Abstract
Acid sphingomyelinase deficiency (ASMD) is a rare, progressive, and often fatal lysosomal storage disease caused by the deficiency of the enzyme acid sphingomyelinase (ASM) resulting in accumulation of sphingomyelin in target tissues. Little is known regarding predictors of disease-related morbidity, healthcare use, and lifestyle impact in adults with chronic disease. A multinational retrospective study collected data on the burden of illness and healthcare resource use for 100 patients across the clinical spectrum of ASMD, including those with rapidly progressive infantile neurovisceral disease (n = 13) and those with the more slowly progressive chronic neurovisceral (n = 6) and chronic visceral (n = 81) disease. Growth was subnormal throughout childhood for all patients with chronic neurovisceral disease and for 50% of patients with chronic visceral disease. Developmental delay, regression, and/or learning disabilities were reported in 40% of patients with chronic neurovisceral ASMD and 21% of patients with chronic visceral ASMD. Outpatient therapy or home healthcare was required for 50% of patients with chronic neurovisceral disease and 12% of patients with chronic visceral disease. Disease-related disability for patients with chronic disease resulted in need for home schooling for 16% of patients and compromised work ability for 22% of patients. Grade school was the highest level of education for 22% of patients older than 13 years of age.
Keywords: Acid sphingomyelinase deficiency, Burden of illness, Disease manifestations, Lysosomal storage disorder, Natural history, Niemann-Pick disease types A and B
Introduction
Acid sphingomyelinase deficiency (ASMD), historically referred to as Niemann-Pick disease (NPD) types A and B, is a rare autosomal recessive lysosomal storage disorder with a clinical spectrum of presentation and outcomes. ASMD is pan-ethnic, and estimates of birth prevalence in different countries range from 0.4 to 0.6 cases per 100,000 live births (Kingma et al. 2015). ASMD results from mutations in the sphingomyelin phosphodiesterase 1 (SMPD1) gene (Schuchman and Desnick 2013) encoding the lysosomal enzyme acid sphingomyelinase (ASM). ASM catalyzes the hydrolysis of sphingomyelin to ceramide and phosphocholine, and deficiency of the enzyme results in progressive accumulation of sphingomyelin and other lipids mainly within macrophages of reticular connective tissue, hepatocytes, and, in severe cases, neurons.
Clinical presentations vary (Schuchman and Wasserstein 2015). Rapidly progressing infantile neurovisceral disease (NPD type A) is the most severe form and is characterized by neurodegeneration, systemic involvement (hepatosplenomegaly, bleeding, respiratory disease), failure to thrive, and death by 3 years of age. Chronic neurovisceral (intermediate form, NPD A/B or NPD B variant) ASMD is characterized by slower progression of neurological degeneration and prolonged survival that distinguishes it from the infantile form. Chronic visceral ASMD (NPD type B) is characterized by variable onset ranging from infancy to adulthood and slowly progressive multisystem manifestations without neurodegeneration. Common clinical features of both chronic visceral and chronic neurovisceral ASMD include delayed growth and start of puberty, hepatosplenomegaly, pro-atherogenic lipid profile, thrombocytopenia, infiltrative lung disease, and liver disease (Schuchman and Wasserstein 2015). Patients may have a normal lifespan or die prematurely from ASMD complications, including respiratory insufficiency and liver disease (Cassiman et al. 2016).
Currently, supportive care is the mainstay of ASMD management, although enzyme replacement therapy (olipudase alfa) has entered late-stage clinical development (Phase 2/3 study NCT02004691) (Wasserstein et al. 2015). Effective disease management depends upon an understanding of the natural history of ASMD and the burden of illness for patients across the ASMD disease spectrum. Several prospective and retrospective studies investigating the natural history of ASMD (Wasserstein et al. 2004; McGovern et al. 2006, 2008, 2013; Hollak et al. 2012; Cassiman et al. 2016) have demonstrated similar findings regarding ages at symptom onset, disease-related morbidities, and causes of death in patients with infantile and chronic ASMD phenotypes (see Table 1). However, less is known regarding predictors of disease-related morbidity, healthcare utilization, and lifestyle impact of chronic ASMD disease.
Table 1.
