Abstract
Biofilms significantly enhance antibiotic resistance by inhibiting penetration of antibiotics and are shielded from the immune system via the formation of an extracellular polymeric matrix. Innovative and novel approaches are required for the inhibition of biofilm formation and treatment of biofilm-associated infectious diseases. In the current study, a biofilm model of Staphylococcus aureus was established in vitro to explore inhibitory effects of brazilin (BN) on biofilm formation and to evaluate damaging effects of BN in the presence and absence of vancomycin (VCM) on the biofilm. Antibiofilm-infection mechanisms of BN were observed. In these experiments, the clinical strain of S. aureus C-4-4 was isolated for biofilm formation. Crystal violet staining and fluorescence microscopy revealed that BN inhibited biofilm formation in vitro and the best effect was observed with two times the minimum inhibitory concentration of BN following 48 h incubation. Additionally, the results demonstrated that BN in combination with VCM enhanced the damage to biofilms, whereas VCM alone did not. The results of the reverse transcription-quantitative polymerase chain reaction analyses demonstrated that BN downregulated gene expression of intercellular adhesion (ica)A and upregulated icaR and the quorum-sensing (QS) system regulator accessory gene regulator A. In summary, BN inhibited S. aureus biofilm formation and destroyed biofilms, while simultaneously increasing permeability to VCM. BN was able to reduce production of the extracellular polymeric matrix and inhibited the QS system. These results support the use of BN as a novel drug and treatment strategy for S. aureus biofilm-associated infections.
Keywords: brazilin, vancomycin, Staphylococcus aureus, biofilm
Introduction
Staphylococcus aureus is a Gram-positive bacterium that typically colonizes the surface of the skin and, as an important pathogen of nosocomial cross-infection, it is also a common pathogenic bacterium of biofilm infections (1,2). More than 80% of human infections are caused by biofilms, including endocarditis, osteomyelitis and implant-associated infections (3). S. aureus-associated infections lead to an increase in hospital stays, in addition to hospital-associated mortality (4). S. aureus biofilms in humans cause chronic persistent infections that are difficult to cure and represent a great challenge for clinical treatment (5).
Biofilms may adhere to surfaces and are enclosed in a self-produced polymeric matrix, which enhances antibiotic resistance and is shielded from the immune system (6). Biofilm-associated infections are difficult to treat and these chronic or relapsing infections typically require increased drug doses or prolonged antibiotic treatment, which is associated with development of drug resistances (7). Biofilm resistance is commonly 10–1,000 times stronger than that of planktonic bacteria (8). At present, vancomycin (VCM) is the most commonly administered drug for S. aureus biofilm-associated infections (9). However, there is cause for concern due to recent developments of VCM-intermediate S. aureus and vancomycin-resistant S. aureus (VRSA) strains (10). Combination of VCM with rifampin, oxacillin, linezolid and tigecycline has been considered in order to improve treatment effectiveness and to reduce resistance (11). Nevertheless, studies (12–14) have indicated that although this combination may be effective against methicillin-sensitive S. aureus, it may not hold promise for use in treating methicillin-resistant S. aureus biofilm infections. Therefore, there is a requirement to identify innovative and novel approaches for the prevention of biofilm formation.
Several studies (15–17) have reported that the use of traditional herbal medicine in the prevention and treatment of biofilm infections is advantageous. Traditional herbal medicine may not only inhibit the formation of biofilms and destroy biofilms, but it may further enhance resistance to infection when combined with antibiotics (18). At the same time, these treatments exhibit little side effects, strong pharmacological action, wide availability and do not easily lead to bacterial drug resistance (19–20). This has become another notable focus of research, distinct from antibiotics. A recent study screened 40 types of traditional herbal medicines common in the Guizhou province of China to test for minimum inhibitory concentration (MIC) for S. aureus ATCC25923 biofilms (21). The results demonstrated that Caesalpinia sappan L. has bacteriostatic properties. These findings were further analyzed in the present study.
