Abstract
Treatment with endothelin receptor antagonists (ERA) can result in adverse hepatic effects in patients with pulmonary arterial hypertension (PAH). We evaluated the hepatic safety of ambrisentan (ABS), an ERA, used as monotherapy, or with tadalafil (TAD), a phosphodiesterase-5 (PDE5) inhibitor as initial combination therapy (ABS + TAD) in the AMBITION trial. This was a retrospective analysis set in academic and private outpatient clinics and research centers. This analysis included 596 patients with PAH who were randomized to ABS or TAD as monotherapy or ABS + TAD as initial combination therapy and received at least one dose of study drug, and who had baseline and follow-up hepatic function data. Treatment options following a clinical failure event included blinded combination therapy (BCT). The proportion of patients with elevations in alanine or aspartate aminotransferases (ALT/AST) > 3 × upper limit of normal (ULN), and those with total bilirubin (TBili) > 2× ULN and ALT or AST > 3 × ULN (referred to as potential Hy’s law), were determined before BCT as well as including time on BCT. Elevations in ALT/AST > 3 × ULN during the study were in the range of 3.4–3.7%, with an annualized incidence of 2.1–2.93%. The majority of patients with elevations in ALT/AST had elevations > 3 to ≤ 5 × ULN. Three patients (0.5%) had ALT/AST > 3 × ULN plus TBili > 2 × ULN. All three patients had probable alternative causes (cardiogenic shock, liver metastases, lymphoma) for the elevations. Our analysis of the AMBITION trial demonstrated that ABS and ABS + TAD were not associated with drug-induced liver injury.
Keywords: pulmonary arterial hypertension, endothelin receptor antagonists, drug-induced liver injury (DILI), Hy’s law, evaluation of drug-induced serious hepatotoxicity (eDISH)
Introduction
Pulmonary arterial hypertension (PAH) is a rare progressive disease characterized by vasoconstriction, hyperproliferation, and thrombosis in situ and, if left untreated, may culminate in right heart failure and death. Prognosis was poor until the introduction of epoprostenol which demonstrated improved mortality and was ultimately approved by the Food and Drug Administration (FDA) in 2000.1,2 D’Alonzo et al. published the first paper in 1991 on results from the National Institutes of Health Registry which demonstrated a median survival in untreated patients with idiopathic PAH of 2.8 years with an estimated five-year survival of 34%.3 There have been significant advancements in the therapeutic armamentarium of PAH over the last 20 years and drug development has yielded the approval of 10 compounds in various dosage forms for the treatment of PAH. Current treatments target three pathways: (1) prostacyclin; (2) endothelin; and (3) nitric oxide, which includes phosphodiesterase-5 (PDE5) and soluble guanylate cyclase as targets within this pathway. The endothelin receptor antagonists (ERA) and PDE5 inhibitors are oral agents and play a major role in the treatment of this devastating disease. These agents have shown improvements in clinical parameters such as functional capacity and clinical worsening.4–7
The AMBITION trial defined initial ambrisentan (ABS, an ERA) in combination with tadalafil (TAD, a PDE5 inhibitor) as the optimal treatment strategy in patients diagnosed with PAH. Despite their important role in the treatment of PAH, some drugs in the ERA class have been associated with adverse hepatic effects. The FDA has used Hy’s law and the evaluation of drug-induced serious hepatotoxicity (eDISH) tool in clinical trials to further evaluate the potential of a drug to cause drug-induced liver injury (DILI).8 Although the requirement of monthly liver function monitoring was removed from the ABS labeling by the FDA in March 2011,9 there continues to be interest in the hepatic safety of ERAs. There are limited data in the literature on the incidence of transaminase elevations with TAD. We sought to evaluate the hepatic safety of ABS as monotherapy, or combination therapy with ABS and TAD (ABS + TAD) in the AMBITION trial.10
Methods
This is a post-hoc analysis of the previously described AMBITION trial.10 Briefly, AMBITION was a Phase III/IV, randomized, double-blind, event-driven trial evaluating the safety and efficacy of aggressive initial therapy with ABS + TAD compared to ABS or TAD in adult patients with right heart catheterization diagnosed World Health Organization Group I PAH and World Health Organization/New York Heart Association functional class II or III symptoms. Patients were randomized 2:1:1 to initial ABS + TAD, ABS, or TAD; TAD was initiated at 20 mg QD and titrated to 40 mg QD after four weeks, and ABS was initiated at 5 mg QD and titrated to 10 mg QD after eight weeks. The primary endpoint was time to first clinical failure event defined as the first occurrence of a composite of death (all-cause), hospitalization for worsening PAH, disease progression, or unsatisfactory long-term clinical response. All reported clinical events were adjudicated by a blinded independent endpoint committee. If a patient experienced a clinical failure event, the protocol specified the option of initiating blinded combination therapy (BCT), which was a continuation of ABS + TAD for patients in the combination arm or initiation of the other monotherapy for patients in the ABS or TAD arms. In all cases, blinding of the initial randomized treatment assignment was maintained.
