Abstract
Background
The aim of this study is to investigate the association between treatment in a closed ICU and survival at discharge in patients with sepsis.
Methods
This is a post hoc analysis utilizing data from the Japan Septic Disseminated Intravascular Coagulation study, including data from patients with sepsis from 2011 to 2013. Multiple logistic regression analysis was used to estimate the association between ICU policy and survival at discharge, and propensity score matching analysis was performed including the same covariates as a sensitivity analysis. Multiple linear regression analysis for the length of ICU stay in surviving patients was also performed with adjustments for the same covariates.
Results
Two thousand four hundred ninety-five patients were analyzed. The median Acute Physiology and Chronic Health Evaluation (APACHE) II score was 22 [17–29], the median Sequential Organ Failure Assessment (SOFA) score was 9 [7–12], and the overall mortality was 33%. There were 979 patients treated in 17 open ICUs and 1516 patients in 18 closed ICUs. In comparison, the APACHE II score and SOFA scores were significantly higher in patients in closed ICUs (closed vs open = 23 [18–29] vs 21 [16–28]; p < .001, 9 [7–13] vs 9 [6–12]; p = 0.004). There was no difference in the unadjusted mortality (closed vs open; 33.1% vs 33.2%), but in multiple logistic regression analysis, treatment in a closed ICU is significantly associated with survival at discharge (odds ratio = 1.59, 95% CI [1.276–1.827], p = .001). The sensitivity analysis (702 pairs of the matching) showed a significantly higher survival rate in the closed ICU (71.8% vs 65.2%, p = 0.011). The length of ICU stay of patients in closed ICUs was significantly shorter (20% less).
Conclusion
This Japanese nationwide analysis of patients with sepsis shows a significant association between treatment in a closed ICU and survival at discharge, and a 20% decrease in ICU stay.
Electronic supplementary material
The online version of this article (10.1186/s40560-018-0322-8) contains supplementary material, which is available to authorized users.
Keywords: Intensive care unit, Sepsis, Intensivist, Mortality
Background
Sepsis is life-threatening organ dysfunction caused by dysregulated host responses to infection, and septic shock is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are sufficiently profound to substantially increase mortality [1]. According to one study, there are more than 750,000 patients annually in the USA with sepsis [2] and the incidence is rising [3]. Septic shock remains lethal even with aggressive management, with a mortality of 20 to 30% [4].
Patients with sepsis are usually treated in the intensive care unit (ICU). Sepsis results from infection, and these patients often develop multiple organ-system failure. Aggressive management, including control of the infection source and support of failing organ-systems, is needed for optimal outcomes. In recent years, guidelines for the treatment of patients with sepsis have been published [5, 6], and intensivists play an important role in the care of these patients.
Intensivists improve patient outcome in the ICU [7], and the ICU organization model influences patient outcomes [8–10]. There are generally two staffing models for ICU treatment, including an “open organization model” and a “closed organization model.” In the open model, there is no intensivist consultation or elective intensivist consultation. Mandatory intensivist consultation is conducted in closed model ICUs [8], in which intensivist directs patient care [11] regardless of the time of day.
However, focusing on sepsis, to the best of our knowledge, the effect of the ICU organization model (open/closed) or directing of care by an intensivist on the mortality of patients is unknown and there are no studies to answer this important clinical question. We hypothesize that a closed ICU improves the outcome of patients with sepsis and septic shock, since a closed ICU is the highest-intensity physician staffing ICU model and intensivists direct care regardless of the time of day in the ICU. The aim of this study is to investigate the association between management in a closed ICU and survival at discharge of patients with sepsis. This is a nationwide study in Japan, including an analysis of clinical data regarding the severity of sepsis, pre-existing co-morbidities, the need for mechanical organ system support, and treatments given.
Methods
Patient selection
>This is a post hoc analysis utilizing the database from the Japan Septic Disseminated Intravascular Coagulation study (JSEPTIC DIC study) (University Hospital Medical Information Network Individual Case Data Repository, UMIN000012543, http://www.umin.ac.jp/icdr/index-j.html), which was a nationwide study in Japan [12]. The JSEPTIC DIC study retrospectively collected data from patients admitted to the ICU for the treatment of sepsis [13] from January 2011 to December 2013, excluding patients younger than 16 years or who developed sepsis after admission to the ICU (the JSEPTIC DIC study used the definitions of sepsis, severe sepsis, and septic shock from [13] as it was performed before publication of the 2016 definitions [1]). The JSEPTIC DIC study was approved by the Institutional Review Boards of all participating hospitals. The requirement for informed consent was waived because of the retrospective nature of the study. Since this database was already anonymized for individual patient data and institutions, the Institutional Review Board waived the need for review of this post hoc study.
Patients were divided into two groups, the closed ICU group (treated in a closed ICU) and the open ICU group (treated in an open ICU). The JSEPTIC DIC study did not demonstrate a clear definition of open ICU or closed ICUs. Each ICU had reported subjective information about their ICU organization model (open/closed/unclassified) at the initiation of the JSEPTIC DIC study. In Japan, the closed ICU is conventionally defined as a unit that transfers all patient care to an intensive care team that directs patient care with primary responsibility for all care and the open ICU is conventionally defined as an ICU where the intensive care team provides expertise via elective or mandatory consultation without assuming primary responsibility for patient care [14]. Patients treated in an ICU which could not clearly be classified as the closed or open were excluded from the final study population.