Natural products may be utilized as raw materials in the development of novel antibacterial substances (18). Brazilin (BN) is isolated from the traditional herbal medicine Caesalpinia sappan L and is a principal active component of it (20). Evidence has indicated that BN exhibits multiple biological properties, including immune system modulatory, antioxidant, anti-inflammatory, antiplatelet, antihepatotoxicity and antitumor activities (19,20). Furthermore, a number of studies have demonstrated that BN is associated with antimicrobial activity (22,23).
The current study aimed to determine whether, and the degree to which, BN may inhibit S. aureus biofilm formation and increase antibiotic susceptibility. Another aim was to demonstrate whether BN was able to reduce secretion of extracellular polysaccharides and interfere with the quorum-sensing (QS) system. To the best of our knowledge, this has not yet been reported and requires further research.
Materials and methods
Bacterial strains
A total of 50 clinical strains of S. aureus were prepared. Samples were collected from The Affiliated Hospital of Zunyi Medical University (Guizhou, China) in January 2017 (Table I) and patients provided written informed consent prior to collection of blood samples and swabs. The present study was approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University. Isolated S. aureus C-4-4 was selected as preliminary studies revealed it had the strongest ability to form biofilms, as determined by Congo red and Crystal violet staining (data not shown) and is the strain discussed subsequently. The S. aureus ATCC25923 standard strain (American Type Culture Collection, Manassas, VA, USA) was used as quality control. S. aureus strains were routinely cultured on blood agar (BA) plates at 37°C and 5% CO2 for 24 h. Single colonies were selected from a culture plate and the bacterial suspension was diluted in Mueller Hinton (M-H) broth (BD Difco™; BD Biosciences, Franklin Lakes, NJ, USA) to an optical density at 600 nm (OD600) of 0.1 prior to use.
Table I.
Samples collected from The Affiliated Hospital of Zunyi Medical University, Zunyi, China.
| No | Strain_ID | Age | Sex | Infection site | Month and year |
|---|---|---|---|---|---|
| 1 | C-3–077 | 2 | Male | Wound | Jan-17 |
| 2 | C-3–078 | 1 | Female | Wound | Jan-17 |
| 3 | C-3–079 | 54 | Male | Abscess | Jan-17 |
| 4 | C-4–013 | 23 | Female | Respiratory | Jan-17 |
| 5 | C-3–080 | 8 | Male | Respiratory | Jan-17 |
| 6 | C-3–081 | 23 | Female | Abscess | Jan-17 |
| 7 | C-3–082 | 55 | Female | Respiratory | Jan-17 |
| 8 | C-3–083 | 41 | Male | Wound | Jan-17 |
| 9 | C-3–084 | 33 | Male | Wound | Jan-17 |
| 10 | C-3–085 | 37 | Male | Respiratory | Jan-17 |
| 11 | C-3–086 | 3 | Female | Wound | Jan-17 |
| 12 | C-3–087 | 39 | Female | Abscess | Jan-17 |
| 13 | C-3–088 | 21 | Female | Abscess | Jan-17 |
| 14 | C-3–089 | 2 | Male | Wound | Jan-17 |
| 15 | C-3–090 | 0 | Male | Respiratory | Jan-17 |
| 16 | C-3–091 | 64 | Female | Respiratory | Jan-17 |
| 17 | C-3–092 | 50 | Male | Wound | Jan-17 |
| 18 | C-3–093 | 57 | Female | Respiratory | Jan-17 |
| 19 | C-3–094 | 75 | Male | Respiratory | Jan-17 |
| 20 | C-3–095 | 0 | Female | Abscess | Jan-17 |
| 21 | C-3–096 | 52 | Male | Respiratory | Jan-17 |