All patients who were randomized and received at least one dose of investigational product (IP) and who had baseline and follow-up hepatic function data were included in this analysis. Patients with baseline alanine or aspartate aminotransferases (ALT/AST) > 3 × upper limit of normal (ULN) were excluded. The incidence of ALT/AST elevations was determined both including time on BCT and excluding time on BCT for patients randomized to monotherapy. Similarly, annualized incidence of ALT/AST elevations was calculated both including and excluding time on BCT by dividing the number of patients with an ALT/AST elevation > 3 × ULN by the total years of study drug exposure. Study drug exposure was calculated from the first day of dosing to the date of the first ALT/AST elevation > 3 × ULN for patients with elevations, and from the first day of dosing to the last day of dosing for patients without elevations both including and excluding time on BCT. The 95% confidence interval (CI) of the annualized incidence rate was calculated using Garwood’s formula.
The AMBITION trial defined potential Hy’s law cases as ALT > 3 × ULN and total bilirubin (TBili) > 2 × ULN (or ALT > 3 × ULN and, if measured, international normalized ratio > 1.5). This analysis defined Hy’s law cases according to the “Guidance for Industry: Drug-Induced Liver Injury - Premarketing Clinical Evaluation” document from the FDA8 and cases meeting all three of the following criteria were identified and characterized further: (a) > 3 × ULN of ALT/AST; (2) > 2 × ULN of TBili; and (3) no other identifiable causes for these elevations.
The eDISH tool is an analytical tool that graphically illustrates peak ALT > or ≤ 3× ULN on the x-axis and peak TBili > or ≤ 2× ULN on the y-axis (using log10 scale) for all patients in a clinical trial.11 We used ALT/AST in the creation of an eDISH graph. The resulting eDISH graph yields four quadrants with three quadrants of interest. The top left quadrant (ALT < 3× ULN and TBili > 2 × ULN) represents patients with hyperbilirubinemia such as is observed in Gilbert’s syndrome, the bottom right quadrant (ALT > 3× ULN, TBili < 2× ULN) represents Temple’s Corollary, and the top right quadrant (ALT > 3× ULN and TBili > 2× ULN), which is of the most interest in the context of DILI, represents potential Hy’s law cases. Specifics such as randomized treatment assignment, treatment at time of elevation of ALT/AST, and clinical failure event(s), if they occurred, were tabulated, and IP dose excursions were examined.
Data are presented for all treatment groups from the first dose of IP to the end of study (final database lock). Descriptive statistics are presented without any formal hypothesis testing.
The study was conducted in accordance with International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice requirements, all applicable subject privacy requirements, and the ethical principles that are outlined in the Declaration of Helsinki 2008. The study protocol was approved by the ethics committee or institutional review board at each site. All patients provided written informed consent before any study-specific procedures were performed.
