Abstract
Various gastrointestinal (GI) disorders have a higher prevalence in women than in men. In addition, estrogen has been demonstrated to have an inhibitory effect on the contractility of GI smooth muscle. Although increased plasma estrogen levels have been implicated in GI disorders, the role of gastric estrogen receptor (ER) in these sex-specific differences remains to be fully elucidated. The present study was designed to investigate the sex-associated differences in the expression of the two ER isoforms, ERα and ERβ, and the effect of estrogen on gastric muscle contraction via the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway. Experiments were performed on single gastric smooth muscle cells (GSMCs) isolated from male and female Sprague Dawley rats. The effect of acetylcholine (ACh), a muscarinic agonist, on the contraction of GSMCs was measured via scanning micrometry in the presence or absence of 1 µM 17β-estradiol (E2), an agonist to the majority of ERs, 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), an ERα agonist, or diarylpropionitrile (DPN), an ERβ agonist. The protein expression levels of ER subtypes in GSMCs were measured using a specifically designed ELISA. GSMCs from female rats had a higher expression of ERα and ERβ protein compared with GSMCs from males. ACh induced less contraction in female that in male GSMCs. Pre-treatment of GSMCs with E2 reduced the contraction of GSMCs from both sexes, but to a greater extent in those from females. PPT and DPN inhibited ACh-induced contraction in GSMCs from females. Furthermore, E2 increased NO and cGMP levels in GSMCs from males and females; however, higher levels were measured in females. Of note, pre-incubation of female GSMCs with Nω-nitro-L-arginine, a NO synthase inhibitor, or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, a guanylyl cyclase inhibitor, reduced the inhibitory effect of estrogen on GSMC contraction. In conclusion, estrogen relaxes GSMCs via an NO/cGMP-dependent mechanism, and the reduced contraction in GSMCs from females by estrogen may be associated with the sex-associated increased expression of ERα and ERβ, and greater production of NO and cGMP, compared with that in GSMCs from males.
Keywords: contraction, estrogen receptor, gastric, sex, smooth muscle, nitric oxide, cyclic guanosine monophosphate
Introduction
Sex differences are becoming increasingly apparent in a range of normal physiological processes, as well as pathological functions in clinical and research settings. These differences have been determined in cardiovascular structure and function, lung health and disease, metabolism and cognition (1,2). Furthermore, there is notable evidence that sex may affect gastrointestinal (GI) motility. For instance, a number of studies have demonstrated that females have an increased probability of GI disturbances, including nausea, vomiting, bloating and constipation, compared with males (3,4). These disturbances may vary during a female's lifetime due to the varying levels of sex hormones during the menstrual cycle, pregnancy and menopause (5,6). In addition, females have an increased probability of being affected by gastroparesis, a chronic gastric motility disorder, in which gastric emptying of solids and liquids is delayed in the absence of obstruction (7). Although the pathogenesis of the disease remains to be fully elucidated, the importance of estrogen in the regulation of gastric motility in females is evident (8). Smooth muscle relaxation is initiated by targeting the 20-kDa regulatory myosin light chain. Most agents cause relaxation by stimulating the production of cyclic adenosine monophosphate (cAMP) and/or cyclic guanosine monophosphate (cGMP). cAMP-activated protein kinase A and cGMP-activated protein kinase G are the major enzymes that induce relaxation in the smooth muscle. Nitric oxide (NO) induces the production of cGMP from guanosine triphosphate via activating the soluble guanylyl cyclase (sGC). cGMP is then rapidly degraded by cGMP-specific phosphodiesterases (9). The elevated levels of circulating estrogen regulate gastric emptying in healthy females by elevating NO levels, an important regulator of gastric motility (10). Furthermore, sex hormones, particularly estrogen, are known to cause GI motility disorders and contribute to irritable bowel syndrome (11). In addition, increased ovarian hormones during pregnancy coincide with a notable increase in numerous GI symptoms, including gastro-esophageal reflux, nausea, vomiting, constipation, bloating, delayed gastric emptying and gall bladder dysfunction (12–15).
The predominant biological effects of estrogen are mediated through two receptors, estrogen receptor (ER)α and ERβ, which have distinct tissue expression patterns. These ERs are expressed at different levels in various regions of the body, including the female reproductive tract, vasculature and the GI tract (16). These ERs may influence each other; therefore, estrogen action in tissues where they are co-expressed is complex, and if one of the receptors is deleted, the resulting changes in physiological function may be unpredictable and difficult to understand (17). Estrogen was also determined to induce a number of rapid-signaling or non-genomic events in a variety of cell types, providing significant functional evidence that surface membrane ERs are also involved in the rapid relaxant effects of estrogen (18). Estrogen was demonstrated to cause relaxation in smooth muscles of the gall bladder (13), trachea (2), urinary bladder (19), blood vessels (20) and colon (21,22). In addition, estrogen induces relaxation of vascular smooth muscle via a process involving the activation of the NO/cGMP pathway (23); however, whether this mechanism underlying estrogen-mediated relaxation occurs in gastric smooth muscle has remained elusive.
In the present study, the hypothesis that sex-associated differences in rat gastric smooth muscle cell (GSMC) contractions exist, which may be a result of differences in the expression and/or activity of ER subtypes, was investigated. The effect of estrogen on the NO/cGMP pathway in the GSMCs was also investigated. Due to motility disorders being major characteristics of numerous GI disturbances, the present study may be of notable importance in understanding the cause of their disproportionate prevalence among females; in addition, it may further pave the way for understanding the ER-mediated smooth muscle contraction-relaxation pathways and thereby establishing novel therapeutic approaches for the treatment of GI disorders.
Materials and methods
Materials
A protein assay kit (cat. no. 500–0119) was obtained from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Rat estrogen β (cat. no. CSB-EL007831RA) and rat 17β-estradiol (E2; cat. no. CSB-E06848r) ELISA kits were obtained from Cusabio Biotech (Newark, DE, USA). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; cat. no. ab120022), Nω-nitro-L-arginine (L-NNA; cat. no. ab141312) and anti-calponin antibodies (cat. no. ab46794) were purchased from Abcam (Cambridge, MA, USA). Diarylpropionitrile (DPN) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The 500-µm Nitex mesh (Sefar Nitex 06–500/38) was from Sefar Inc. (Thal, Switzerland). All remaining chemicals were from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). A stock solution of E2 was prepared in 100% ethanol. Stock solutions of 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), DPN, L-NNA and ODQ were prepared in dimethyl sulfoxide (DMSO). The final concentration of ethanol and DMSO used was 1% (volume/volume).