| 22 | C-3–097 | 63 | Male | Respiratory | Jan-17 |
| 23 | C-3–098 | 3 | Female | Wound | Jan-17 |
| 24 | C-3–099 | 23 | Female | Abscess | Jan-17 |
| 25 | C-3–100 | 2 | Female | Wound | Jan-17 |
| 26 | C-4–001 | 0 | Male | Respiratory | Jan-17 |
| 27 | C-4–020 | 71 | Female | Respiratory | Jan-17 |
| 28 | C-4–003 | 0 | Female | Respiratory | Jan-17 |
| 29 | C-4–004 | 46 | Female | Abscess | Jan-17 |
| 30 | C-4–005 | 4 | Female | Wound | Jan-17 |
| 31 | C-4–006 | 47 | Male | Wound | Jan-17 |
| 32 | C-4–007 | 50 | Male | Wound | Jan-17 |
| 33 | C-4–008 | 39 | Male | Wound | Jan-17 |
| 34 | C-4–009 | 60 | Female | Wound | Jan-17 |
| 35 | C-4–010 | 0 | Female | Respiratory | Jan-17 |
| 36 | C-4–011 | 1 | Male | Wound | Jan-17 |
| 37 | C-4–012 | 64 | Female | Wound | Jan-17 |
| 38 | D-1–078 | 45 | Male | Wound | Jan-17 |
| 39 | D-1–079 | 19 | Male | Abscess | Jan-17 |
| 40 | D-1–080 | 5 | Female | Bacteremia | Jan-17 |
| 41 | D-1–081 | 48 | Male | Respiratory | Jan-17 |
| 42 | D-1–082 | 37 | Male | Abscess | Jan-17 |
| 43 | D-1–083 | 51 | Male | Wound | Jan-17 |
| 44 | D-1–084 | 51 | Male | Abscess | Jan-17 |
| 45 | D-1–085 | 5 | Male | Bacteremia | Jan-17 |
| 46 | D-1–086 | 51 | Male | Wound | Jan-17 |
| 47 | D-1–087 | 75 | Male | Respiratory | Jan-17 |
| 48 | D-1–088 | 41 | Male | Cerebrospinal fluid | Jan-17 |
| 49 | D-1–089 | 49 | Male | Bacteremia | Jan-17 |
| 50 | D-1–090 | 59 | Female | Cerebrospinal fluid | Jan-17 |
Determination of the effect of BN on S. aureus
BN (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) purity was confirmed to >98% using high-performance liquid chromatography. BN (1,024 µg/ml) was dissolved in deionized water and stored at 4°C until use. The pH of the solution was maintained at 7.2–7.4. Antimicrobial susceptibility testing of BN and VCM was performed using the standardized broth microdilution method in 96-well U-bottom plates (Costar 3590; Corning Incorporated, Corning, NY, USA) using M-H broth, according to the 2015 Clinical and Laboratory Standards Institute guidelines (24). VCM (0.5 µg/ml; Sigma-Aldrich; Merck KGaA) was used as a positive control.
Crystal violet staining elaborating the effect of BN on S. aureus biofilm formation
S. aureus strains were cultured on BA plates at 37°C and 5% CO2 overnight. The following day, the bacterial suspension was diluted in M-H broth to OD600=0.1. Bacterial suspension (100 µl) was applied to sterile polystyrene 96-well plates and 100 µl BN (1/2, 1 or 2 MIC) were added to final concentrations of 1/4, 1/2 and 1 MIC, respectively. VCM (100 µl, 2 MIC) was used as positive control and deionized water as blank. Suspensions were cultivated at 37°C and 5% CO2 for 6, 12, 24 and 48 h. Unbound cells from S. aureus 6-, 12-, 24- and 48-h incubation plates were removed by washing with PBS (3×). The biomass of each slice was air-dried for 20 min at 37°C and stained for 20 min at 25°C with 200 µl filtered 0.25% crystal violet, washed with PBS (3×) to remove unbound stain, dried at 37°C for 20 min and solubilized in 200 µl 95% ethanol for 15 min at 25°C. A reading at 570 nm using an ELISA microplate reader (Multiskan FC; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was obtained. Experiments were performed six times.