Results
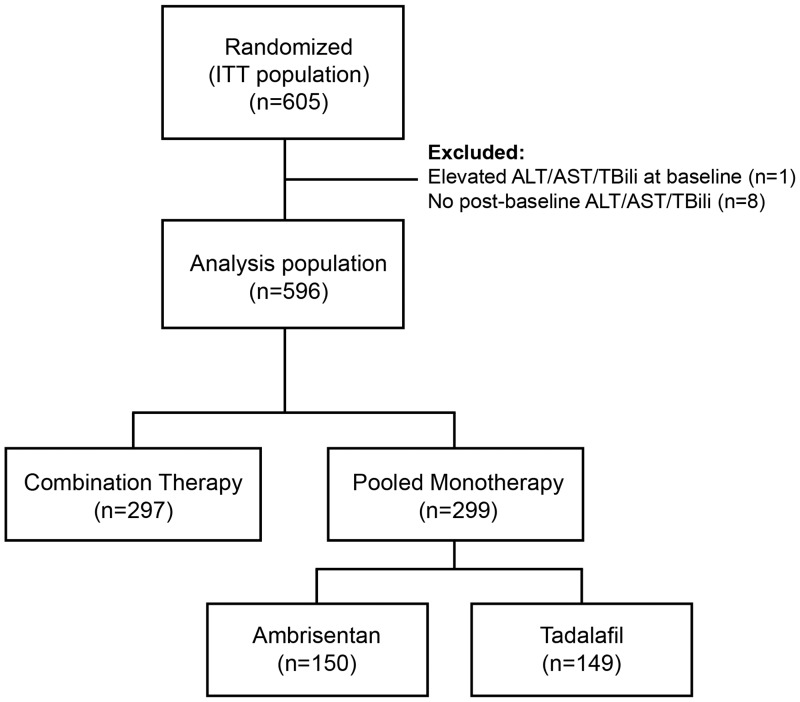
The study population evaluated for this analysis consisted of 596 patients (Fig. 1). The results of the incidence of elevations in aminotransferases are presented in Table 1. Excluding time on BCT, the total IP exposure was 946 years, and 20/596 patients (3.4%) had ALT/AST elevations. The annualized incidence of ALT/AST elevations based on this exposure was 2.12%. The majority of ALT/AST elevations were > 3× ULN – ≤ 5× ULN which occurred in 17 patients (2.9%). Two patients (0.3%) had ALT/AST elevations > 5× ULN – ≤ 8× ULN and one patient (0.2%) had ALT/AST elevations > 8× ULN. Including time on BCT, the total IP exposure was 1031 years and 22/596 patients (3.7%) had ALT/AST elevations. The annualized incidence of ALT/AST elevations based on this exposure was 2.13%. The majority of ALT/AST elevations were > 3× ULN – ≤ 5× ULN which occurred in 17 patients (2.9%). Three patients (0.5%) had ALT/AST elevations > 5× ULN – ≤ 8× ULN and two patients (0.3%) had ALT/AST elevations > 8× ULN. The annualized incidence of ALT/AST elevations > 3× ULN, excluding time on BCT, was 2.10% in the ABS + TAD group, 2.93% in the ABS monotherapy group, and 1.39% in the TAD monotherapy group. The corresponding results including time on BCT were 2.10%, 2.78%, and 1.57%, respectively.
Fig. 1.
Patient disposition. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ITT, intention-to-treat; TBili, total bilirubin.
Table 1.
Incidence of aminotransferase (ALT/AST) elevations and potential Hy’s law.
| Combination ambrisentan + tadalafil (n = 297) | Ambrisentan monotherapy (n = 150) | Tadalafil monotherapy (n = 149) | Total (n = 596) | |
|---|---|---|---|---|
| Excluding time on blinded combination therapy for monotherapy groups | ||||
| ALT/AST elevations | ||||
| Any > 3 × ULN | 11 (3.7) | 6 (4.0) | 3 (2.0) | 20 (3.36) |
| >3 × ULN - ≤5× ULN | 9 (3.0) | 5 (3.3) | 3 (2.0) | 17 (2.9) |
| >5 × ULN - ≤8× ULN | 1 (0.3) | 1 (0.7) | 0 | 2 (0.3) |
| >8× ULN | 1 (0.3) | 0 | 0 | 1 (0.2) |
| Total exposure (patient-years) | 524 | 205 | 217 | 946 |
| Annualized incidence of ALT/AST > 3× ULN (% [95%]) | 2.10 (1.05–3.76) | 2.93 (1.07–6.37) | 1.39 (0.29–4.05) | 2.12 (1.29–3.27) |
| ALT/AST > 3× ULN + TBili > 2× ULN (potential Hy’s law) | 0 | 1 (0.7) | 0 | 1 (0.2) |
| Including time on blinded combination therapy | ||||
| ALT/AST elevations | ||||
| Any > 3× ULN | 11 (3.7) | 7 (4.7) | 4 (2.7) | 22 (3.7) |
| >3 × ULN – ≤ 5 × ULN | 9 (3.0) | 5 (3.3) | 3 (2.0) | 17 (2.9) |
| >5 × ULN – ≤ 8 × ULN | 1 (0.3) | 2 (1.3) | 0 | 3 (0.5) |
| >8 × ULN | 1 (0.3) | 0 | 1 (0.7) | 2 (0.3) |
| Total exposure (patient-years) | 524 | 252 | 255 | 1031 |
| Annualized incidence of ALT/AST > 3× ULN (% [95%]) | 2.10 (1.05–3.76) | 2.78 (1.12–5.73) | 1.57 (0.43–4.01) | 2.13 (1.34–3.23) |
| ALT/AST > 3 × ULN + TBili > 2× ULN (potential Hy’s law) | 0 | 2 (1.3) | 1 (0.7) | 3 (0.5) |
Data are n (%) unless otherwise specified.