Animals
Young Sprague Dawley rats [age, 12 weeks; weight, 250–300 g; n=93 (49 males and 44 females)] were supplied by the animal center of Jordan University of Science and Technology (Irbid, Jordan). Rats were euthanized by CO2 inhalation and euthanasia was further confirmed by incising the diaphragm with a scalpel blade. The present study was approved by the Animal Care and Use Committee of Jordan University of Science and Technology (Irbid, Jordan). All experimental procedures followed the NIH's guidelines.
Preparation of dispersed GSMCs
The stomach was rapidly excised following euthanasia. GSMCs were isolated from the circular muscle layer of the rat stomach by sequential enzymatic digestion, filtration and centrifugation, as previously described (24). Sections of circular muscle from the stomach were dissected and incubated at 31°C for 30 min in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) medium (pH was adjusted to 7.4), containing 120 mM NaCl, 4 mM KCl, 2.0 mM CaCl2, 2.6 mM KH2PO4, 0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose, 2.1% Eagle's essential amino acid mixture (Sigma-Aldrich; Merck KGaA), 0.1% collagenase (Sigma-Aldrich; Merck KGaA) and 0.01% soybean trypsin inhibitor. The tissue was continuously exposed to 100% oxygen during the entire isolation procedure. Subsequently, the partially digested sections were washed twice with 50 ml enzyme-free HEPES medium, and the GSMCs were then incubated at room temperature for spontaneous dispersion for 30 min. The cells were harvested via filtration through 500-µm Nitex mesh and centrifuged twice at 350 × g for 10 min to remove any broken cells and organelles. The cell isolation procedure consistently yielded spindle-shaped, viable GSMCs that exhibited significant contraction in response to contractile stimuli. All the experiments were performed within 2–3 h of cell dispersion.
Identification of GSMCs
The identity of the rat GSMCs was verified by immunohistochemical staining. Cells were added to adhesive-coated slides to enhance attachment and air-dried for 15 min. Slides were then fixed with 4% formaldehyde in PBS solution for 4 min at 4°C, and then washed twice for 5 min in fresh PBS. A blocking solution consisting of 5 mM ethylenediaminetetraacetic acid in PBS with 5% goat serum and 1% bovine serum albumin was applied for 20 min at room temperature. Following this, the blocking solution was drained from each slide and the anti-calponin antibody (150 µl per slide; 1:100) was added. The slides were then incubated for 1 h at 4°C. Subsequently, slides were washed twice in fresh PBS solution for 5 min at room temperature. Goat anti rabbit Immunoglobulin G antibodies (cat. no. A0545, 1:0; Sigma-Aldrich, Merck KGaA) were diluted in PBS and added to sections for 30 min at room temperature, with two 5 min washes with PBS. Then an avidin-biotin-horseradish peroxidase complex was added for 30 min at room temperature. Sections were then washed, covered with diaminobenzidine chromogen and counter-stained with hematoxylin for 10 min at room temperature. Samples were dehydrated in a graded series of alcohols and mounted with cover slips and sealed. All Slides, chemicals and reagents used for immunohistochemical staining were purchased from Dako (Agilent Technologies, Inc., Santa Clara, CA, USA).
Expression of ERα and ERβ via ELISA
GSMCs collected from 10 ml muscle cell suspension (3×106 cells/ml) were centrifuged (20,000 × g at 4°C for 1 min) and the pellet was snap-frozen in liquid nitrogen and homogenized using a Teflon glass pestle in 400 µl ice-cold distilled water. Following the centrifugation of the lysates at 20,000 × g at 4°C for 10 min, the protein concentration in the supernatant was determined with a Dc protein assay kit (Bio-Rad Laboratories, Inc.). Samples containing equal amounts of protein were used for quantification of ERα and ERβ using the ELISA kits according to the manufacturer's protocol.
Measurement of smooth muscle NO
The concentration of NO in basal and E2-treated (1 µM for 10 min) smooth muscle samples was indirectly measured by determining nitrate and nitrite levels utilizing an NO (NO2−/NO3−) assay kit (cat. no. 23479; Sigma-Aldrich; Merck KGaA), following the manufacturer's protocol. The assay determined the NO concentration based on the enzymatic conversion of nitrate to nitrite by nitrate reductase. The reaction was followed by colorimetric detection of nitrite as a product of the Griess reaction, based on the diazotization reaction, in which acidified NO2− produced a nitrosylating agent that reacted with sulfanilic acid to yield the diazonium ion. This ion was then combined with N-(1-naphthyl) ethylenediamine to form a chromophoric azo derivative, which absorbs light at 540 nm. Protein interference was avoided by treating samples with zinc sulfate and centrifugation at 4°C for 10 min at 2,000 × g. Samples were spectrophotometrically quantified using an ELISA microplate reader (elx-800; BioTek Instruments, Winooski, VT, USA) at 540 nm. NaNO2 was used as a standard and a curve of the nitrite concentration against the optical density was plotted.
Measurement of smooth muscle cGMP
The level of cGMP in control and E2-treated (1 µM for 10 min) smooth muscle cell samples was measured using an ELISA kit (cat. no. STA-505; Cell Biolabs, Inc., San Diego, CA, USA) according to the manufacturer's protocol.