Fluorescence microscopy elaborating the effect of BN on S. aureus biofilm formation
A polystyrene carrier (10×10 mm) was placed in a 24-well plate and 100 µl bacterial suspension were applied along with 100 µl BN (1/2, 1 and 2 MIC). VCM (100 µl; 2 MIC) was used as positive control and deionized water as blank. Plates were incubated at 37°C and 5% CO2 for 6, 12, 24 and 48 h. Unbound cells from S. aureus 6-, 12-, 24- and 48-h incubation plates were removed by washing with PBS (3×). Biofilms were stained with fluorescein isothiocyanate-conjugated concanavalin A (FITC-ConA) for 20 min at 25°C and washed with PBS to remove stain. Fluorescent images were obtained using BX53 fluorescence microscopy (Olympus Corporation, Tokyo, Japan).
Crystal violet staining elaborating the combined effect of BN and VCM on S. aureus biofilms
Bacterial suspension (200 µl) was added to 96-well plates and cultivated at 37°C and 5% CO2 for 48 h to mature the biofilm. Plates were gently washed with PBS (3×). BN (100 µl) with or without VCM was added to each well and incubated at 37°C in 5% CO2 for 6, 12, 24 or 48 h. The final concentrations of BN were 1/2, 1 and 2 MIC (16, 32 and 64 µg/ml) and VCM were 1/2, 1 and 2 MIC (0.25, 0.5 and 1 µg/ml). Plates were cultivated at 37°C for 6, 12, 24 and 48 h. Plates were washed with PBS to remove planktonic cells. The biomass of each slice was air-dried at 37°C for 20 min and stained for 20 min at 25°C with 200 µl filtered 0.25% crystal violet, washed with PBS (3×) to remove unbound stain, dried for 20 min at 37°C and solubilized in 200 µl 95% ethanol for 15 min at 25°C. A reading at 570 nm using an ELISA microplate reader was obtained. Experiments were performed six times.
Fluorescence microscopy elaborating the combined effect of BN and VCM on S. aureus biofilms
Biofilms were prepared in 24-well plates containing a polystyrene slice as described. Slices were gently washed with PBS (2×). BN (100 µl) with or without VCM was added to each well. Concentrations of BN were 1/2, 1 and 2 MIC (16, 32 and 64 µg/ml) and VCM were 1/2, 1 and 2 MIC (0.25, 0.5 and 1 µg/ml). Plates were incubated at 37°C for 6, 12, 24 and 48 h. Slices were washed with PBS to remove planktonic cells. Biofilms were stained with FITC-ConA for 20 min at 25°C and washed with PBS to remove stain. Fluorescent images were captured by fluorescence microscopy. Experiments were performed in triplicate.
Biofilm bacterial colony-forming unit (CFU) counts
Bacterial suspension (200 µl) was added to 96-well plates and cultivated at 37°C and 5% CO2 for 48 h to mature the biofilms. The mature biofilms were treated with 100 µl 1/2, 1 and 2 MIC BN with or without 1/2, 1 and 2 MIC VCM and incubated at 37°C for 6, 12, 24 and 48 h. Slices were washed with PBS to remove planktonic cells and sonicated to disrupt cell clumps. Biofilm bacterial CFU counts were conducted by plating serial dilutions on TSB agar. Experiments were performed in triplicate.