Exposure and outcome variables
The present study used all variables collected in the JSEPTIC DIC study. Variables for which the proportion of missing data was above 10% (fibrinogen, fibrin/fibrinogen degradation products, D-dimer, antithrombin, and lactate) and primary infection site are not included. The main exposure variable was set as the closed ICU or open ICU. The primary outcome measure was survival at discharge. Due to the healthcare system in Japan, no patients were discharged to hospice care.
Covariates for patient characteristics included age, gender, weight, pre-existing organ dysfunction and hemostatic disorders (comorbidity), Acute Physiology and Chronic Health Evaluation (APACHE) II score [15] (day 1), Sequential Organ Failure Assessment (SOFA) score [16] (day 1), systemic inflammatory response syndrome score [17] (days 1), the identification of microorganisms responsible for sepsis, blood culture results, and results of laboratory tests on day 1 including white blood cell count, platelet count, hemoglobin level, and prothrombin time-international normalized ratio (PT-INR). Treatment variables reviewed included administration of medications, including anti-disseminated intravascular coagulation (DIC) or anti-thrombotic drugs, immunoglobulins, low-dose steroids, and transfusion of blood products during the first week after ICU admission. Other therapeutic interventions reviewed included renal replacement therapy, renal replacement therapy for non-renal indications, plasma exchange, polymyxin B direct hemoperfusion (PMX-DHP), extracorporeal membrane oxygenation (ECMO), and use of the intra-aortic balloon pump during the first week after ICU admission.
Statistical analysis
Baseline characteristics were compared before and after the follow-up period for patients in both the closed and open ICU groups. Distributed continuous variables without a normal distribution are presented as median with interquartile range (IQR). Categorical data are summarized using numbers or percentages. The Mann-Whitney U test was used for comparing continuous variables, and the Fisher’s exact test was used for categorical data. As for the main result, multiple logistic regression analysis was performed to estimate the association between the closed/open ICU and survival at discharge from the hospital, adjusted by baseline patient characteristics and treatment variables. As a sensitivity analysis, propensity score matching analysis including the same covariate was performed. For propensity score matching analysis, we use calipers of width equal to 0.1 of the standard deviation. As for sub-analysis, multiple linear regression analysis for the log-transformed values of the length of ICU stay and hospital stay in survived patients was performed with adjustments for the same covariates, and estimated the ratio of the length of stay between the closed ICU and open ICU. The level of significance was set at p < 0.05. All statistical analyses were performed with SAS (Version 9.4, SAS Institute Inc., Cary, North Carolina, USA).
Results
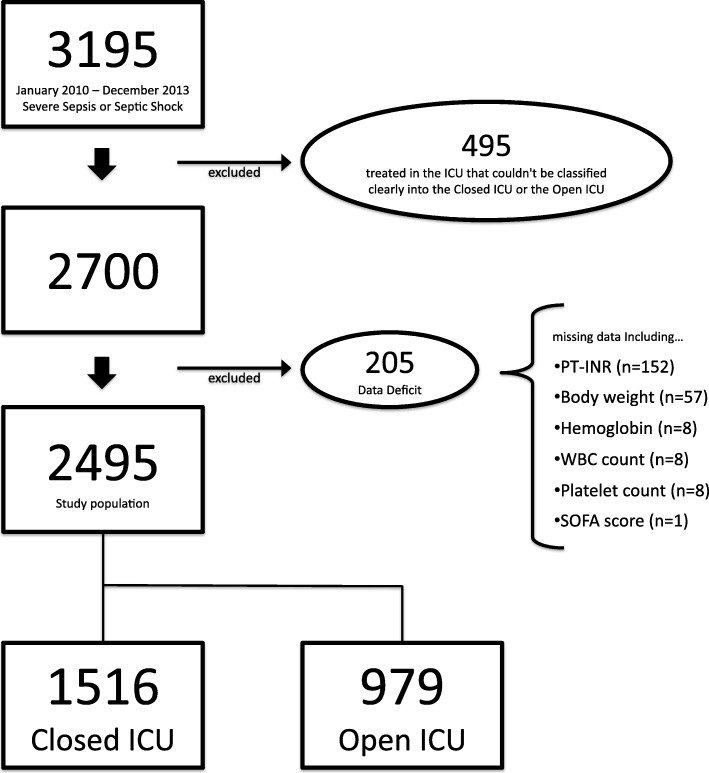
A total of 3195 patients were included in the JSEPTIC DIC study, and 2700 patients were enrolled in this study after excluding patients treated in ICUs not clearly classified as closed or open. There were data deficits in 205 patients, and data for the remaining 2495 patients were analyzed (Fig. 1). The mean age was 72 years, 59.7% male, the median APACHE II score was 23 [17–29], and the median SOFA score (day 1) was 9 [7–12]. The rate of survival at discharge was 66.8% (Table 1). Participating ICUs included 17 (49%) closed and 18 (51%) open.
Fig. 1.
Patient inclusion flow chart. Data for 3195 patients were reviewed and 495 patients did not meet inclusion criteria. There were data deficits for 205 patients, leaving a final study group of 2495 patients. The number of patients with missing data is not considered regarding duplication. PT-INR: prothrombin time international normalized ratio; WBC: white blood cell; SOFA score: Sequential Organ Failure Assessment score
Table 1.