Study design and major findings in ASMD natural history studies
| Parameter | Current study | McGovern et al. (2006) | McGovern et al. (2008) | McGovern et al. (2013) | Wasserstein et al. (2004) | Hollak et al. (2012) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | N = 100 | N = 10 | N = 59 | N = 103 | N = 29 | N = 25 | ||||||
| Phenotype; number of patients (n); median age at diagnosis | ||||||||||||
| Infantile neurovisceral | n = 13 | 6 months | n = 10 | 6 months | 0 | 0 | 0 | n = 4 | 6 months | |||
| Chronic neurovisceral | n = 6 | 8.9 years | 0 | 0 | n = 8 | 0 | n = 6 | 3 years | ||||
| Chronic visceral | n = 81 | 9.3 years | 0 | n = 59 | 9.8 years | n = 95 | 8.1 years | n = 29 | NA | n = 15 | 32 years | |
| Type of study | Retrospective chart review | Case series | Prospective cross-sectional survey | Retrospective chart review | 10-year longitudinal study | Retrospective chart review with prospective follow-up of chronic disease | ||||||
| Study centers | Brazil (n = 46), Canada (n = 13), United States (n = 41) |
United States | Brazil (n = 13), France (n = 7), Germany (n = 5), Italy (n = 8), United States (n = 26) |
United States | United States | The Netherlands and Belgium | ||||||
| Mortality | Infantile neurovisceral: ten deaths Chronic visceral: two deaths |
Ten deaths (100%) | Chronic neurovisceral: 7 deaths Chronic visceral: 11 deaths |
Three deaths | Infantile neurovisceral: four deaths Chronic ASMD: five deaths |
|||||||
| Causes of death | Respiratory failure (n = 3), pneumonia (n = 3), lung disorder (n = 1), hepatic failure (n = 2), unknown (n = 3) | Respiratory failure (n = 9) and complications from bleeding (n = 1) | Pneumonia (n = 5), liver failure (n = 3), hemorrhage (n = 3), other (n = 7) | One each due to traumatic subdural hematoma, liver failure, failed bone marrow transplant complications | Pulmonary disease (n = 4), progressive neurological symptoms (n = 1), malignant edema with subdural hematoma (n = 1), malignancy (n = 1), unknown cause (n = 2) | |||||||
| Major morbidities | HS, GI disorders, respiratory disorders, infections | HS, GI symptoms, respiratory symptoms | HS, respiratory infections, ILD, bleeding | HS, TCP, bleeding, ILD, liver disease | HS, TCP, atherogenic lipid profile, pulmonary disease | HS, ILD, TCP | ||||||
| Changes over time in chronic ASMD | Chronic visceral disease: platelet counts decreased over time, WBC decreased, total bilirubin increased, no statistically significant changes in lipid profiles | NA | ND | ND | Progressive hypersplenism, worsening atherogenic profile, gradual deterioration in pulmonary function, decrease in platelet and WBC counts | Gradual decrease in platelet count in some patients with chronic neurovisceral and chronic visceral disease; decreased bone marrow fat fractions in chronic visceral disease | ||||||
| Predictors of major morbidity | No statistically significant predictors for infantile neurovisceral form Earlier age at diagnosis and younger gestational age for patients with chronic disease |
ND | ND | ND | Phenotypic severity correlated with certain genotypes | ND | ||||||
| Resource use and lifestyle limitations | Hospitalization, mobility status, disability status, schooling, work status, med., procedures, medications: see text for results | ND | Diminished QoL in pediatric patients (multiple scores) and adults (general health) | ND | ND | Follow-up of 6 patients with chronic visceral disease: 1/6 able to work full time | ||||||
GI gastrointestinal, HS hepatosplenomegaly, ILD interstitial lung disease, med. medical, NA not available, ND not determined, QoL quality of life, TCP thrombocytopenia, WBC white blood cell
This multinational retrospective study collected data on the burden of illness and healthcare resource use for patients across the clinical spectrum of ASMD. Novel data include healthcare resource use including hospitalizations and surgical procedures, assessment of morbidity predictors, growth pattern across all phenotypes, and lifestyle data including mobility and disability status, school attendance, and work status.
Patients and Methods
Study Design and Patients
This multicenter historical cohort study was conducted at three sites in North and South America (the United States, Brazil, and Canada) between January 2006 and February 2008. Patients diagnosed with ASMD by enzymatic assay or SMPD1 genotype were identified through a systematic review of records spanning 25 years. Invited patients/families were sent a patient package by the site investigator that included study information and consent forms. Upon patient recruitment, patient medical records were requested from diagnostic laboratories, referring physicians, and all treating physicians. The Institutional Review Board or Ethics Committee at each site approved the protocol. All patients participating in the study provided signed ICFs, medical record release forms, treatment history forms, and, in the United States, HIPAA forms.
Medical records of participating patients were abstracted and data collected for the following: demographics, diagnostic history, ASMD phenotype, developmental history (cognitive assessments, pubertal history, and growth), medical history (symptoms, conditions, surgical procedures, hospitalizations and ER visits, outpatient services, respiratory support, ambulation aids, and laboratory test results), and lifestyle history (mobility, school attendance, work status, and disability status).