Confocal laser scanning microscopy (CLSM)
The biofilm was determined using a double live/dead staining kit (L13152 LIVE/DEAD® BacLight™ Bacterial Viability kits; Thermo Fisher Scientific, Inc.), containing nucleic acid stains SYTO9 (green) and propidium iodide (PI, red), which differ in their ability to penetrate healthy bacterial cells. SYTO9 stain labels live bacteria, whereas PI penetrates bacteria with damaged membranes. Bacterial suspension (4 ml) was added to the bottom of a 24-well plate. Following 48 h incubation at 37°C, plates were washed with PBS and 1/2, 1 and 2 MIC BN with or without 1/2, 1 and 2 MIC VCM was added followed by incubation at 37°C for 48 h. Slices were washed with PBS. According to the manufacturer's protocol, the slices were stained with SYTO9/PI for 20 min at 25°C in the dark. A TCS SP5 microscope (Leica Microsystems GmbH, Wetzlar, Germany) was used to investigate S. aureus biofilms.
Scanning electron microscopy (SEM)
Polystyrene plates (24×24 mm) were sterilized and placed into 6-well plates for 48 h to form the biofilm. BN (2 MIC, 64 µg/ml) with or without VCM (2 MIC, 1 µg/ml) were added to each well, followed by incubation at 37°C for 48 h. Slices were washed with PBS and fixed at 4°C with 2.5% glutaraldehyde for 8 h. Surfaces were rinsed three times and fixed with 0.1% osmium tetraoxide for 1 h. Samples were dehydrated through a graded ethanol series (30, 50, 70, 80, 90, 95 and 100%) for 15 min each at room temperature and coated with gold. Images were obtained using an SU8010 SEM (Hitachi, Ltd., Tokyo, Japan) (25).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis of genes forming biofilms
Biofilms were prepared and exposed to BN with or without VCM in 6-well plates as described previously. Total RNA was extracted using the MiniBEST Universal RNA Extraction kit (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's protocol. Biofilms were centrifuged at 7,104 × g at 4°C for 2 min, 600 µl Buffer RL (Takara Biotechnology Co., Ltd.) were added and incubated for 2 min at room temperature. The pyrolysis liquid was transferred into a gDNA Eraser Spin Column and centrifuged at 13,400 × g at 4°C for 1 min. To the filtrate an equal amount of 70% ethanol was added. Immediately, the mixture was transferred into an RNA Spin Column and centrifuged at 13,400 × g at 4°C for 1 min. Buffer RWA (500 µl) and Buffer RWB (600 µl) were added to a RNA Spin Column and spun at 13,400 × g at 4°C for 30 sec. RNA pellets were suspended in 100 µl RNase-free water. cDNA was synthesized using EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix kit (Generon, Slough, UK). RT reaction mixtures contained total RNA sample (5 µl,) anchored Oligo(dT)18 Primer (0.5 µg/µl; 1 µl), 2× ES Reaction mix (10 µl), EasyScript® RT/RI Enzyme mix (1 µl), gDNA Remover (1 µl) and RNase-free water to a final volume of 20 µl. Reverse transcriptase was inactivated by incubation at 42°C for 15 min, then 85°C for 5 sec, followed by cooling at 4°C and storage at −80°C. Primer sequences were as follows: Intercellular adhesion (ica)A, (189 bp) forward 5′-GCAGTTGTCGATGTTGGCTA-3′ and reverse 5′-TACTTCGTGTCCCCCTTGAG-3′; icaR (186 bp), forward 5′-TACGCCTGAGGAATTTTCTG-3′ and reverse 5′-CCAAATTTTTGCGAAAAGGA-3′; accessory gene regulator (agr) A (82 bp), forward 5′-ACGTGGCAGTAATTCAGTGTATGTT-3′ and reverse 5′-GGCAATGAGTCTGTGAGATTTTGT-3′; and 16SrRNA (201 bp), forward 5′-TATTTTTCCGGTTGGTCGTC-3′ and reverse 5′-GGCTTAACCTTGCCATCAGA-3′. qPCR was performed according to the manufacturer's protocol (Takara Biotechnology Co., Ltd.), in 20 µl reactions, containing SYBR Premix Ex TaqII (10 µl), forward and reverse primers (0.8 µl), cDNA (2 µl), ROX Reference Dye II (0.4 µl) and deionized water (6 µl). An Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems, Thermo Fisher Scientific, Inc.) was used. Cycling parameters were as follows: 95°C for 30 sec; 95°C for 5 sec and 60°C for 34 sec for 40 cycles and one melt curve at 95°C for 15 sec, followed by 60°C for 60 sec and 95°C for 15 sec. Experiments were performed in duplicate and analyzed in triplicate; 16SrRNA was used as internal control. RT-qPCR data were analyzed using the relative quantitative (2−ΔΔCq) method (26).