Comparison for patient characteristics and treatments (n = 2495)
| All patients (n = 2495) | Closed ICU (n = 1516) | Open ICU (n = 979) | p value | |
|---|---|---|---|---|
| In-hospital surgical ICU | 50 (2.0%) | 50 (3.3%) | 0 (0.0%) | < 0.001* |
| In-hospital general ICU | 1168 (46.8%) | 644 (42.5%) | 5234 (53.5%) | < 0.001* |
| Emergency ICU | 1277 (51.2%) | 822 (54.2%) | 455 (46.5%) | < 0.001* |
| Number of beds (IQR†) | 12 [8–18] | 10 [6–18] | 12 [10–20] | < 0.001* |
| SOFA score (IQR†) | 9 [7–12] | 9 [7–13] | 9 [6–12] | 0.004* |
| APACHE II score (IQR†) | 22 [17–29] | 23 [18–29] | 21 [16–28] | < 0.001* |
| SIRS score (IQR†) | 3 [2–4] | 3 [2–4] | 3 [2–4] | 0.058 |
| Age (IQR†, year old) | 72 [62–80] | 72 [62–80] | 73 [63–81] | 0.007* |
| Sex (male, %) | 1490 (59.7%) | 916 (60.4%) | 574 (58.6%) | 0.373 |
| Body weight (IQR†, kg) | 54.5 [46.3–64] | 54.7 [46.5–65] | 54.0 [46.0–63] | 0.208 |
| White blood cell count (IQR†, × 103) | 11.2 [4.5–17.8] | 11.00 [4.40–17.76] | 11.66 [4.85–17.80] | 0.199 |
| Hemoglobin (IQR†, g/dl) | 10.6 [9–12.4] | 10.5 [8.9–12.4] | 10.6 [9.1–12.5] | 0.055 |
| Platelet count (IQR†, × 103) | 120 [64–191] | 121 [64–194] | 118 [64–186] | 0.498 |
| Prothrombin time international normalize ratio (IQR†) | 1.34 [1.17–1.61] | 1.35 [1.18–1.63] | 1.32 [1.16–1.56] | 0.057 |
| Co-morbidities | ||||
| Liver failure (yes, %) | 104 (4.2%) | 68 (4.5%) | 36 (3.7%) | 0.324 |
| Respiratory failure (yes, %) | 98 (3.9%) | 64 (4.2%) | 34 (3.5%) | 0.347 |
| Cardiac failure (yes, %) | 134 (5.4%) | 82 (5.4%) | 52 (5.3%) | 0.916 |
| Renal failure (yes, %) | 217 (8.7%) | 114 (7.5%) | 103 (10.5%) | 0.009* |
| Immunological disorder (yes, %) | 381 (15.3%) | 263 (17.3%) | 118 (12.1%) | < 0.001* |
| Hematologic disorder | ||||
| Cirrhosis (yes, %) | 97 (3.9%) | 63 (4.2%) | 34 (3.5%) | 0.389 |
| Hematologic malignancy (yes, %) | 84 (3.4%) | 58 (3.8%) | 26 (2.7%) | 0.113 |
| Chemotherapy (yes, %) | 114 (4.6%) | 79 (5.2%) | 35 (3.6%) | 0.056 |
| Warfarin intake (yes, %) | 113 (4.5%) | 73 (4.8%) | 40 (4.1%) | 0.392 |
| Others (yes, %) | 48 (1.9%) | 33 (2.2%) | 15 (1.5%) | 0.252 |
| Positive blood culture | 1111 (44.5%) | 679 (44.8%) | 432 (44.1%) | 0.745 |
| Negative blood culture | 1240 (49.7%) | 778 (51.3%) | 462 (47.2%) | 0.044* |
| No blood culture | 144 (5.8%) | 59 (3.9%) | 85 (8.7%) | < 0.001* |
| Viral infection | 22 (0.9%) | 18 (1.2%) | 4 (0.4%) | 0.042* |
| GNR infection | 912 (36.6%) | 528 (34.8%) | 384 (39.2%) | 0.026* |
| GPC infection | 586 (23.5%) | 344 (22.7%) | 242 (24.7%) | 0.243 |
| Fungal infection | 38 (1.5%) | 27 (1.8%) | 11 (1.1%) | 0.190 |
| Mixed infection | 339 (13.6%) | 246 (16.2%) | 93 (9.5%) | < 0.001* |
| Other infection | 45 (1.8%) | 33 (2.2%) | 12 (1.2%) | 0.081 |
| Unknown infection | 553 (22.2%) | 320 (21.1%) | 233 (23.8%) | 0.114 |
| Red blood cell transfusion (IQR†, units) | 0 [0–4] | 0 [0–4] | 0 [0–4] | < 0.001* |
| Fresh frozen plasma transfusion (IQR†, units) | 0 [0–4] | 0 [0–5] | 0 [0–4] | 0.002* |
| Platelet concentration transfusion (IQR†, units) | 0 [0–0] | 0 [0–10] | 0 [0–0] | < 0.001* |
| Treatment for DIC (yes, %) | 1074 (43.0%) | 656 (43.3%) | 418 (42.7%) | 0.777 |
| Antithrombin III (yes, %) | 726 (29.1%) | 447 (29.5%) | 279 (28.5%) | 0.596 |
| rhsTM (yes, %) | 636 (25.5%) | 420 (27.7%) | 216 (22.1%) | 0.002* |
| Nafamostat (yes, %) | 880 (35.3%) | 533 (35.2%) | 347 (35.5%) | 0.884 |
| Heparin (yes, %) | 453 (18.2%) | 247 (16.3%) | 206 (21.0%) | 0.003* |
| Warfarin (yes, %) | 39 (1.6%) | 15 (1.0%) | 24 (2.5%) | 0.004* |
| Antiplatelet (yes, %) | 56 (2.2%) | 37 (2.4%) | 19 (1.9%) | 0.411 |
| Others (yes, %) | 15 (0.6%) | 7 (0.5%) | 8 (0.8%) | 0.262 |
| Specific treatment | ||||
| Immunoglobulin (yes, %) | 743 (29.8%) | 447 (29.5%) | 296 (30.2%) | 0.689 |