Measurement of contraction of dispersed GSMCs
Contraction of recently dispersed GSMCs was determined by scanning micrometry, as previously described (4). Aliquots (0.4 ml) of cell suspension containing ~104 cells/ml were prepared. Cells were pooled from different animals of the same sex to enhance the cell number. Aliquots were randomly distributed into the control, E2, PPT [a selective ERα agonist (24)], DPN [a selective ERβ agonist (25)], ODQ (a guanylyl cyclase inhibitor), or L-NNA (an NO synthase inhibitor) treatment groups. Aliquots designated for treatment were incubated with 1 µM E2, PPT, DPN, L-NNA or ODQ for 10 min. Cells were stimulated with acetylcholine (ACh; 0.1 µM) for 10 min at room temperature in the presence or absence of ER modulator treatment, and the reaction was terminated with 1% acrolein at a final concentration of 0.1%. Acrolein kills and fixes cells without affecting the cell length. The cells were viewed using a ×10, ×20 and ×40 magnification with an inverted Nikon TMS-f microscope (Nikon, Tokyo, Japan), and cell images were captured using a Canon digital camera (cat. no. DS126291; Canon, Inc., Tokyo, Japan) and ImageJ software (version 1.45s; National Institutes of Health, Bethesda, MA, USA). The resting cell length was determined in control experiments, in which muscle cells were not treated with ACh. The mean length of 50 GSMCs from each group was measured using ImageJ software. An aliquot of cells fixed with acrolein was placed on a slide under a coverslip. Images were captured for each slide with the microscope-connected camera and the lengths of the first 50 randomly encountered cells in successive microscopic fields were measured using the ImageJ software. The contractile response to ACh was defined as a decrease in the mean length of 50 cells, and expressed as the percentage change in length relative to the average resting length.
Statistical analysis
The results were expressed as the mean ± standard error of the mean. Each experiment was performed on cells obtained from rats of same sex. P-values were determined by an unpaired Student's t-test when comparing two samples, or by one-way analysis of variance followed by Tukey's post-hoc test when comparing >2 samples, using Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Verification of the identity of GSMCs
Freshly dispersed and isolated GSMCs appeared to be spindle-shaped with various lengths, as determined using phase contrast microscopy; an example of a singular male GSMCs is depicted in Fig. 1A. The identity of the rat GSMCs was verified by immunohistostaining with anti-calponin antibodies (Fig. 1B). Of the cells collected, >95% stained positive for h1-calponin, a protein whose expression is specific for differentiated SMCs (26).
Figure 1.
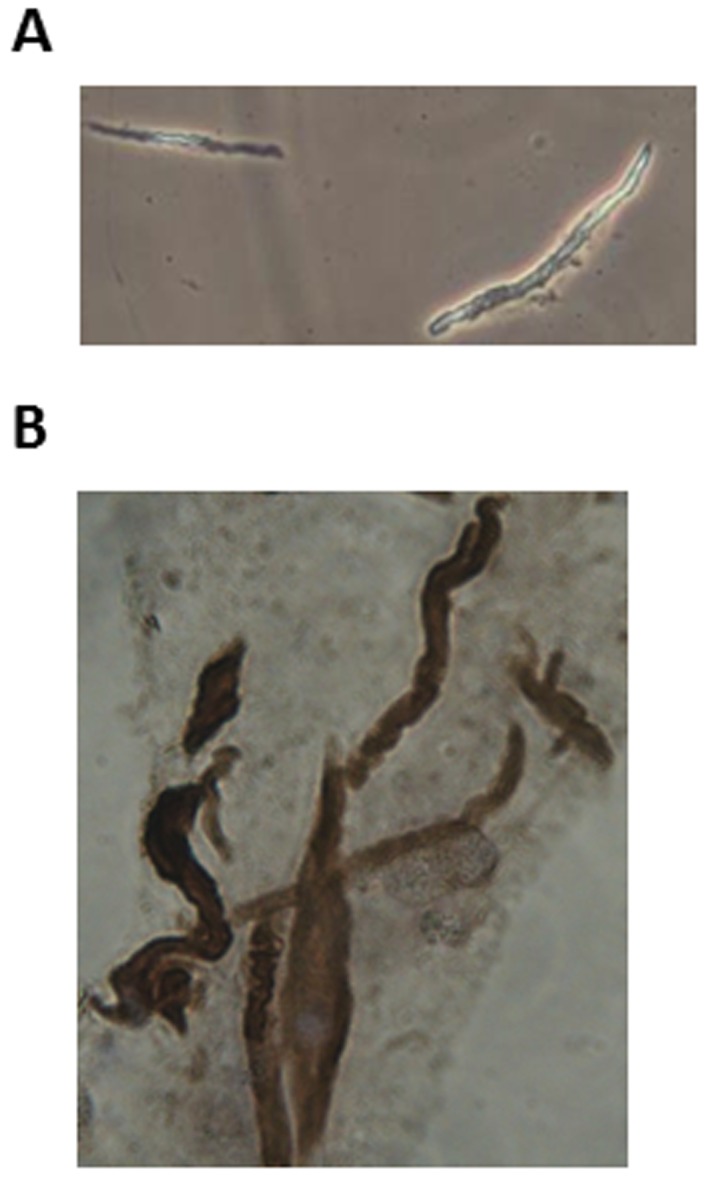
Identification of rat GSMCs. (A) Each GSMC had a spindle-like shape, as determined by phase contrast microscopy. The mean length of the GSMCs at rest was similar in males and females. (B) Immunohistochemical staining of dispersed rat GSMCs using anti-h1-calponin antibodies. GSMC, gastric smooth muscle cells. Pictures were viewed at ×40 magnification.
ER expression
The ELISAs revealed that the protein expression of ERα and ERβ (P<0.05) was significantly increased in the GSMCs from females compared with those from males (Fig. 2A and B, respectively).
Figure 2.
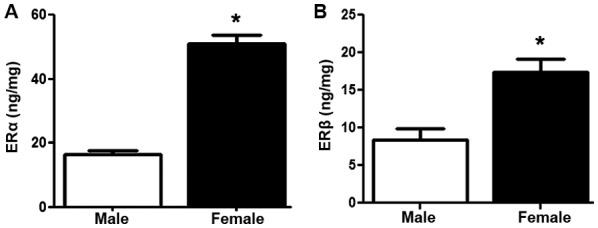
Expression of ERα and ERβ protein in GSMCs from male and female rats. Protein expression levels of (A) ERα and (B) ERβ in male and female GSMCs. ERα and ERβ proteins were more highly expressed in GSMCs from female rats compared with those in GSMCs from male rats. The values are representative of the mean of four independent experiments performed in triplicate. Samples were collected from 14 male and 9 female rats. Values are expressed as the mean ± standard error of the mean. *P<0.05 vs. male. ERα, estrogen receptor α; GSMCs, gastric smooth muscle cells.