Statistical analysis
All data were analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA) and are expressed as the mean ± standard deviation. Statistical comparisons between groups were made using one-way analysis of variance followed by Fisher's LSD test. P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibition of S. aureus by BN
MICs of BN for C-4-4 and ATCC25923 strains were 32 µg/ml. The MIC of VCM for the two strains was 0.5 µg/ml. ATCC25923 was used as control strain; VCM as positive control.
Effects of BN on S. aureus biofilm formation in vitro
Biofilms were stained with crystal violet and FITC-ConA to study effects of BN on S. aureus biofilm formation. It was identified that compared with the control group, varying treatment times and concentrations of BN had an effect on biofilm formation, in addition to 6 and 12 h 1/4 MIC BN (P<0.05; Table II). With an increase in BN concentration, the effect became more pronounced. The strongest decreases in OD measurements were observed with 1 MIC BN following 24 and 48 h (Table II).
Table II.
Effect of BN on S. aureus biofilm formation measured using crystal violet staining.
| OD570 | ||||
|---|---|---|---|---|
| Group | 6 h | 12 h | 24 h | 48 h |
| Control | 0.274±0.056 | 0.439±0.059 | 0.726±0.119 | 1.133±0.283 |
| 1 MIC VCM | 0.195±0.030a | 0.274±0.053a | 0.356±0.066a | 0.470±0.125a |
| 1/4 MIC BN | 0.244±0.049 | 0.336±0.012 | 0.535±0.099a | 0.672±0.163a |
| 1/2 MIC BN | 0.221±0.028a | 0.308±0.064a | 0.441±0.095a | 0.510±0.108a |
| 1 MIC BN | 0.202±0.033a | 0.281±0.075a | 0.346±0.127a | 0.450±0.121a |
P<0.05 vs. control. BN, brazilin; VCM, vancomycin; MIC, minimum inhibitory concentration; OD, optical density.
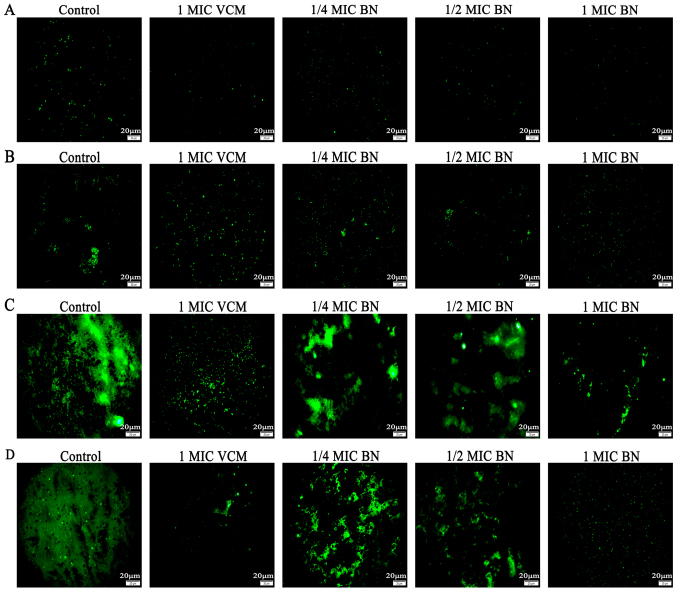
FITC-ConA produces green fluorescence by combining with polysaccharides in the biofilm. The stronger the biofilm, the greater the green fluorescence (17). The current study used FITC-ConA-tagged fluorescence microscopy imaging to evaluate effects of BN on biofilm formation. The results revealed that 1/4, 1/2 and 1 MIC BN for 12, 24 and 48 h inhibited biofilm formation, whereas only minor effects were observed at 6 h (Fig. 1). At 48 h, the effect of 1 MIC BN was most pronounced.