| Low dose steroid (yes, %) | 600 (24.0%) | 413 (27.2%) | 187 (19.1%) | < 0.001* |
| Renal replacement therapy (yes, %) | 664 (26.6%) | 452 (29.8%) | 212 (21.7%) | < 0.001* |
| Renal replacement therapy not for renal indication (yes, %) | 165 (6.6%) | 58 (3.8%) | 107 (10.9%) | < 0.001* |
| Polymyxin B direct hemoperfusion (yes, %) | 547 (21.9%) | 316 (20.8%) | 231 (23.6%) | 0.105 |
| Plasma exchange (yes, %) | 20 (0.8%) | 9 (0.6%) | 11 (1.1%) | 0.147 |
| Veno-arterial ECMO (yes, %) | 23 (0.9%) | 20 (1.3%) | 3 (0.3%) | 0.010* |
| Veno-venus ECMO (yes, %) | 27 (1.1%) | 26 (1.7%) | 1 (0.1%) | < 0.001* |
| Intra-aortic balloon pumping (yes, %) | 11 (0.4%) | 7 (0.5%) | 4 (0.4%) | 0.845 |
| Mechanical ventilation support (yes, %) | 1799 (72.1%) | 1184 (78.1%) | 615 (62.8%) | < 0.001* |
| Survival discharge (yes, %) | 1668 (66.9%) | 1014 (66.9%) | 654 (66.8%) | 0.965 |
IQR† median [25%, 75%] for continuous variables, ICU intensive care unit, SOFA score Sequential Organ Failure Assessment score, APACHE II score Acute Physiology and Chronic Health Evaluation II score, SIRS score systemic inflammatory response syndrome score, GNR gram-negative rods, GPC gram-positive coccus, DIC disseminated intravascular coagulation, rhsTM recombinant human soluble thrombomodulin, ECMO extracorporeal membrane oxygenation
*p < 0.05
There were 979 patients treated in open ICUs and 1516 patients in closed ICUs. A comparison of the characteristics of these two groups is shown in Table 1. The closed ICU group included fewer patients with renal failure than the open ICU group (closed vs open = 7.5% vs 10.5%, p = 0.009), and the closed ICU group included significantly more patients with immunological disorders (closed vs open = 17.4% vs 12.1%, p < .001). The APACHE II and SOFA scores (day 1) of patients in the closed ICU group is significantly higher than that in the open ICU (APACHE II score: closed vs open = 23 [18–29] vs 21 [16–28], p < 0.001, SOFA score: closed vs open = 9 [7–13] vs 9 [6–12], p = 0.004). Patients in the closed ICU group were more severely ill than patients in the open ICU group, based on these scores.
The value of variables examined was different comparing treatments used during follow-up in the closed and open groups. Recombinant human soluble thrombomodulin (rhsTM) is used more frequently in the closed ICU group (closed vs open = 27.7% vs 22.1%, p = 0.002), but heparin and warfarin are used more often in the open ICU group (heparin; closed vs open = 16% vs 21%, p = 0.003), (warfarin; closed vs open = 1.0% vs 2.5%, p = 0.004). The use of low-dose steroids in patients with sepsis (closed vs open = 27.2% vs 19.1%, p < 0.001) and renal replacement therapy was more frequent in the closed ICU group (closed vs open = 29.8% vs 21.7%, p < 0.001), but renal replacement therapy for non-renal indications was more often performed in the open ICU group (closed vs open = 3.8% vs 10.9%, p < 0.001). Mechanical ventilation was more frequently used in the closed ICU group (closed vs open = 78.1% vs 62.8%, p < 0.001).
In the main analysis, the crude, the unadjusted analysis did not show a significant difference in survival at discharge between the closed and open ICU models (closed vs open = 66.9% vs 66.8%, p = 0.97). However, in multiple logistic regression analysis adjusted by baseline, patient characteristics and treatment variables had a significant association between treatment in a closed ICU and survival at discharge (odds ratio = 1.59, 95% CI [1.276–1.827], p = 0.001) (Table 2). In sensitivity analysis (702 pairs after propensity score matching), the closed ICU group showed a significantly higher survival rate, compared to the open ICU (71.8% vs 65.2%, odds ratio = 1.41 (95% CI [1.12–1.77]), p = 0.011) (Table 3, Additional file 1: Table S1).
Table 2.