Effect of estrogen on muscle cell contraction
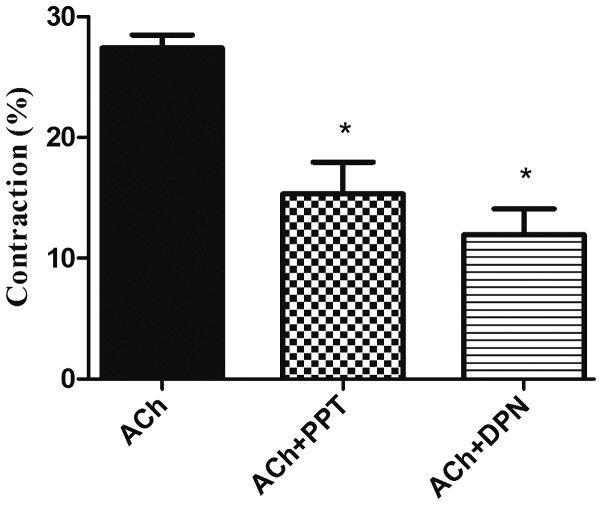
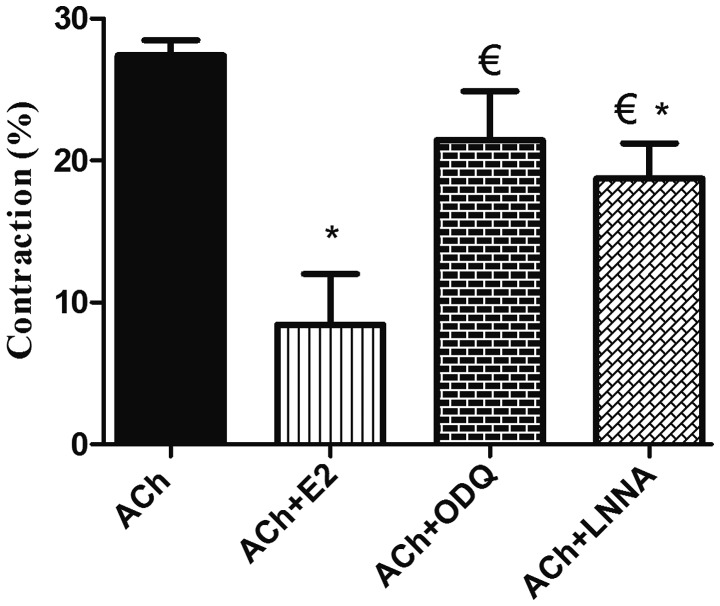
Recently isolated and dispersed GSMCs from both sexes were treated with ACh, and scanning micrometry was performed to measure the decrease in muscle cell length. Resting muscle length was identical in male and female cells. ACh caused muscle cell contraction in both sex groups. Contraction in response to ACh was significantly reduced in the female cells compared with that in male cells (P<0.05). Of note, pre-incubation of GSMCs from males and females with E2 significantly decreased the ACh-induced contraction (P<0.05). Furthermore, the estrogen-induced relaxation was greater in female cells compared with that in male cells (50 vs. 70% reduction in contraction of males and females, respectively) (P<0.05; Fig. 3). Due to the increased effect of estrogen in female GSMCs, an investigation into the effect of various ER agonists on the muscle contraction of female GSMCs was then pursued. The ERα agonist PPT and the ERβ agonist DPN reduced ACh-induced contraction. DPN induced relaxation to a greater extent than PPT, although this result was not statistically significant (Fig. 4).
Figure 3.
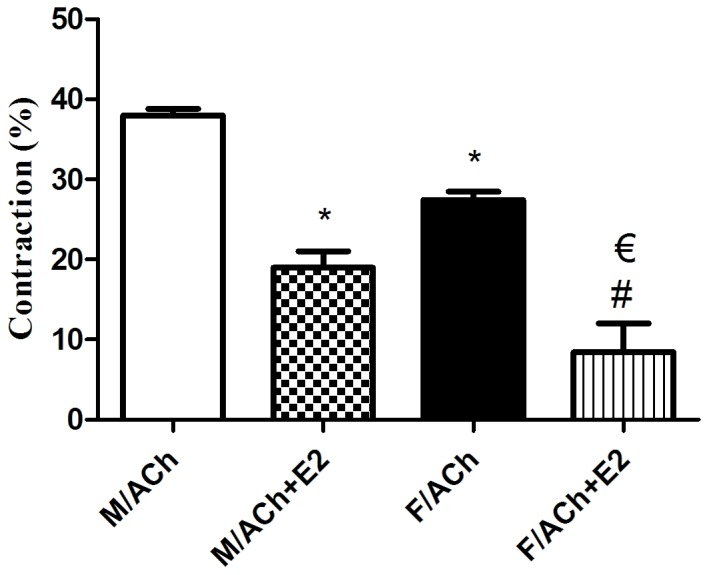
Effect of estrogen on ACh-induced contraction of GSMCs in male versus female rats. GSMCs from male and female rats were stimulated with ACh in the presence or absence of E2, an activator of the majority of ERs, and observed under a microscope. Images of treated and non-treated single cells were acquired and the extent of cell contraction was measured. ACh-induced contraction was significantly reduced in female cells compared with that in male cells. E2 significantly reduced ACh-induced contraction in cells from both sexes, but to a greater extent in GSMCs from females compared with those from males. Values are expressed as the mean ± standard error of the mean (n=50 cells from 10 male or 10 female rats). *P<0.05 vs. M/ACh; #P<0.05 vs. F/ACh; €P<0.05 vs. M/ACh+E2. ACh, acetylcholine; GSMCs, gastric smooth muscle cells; E2, 17β-estradiol; M, male group; F, female group.
Figure 4.
Effect of ER modulators on ACh-induced contraction in GSMCs from female rats. GSMCs of female rats were stimulated with ACh in the presence or absence of PPT, an ERα agonist, or DPN, an ERβ agonist. Pre-incubation with PPT or DPN significantly reduced ACh-induced contraction in the GSMCs. Values are expressed as the mean ± standard error of the mean (n=50 cells from 10 female rats). *P<0.05 vs. ACh. ER, estrogen receptor; ACh, acetylcholine; GSMCs, gastric smooth muscle cells; PPT, 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole; DPN, diarylpropionitrile.