Data Analysis
Descriptive statistics were calculated for continuous variables and frequencies and percentages for discrete variables. Inferential tests were performed at 5% significance level. Laboratory results were analyzed by time from diagnosis of ASMD (years) overall and by disease type. Patient growth (weight, length/height, and head circumference) for patients 0–18 years of age was summarized and compared with the National Center for Health Statistics/World Health Organization (NCHS/WHO) international reference data. Z-scores were calculated and the number and percentage of patients below normal growth (<5th percentile of the reference population) presented for each age category. Body mass index (BMI) was calculated for patients older than 18 years of age.
Major morbidity was defined as respiratory insufficiency/failure, pneumonia/aspiration, myocardial infarction, congestive heart failure, valvular heart disease, pulmonary hypertension, cirrhosis/liver failure, hemorrhage, neurodegeneration, seizure, or failure to thrive/malnutrition. The Kaplan-Meier method was used to estimate cumulative morbidity rates with corresponding 95% confidence interval (CI) as a function of age. The Cox proportional hazard model was used to identify the predictors of major morbidity for overall patients and by disease type. The predictors included age at time of morbidity, gender, race, age at ASMD diagnosis, birth gestational age, and region.
The mean number of emergency room visits and hospital stays per year was calculated for each patient subsequent to the initial diagnosis of ASMD. Frequencies and percentages were presented for mobility status, disability status, whether patient was ever home schooled for medical reasons, highest grade level achieved, and work status by disease type. For lost ambulation, caregiver assistance required due to disability, work part time due to disability, and work stopped due to disability, the Kaplan-Meier method was used to estimate cumulative rates as a function of age.
Results
Patients
One hundred patients were enrolled, and data abstraction was completed for all. Demographics and disease characteristics are summarized in Table 2. Most patients were from Brazil, 46 (46%), 41 (41%) were from the United States, and 13 (13%) were from Canada. The majority were female (62%), were Caucasian (68%), and were diagnosed with chronic visceral ASMD (81%).
Table 2.
Demographics, patient characteristics, and resource use
| Overall | Disease phenotype | |||
|---|---|---|---|---|
| Infantile neurovisceral | Chronic neurovisceral | Chronic visceral | ||
| N = 100 | N = 13 | N = 6 | N = 81 | |
| Study site, n (%) | ||||
| Brazil United States Canada |
46 (46.0) 41 (41.0) 13 (13.0) |
2 (15.4) 3 (23.1) 8 (61.6) |
1 (16.7) 5 (83.3) 0 (0.0) |
43 (53.1) 33 (40.7) 5 (6.1) |
| Gender, n (%) | ||||
| Male Female |
38 (38.0) 62 (62.0) |
6 (46.2) 7 (53.8) |
3 (50.0) 3 (50.0) |
29 (35.8) 52 (64.2) |
| Race, n (%) | ||||
| Caucasian Black Asian Other |
68 (68.0) 1 (1.0) 5 (5.0) 26 (26.0) |
11 (84.6) 0 (0.0) 0 (0.0) 2 (15.4) |
3 (50.0) 0 (0.0) 1 (16.7) 2 (33.3) |
54 (66.7) 1 (1.2) 4 (4.9) 22 (27.2) |
| Ethnicity, n (%) | ||||
| Hispanic Ashkenazi Jewish Other Not available |
21 (21.0) 2 (2.0) 20 (20.0) 57 (57.0) |
3 (23.1) 0 (0.0) 6 (46.2) 4 (30.8) |
0 (0.0) 1 (16.7) 2 (33.3) 3 (50.0) |
18 (22.2) 1 (1.2) 12 (14.8) 50 (61.7) |
| Age (years) at last visit or death | ||||
| n Mean (SD) Median (min, max) |
12 1.9 (0.5) 1.9 (1.1, 2.6) |
6 22.5 (18.4) 22.5 (1.4, 50.8) |
81 19.8 (14.3) 15.4 (1, 69.6) |
|
| Age distribution [n (%)] at last visit or death | ||||
| n <18 years ≥18 years |
12 12 (100) 0 |
6 2 (33) 4 (67) |
81 46 (57) 35 (43) |
|
| Age (years) at diagnosis | ||||