For patients with an ALT/AST elevation, exposure (days) is calculated as expdays = date of first elevation – first dose date + 1. For patients without an ALT/AST elevation, exposure is calculated as last dose date – first dose date + 1. Total exposure (years) is calculated by summing expdays across all patients and dividing by 365.25. Annualized incidence is calculated as the number of patients with an ALT/AST elevation divided by the total exposure.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; TBili, total bilirubin.
Including time on BCT, of the 22 patients with elevations in ALT/AST, five had confirmed elevations (defined as a repeat test within seven days). Of the five patients with confirmed elevations, one had no IP dose excursions, three had dose interruptions of IP with all patients restarting IP at full doses, while one discontinued IP but remained in the study, with the reason for discontinuation listed as “exacerbation of Still’s disease.” This patient eventually died of a pulmonary embolism. Of the remaining 17 patients without confirmed elevations in ALT/AST, four had IP dose excursions. Of the four patients with dose excursions, one interrupted IP for one day due to bowel perforation, one did not up-titrate ABS from 5 mg to 10 mg QD due to an adverse event (however, the description of the adverse event was not provided), one interrupted IP for 12 days due to elevated ALT/AST but restarted ABS 10 mg QD and TAD 20 mg QD, and one interrupted IP for 1 day with no reason provided for the interruption. A detailed description of all 22 patients with randomized treatment, day of elevation in ALT/AST after IP initiation, treatment at time of elevation, and clinical failure events that occurred during the trial are provided in Table 2.
Table 2.
Detailed description of the 22 patients who experienced elevations in alanine and aspartate aminotransferases and clinical failure event(s), if any, during the AMBITION trial.
| Patient ID | Randomized treatment | Treatment at time of first elevation in ALT/AST | Days after IP initiation first ALT/AST elevation observed | Description of clinical failure event(s), if applicable |
|---|---|---|---|---|
| 313 | Combination | Combination | 59 | Not applicable |
| 1563 | Combination | Combination | 225 | Not applicable |
| 191 | Combination | Combination | 699 | Not applicable |
| 192 | Combination | Combination | 288 | Death |
| 242 | Combination | Combination | 83 | Hospitalization, disease progression, unsatisfactory long-term clinical response |
| 872 | Combination | Combination | 60 | Disease progression |
| 1158 | Combination | Combination | 699 | Not applicable |
| 1073 | Combination | Combination | 372 | Not applicable |
| 588 | Combination | None: Combination IP stopped on day 9 | 29 | Death |
| 771 | Combination | Combination | 412 | Not applicable |
| 794 | Combination | Combination | 15 | Hospitalization, death |
| 1107 | Ambrisentan | Ambrisentan | 106 | Not applicable |
| 621 | Ambrisentan | Ambrisentan | 814 | Disease progression, hospitalization, unsatisfactory long-term clinical response |
| 631 | Ambrisentan | Ambrisentan | 141 | Disease progression, hospitalization |
| 385 | Ambrisentan | Ambrisentan | 507 | Not applicable |
| 144 | Ambrisentan | Ambrisentan | 562 | Not applicable |
| 1539 | Ambrisentan | BCT | 127 (AST elevation) | Hospitalization, death |
| 142 (AST and TBili elevation) | ||||
| 153 | Ambrisentan | Ambrisentan | 765 | Death |
| 41 | Tadalafil | Tadalafil | 142 | Not applicable |
| 1188 | Tadalafil | Tadalafil | 158 | Not applicable |
| 1181 | Tadalafil | Tadalafil | 143 | Not applicable |
| 583 | Tadalafil | None: BCT IP stopped on day 874 | 901 | Disease progression, hospitalization, death |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCT, blinded combination therapy; IP, investigational product; TBili, total bilirubin.