Effect of estrogen on NO formation in singular GSMCs
Basal NO levels were similar in male and female singular GSMCs (P>0.05), with mean values of 2.27±0.40 and 3.89±2.33 µmol/mg protein, respectively. Treatment of the GSMCs with E2 significantly increased the NO levels in male and female cells (P<0.05). Of note, the E2-induced increase in NO levels in female cells was significantly greater than that in male cells (>3-fold; P<0.05; Fig. 5).
Figure 5.
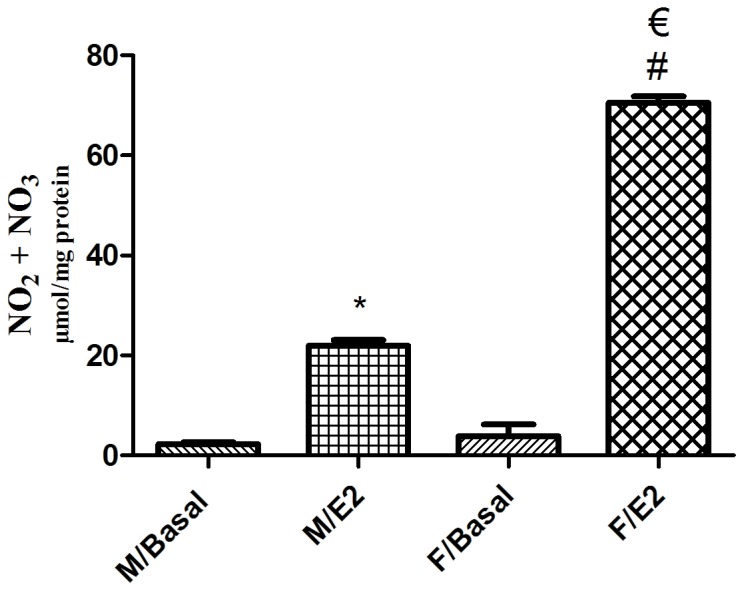
Effect of estrogen on the NO levels in single GSMCs from male vs. female rats. Total NO metabolites (nitrate + nitrite) were measured as indicators of NO levels. Pre-incubation of the GSMCs with E2 significantly increased NO levels in GSMCs from both sexes. Of note, E2 elevated NO levels in female cells to a greater extent than in male cells. The values are representative of the mean of at least four independent experiments performed in triplicate. Samples were collected from 25 male and 25 female rats. Values are expressed as the mean ± standard error of the mean. *P<0.05 vs. M/Basal; #P<0.05 vs. F/Basal; €P<0.05 vs. M/E2. NO, nitric oxide; GSMCs, gastric smooth muscle cells; E2, 17β-estradiol; M, male group; F, female group.
Effect of estrogen on cGMP formation in singular GSMCs
The mean basal cGMP levels in singular male and female GSMCs were 16.75±12.33 and 21.36±7.97 pmol/mg protein, respectively. Treatment of the male and female GSMCs with E2 significantly increased the cGMP levels (P<0.05). Of note, the E2-induced increase in cGMP levels in female cells was significantly greater than that in male cells (~1.9-fold; P<0.05; Fig. 6).
Figure 6.
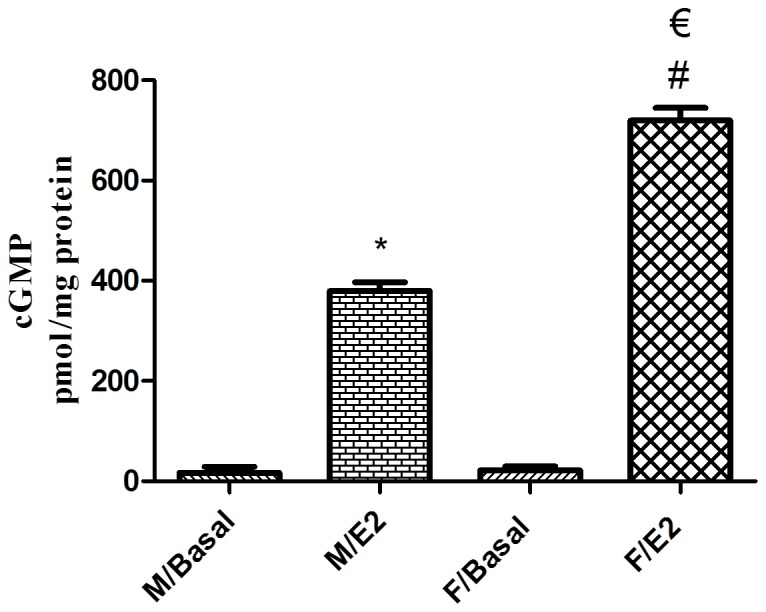
Effect of estrogen on cGMP levels in single GSMCs from male vs. female rats. Treatment of GSMCs with E2 significantly increased cGMP levels in GSMCs from both sexes. Of note, E2 elevated cGMP levels in female cells, compared with that in male cells. Values are representative of the mean of at least four independent experiments performed in triplicate. Samples were collected from 25 male and 25 female rats. Values are expressed as the mean ± standard error of the mean. *P<0.05 vs. M/Basal; #P<0.05 vs. F/Basal; €P<0.05 vs. M/E2. cGMP, cyclic guanosine monophosphate; GSMC, gastric smooth muscle cells; E2, 17β-estradiol; M male group; F, female group.
Effect of the blockade of NO synthase and sGC on E2-induced relaxation
As the production of NO and cGMP stimulated by estrogen was greater in female cells, the focus was on investigating the effect of the NO synthase blocker L-NNA and the sGC blocker ODQ on the E2-induced inhibition of muscle contraction in female cells. L-NNA and ODQ significantly reduced the E2-induced inhibition of GSMC contraction (P<0.05; Fig. 7).
Figure 7.
Effect of blockade of NO synthase and sGC on estrogen-induced relaxation in GSMCs from female rats. Cells were treated with ACh and contraction was expressed relative to control-cell contraction. ACh induced GSMC contraction, whereas pre-treatment of the GSMCs with E2 significantly reduced ACh-induced contraction. Relaxation induced by E2 was significantly inhibited in muscle cells pre-incubated with ODQ or L-NNA. Cumulative data (n=50 cells from 10 female rats) are presented as the mean ± standard error of the mean. *P<0.05 vs. ACh; €P<0.05 vs. ACh+E2. NO, nitric oxide; sGC, soluble guanylyl cyclase; GSMC, gastric smooth muscle cells; ACh, acetylcholine; E2, 17β-estradiol; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; L-NNA, Nω-nitro-L-arginine.