Figure 1.
Fluorescence microscopy based on interactions with polysaccharides of the S. aureus biofilm (magnification, ×400). Experiments used fluorescein isothiocyanate-conjugated concanavalin A to evaluate the effect of BN at 1/4, 1/2 and 1 MIC on biofilm formation following treatment for (A) 6, (B) 12, (C) 24 and (D) 48 h. Scale bars, 20 µm. BN, brazilin; MIC, minimum inhibitory concentration; VCM, vancomycin.
BN combined with VCM damages the biofilm
The current study aimed to compare damaging effects of BN on S. aureus biofilms in the presence and absence of VCM, in order to assess synergistic effects. According to the crystal violet staining results, VCM alone only eradicated biofilms at 2 MIC following 48 h. BN and BN + VCM reduced biofilm formation in a concentration-dependent manner (Table III).
Table III.
Effect of BN combined with VCM on S aureus biofilms analyzed using crystal violet staining.
| OD570 | ||||
|---|---|---|---|---|
| Group | 6 h | 12 h | 24 h | 48 h |
| Blank | 1.224±0.246 | 1.291±0253 | 1.226±0.194 | 1.283±0.272 |
| 1/2 MIC VCM | 1.197±0.262 | 1.269±0.182 | 1.168±0.234 | 1.156±0.154 |
| 1 MIC VCM | 1.211±0.287 | 1.264±0.267 | 1.151±0.163 | 1.112±0.116 |
| 2 MIC VCM | 1.140±0.296 | 1.224±0.311 | 1.105±0.121 | 1.041±0.136a |
| 1/2 MIC BN | 1.211±0.291 | 1.021±0.180 | 0.961±0.067a | 0.882±0.121a |
| 1 MIC BN | 1.112±0.287 | 1.048±0.196 | 0.904±0.127a,c | 0.810±0.134a,c |
| 2 MIC BN | 0.965±0.134a | 0.965±0.188a | 0.877±0.176a,c | 0.711±0.201a,c |
| 1/2 MIC VCM+1/2 MIC BN | 0.796±0.060a | 0.916±0.160a | 0.854±0.121a,c | 0.747±0.146a,c |
| 1/2 MIC VCM+1 MIC BN | 0.945±0.149a,c | 0.880±0.075a,c | 0.802±0.876a,c | 0.678±0.173a,c |
| 1/2 MIC VCM+2 MIC BN | 0.903±0.159a,c | 0.857±0.160a,c | 0.772±0.169a,c | 0.644±0.170a,c |
| 1 MIC VCM+1/2 MIC BN | 0.882±0.148a,c | 0.833±0.211a,c | 0.757±0.120a,c | 0.584±0.214a–c |
| 1 MIC VCM+1 MIC BN | 0.863±0.219a–c | 0.805±0.177a–c | 0.720±0.170a–c | 0.526±0.222a–c |
| 1 MIC VCM+2 MIC BN | 0.833±0.199a–c | 0.816±0.070a–c | 0.654±0.182a–c | 0.498±0.144a–c |
| 2 MIC VCM+1/2 MIC BN | 0.831±0.150a–c | 0.776±0.093a–c | 0.597±0.138a–c | 0.469±0.158a–c |
| 2 MIC VCM+1 MIC BN | 0.768±0.149a–c | 0.732±0.157a–c | 0.553±0.223a–c | 0.404±0.171a–c |
| 2 MIC VCM+2 MIC BN | 0.756±0.112a–c | 0.645±0.116a–c | 0.507±0.209a–c | 0.390±0.160a–c |
P<0.05 vs. control
P<0.05 vs. 1/2 and 1 MIC BN
P<0.05 vs. VCM. BN, brazilin; VCM, vancomycin; MIC, minimum inhibitory concentration; OD, optical density.