Multiple logistic regression analysis adjusted for baseline patient characteristics and treatment (n = 2495)
| Coefficient | Adj OR | 95% CI | p value | ||||
|---|---|---|---|---|---|---|---|
| Closed ICU | (Ref: open ICU) | 0.463 | 1.589 | 1.276 | – | 1.827 | 0.001* |
| Type of ICU | (Ref: in-hospital general ICU) | ||||||
| In-hospital surgical ICU | − 0.525 | 0.592 | 0.286 | – | 1.223 | 0.157 | |
| Emergency ICU | 0.101 | 1.106 | 0.9 | – | 1.36 | 0.338 | |
| Number of beds | (Continuous) | − 0.002 | 0.998 | 0.981 | – | 1.016 | 0.846 |
| Blood culture | (Ref: no blood culture) | ||||||
| Positive | − 0.785 | 0.456 | 0.279 | – | 0.746 | 0.002* | |
| Negative | − 0.407 | 0.666 | 0.419 | – | 1.059 | 0.086 | |
| Infection type | (Ref: unknown infection) | ||||||
| Viral infection | 0.447 | 1.563 | 0.48 | – | 5.085 | 0.458 | |
| GNR infection | 0.606 | 1.833 | 1.349 | – | 2.492 | < 0.001* | |
| GPC infection | 0.224 | 1.251 | 0.898 | – | 1.743 | 0.185 | |
| Fungal infection | − 0.490 | 0.613 | 0.284 | – | 1.321 | 0.211 | |
| Mixed infection | 0.015 | 1.015 | 0.715 | – | 1.442 | 0.933 | |
| Other infection | 0.656 | 1.926 | 0.908 | – | 4.086 | 0.088 | |
| SOFA score | (Continuous) | − 0.133 | 0.876 | 0.845 | – | 0.908 | < 0.001* |
| APACHE II score | (Continuous) | − 0.018 | 0.982 | 0.969 | – | 0.996 | 0.010* |
| SIRS score | (Continuous) | − 0.115 | 0.892 | 0.796 | – | 0.999 | 0.048* |
| Sex, female | (Ref: male) | 0.373 | 1.451 | 1.171 | – | 1.799 | < 0.001* |
| Age | (Continuous) | − 0.0238 | 0.976 | 0.969 | – | 0.985 | < 0.001* |
| Body weight | (Continuous) | 0.0193 | 1.020 | 1.011 | – | 1.028 | < 0.001* |
| White blood cell count (× 103) | (Ref: 3.5~9) | ||||||
| 0~3.5 | 0.267 | 1.306 | 0.741 | – | 2.302 | 0.355 | |
| 9~ | − 1.407 | 0.245 | 0.019 | – | 3.107 | 0.278 | |
| Hemoglobin | (Continuous) | 0.049 | 1.05 | 1.006 | – | 1.097 | 0.026* |
| Platelet count (per 104) | (Continuous) | 0.003 | 1.003 | 0.991 | – | 1.015 | 0.624 |
| PT-INR | (Continuous) | − 0.0614 | 0.94 | 0.868 | – | 1.019 | 0.135 |
| Co-morbidities | (Ref: no) | ||||||
| Liver failure | − 0.724 | 0.485 | 0.288 | – | 0.817 | 0.007* | |
| Respiratory failure | − 0.434 | 0.648 | 0.404 | – | 1.038 | 0.071 | |
| Cardiac failure | − 0.486 | 0.615 | 0.403 | – | 0.938 | 0.024* | |
| Renal failure | − 0.640 | 0.527 | 0.368 | – | 0.756 | < 0.001* | |
| Immunological disorder | − 0.547 | 0.579 | 0.43 | – | 0.779 | < 0.001* | |
| Hematologic disorder | (Ref: no) | − 0.355 | 0.701 | 0.516 | – | 0.952 | 0.023* |
| Red blood cell transfusion | (Continuous) | − 0.022 | 0.978 | 0.956 | – | 1.001 | 0.061 |
| Fresh frozen plasma transfusion | (Continuous) | − 0.010 | 0.99 | 0.98 | – | 1.001 | 0.083 |
| Platelet concentration transfusion | (Continuous) | 0.002 | 1.002 | 0.996 | – | 1.008 | 0.529 |
| Treatment for DIC | (Ref: no) | ||||||
| Antithrombin III | 0.185 | 1.203 | 0.938 | – | 1.544 | 0.145 | |
| rhTM | 0.294 | 1.341 | 1.043 | – | 1.725 | 0.022* | |
| Nafamostat | 0.152 | 1.165 | 0.869 | – | 1.561 | 0.308 | |
| Heparin | 0.334 | 1.396 | 1.06 | – | 1.839 | 0.018* | |
| Warfarin | − 0.148 | 0.862 | 0.400 | – | 1.861 | 0.706 | |
| Antiplatelet | 0.318 | 1.374 | 0.679 | – | 2.778 | 0.377 | |
| Others | 0.303 | 1.354 | 0.318 | – | 5.768 | 0.682 | |
| Specific treatment | (Ref: no) | ||||||
| Immunoglobulin | 0.147 | 1.158 | 0.909 | – | 1.476 | 0.236 | |
| Low-dose steroid | − 0.534 | 0.586 | 0.462 | – | 0.744 | < 0.001* | |
| Renal replacement therapy | − 0.390 | 0.677 | 0.495 | – | 0.926 | 0.015* | |
| Renal replacement therapy not for renal indication | − 0.279 | 0.756 | 0.496 | – | 1.153 | 0.195 | |
| Polymyxin B direct hemoperfusion | 0.365 | 1.44 | 1.095 | – | 1.894 | 0.009* | |
| Plasma exchange | − 0.457 | 0.633 | 0.189 | – | 2.118 | 0.459 | |
| Veno-arterial ECMO | − 2.475 | 0.084 | 0.021 | – | 0.341 | < 0.001* | |
| Veno-venus ECMO | − 1.169 | 0.311 | 0.111 | – | 0.865 | 0.025* | |
| Ventilation support | − 0.815 | 0.443 | 0.339 | 0.578 | < 0.001* | ||
| Intra-aortic balloon pumping | 0.993 | 2.699 | 0.467 | – | 15.608 | 0.268 | |
White blood cell count (× 103)
adj OR adjusted odds ratio, CI confidence intervals, ICU intensive care unit, GNR gram-negative rods, GPC gram-positive coccus, SOFA score Sequential Organ Failure Assessment score, APACHE II score Acute Physiology and Chronic Health Evaluation II score, SIRS score systemic inflammatory response syndrome score, PT-INR prothrombin time-international normalized ratio, DIC disseminated intravascular coagulation, rhsTM recombinant human soluble thrombomodulin, ECMO extracorporeal
*p < 0.05
Table 3.