Discussion
In the present study, an increased expression of ERα and ERβ, and a decreased contraction of GSMCs from females compared with those from males was demonstrated. Estrogen induced a greater extent of relaxation in the GSMCs from females compared with those from males, probably via the increased production of NO and cGMP. Previous studies demonstrated sex-specific differences in smooth muscle, which has functions in a number of different organs and in various species (27,28). It was recently determined that the extent of activation of the small G protein Ras homolog gene family (Rho), member A and its downstream effector, Rho-associated protein kinase, members of an important pathway in developing smooth muscle tone, is elevated in response to the muscarinic agonist ACh, and thus, the contraction of male GSMCs is greater compared with that of female GSMCs (29). Numerous studies have investigated the effect of sex steroids on the function of the GI tract, indicating that sex differences may be due to differences in the expression/activity of estrogen and its receptors (30–32). For instance, previous studies demonstrated that circulating levels of estrogen, which fluctuate during the various stages of the ovarian cycle, may serve a role in gastric motility, GI transit times and GSMC reactivity (10,33). Previous studies have also indicated that estrogen affects gastric motility at the tissue level, with an evident effect on the neuronal NO synthase of non-adrenergic non-cholinergic neurons (33). Taking into consideration that the multi-cellular composition of the stomach makes it difficult to differentiate between the specific roles of cells, recently dispersed GSMC were used and the contraction of singular cells in response to ACh, the major contractile agonist in the GI tract, was determined. Cells were isolated from different animals of the same sex to enhance the number of cells collected. However, improving the isolation procedure in the future may enhance the amount of cells collected, even from a single animal. Of note, a reduced ACh-induced contraction was observed in GSMCs of female rats compared with that in GSMCs from male rats, which is consistent with the results of previous studies by our group (29,34) and with observations made in non-GI smooth muscle regions (20).
A number of the effects of estrogen on muscles are mediated via its classical receptors. ER subtypes have been identified in the female reproductive tract, mammary glands and blood vessels, and throughout the GI tract of humans and experimental animals (16). The present study provided evidence for sex-associated differences in the amount of gastric ERα and ERβ in GSMCs, with a greater amount in female compared with that in male GSMCs; however, a number of studies have reported that ERs are only present in the gastric mucosa and not in the muscular layer (35). This variation may be due to differences in sensitivity of the techniques applied, as in situ hybridization was used, as well as differences in species, due to the studies being performed on human tissue samples.
Functionally, estrogen has been demonstrated to have an inhibitory effect on the contractility of smooth muscle. Consistent with previous studies on other parts of the GI tract (13,15,21,13), it was determined that estrogen, an agonist for both ER subtypes, inhibited muscle contraction in both sexes. Of note, the extent of the relaxation effect induced by estrogen was greater in female GSMCs compared with that in male GSMCs, in parallel with the ER expression pattern in males and females. The next aim was to examine the contribution of each specific ER subtype to the effect of E2 using ER type-specific agonists. As ER expression and the effect of estrogen were greater in female GSMCs compared with those in male GSMCs, the effect of ER agonists on female GSMCs was further investigated. PPT and DPN inhibited the ACh-induced contraction of the GSMCs. DPN induced a greater extent of relaxation compared with PPT, although this difference was not statistically significant. This may be due to differences in the expression of ER subtypes in GSMCs. The results of the present study are in accordance with a previous study, which indicated that ERβ serves a predominant role in inhibiting colonic contractility (37). Future contraction studies examining the effect of various ER agonists on muscle contraction to test the sensitivity of each receptor may be required. The rapid time-course (≤10 min) of the muscle relaxant action of estrogen in the present study indicated the non-genomic effect of the hormone, as a characteristic genomic effect involves time-consuming transcription and translation processes (38). This is supported by the fact that membrane ERs are implicated in the rapid vasodilation effects of estrogen (18).
It is notable that the concentrations of E2 used in the present experiments are far greater than the picomolar-to-nanomolar levels of free hormone present in the plasma under normal physiological conditions (i.e., in the absence of pregnancy). As estrogen is lipophilic, its plasma levels may not reflect its gastric tissue levels, and prolonged exposure to small estrogen concentrations in vivo may result in gradual tissue accumulation, eventually reaching levels similar to those used in acute studies; thus, in vitro studies may require higher E2 concentrations than those usually encountered in vivo to bind plasma membrane lipids and ERs (39). After reviewing the E2 dose-response curve from a previous study (40), it was determined that a concentration of 1 µM, which lies in the middle of the curve, is adequate. Furthermore, the concentrations of the various agonists used in this study were found to properly affect muscle contraction in previous experiments. In addition, previous research reported that ethanol and DMSO had no effect on muscle tone at a final concentration of 1% (volume/volume) (20,41,42). However, including vehicles of the dissolved reagents would further strengthen these results.
Based on estrogen-associated studies in the muscle of other body regions, it was hypothesized that the mechanism underlying the effect of estrogen on muscle contraction may result from activation of the NO/cGMP pathway. Numerous studies have demonstrated the stimulatory effect of estrogen on the production of NO, an important regulatory neurotransmitter that controls gastric motility, and the downstream Cgmp (23,33). In addition, the present study determined that estrogen enhanced the production of NO in male and female GSMCs, and that the effect was greater in female cells. NO is a potent relaxant due to its stimulatory effect on smooth muscle guanylate cyclase and the production of cGMP (43), and is produced by the enzyme NO synthase. As the present study used singular GSMCs, the elevated NO production was primarily due to the activation of the constitutive NO synthase isoform in SMCs (44). This estrogen-induced NO production was paralleled by an increased production of cGMP in GSMCs from both sexes, which was increased in females compared with that in males. To prove the contribution of the NO/cGMP pathway in the estrogen-mediated relaxation of GSMCs, female GSMCs were treated with inhibitors of NO synthase and guanylyl cyclase. Consistent with other studies, the blockade of NO synthase by L-NNA or guanylyl cyclase by ODQ significantly inhibited estrogen-induced relaxation (23,45–47). These results provide evidence for the involvement of NO and cGMP in gastric estrogen action. cGMP may also induce relaxation through its well-established ability to reduce the cytosolic Ca2+ concentration (48) and modulate the activity of potassium channels (49). Taking into consideration the possible effect of estrogen on these and other possible muscle targets may explain the disproportionate difference in E2-induced relaxation and the E2-induced activation of NO and GC activity between female and male GSMCs. Further studies are required to investigate the signaling pathway mediation of estrogen-induced SMC relaxation downstream of NO/cGMP.