| n Mean (SD) Median (min, max) |
13 0.52 (0.644) 0.60 (−0.6a, 1.7) |
6 8.87 (16.680) 2.90 (0.4, 42.8) |
77 9.32 (9.420) 6.00 (0.5, 40.6) |
|
| Developmental delay n (%) |
n = 13 12 (92) |
n = 5 2 (40) |
n = 59 12 (21) |
|
| Developmental regression n (%) |
n = 11 7 (63) |
n = 5 2 (40) |
n = 54 1 (2) |
|
| Learning disability n (%) |
n = 1 0 |
n = 5 2 (40) |
n = 55 7 (13) |
|
| Mental retardation n (%) |
n = 6 1 (17) |
n = 6 1 (17) |
n = 66 1 (2) |
|
| Genotypeb n (%) | ||||
| Not available R608del/R608del R608del/H575L R608del/P475L R608del/R474W R608del/R496L R608del/Q21X R608del/fsP330 R608del/fsL269 R608del/R600H Otherc |
11 (84.6) 0 0 0 0 0 0 0 0 0 2 (15.4) |
1 (16.7) 0 0 0 0 0 0 0 0 0 5 (83.3) |
50 (61.7) 1 (1.2) 1 (1.2) 2 (2.5) 1 (1.2) 2 (2.4) 2 (2.4) 1 (1.2) 1 (1.2) 2 (2.4) 18 (22.2) |
|
| Resource use | ||||
| Hospital admissions/year | ||||
| n Mean (SD) Median (min, max) |
10 2.23 (2.17) 1.93 (0, 6.3) |
6 0.42 (0.49) 0.24 (0, 1.3) |
59 0.41 (0.89) 0.13 (0, 6) |
|
| Emergency room visits/year | ||||
| N Mean (SD) Median (min, max) |
10 0.72 (0.94) 0.47 (2.5) |
6 0 0 (0, 2) |
59 0.07 (0.28) 0 (0,0) |
|
| Most commonly reported surgical procedures n (%) | ||||
| Tonsillectomy | 0 | 0 | 12 (15) | |
| Ear tube insertion | 1 (8) | 1 (17) | 8 (10) | |
| Adenoidectomy | 1 (8) | 0 | 8 (10) | |
| Liver biopsy | 1 (8) | 1 (17) | 5 (6) | |
| Cholecystectomy | 0 | 1 (17) | 5 (6) | |
| Splenectomy | 0 | 1 (17) | 5 (6) | |
| Gastrostomy tube insertion | 4 (31) | 0 | 1 (1) | |
| Medication use reported by >50% of patients in any group n (%) | ||||
| At least one medication/therapy | 11 (85) | 4 (67) | 56 (69) | |
| Pain medications | 10 (77) | 3 (50) | 35 (43) | |
| Antibiotics | 11 (85) | 3 (50) | 44 (54) | |
| Respiratory drugs | 8 (62) | 0 (0) | 20 (25) | |
| Outpatient health services n (%) | ||||
| Physical therapy, occupational therapy, speech therapy, respiratory therapy, home healthcare, massage therapy, psychotherapy, dietary/nutritional consultation | 4 (31) | 3 (50) | 10 (12) | |
| Devices n (%) | ||||
| Respiratory support devices | 6 (46) | 0 (0) | 5 (6) | |
| Ambulation aids | 1 (8) | 0 (0) | 5 (6) | |
aIndicates prenatal diagnosis
bBased on GenBank Accession Number M81780.1 reference sequence
cThere was no overlap across the disease phenotypes among the “Other” genotypes
Median age at diagnosis was earliest for patients with infantile neurovisceral disease (mean age 0.6 years) and later for patients with chronic neurovisceral (2.9 years) or chronic visceral (6 years) disease. Almost all patients (98/100) had enzyme assays performed as part of their diagnostic assessments. SMPD1 genotypes were available for 38 patients (Table 2). Missing genotype information was a consequence of several factors, including patients being deceased, patients not enrolled in research studies where genotyping was provided, and lack of funds for genotyping. Genotypes reflect the numbering system used in the GenBank Accession Number M81780.1 reference sequence. Genotypes were highly heterogeneous with no more than two patients sharing the same genotype. Among patients with chronic visceral ASMD, the mild R608del mutation was present in 42% (13/31) of cases. There was no overlap of genotypes between the chronic visceral and the two neurovisceral phenotypes.
Mortality
Overall, 12 patients were deceased prior to data abstraction. Ten patients with infantile neurovisceral ASMD died at a mean age of 2.4 years from lung disorder (n = 1), respiratory failure (n = 2), pneumonia (n = 3), hepatic failure (n = 1), or unknown causes (n = 3). Two patients with chronic visceral ASMD died, one from hepatic failure at 2 years of age and the other from respiratory failure at 42 years of age.