Cross table after propensity score matching
| Open ICU | Closed ICU | ||
|---|---|---|---|
| Death | 244 | 198 | 442 |
| Survive | 458 | 504 | 962 |
| 702 | 702 | 1404 |
P = 0.011 (chi-squared test). OR = 1.41 (95% CI 1.12–1.77)
OR odds ratio, CI confidence intervals
In sub-analysis, multiple linear regression analysis using the data about the length of ICU stay and hospital stay as an objective variable showed that the length of ICU stay of patients treated in closed ICUs was significantly shorter (20% less) than patients in open ICUs, but this significance was not seen in the length of hospital stay (Table 4).
Table 4.
The multiple liner regression analysis for the log-transformed values of the length of ICU stay and hospital stay in survived patients
| Ratio | 95% confidence interval | p value | ||
|---|---|---|---|---|
| Sub-analysis 1: multiple linear regression in survived patients for the length of ICU stay | ||||
| (Closed ICU/open ICU) * | 0.80 | 0.75 | 0.87 | < 0.001 |
| Sub-analysis 2: multiple linear regression in survived patients for the length of hospital stay | ||||
| (Closed ICU/open ICU) * | 0.98 | 0.90 | 1.07 | 0.642 |
*Adjusted by both patient backgrounds and treatment variables (exactly the same covariates in main analysis)
Discussion
In this analysis of a large nationwide Japanese cohort of patients with sepsis and septic shock, patient management in a closed ICU is significantly associated with improved rate of survival at discharge and a decrease in the length of ICU stay.
Efficacy of the treatment of sepsis in a closed ICU
According to multiple logistic regression analysis adjusted for baseline characteristics and treatment variables, treatment in a closed ICU had a significant association with improved survival at discharge (odds ratio = 1.59, 95% CI [1.276–1.827], p = 0.001) (Table 2). This result suggests that the efficacy of care in a closed ICU may depend on the management of sepsis conducted in a closed ICU. However, according to the crude comparison (Table 1), the patient cohorts were different in the closed and open ICUs. We additionally performed propensity score matching analysis as a sensitivity analysis, and the closed ICU group has a significantly higher survival rate, compared to open ICU (Table 3, Additional file 1: Table S1). The result of the main analysis is fully supported by the sensitivity analysis. There is a significant association between treatment in a closed ICU and improved survival at discharge.
A significant difference in the rate of using various treatment modalities in closed and open ICUs is identified in this study (Table 1). Although these treatments are adjuvant therapies for sepsis and their efficacy is controversial, the difference in the rate of using these treatments performed in each ICU may be partly responsible for the observed significant association between treatment in a closed ICU and survival at discharge. The present study demonstrates a significant association between prognosis and each specific treatment for sepsis including use of recombinant human soluble thrombomodulin (rhsTM), low-dose steroid therapy, or PMX-DHP.
Some DIC treatment guidelines recommend the use of rhsTM rather than heparin for patients with DIC due to sepsis [18], and both positive and negative evidence regarding the survival benefit of rhsTM have been reported [19, 20]. The result of a phase 3 trial of rhsTM [21] is awaited. The JSEPTIC DIC study using propensity score analysis reported a survival benefit with the use of rhsTM [12]. The present study, which uses the same data as the JSEPTIC DIC study, also shows a significant association between survival at discharge and rhsTM, using a different statistical analysis, and the use of rhsTM was more frequent in closed ICUs and the use of heparin was more frequent in open ICUs. This difference may be partly responsible for the association between improved survival at discharge and treatment in a closed ICU.
The efficacy of steroid therapy to reduce mortality in patients with sepsis has been controversial [22, 23]. In the present study, the use of low-dose steroids (which is more frequent in a closed ICU) was significantly associated with in-hospital mortality, but the route of administration, timing, and presence of side effects were not reviewed. A significant association between the use of PMX-DHP and survival at discharge was also shown, but there was no significant difference of the using rate of PMX-DHP between the closed and open ICUs. Some trials showed a survival benefit with the use of PMX-DHP [24], but others have not [25]. The effect of PMX-DHP remains controversial, and the results of another prospective multicenter randomized controlled trial is awaited, including a phase 3 trial of PMX-DHP [26].