A novel transmembrane ER known as G-protein-coupled ER (GPR30) is implicated in various physiological processes in the reproductive, nervous, endocrine, immune and cardiovascular systems, as well as pathological processes in a diverse array of disorders (50–52). This third ER type may serve an important role in the regulation of muscle tone, works solely through non-genomic pathways, and also stimulates NO and cGMP production in various cell types, including SMCs (51,53,54). In addition to the activation of ERα, PPT has also been demonstrated to activate GPR30 in a range of contexts, particularly when used in a high dose range (55). Whether GPR30 is expressed in GSMCs and whether it displays any sex-associated differences remains elusive; therefore, further studies are required to investigate its contribution.
In conclusion, the present study demonstrated an increased expression of ERα and ERβ in GSMCs from females compared with those from males. The greater reduction in contraction of female GSMCs following estrogen treatment may be due to the sex-associated increases in the expression of ERα and ERβ, resulting in a greater activation of the NO/cGMP pathway. ERs may potentially exert non-genomic effects as well as genomic effects on the contraction-relaxation pathway in SMCs. The exact mechanisms by which ERs may affect smooth muscle contraction should be further investigated. Sex-associated differences are present in the GI system, with ER expression and sensitivity serving a pivotal role in GI function. An improved understanding of the role of sex hormones and their receptors in modulating the normal and pathophysiological GI tract function may provide the possibility for more effective and sex-specific therapeutic approaches for various GI diseases.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ACh
acetylcholine
- cGMP
cyclic guanosine monophosphate
- DPN
diarylpropionitrile
- E2
17β-estradiol
- ER
estrogen receptor
- GI
gastrointestinal
- GSMC
gastric smooth muscle cells
- L-NNA
Nω-nitro-L-arginine
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PPT
1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole
Funding
The present work was supported by Jordan University of Science and Technology (Irbid, Jordan; grant no. 20140234).
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
Conception and design of the study were performed by OAA. Acquisition of data and drafting of the manuscript were performed by OAA, MSN and AAO. Analysis and interpretation of data and critical revision of the manuscript for important intellectual content were performed by OAA, MSN, AGM, ANA, MoAA1, AAO, MaAA2 and MIA. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The current study protocols were approved by and followed the guidelines of the Animal Care and Use Committee of Jordan University of Science and Technology (Irbid, Jordan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615–624. doi: 10.1096/fasebj.10.5.8621060. [DOI] [PubMed] [Google Scholar]
- 2.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonenne J, Esfandyari T, Camilleri M, Burton DD, Stephens DA, Baxter KL, Zinsmeister AR, Bharucha AE. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol Motil. 2006;18:911–918. doi: 10.1111/j.1365-2982.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 4.Matchock RL, Levine ME, Gianaros PJ, Stern RM. Susceptibility to nausea and motion sickness as a function of the menstrual cycle. Womens Health Issues. 2008;18:328–335. doi: 10.1016/j.whi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull GK, Thompson DG, Day S, Martin J, Walker E, Lennard-Jones JE. Relationships between symptoms, menstrual cycle and orocaecal transit in normal and constipated women. Gut. 1989;30:30–34. doi: 10.1136/gut.30.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Liu R, Dong Y. Regulative effects of ovarian steroids on rat gastric motility and sensitivity. Sheng Li Xue Bao. 2006;58:275–280. [PubMed] [Google Scholar]
- 7.Rao JN. Estrogens and gastroparesis: A clinical relevance. Dig Dis Sci. 2013;58:1449–1451. doi: 10.1007/s10620-013-2683-0. [DOI] [PubMed] [Google Scholar]
- 8.Ravella K, Al-Hendy A, Sharan C, Hale AB, Channon KM, Srinivasan S, Gangula PR. Chronic estrogen deficiency causes gastroparesis by altering neuronal nitric oxide synthase function. Dig Dis Sci. 2013;58:1507–1515. doi: 10.1007/s10620-013-2610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 10.Gangula PR, Sekhar KR, Mukhopadhyay S. Gender bias in gastroparesis: Is nitric oxide the answer? Dig Dis Sci. 2011;56:2520–2527. doi: 10.1007/s10620-011-1735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulak A, Taché Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20:2433–2448. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill SK, Maltepe C, Koren G. The effect of heartburn and acid reflux on the severity of nausea and vomiting of pregnancy. Can J Gastroenterol. 2009;23:270–272. doi: 10.1155/2009/678514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riezzo G, Pezzolla F, Darconza G, Giorgio I. Gastric myoelectrical activity in the first trimester of pregnancy: A cutaneous electrogastrographic study. Am J Gastroenterol. 1992;87:702–707. [PubMed] [Google Scholar]
- 14.Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin North Am. 1992;21:751–776. [PubMed] [Google Scholar]
- 15.Kline L, Karpinski E. A comparison of the effects of various sex steroids on cholecystokinin- and KCl-induced tension in female guinea pig gallbladder strips. Gen Comp Endocrinol. 2013;185:37–43. doi: 10.1016/j.ygcen.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 18.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90:3F–6F. doi: 10.1016/S0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 19.Dambros M, van Koeveringe GA, Bast A, van Kerrebroeck PE. Relaxant effects of estradiol through non-genomic pathways in male and female pig bladder smooth muscle. Pharmacology. 2004;72:121–127. doi: 10.1159/000080184. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Qiao X, Falone AE, Reslan OM, Sheppard SJ, Khalil RA. Gender-specific reduction in contraction is associated with increased estrogen receptor expression in single vascular smooth muscle cells of female rat. Cell Physiol Biochem. 2010;26:457–470. doi: 10.1159/000320569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan AM, Kennelly R, Collins D, Baird AW, Winter DC. Oestrogen inhibits human colonic motility by a non-genomic cell membrane receptor-dependent mechanism. Br J Surg. 2009;96:817–822. doi: 10.1002/bjs.6612. [DOI] [PubMed] [Google Scholar]