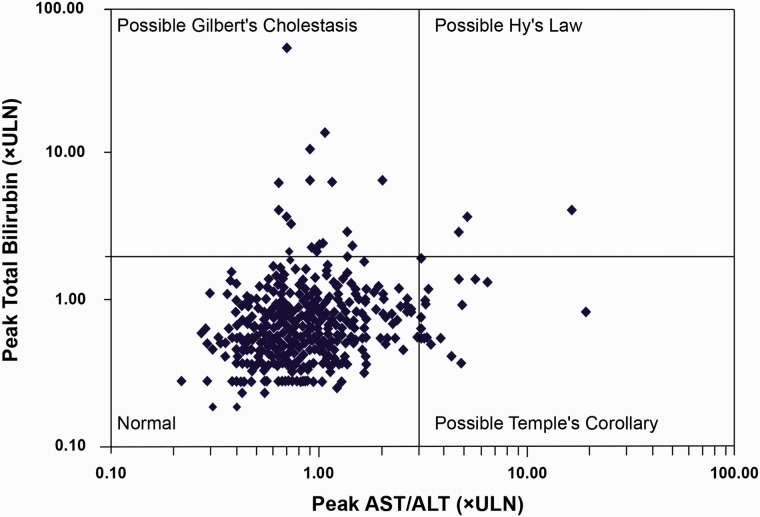
Three patients met two of the three criteria for Hy’s law. These three patients are shown in the right upper quadrant in Fig. 2. Further examination of these patients showed alternative reasons for the elevations. A brief description of the three patients is provided below. The time course of ALT/AST elevations for these three patients is provided in Fig. 3.
Fig. 2.
eDISH plot: peak AST/ALT vs. peak total bilirubin (log10 scale). ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
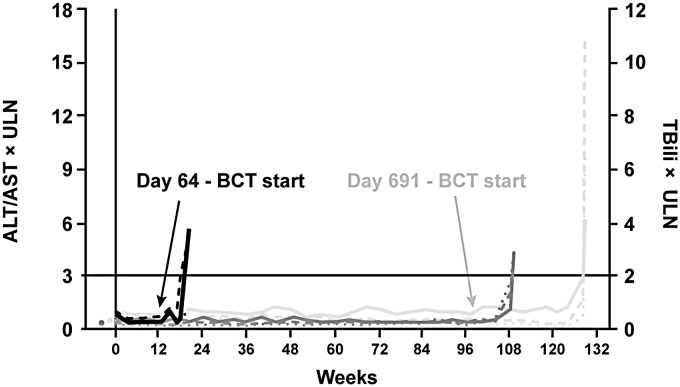
Fig. 3.
Time course of changes in ALT, AST, and TBili for the three potential
Hy’s law patients.  Patient 1539 ALT,
Patient 1539 ALT,
 Patient 1539 AST,
Patient 1539 AST,  Patient 1539 TBili,
Patient 1539 TBili,
 Patient 153 ALT,
Patient 153 ALT,  Patient 153 AST,
Patient 153 AST,
 Patient 153 TBili,
Patient 153 TBili,  Patient 583 ALT,
Patient 583 ALT,
 Patient 583 AST,
Patient 583 AST,  Patient 583 TBili.
Patient 1539 – randomized to ambrisentan monotherapy. Initiated BCT at
day 64 with elevations in AST/TBili at day 142. Patient 583 – randomized
to tadalafil monotherapy. Initiated BCT at day 691, with elevations in
ALT/AST/TBili at day 901. Patient 153 – randomized to ambrisentan
monotherapy. Elevations in ALT/AST/TBili at day 765. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; BCT, blinded
combination therapy; TBili, total bilirubin.
Patient 583 TBili.
Patient 1539 – randomized to ambrisentan monotherapy. Initiated BCT at
day 64 with elevations in AST/TBili at day 142. Patient 583 – randomized
to tadalafil monotherapy. Initiated BCT at day 691, with elevations in
ALT/AST/TBili at day 901. Patient 153 – randomized to ambrisentan
monotherapy. Elevations in ALT/AST/TBili at day 765. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; BCT, blinded
combination therapy; TBili, total bilirubin.
Patient 153 was a 49-year-old woman randomized to ABS monotherapy who continued on IP after being diagnosed with small cell lung carcinoma 551 days after IP initiation. The patient was treated with chemotherapy and concurrent radiation therapy and developed liver metastases 764 days after IP initiation. Elevations in ALT (4.71 × ULN), AST (4.40 × ULN), and TBili (2.91 × ULN) were noted 765 days after IP initiation while on ABS. IP was discontinued 766 days after IP initiation and the patient died a few days later due to liver metastases.
Patient 583 was a 60-year-old woman with numerous medical conditions who was randomized to TAD monotherapy. The patient initiated BCT 691 days after IP initiation; 874 days after IP initiation, the patient developed intermittent moderate lower abdominal pain, and IP (ABS + TAD) was discontinued. Seven days after IP discontinuation, the patient was diagnosed with cardiomegaly, pulmonary vascular congestion, moderate atrial fibrillation, moderate azotemia, and elevated blood urea nitrogen and serum creatinine. Elevations in TBili (1.91 × ULN) were noted 22 days after IP discontinuation and elevations in ALT (9.85 × ULN), AST (16.43 × ULN), and TBili (4.09 × ULN) were noted 26 days after IP discontinuation. The patient died soon after due to severe cardiogenic shock.