Growth and Development
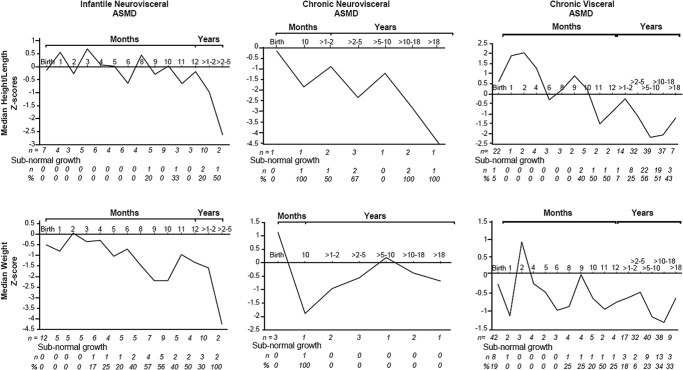
Mean patient Z-scores for weight and height/length decreased over time from birth to 5 years of age across all three phenotypes (Fig. 1). Patients with infantile neurovisceral disease had lower mean weight and height/length Z-scores compared to the chronic disease phenotypes at all time points. Mean (SD) linear growth Z-scores for children 1–2 years of age were −1.33 (1.95), −0.90 (1.39), and −0.23 (0.98) for patients with infantile neurovisceral, chronic neurovisceral, and chronic visceral disease, respectively. Mean linear growth Z-scores for patients between 2 and 5 years were −2.62 (2.69), −1.76 (1.28), and −1.06 (1.13), respectively. Mean head circumference Z-scores remained within normal limits in all groups. Mean BMI remained constant in adult patients with chronic ASMD as a function of age. The numbers and percent of patients with below normal weight and height/length at each time point are also shown in Fig. 1.
Fig. 1.
Z-scores for height/length and weight over time. Z-scores are plotted for the indicated time points across all three ASMD phenotypes. Number of patients (n) and number and percent with subnormal growth (defined as <5th percentile of the reference population based on NCHS/WHO international reference data for weight and height of children) at each time point are indicated below the graphs
Developmental Delay and Learning Disabilities
Developmental delay (92%) and regression (63%) were noted in the majority of patients with infantile neurovisceral disease with available data (Table 2). Developmental delay, developmental regression, and learning disabilities were reported for 40% of patients with chronic neurovisceral ASMD and were less common in patients with chronic visceral ASMD (21%, 2%, and 13%, respectively).
Morbidity and Predictors of Morbidity
The most frequently reported morbidity was hepatosplenomegaly in 92% of patients with infantile neurovisceral disease, 83% of patients with chronic neurovisceral disease, and 80% of patients with chronic visceral disease. Other common morbidities (observed in greater than 75% of patients) were respiratory disorders, infections, and gastrointestinal (GI) disorders. Cumulative morbidity rates increased as a function of age for all three phenotypes, and all patients experienced at least one major disease-related morbidity. Patients with either infantile or chronic neurovisceral ASMD disease had the earliest onset of disease-related morbidities (prior to 1 year of age). Patients with chronic visceral ASMD had later onset, but 50% had major disease-related morbidity by 2 years of age. Statistically significant predictors of overall morbidity in patients with chronic visceral disease included younger age at diagnosis (hazards ratio [HR] 0.954, p = 0.0032) and younger gestational age (HR 0.779, p = 0.044).
Laboratory Evaluations over Time
Laboratory evaluations included hematological and liver assessments. There were no clinically relevant changes over time for any of the assessments, although platelet counts decreased in patients with chronic visceral disease from the year prior to diagnosis to the year of diagnosis (mean change −29.1 × 103/μL, p = 0.0483). Other statistically significant changes for patients with chronic visceral disease included an increase in total bilirubin prior to diagnosis to age 4 years (0.46 mg/dL, p = 0.0020) and decreases in WBC counts prior to diagnosis to age 1 (p = 0.013) and 3 years (p = 0.049) of −1.59 and −2.87 × 103/μL, respectively.
Medical Service Needs
Surgeries, number of hospitalizations and emergency room visits, outpatient resource use, and medication use subsequent to a diagnosis of ASMD are summarized for patients in all groups in Table 2. The annual rate of hospitalization was higher for patients with infantile neurovisceral ASMD (mean of 2.23 hospital admissions/year) compared to patients with chronic neurovisceral disease (0.42/year) or chronic visceral disease (0.41/year). Similarly, emergency room visits per year was greater for patients with infantile neurovisceral ASMD than those with chronic disease.
At least one surgical procedure was performed in 54% (n = 7) of patients with infantile neurovisceral disease, 83% (n = 5) of patients with chronic neurovisceral disease, and 52% (n = 42) of patients with chronic visceral disease. While some surgeries reflected those common to the pediatric population (e.g., tonsillectomy, adenoidectomy, and ear tube insertion), others were ASMD-related (i.e., liver biopsy, cholecystectomy, splenectomy, and gastrostomy tube insertion).
