, Lee PJ, Hughes DA, et al. Depression in adults with Fabry disease: a common and under-diagnosed problem. J Inherit Metab Dis. 2007;30(6):943–951. doi: 10.1007/s10545-007-0708-6. [DOI] [PubMed] [Google Scholar]
- Colombi A, Kostyal A, Bracher R, et al. Angiokeratoma corporis diffusum – Fabry’s disease. Helv Med Acta. 1967;34:67–83. [PubMed] [Google Scholar]
- Deegan PB, Baehner AF, Barba Romero MA, et al. Natural history of Fabry disease in females in the Fabry outcome survey. J Med Genet. 2006;43:347–352. doi: 10.1136/jmg.2005.036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnick RJ, Ioannou YA, Eng CM (2001) α-Galactosidase a deficiency: Fabry disease. In: Scriver CR (ed) The metabolic and molecular bases of inherited disease. McGraw Hill, New York, NY, pp 3733–3774
- Germain DP, Waldek S, Banikazemi M, et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol. 2007;18:1547–1557. doi: 10.1681/ASN.2006080816. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ries M, Kotsopoulos S, et al. The relationship of vascular glycolipid storage to clinical manifestations of Fabry disease: a cross-sectional study of a large cohort of clinically affected heterozygous women. Medicine (Baltimore) 2005;84:261–268. doi: 10.1097/01.md.0000178976.62537.6b. [DOI] [PubMed] [Google Scholar]
- Hopkin RJ, Bissler J, Banikazemi M, et al. Characterization of Fabry disease in 352 pediatric patients in the Fabry Registry. Pediatr Res. 2008;64:550–555. doi: 10.1203/PDR.0b013e318183f132. [DOI] [PubMed] [Google Scholar]
- Kolodny EH, Pastores GM. Anderson-Fabry disease: extrarenal, neurologic manifestations. J Am Soc Nephrol. 2002;13(Suppl 2):S150–S153. [PubMed] [Google Scholar]
- Lidove O, Zeller V, Chicheportiche V, et al. Musculoskeletal manifestations of Fabry disease: a retrospective study. Joint Bone Spine. 2016;83(4):421–426. doi: 10.1016/j.jbspin.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Lien YH, Lai LW. Bilateral femoral head and distal tibial osteonecrosis in a patient with Fabry disease. Am J Orthop (Belle Mead NJ) 2005;34(4):192–194. [PubMed] [Google Scholar]
- MacDermot KD, Holmes A, Miners AH. Anderson–Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38:769–775. doi: 10.1136/jmg.38.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger B, Mengel E, Schaefer RM. Rheumatologic aspects of lysosomal storage diseases. Clin Rheumatol. 2007;26:335–341. doi: 10.1007/s10067-006-0299-x. [DOI] [PubMed] [Google Scholar]
- Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry outcome survey. Eur J Clin Investig. 2004;34(3):236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- Mersebach H, Johansson JO, Rasmussen AK, et al. Osteopenia: a common aspect of Fabry disease. Predictors of bone mineral density. Genet Med. 2007;9(12):812–818. doi: 10.1097/GIM.0b013e31815cb197. [DOI] [PubMed] [Google Scholar]
- Morgan SH, Crawfurd MA. Anderson-Fabry disease. BMJ. 1988;297:872–873. doi: 10.1136/bmj.297.6653.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- NHS Digital (2015) National Statistics. Health Survey for England, 2014. http://content.digital.nhs.uk/catalogue/PUB19295
- Paira SO, Roverano S, Iribas JL, et al. Joint manifestations of Fabry’s disease. Clin Rheumatol. 1992;11(4):562–565. doi: 10.1007/BF02283120. [DOI] [PubMed] [Google Scholar]
- Ross G, Kuwamura F, Goral A. Association of Fabry’s disease with femoral head avascular necrosis. Orthopedics. 1993;16(4):471–473. doi: 10.3928/0147-7447-19930401-12. [DOI] [PubMed] [Google Scholar]
- Sadek J, Shellhaas R, Camfield CS, et al. Psychiatric findings in four female carriers of Fabry disease. Psychiatr Genet. 2004;14(4):199–201. doi: 10.1097/00041444-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Askari H, Timmons M, et al. Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol. 2007;18:1576–1583. doi: 10.1681/ASN.2006111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley ED, Shelley WB, Kurczynski TW. Painful fingers, heat intolerance, and telangiectases of the ear: easily ignored childhood signs of Fabry disease. Pediatr Dermatol. 1995;12:215–219. doi: 10.1111/j.1525-1470.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Sims K, Politei J, Banikazemi M, et al. Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events. Natural history data from the Fabry Registry. Stroke. 2009;40:788–794. doi: 10.1161/STROKEAHA.108.526293. [DOI] [PubMed] [Google Scholar]
- von Scheidt W, Eng CM, Fitzmaurice TF, et al. An atypical variant of Fabry’s disease with manifestations confined to the myocardium. N Engl J Med. 1991;324:395–399. doi: 10.1056/NEJM199102073240607. [DOI] [PubMed] [Google Scholar]
- Wang RY, Lelis A, Mirocha J, et al. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med. 2007;9:34–45. doi: 10.1097/GIM.0b013e31802d8321. [DOI] [PubMed] [Google Scholar]
- Weidemann F, Niemann M, Breunig F, et al. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;119:524–529. doi: 10.1161/CIRCULATIONAHA.108.794529. [DOI] [PubMed] [Google Scholar]
- Whybra C, Kampmann C, Krummenauer F, et al. The Mainz severity score index: a new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet. 2004;65:299–307. doi: 10.1111/j.1399-0004.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Yoshitama T, Nakao S, Takenaka T, et al. Molecular genetic, biochemical, and clinical studies in three families with cardiac Fabry’s disease. Am J Cardiol. 2001;87:71–75. doi: 10.1016/S0002-9149(00)01275-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplots of fatigue scores for Fabry patients and healthy controls. Upper graph displays data on females, lower graph on males (TIFF 114 kb)
Response to Reveiwers Comments R2 (DOCX 17 kb)