Patient 1539 was a 44-year-old woman randomized to ABS monotherapy. She initiated BCT on day 64. The patient was diagnosed with a grade 2 or moderate intestinal perforation and severe perforated diverticulitis 105 and 126 days, respectively, after IP initiation. Elevations in AST (3.2 × ULN) were noted 127 days after IP initiation and IP was discontinued 139 days after IP initiation. The patient was diagnosed with severe lymphoma two days after IP discontinuation and elevations in AST (5.17 × ULN) and TBili (3.75 × ULN) were noted a day later. The patient was diagnosed with Stage IV non-Hodgkin’s large B-cell lymphoma and died soon after due to lymphoma.
Discussion
The AMBITION trial defined initial ABS + TAD as the optimal treatment strategy in patients diagnosed with PAH. Based on results from the AMBITION trial, (a) the combination of ABS + TAD was approved by the FDA for the treatment of PAH making it the only ERA + PDE5 inhibitor combination to be indicated in this population12 and (b) the recent European Society of Cardiology (ESC)/European Respiratory Society (ERS) Guidelines for the diagnosis and treatment of PAH have given ABS + TAD a level IB recommendation for the efficacy of initial combination therapy for PAH.13
Despite their important role in the treatment of PAH, ERAs have been associated with drug-related hepatic adverse effects. Detection of drug-induced hepatic adverse effects in PAH is complicated by its association with portopulmonary hypertension with or without cirrhosis, and in severe PAH, with hepatic congestion, cardiogenic shock, hypotension, and right heart failure. Currently available ERAs include bosentan, ambrisentan, and macitentan. Sitaxsentan was withdrawn from the market in Europe and from development in the USA in 2010 after concerns of liver toxicity.14 Chemically, bosentan and sitaxsentan are sulfonamides, macitentan is a sulfamide, and ambrisentan is a propanoic acid derivative. Furthermore, bosentan and macitentan are non-selective for the endothelin A and B receptor, while sitaxsentan and ambrisentan are considered selective for the endothelin A receptor. Bosentan has also been associated with hepatic injury with the incidence of elevations in ALT/AST > 3× ULN reported as 4–14% in clinical trials and in post-marketing surveillance.7,15–19 No patients in the pivotal 12-week ARIES-1 and ARIES-2 trials who were treated with ABS experienced elevations in ALT/AST.6 Despite this, ABS was FDA-approved with a class-labeling for monthly liver function monitoring which was subsequently removed by the FDA in March 20119 after post-marketing surveillance in over 10,000 patients treated with ABS showed the incidence of ALT/AST > 3× ULN to be 0.72% with no cases of confirmed DILI.20 Macitentan, the newest ERA, is a sulfamide dual ERA and the incidence of elevations > 3× ULN in the macitentan 10 mg QD and placebo groups were 3.4% and 4.5%, respectively, while elevations > 8× ULN were 2.1% and 0.4%, respectively.21 A search of the literature identified a recent case of fulminant hepatitis in association with macitentan reported through the FDA Adverse Event Reporting System.22 The OPsumit USers registry, a post-approval commitment requested by the FDA, is an ongoing, prospective observational drug registry to further characterize the safety profile of macitentan.