We consider that a significant association between treatment in a closed ICU and survival at discharge can be related to the high-quality care provided in a closed ICU. A high rate of compliance with guidelines is one element of “high quality” intensive care. This element affects the patients’ prognosis, and compliance is different in open and closed ICUs. Some reports show that patients with acute respiratory distress syndrome (ARDS) cared for in a closed ICU had lower hospital mortality due to the increased rate of compliance with guideline-recommended lung-protective ventilation [27] (a safety management was given for the patients with ARDS in the closed ICU). Considering the present study, a significantly lower rate of obtaining blood cultures (blood cultures are strongly recommended in the sepsis guideline [5, 6]) in the open ICU (Table 1) suggests that compliance with the guidelines in an open ICU may be lower than that in closed ICUs and the lower compliance may contribute to the higher mortality in open ICUs. Patient with sepsis commonly need ICU care, and in this decade, guidelines for the treatment of sepsis have been published from international societies [5]. The management of patients with sepsis has been qualified and standardized throughout the world, and the closed ICU can provide standardized intensive care for patients with sepsis based on the guidelines, which can improve the outcomes [28].
The safety management, prevention, or rapid and optimal response to complications is an element of “high quality” intensive care. Another report from the Leapfrog Group reported that applying ICU physician safety staffing standards could save more than 54,000 lives in the USA each year [29]. Intensivists can be an expert in safety management and complication management (prevention or appropriate response) in the ICU. Although the present study did not investigate the differences in incidence of complications or other safety management issues between closed and open ICUs, this potential difference may contribute to the significant association between treatment in a closed ICU and a higher survival rate at discharge. Future study should be focused on the differences in these factors.
Study limitations
This is a sub-analysis of the JSEPTIC DIC study, which used retrospective data. The 205 patients excluded because of data insufficiency may have an impact on the results although these excluded patients represent less than 10% of the study population. The J-SEPTIC DIC study did not clearly define open and closed ICUs, and each ICU had reported subjective information about their ICU model (open/closed/unclassified). This study comparing the outcomes of patients with sepsis in open and closed ICUs was conducted based on a definition that was subjectively reported from each institution. Although the present study demonstrates the potential of improved efficacy of the treatment of sepsis in a closed ICU, this result may be unreliable because of an unclear definition of the types of ICUs. Further study should clearly define open and closed ICUs to investigate the association between the outcome of patients with sepsis and ICU organization models.
This study focuses only on the association between outcomes and the type of ICU model. Details of differences in ICU treatment and quality of care in each institution have not been analyzed. Although many kinds of ICU staffing components influence ICU outcomes, the information such as hospital characteristics, general/university; the staffing numbers for open or closed ICU’s in this study, nursing/junior doctors/fellows/certificated specialist intensivists/non-certificated intensivist; or nighttime coverage by certificated specialist intensivists was not available in this post hoc analysis. These staffing patterns must influence the outcomes of patients in the ICU, but this information was unavailable in this study. This is a severe limitation of the present study. Future studies should include staffing information to investigate a relationship between the outcomes of patients with sepsis and treatment using each ICU model (open/closed).
Although many kinds of guidelines or safety management protocols in the ICU such as hand hygiene protocols, ventilation-associated pneumonia bundles, and catheter-related blood stream infection management impact outcomes in the care of patients with sepsis, compliance with these care bundles was not evaluated in this study. Future studies should focus on compliance with sepsis and other care guidelines, treatment differences, and quality of care in the ICU. To further define the benefit of a closed ICU, additional prospective multicenter studies are warranted.
Conclusions
This nationwide retrospective post hoc analysis of patients with sepsis in Japan shows a significant association between treatment in a closed ICU and improved survival at discharge, although there are acknowledged study limitations. Future prospective trials are indispensable to evaluate the efficacy of treatment in a closed ICU for the treatment of patients with sepsis. These studies should define the ICU models (closed/open) clearly and focus on staffing patterns, compliance with care guidelines, treatment differences, and quality of care in ICUs with both closed and open ICU models.
Additional file
Table S1. Comparison for patient characteristics and treatments after matching. (DOCX 25 kb)
Acknowledgements
We wish to express our gratitude to all of the J-Septic DIC study contributors: Mineji Hayakawa, Kazuma Yamakawa, Shinjiro Saito, Shigehiko Uchino, Daisuke Kudo, Yusuke Iizuka, Masamitsu Sanui, Kohei Takimoto, Toshihiko Mayumi, Kota Ono, Takeo Azuhata, Fumihito Ito, Shodai Yoshihiro, Katsura Hayakawa, Tsuyoshi Nakashima, Eiichiro Noda, Ryosuke Sekine, Yoshiaki Yoshikawa, Motohiro Sekino, Keiko Ueno, Yuko Okuda, Masayuki Watanabe, Akihito Tampo, Nobuyuki Saito, Yuya Kitai, Hiroki Takahashi, Iwao Kobayashi, Yutaka Kondo, Wataru Matsunaga, Sho Nachi, Toru Miike, Hiroshi Takahashi, Shuhei Takauji, Kensuke Umakoshi, Takafumi Todaka, Hiroshi Kodaira, Kohkichi Andoh, Takehiko Kasai, Yoshiaki Iwashita, Hideaki Arai, Masato Murata, Masahiro Yamane, Kazuhiro Shiga, and Naoto Hori.