Overall, 71% of patients received at least one medication or therapy. The percentage of patients using at least one medication was highest among patients with infantile neurovisceral disease (85%). Patients with chronic visceral and neurovisceral disease had similar use of at least one medication (67% and 69%, respectively). Medications used by more than 50% in any group included analgesics, antibiotics, and respiratory medications.
Outpatient health resource use (including outpatient therapy, home healthcare, respiratory support devices, and ambulation aids) was greatest in patients with chronic neurovisceral disease (50% of patients), but 31% of patients with infantile neurovisceral disease and 12% of patients with chronic visceral disease required some form of outpatient therapy or home healthcare.
Lifestyle Limitations
Mobility and Assistance Needs
Four patients with infantile neurovisceral disease reached an age where walking could be assessed. Two of these patients were not ambulatory, one patient was ambulatory with an ambulatory device aid, and one patient was ambulatory without assistance. Two patients with infantile neurovisceral disease required caregiver assistance. All patients with chronic visceral disease were ambulatory and did not require caregiver assistance, but as reported in Table 2, ambulatory aids were used by five patients. Five of six patients (83%) with chronic neurovisceral disease were ambulatory, and one patient required caregiver assistance.
School and Work Status
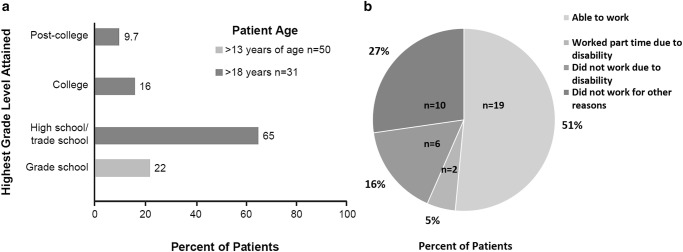
Schooling and work status were not applicable for patients with infantile neurovisceral disease due to their young age or deaths in early childhood. Among patients with chronic visceral disease and information on type of education received (n = 68), 16% (11/68) had received some home schooling. The highest grade level attained was available for 71 patients with chronic ASMD. Forty patients were 18 years of age or younger and 31 patients were older than 18 years of age. Fifty patients were older than age 13 and were eligible to have completed grade school. The highest grade levels by age distribution are shown in Fig. 2a for patients eligible to have completed grade school, high school/trade school, college, or post-college. Grade school was the highest level of education for 22% of patients older than 13 years of age. Among patients older than age 18, 65% completed high school/trade school as the highest education level, and 26% attained higher level education.
Fig. 2.
Burden of chronic ASMD on patient lifestyle. (a) Highest grade levels attained by patients with chronic ASMD. (b) Impact of disability on ability to work in patients with chronic visceral ASMD
Work status was available for 37 adult patients with chronic visceral disease (Fig. 2b). Nineteen of 37 (51%) worked full time or elected to work part time. Two patients (5%) worked part time due to disability, and six patients (16%) did not work at all due to disability. Ten patients did not work for other reasons unrelated to disability or disease. For three patients with chronic neurovisceral disease, two worked full or part time and one did not work due to psychosis.
Discussion
This study provides data on the burden of disease experienced by patients with ASMD phenotypes ranging from infantile neurovisceral disease to a more slowly progressing chronic visceral disease. Previous studies (described in Table 1) have documented ages at diagnosis, disease manifestations, and causes of death in patients with ASMD, and patients in our study had similar characteristics and diagnostic delays as noted in these previous reports. Due to the small patient population worldwide, there may be some overlap of US patients in these studies; however, the data for patients from Canada and Brazil have not been previously reported. Importantly, the present study reports new data related to longitudinal growth, burden of illness and predictors of disease-related morbidity, and healthcare use for patients with the chronic disease phenotypes that have not been previously studied in a cohort of this size.
Linear growth and weight gain were progressively impaired over time in patients with chronic disease with linear growth below normal for greater than 50% of patients with chronic visceral disease at age 5 years and older. A previous study analyzing growth in 23 children and adolescents with chronic ASMD determined that homozygosity for the R608del mutation was associated with normal growth (Wasserstein et al. 2003). Only one patient from the current study with chronic visceral disease was homozygous for the R608del mutation.