- 22.Zielińska M, Fichna J, Bashashati M, Habibi S, Sibaev A, Timmermans JP, Storr M. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol Motil. 2017;29:e13025. doi: 10.1111/nmo.13025. [DOI] [PubMed] [Google Scholar]
- 23.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272:H2765–H2773. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 24.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: Structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 25.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/S0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 26.Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol. 2003;284:C156–C167. doi: 10.1152/ajpcell.00233.2002. [DOI] [PubMed] [Google Scholar]
- 27.Hogg ME, Vavra AK, Banerjee MN, Martinez J, Jiang Q, Keefer LK, Chambon P, Kibbe MR. The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res. 2012;173:e1–e10. doi: 10.1016/j.jss.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wierman ME. Sex steroid effects at target tissues: Mechanisms of action. Adv Physiol Educ. 2007;31:26–33. doi: 10.1152/advan.00086.2006. [DOI] [PubMed] [Google Scholar]
- 29.Al-Shboul O. The role of the RhoA/ROCK pathway in gender-dependent differences in gastric smooth muscle contraction. J Physiol Sci. 2016;66:85–92. doi: 10.1007/s12576-015-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Shboul O, Mustafa A, Al-hashimi F. Non-genomic effects of progesterone on Rho kinase II in rat gastric smooth muscle cells. J Smooth Muscle Res. 2013;49:55–62. doi: 10.1540/jsmr.49.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol. 1995;268:G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 32.Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: Potential mechanisms of sex hormones. World J Gastroenterol. 2014;20:6725–6743. doi: 10.3748/wjg.v20.i22.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: Implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1546–R1554. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- 34.Al-Shboul OA, Al-Dwairi AN, Alqudah MA, Mustafa AG. Gender differences in the regulation of MLC20 phosphorylation and smooth muscle contraction in rat stomach. Biomed Rep. 2018;8:283–288. doi: 10.3892/br.2018.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 36.Kline LW, Karpinski E. 17β-Estradiol relaxes cholecystokinin- and KCl-induced tension in male guinea pig gallbladder strips. Steroids. 2011;76:553–557. doi: 10.1016/j.steroids.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Hogan AM, Collins D, Sheehan K, Zierau O, Baird AW, Winter DC. Rapid effects of phytoestrogens on human colonic smooth muscle are mediated by oestrogen receptor beta. Mol Cell Endocrinol. 2010;320:106–110. doi: 10.1016/j.mce.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Bayard F, Clamens S, Meggetto F, Blaes N, Delsol G, Faye JC. Estrogen synthesis, estrogen metabolism, and functional estrogen receptors in rat arterial smooth muscle cells in culture. Endocrinology. 1995;136:1523–1529. doi: 10.1210/endo.136.4.7895662. [DOI] [PubMed] [Google Scholar]
- 39.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–942. doi: 10.1161/01.RES.77.5.936. [DOI] [PubMed] [Google Scholar]
- 40.Andersen HL, Weis JU, Fjalland B, Korsgaard N. Effect of acute and long-term treatment with 17-beta-estradiol on the vasomotor responses in the rat aorta. Br J Pharmacol. 1999;126:159–168. doi: 10.1038/sj.bjp.0702289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patkar S, Farr TD, Cooper E, Dowell FJ, Carswell HV. Differential vasoactive effects of oestrogen, oestrogen receptor agonists and selective oestrogen receptor modulators in rat middle cerebral artery. Neurosci Res. 2011;71:78–84. doi: 10.1016/j.neures.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Raffetto JD, Qiao X, Beauregard KG, Khalil RA. Estrogen receptor-mediated enhancement of venous relaxation in female rat: Implications in sex-related differences in varicose veins. J Vasc Surg. 2010;51:972–981. doi: 10.1016/j.jvs.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. doi: 10.1096/fasebj.6.12.1381691. [DOI] [PubMed] [Google Scholar]
- 44.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342–G351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98:2158–2166. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong CM, Au CL, Tsang SY, Lau CW, Yao X, Cai Z, Chung AC. Role of inducible nitric oxide synthase in endothelium-independent relaxation to raloxifene in rat aorta. Br J Pharmacol. 2017;174:718–733. doi: 10.1111/bph.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang JJ, Xu XB, Li HF, Zhang XY, Zheng TZ, Qu SY. Inhibition of beta-estradiol on trachea smooth muscle contraction in vitro and in vivo. Acta Pharmacol Sin. 2002;23:273–277. [PubMed] [Google Scholar]
- 48.Twort CH, van Breemen C. Cyclic guanosine monophosphate-enhanced sequestration of Ca2+ by sarcoplasmic reticulum in vascular smooth muscle. Circ Res. 1988;62:961–964. doi: 10.1161/01.RES.62.5.961. [DOI] [PubMed] [Google Scholar]
- 49.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tropea T, De Francesco EM, Rigiracciolo D, Maggiolini M, Wareing M, Osol G, Mandalà M. Pregnancy augments G protein estrogen receptor (GPER) induced vasodilation in rat uterine arteries via the nitric oxide-cGMP signaling pathway. PloS One. 2015;10:e0141997. doi: 10.1371/journal.pone.0141997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prossnitz ER, Hathaway HJ. What have we learned about GPER function in physiology and disease from knockout mice? J Steroid Biochem Mol Biol. 2015;153:114–126. doi: 10.1016/j.jsbmb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids. 2014;81:99–102. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HJ, Suh JK, Song HH, Jeong MA, Yeom JH, Kim DW. Antioxidant effects of methylprednisolone and hydrocortisone on the impairment of endothelium dependent relaxation induced by reactive oxygen species in rabbit abdominal aorta. Korean J Anesthesiol. 2013;64:54–60. doi: 10.4097/kjae.2013.64.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, Smith HO, Hathaway HJ, Prossnitz ER. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet Gynecol Int. 2013;2013:472720. doi: 10.1155/2013/472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.