In this post-hoc analysis, the 95% CI for the annualized incidence rate of ALT/AST elevations > 3× ULN was 0.29–4.05 for TAD monotherapy, 1.07–6.37 for ABS monotherapy, and 1.05–3.76 for ABS + TAD. The AMBITION study was not designed to compare monotherapy groups; however, the 95% CIs suggest that there is not a statistically significant difference between the groups. Results from the VOLibris Tracking (VOLT) study, an open-label, prospective, observational registry that included 998 patients indicated ambrisentan was not associated with an increase in AST or ALT > 3× ULN above an assumed background incidence of 1.5% per year.23 There are no data in the literature on the incidence or annualized incidence of elevations in ALT/AST with TAD. An analysis of data collected between 1999–2002 as part of the National Health and Nutrition Examination Survey showed the overall prevalence of elevations in ALT or AST in the US population was 9.8%.24
There are no specific biomarkers for DILI. The FDA has provided a guidance document for pre-marketing clinical evaluation of DILI.8 Serum ALT/AST are not markers of liver function but rather liver injury. Although some drugs have the potential to cause hepatocellular injury, injury significant enough to affect the liver’s functional ability – as evidenced by elevations in TBili – resulting in clinically significant disease is rare. Injury alone which manifests as elevations in ALT/AST, is a sensitive but not specific indicator of a drug’s potential to cause severe DILI. The late Hyman Zimmerman made the observation that hepatocellular injury accompanied by alterations in liver function as shown by jaundice may predict a serious and potentially fatal outcome.8 This observation later referred to as Hy’s law by Robert Temple, has been used by the FDA for many years to identify drugs with a potential to cause hepatotoxicity.8 The eDISH tool enhances early identification of Hy’s law cases and identification of drugs with a potential for adverse effects on the liver.10 In clinical trials, frequent liver function testing allows early identification of patients in the Temple’s Corollary quadrant (AST/ALT elevations without concomitant TBili elevation) before injury is severe enough to cause impairment of liver function where patients have elevated ALT/AST and TBili (Hy’s law quadrant). Based on Hy Zimmerman’s observation, an elevated ALT/AST > 3× ULN along with an elevated TBili > 2× ULN constitutes a serious liver safety signal and requires further investigation for other causes of biochemical abnormality. Our analysis identified three patients with potential Hy’s law. All three patients had alternative reasons for elevations in ALT/AST > 3× ULN and TBili > 2× ULN and included liver metastases, severe cardiogenic shock, and non-Hodgkin’s lymphoma. Therefore, ABS used as monotherapy or in combination with TAD was not determined to be associated with DILI.
While each of the ERAs is structurally distinct, the impact of each ERA on hepatocyte membrane transporters may provide a rationale for observed differences in transaminase elevations and in DILI. The liver is a central clearing house for drugs and much has been learned about the role of hepatocyte transporter proteins in drug disposition. The processing of bile acids into and out of the hepatocyte is dependent on hepatocyte membrane transporters. The two most important families of membrane transporters in the liver are the solute carrier (SLC) and the ATP-binding cassette superfamilies.25 Transporters found on the basolateral (sinusoidal) membrane of the hepatocyte include the organic anion transporter protein and sodium taurocholate cotransporting polypeptide (NTCP) and play an important role in the uptake of xenobiotics into the hepatocyte for further processing.25 Those found on the apical (canalicular) membrane include the bile salt export pump, p-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein and are involved in the efflux of bile salts, xenobiotics, and metabolites into bile.25 Inhibition of these transporters by drugs can lead to potential liver safety issues.25 Studies in sandwich-cultured human hepatocytes have shed some light on the differences in adverse effects observed clinically between ERAs.26,27 Hartmann et al.26 and Lepist et al.27 evaluated ambrisentan, darusentan, bosentan, and sitaxsentan, and ambrisentan, bosentan, macitentan, and sitaxsentan, respectively. These studies showed inhibition of NTCP-mediated influx by both bosentan and sitaxsentan, 78% reduction in bile salt export pump transport by bosentan and macitentan, inhibition of organic anion transporter protein and NTCP by macitentan, and minimal inhibition of organic anion transporter protein and NTCP and no inhibition of bile salt export pump by ambrisentan. Burbank et al. evaluated bosentan, ambrisentan, sitaxsentan, and macitentan in human HepaRG cells. This study found macitentan to share cholestatic features with bosentan, and sitaxsentan to have only cytotoxic effects, but no hepatotoxic effects were observed with ambrisentan across various concentrations.28
Our analysis has a number of strengths, including the relatively large sample size and long duration of the trial. There are some limitations of our analysis. It is a post-hoc analysis and as such makes it difficult to determine if selection bias altered the results of our analysis although we evaluated all patients with normal baseline hepatic function data. It does not include a placebo group thus making it difficult to determine the incidence of transaminase elevations in a PAH population not treated with an ERA; however, we did evaluate the incidence of transaminase elevations in patients treated with TAD. Information about co-morbid conditions and concomitant medications that may have contributed to transaminase elevations was limited to the specific data gathered during the study on the case report forms, so it is possible that not all relevant information was captured.
The incidence of ALT/AST > 3× ULN with ABS used as monotherapy or as combination therapy with TAD in the AMBITION trial was consistent with findings from previous trials of ABS. ABS and ABS + TAD in the AMBITION trial were not associated with DILI. ERAs are structurally different and although adverse hepatic effects were initially thought to be a class effect, data from the ABS trials including the AMBITION trial have shown there are differences between ERAs with regard to adverse hepatic effects.
Acknowledgments
Support for formatting and submission was provided by C4 MedSolutions, LLC (Yardley, PA), a CHC Group company, and was funded by Gilead.