Disease-related morbidity, including respiratory infections and GI disorders, occurred early in patients with chronic visceral disease, and 50% of patients had major disease-related morbidity by the age of 2. Predictors for overall and individual morbidities in patients with chronic disease included earlier age at diagnosis, likely correlating with disease severity. Early gestational age at birth was associated with greater overall morbidity and morbidity in several individual organ systems in patients with chronic disease. It is known that preterm birth is associated with increased morbidity, and even late preterm births are associated with significantly increased neonatal mortality and morbidity compared with births at full term (McIntire and Leveno 2008). While patients with chronic neurovisceral disease have developmental delays and/or developmental regression, patients with chronic visceral disease typically do not have a neurological component. The developmental delay identified in several patients with chronic visceral ASMD may have resulted from disease-related morbidities, and the frequency of learning disabilities in patients with chronic visceral disease was similar to what has been reported in the general population (Cortiella and Horowitz 2014). Developmental regression and mental retardation reported in one case may have resulted from other causes or from misdiagnosis.
The analyses of healthcare resource utilization and lifestyle limitations showed that a significant proportion of patients with chronic disease required outpatient healthcare resources including physical therapy or home healthcare. The need for home schooling was also prominent for patients with chronic ASMD. In addition, the majority of patients did not achieve education past grade school or high school. While multiple factors affect the level of education, it is striking that almost 25% of patients older than 13 years of age achieved only a grade school level education. Almost a quarter of patients with chronic disease who were eligible to work either limited their work hours or could not work due to ASMD-related disability. Data assessing the impact of chronic ASMD on lifestyle are very limited. Hollak et al. (2012) reported that among six patients with chronic visceral ASMD, five were unable to work due to their disease. In general, quality of life is impacted among patients with attenuated forms of lysosomal storage diseases (Ulmer et al. 2009; Kanters et al. 2011; Raluy-Callado et al. 2013; Arends et al. 2015; Guffon et al. 2015; Needham et al. 2015), although assessments using disease-specific questionnaires are limited. Pediatric patients with chronic visceral ASMD were shown to have diminished quality of life in several areas, including physical functioning, mental health, and general health perceptions (McGovern et al. 2008), while adult patients had minimal impairment except in the general health domain, which may reflect the lack of sensitivity of the generic quality of life instrument that was used. Our study indicates significant impact of chronic disease on lifestyle and healthcare use.
Study limitations included the retrospective design and voluntary participation of patients, both of which affect the completeness of the data collection. Natural history studies of patients with rare diseases are inherently difficult to perform given the limited numbers of patients and difficulty in obtaining complete medical records. Nevertheless, all available clinical records were obtained for each patient in the study.
This is the largest cohort study to assess lifestyle limitations and resource use in patients with chronic ASMD, and the results expand our understanding of the burden of illness in patients with chronic disease. Information on the burden of disease for rare diseases such as ASMD is needed to ensure that appropriate monitoring is implemented early and sufficiently to manage disease-related morbidities and lifestyle limitations. Symptom treatment and supportive care are currently the only available management strategies for ASMD, although enzyme replacement therapy (olipudase alfa) is in late-stage clinical development (Wasserstein et al. 2015).
In conclusion, while infantile neurovisceral ASMD is fatal in early childhood, chronic forms of ASMD are associated with significant burden of illness in both pediatric and adult patients, which impact healthcare use and quality of life.
Acknowledgments
The authors thank the patients and their families for participating in this study and Dr. Joseph Clarke, University of Toronto, Canada, for contributing patient data.
Funding for this study was provided by Sanofi Genzyme. Patrice C Ferriola, PhD (KZE PharmAssociates, LLC, Chapel Hill NC) provided assistance in preparation of the manuscript and was funded by Sanofi Genzyme.
Synopsis
While infantile neurovisceral ASMD is fatal in early childhood, there is a significant burden of illness throughout life for patients with chronic forms of ASMD that impacts healthcare use and quality of life.
Compliance with Ethics Guidelines
Details of Funding
Sanofi Genzyme was the sponsor and provided support for this study, including support for medical writing. The authors confirm independence from the sponsor and the content of the article was not influenced by the sponsors.
Author Contributions
All authors were involved in the planning, conducting, and reporting of the study and contributed to the development, writing, and editing of the manuscript.
Conflicts of Interest
GC was a former employee of Sanofi Genzyme and holds stock from Genzyme Corp and Sanofi Genzyme.
RG has no financial relationships that present a potential conflict of interest and has received support as a speaker for travel, for consultancy, and for board membership.
LC has no financial relationships that present a potential conflict of interest and has received support for consultancy and travel.
MMM has no financial relationships that present a potential conflict of interest and has received honoraria for consultancy.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. All patients provided informed consent for their information to be included in the study (invited patients/families were sent a patient package by the site investigator that included study information and consent forms).