Availability of data and materials
The dataset generated and analyzed during the current study is not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- APACHE II score
Acute Physiology and Chronic Health Evaluation II score
- ARDS
Acute respiratory distress syndrome
- DIC
Disseminated intravascular coagulation
- ECMO
Extracorporeal membrane oxygenation
- ICU
Intensive care unit
- JSEPTIC DIC study
Japan Septic Disseminated Intravascular Coagulation study
- PMX-DHP
Polymyxin B direct hemoperfusion
- PT-INR
Prothrombin time-international normalized ratio
- SOFA score
Sequential Organ Failure Assessment score
Authors’ contributions
TO conceived and designed this study, contributed to acquisition, analysis, and interpretation of the data, and was responsible for the drafting, editing, and submission of the manuscript. YN and DK contributed to interpretation of the data and drafting of the manuscript. SM, KT, and KN played a significant role in the analysis of the data and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the ethics committee of all the participating hospitals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takayuki Ogura, Phone: +81-27-224-4585, Email: alongthelongestway2003@yahoo.co.jp.
Yoshihiko Nakamura, Email: pdmxy827@yahoo.co.jp.
Kunihiko Takahashi, Email: kunihiko@med.nagoya-u.ac.jp.
Kazuki Nishida, Email: nishida@med.nagoya-u.ac.jp.
Daisuke Kobashi, Email: daisuke_kobashi_8231@yahoo.co.jp.
Shigeyuki Matsui, Email: smatsui@med.nagoya-u.ac.jp.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Lagu T, Rothberg MB, Shieh MS, Pe-kow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 4.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 6.Oda S, Aibiki M, Ikeda T, Imaizumi H, Endo S, Ochiai R, Sepsis Registry Committee of The Japanese Society of Intensive Care Medicine et al. The Japanese guidelines for the management of sepsis. J Intensive Care. 2014;2:55. doi: 10.1186/s40560-014-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JL. Need for intensivists in intensive-care units. Lancet. 2000;356:695–696. doi: 10.1016/S0140-6736(00)02622-2. [DOI] [PubMed] [Google Scholar]
- 8.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DJ, Angus DC, Barnato AM, Kramer AA, Khan JM. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366:2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox ME, Chong CA, Niven DJ, Rubenfeld GD, Rowan KM, Wunsch H, et al. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Crit Care Med. 2013;41:2253–2274. doi: 10.1097/CCM.0b013e318292313a. [DOI] [PubMed] [Google Scholar]
- 11.Carson SS, Stocking C, Podsadecki T, Christenson J, Pohlman A, MacRae S, et al. Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of ‘open’ and ‘closed’ formats. JAMA. 1996;276:322–328. doi: 10.1001/jama.1996.03540040066035. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa M, Yamakawa K, Saito S, Uchino S, Kudo D, Iizuka Y, Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study group et al. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost. 2016;115:1157–1166. doi: 10.1160/TH15-12-0987. [DOI] [PubMed] [Google Scholar]
- 13.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 14.Ono Y, Tanigawa K, Shinohara K, Yano T, Sorimachi K, Sato L, et al. Difficult airway management resources and capnography use in Japanese intensive care units: a nationwide cross-sectional study. J Anesth. 2016;30:644–652. doi: 10.1007/s00540-016-2176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793–1800. [DOI] [PubMed]
- 17.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Wada H, Thachil J, Di Nisio M, Mathew P, Kurosawa S, Gando S, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013; 10.1111/jth.12155. [Epub ahead of print] [DOI] [PubMed]
- 19.Yamakawa K, Ogura H, Fujimi S, Morikawa M, Ogawa Y, Mohri T, et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med. 2013;39:644–652. doi: 10.1007/s00134-013-2822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Recombinant human soluble thrombomodulin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2015;13:31–40. doi: 10.1111/jth.12786. [DOI] [PubMed] [Google Scholar]
- 21.Phase 3 safety and efficacy study of ART-123 in subjects with severe sepsis and coagulopathy. http://clinicaltrials.gov/ct2/show/NCT01598831?term=ART-123&rank=2 of subordinate document. Accessed 1st July 2015.
- 22.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al: CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008;358:111–124. [DOI] [PubMed]
- 23.Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Crit Care. 2010;14:R134. doi: 10.1186/cc9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 25.Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, ABDOMIX group et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975–984. doi: 10.1007/s00134-015-3751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein DJ, Foster D, Schorr CA, Kazempour K, Walker PM, Dellinger RP. The EUPHRATES trial (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock): study protocol for a randomized controlled trial. Trials. 2014;15:218. doi: 10.1186/1745-6215-15-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treggiari MM, Martin DP, Yanez ND, Caldwell E, Hudson LD, Rubenfeld GD. Effect of intensive care unit organizational model and structure on outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2007;176:685–690. doi: 10.1164/rccm.200701-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40:1623–1633. doi: 10.1007/s00134-014-3496-0. [DOI] [PubMed] [Google Scholar]
- 29.LEAPFROG PATIENT SAFETY STANDARDS. The Potential Benefits of Universal Adoption. 2000. http://www.safetyleaders.org/SafePracticeArticles/leapfrog_patient_safety_standards.pdf. Accessed 1st Apr 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison for patient characteristics and treatments after matching. (DOCX 25 kb)
Data Availability Statement
The dataset generated and analyzed during the current study is not publicly available but are available from the corresponding author on reasonable request.