Conflict of interest
At the time of analysis and manuscript writing, all authors (KRP, CJB, JDT) were employees of Gilead Sciences Inc. KRP, CJB, and JDT hold stock options in Gilead Sciences Inc.
Funding
This study was supported by Gilead Sciences Inc. and GlaxoSmithKline.
References
- 1.GlaxoSmithKline. FLOLAN (package insert). Research Triangle Park, NC: GlaxoSmithKline, 2008.
- 2.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–301. [DOI] [PubMed] [Google Scholar]
- 3.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–3019. [DOI] [PubMed] [Google Scholar]
- 7.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation, Washington, DC: FDA, 2009. . Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf (accessed 22 February 2018). [Google Scholar]
- 9.US Food and Drug Administration. FDA Drug Safety Communication: liver injury warning to be removed from Letairis (ambrisentan) tablets. Washington, DC: US FDA, 2011. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm245852.htm (accessed 22 February 2018).
- 10.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 11.Pauls LL, Senior JR. Serious drug induced liver injury, Washington, DC: US FDA, 2013. . Available at: https://www.fda.gov/downloads/training/clinicalinvestigatortrainingcourse/ucm378686.pdf (accessed 22 February 2018). [Google Scholar]
- 12.Gilead Sciences, Inc. LETAIRIS (package insert), Foster City, CA: Gilead Sciences, Inc., 2014. [Google Scholar]
- 13.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 14.Pfizer Canada Inc. Thelin (sitaxsentan sodium) - Worldwide Product Withdrawal - For the Public. Kirkland, QC: Pfizer, 2010. Available at: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2010/16113a-eng.php (accessed 22 February 2018).
- 15.Don GW, Joseph F, Celermajer DS, et al. Ironic case of hepatic dysfunction following the global withdrawal of sitaxentan. Intern Med J 2012; 42: 1351–1354. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson C, Gustavsson A, Kronvall T, et al. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension: a case-report and review of the literature. J Gastrointestin Liver Dis 2011; 20: 77–80. [PubMed] [Google Scholar]
- 17.Humbert M. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004; 24: 353–359. [DOI] [PubMed] [Google Scholar]
- 18.Humbert M, Segal ES, Kiely DG, et al. Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J 2007; 30: 338–344. [DOI] [PubMed] [Google Scholar]
- 19.Mulchey K, Bshouty Z. An atypical presentation of liver enzyme elevation resulting from bosentan use. Can Respir J 2009; 16: e54–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Yehuda O, Pizzuti D, Brown A, et al. Long-term hepatic safety of ambrisentan in patients with pulmonary arterial hypertension. J Am Coll Cardiol 2012; 60: 80–81. [DOI] [PubMed] [Google Scholar]
- 21.Actelion Pharmaceuticals US, Inc. OPSUMIT (package insert), South San Francisco, CA: Actelion Pharmaceuticals US, Inc, 2017. [Google Scholar]
- 22.Tran TT, Brinker AD, Munoz M. Serious liver injury associated with macitentan: a case report. Pharmacotherapy 2018; 38: e22–e24. [DOI] [PubMed] [Google Scholar]
- 23.Vachiéry J-L, Hoeper MM, Peacock AJ, et al. Ambrisentan use for pulmonary arterial hypertension in a post-authorization drug registry: The VOLibris Tracking Study. J Heart Lung Transplant 2017; 36: 399–406. [DOI] [PubMed] [Google Scholar]
- 24.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol 2006; 101: 76–82. [DOI] [PubMed] [Google Scholar]
- 25.International Transporter Consortium Giacomini KM, Huang SM, et al. Membrane transporters in drug development. Nat Rev Drug Discov 2010; 9: 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartman JC, Brouwer K, Mandagere A, et al. Evaluation of the endothelin receptor antagonists ambrisentan, darusentan, bosentan, and sitaxsentan as substrates and inhibitors of hepatobiliary transporters in sandwich-cultured human hepatocytes. Can J Physiol Pharmacol 2010; 88: 682–691. [DOI] [PubMed] [Google Scholar]
- 27.Lepist E-I, Gillies H, Smith W, et al. Evaluation of the endothelin receptor antagonists ambrisentan, bosentan, macitentan, and sitaxsentan as hepatobiliary transporter inhibitors and substrates in sandwich-cultured human hepatocytes. PLoS ONE 2014; 9: e87548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burbank MG, Sharanek A, Burban A, et al. From the cover: mechanistic insights in cytotoxic and cholestatic potential of the endothelial receptor antagonists using hepaRG cells. Toxicol Sci 2017; 157: 451–464. [DOI] [PubMed] [Google Scholar]