Contributor Information
Gerald F. Cox, Email: gerald.cox@editasmed.com
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Arends M, Hollak CE, Biegstraaten M. Quality of life in patients with Fabry disease: a systematic review of the literature. Orphanet J Rare Dis. 2015;10:77. doi: 10.1186/s13023-015-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiman D, Packman S, Bembi B, et al. Cause of death in patients with chronic visceral and chronic neurovisceral acid sphingomyelinase deficiency (Niemann-Pick disease type B and B variant): literature review and report of new cases. Mol Genet Metab. 2016;118(3):206–213. doi: 10.1016/j.ymgme.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Cortiella C, Horowitz S. The state of learning disabilities: facts, trends, and emerging issues. 3. New York: National Center for Learning Disabilities; 2014. [Google Scholar]
- Guffon N, Heron B, Chabrol B, Feillet F, Montauban V, Valayannopoulos V. Diagnosis, quality of life, and treatment of patients with Hunter syndrome in the French healthcare system: a retrospective observational study. Orphanet J Rare Dis. 2015;10:43. doi: 10.1186/s13023-015-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollak CE, de Sonnaville ES, Cassiman D, et al. Acid sphingomyelinase (Asm) deficiency patients in The Netherlands and Belgium: disease spectrum and natural course in attenuated patients. Mol Genet Metab. 2012;107(3):526–533. doi: 10.1016/j.ymgme.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Kanters TA, Hagemans ML, van der Beek NA, Rutten FF, van der Ploeg AT, Hakkaart L. Burden of illness of Pompe disease in patients only receiving supportive care. J Inherit Metab Dis. 2011;34(5):1045–1052. doi: 10.1007/s10545-011-9320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma SD, Bodamer OA, Wijburg FA. Epidemiology and diagnosis of lysosomal storage disorders; challenges of screening. Best Pract Res Clin Endocrinol Metab. 2015;29(2):145–157. doi: 10.1016/j.beem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- McGovern MM, Aron A, Brodie SE, Desnick RJ, Wasserstein MP. Natural history of type A Niemann-Pick disease: possible endpoints for therapeutic trials. Neurology. 2006;66(2):228–232. doi: 10.1212/01.wnl.0000194208.08904.0c. [DOI] [PubMed] [Google Scholar]
- McGovern MM, Wasserstein MP, Giugliani R, et al. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics. 2008;122(2):e341–e349. doi: 10.1542/peds.2007-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern MM, Lippa N, Bagiella E, Schuchman EH, Desnick RJ, Wasserstein MP. Morbidity and mortality in type B Niemann-Pick disease. Genet Med. 2013;15(8):618–623. doi: 10.1038/gim.2013.4. [DOI] [PubMed] [Google Scholar]
- McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- Needham M, Packman W, Quinn N, et al. Health-related quality of life in patients with MPS II. J Genet Couns. 2015;24(4):635–644. doi: 10.1007/s10897-014-9791-7. [DOI] [PubMed] [Google Scholar]
- Raluy-Callado M, Chen WH, Whiteman DA, Fang J, Wiklund I. The impact of Hunter syndrome (mucopolysaccharidosis type II) on health-related quality of life. Orphanet J Rare Dis. 2013;8:101. doi: 10.1186/1750-1172-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman EH, Desnick RJ, et al. Niemann-Pick disease types A and B: acid sphingomyelinase deficiencies. In: Valle D, Beaudet A, Vogelstein B, et al., editors. OMMBID-the online metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2013. [Google Scholar]
- Schuchman EH, Wasserstein MP. Types A and B Niemann-Pick disease. Best Pract Res Clin Endocrinol Metab. 2015;29(2):237–247. doi: 10.1016/j.beem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Ulmer FF, Landolt MA, Vinh RH, et al. Intellectual and motor performance, quality of life and psychosocial adjustment in children with cystinosis. Pediatr Nephrol. 2009;24(7):1371–1378. doi: 10.1007/s00467-009-1149-2. [DOI] [PubMed] [Google Scholar]
- Wasserstein MP, Larkin AE, Glass RB, Schuchman EH, Desnick RJ, McGovern MM. Growth restriction in children with type B Niemann-Pick disease. J Pediatr. 2003;142(4):424–428. doi: 10.1067/mpd.2003.113. [DOI] [PubMed] [Google Scholar]
- Wasserstein MP, Desnick RJ, Schuchman EH, et al. The natural history of type B Niemann-Pick disease: results from a 10-year longitudinal study. Pediatrics. 2004;114(6):e672–e677. doi: 10.1542/peds.2004-0887. [DOI] [PubMed] [Google Scholar]
- Wasserstein MP, Jones SA, Soran H, et al. Successful within-patient dose escalation of olipudase alfa in acid sphingomyelinase deficiency. Mol Genet Metab. 2015;116(1–2):88–97. doi: 10.1016/j.ymgme.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]