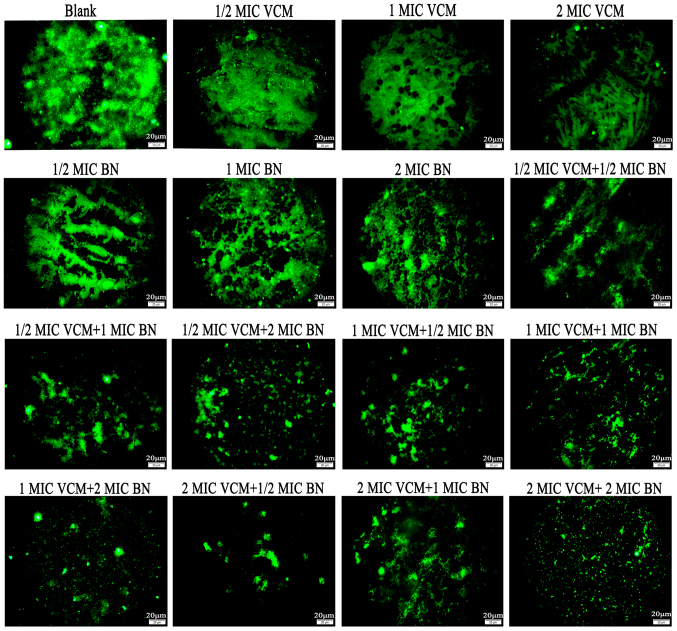
Fluorescence microscopy demonstrated that VCM had no marked effect on S. aureus biofilms compared with the control group, which exhibited large areas of green fluorescence. With an increase in BN concentration, mature biofilms were damaged; with increasing concentrations of BN + VCM, decreasing green fluorescence was observed (Fig. 2).
Figure 2.
Fluorescence microscopy highlights effects of BN and VCM on S. aureus biofilms (magnification, ×400). Effects of varying concentrations of BN (1/2, 1 and 2 MIC) in the presence and absence of VCM (1/2, 1 and 2 MIC) on mature biofilms were evaluated for 48 h. Biofilms were stained with fluorescein isothiocyanate-conjugated concanavalin A. Scale bars, 20 µm. BN, brazilin; VCM, vancomycin; MIC, minimum inhibitory concentration.
Biofilm bacterial CFU counts
VCM alone only reduced bacterial counts at 2 MIC following 48 h treatment. BN at 1 or 2 MIC for 6 and 12 h and at 1/2, 1 or 2 MIC following 48 h was able to decrease bacterial counts. The most distinct effect was observed with 2MIC BN + 2MIC VCM at 48 h (Table IV).
Table IV.
Influence of BN and VCM of bacteria counts.
| ×106 colony-forming unit/ml | ||||
|---|---|---|---|---|
| Group | 6 h | 12 h | 24 h | 48 h |
| Blank | 256.667±24.502 | 255.000±27.074 | 256.333±14.503 | 256.333±13.204 |
| 1/2 MIC VCM | 239.667±27.502 | 246.333±30.534 | 248.667±18.148 | 241.333±21.221 |
| 1 MIC VCM | 241.667±10.017 | 242.333±30.616 | 239.000±13.454 | 233.333±31.390 |
| 2 MIC VCM | 239.667±23.029 | 238.000±19.519 | 236.333±8.622 | 199.000±19.313a |
| 1/2 MIC BN | 229.333±16.563 | 226.667±23.007 | 215.333±12.897a | 198.333±5.695a |
| 1 MIC BN | 223.000±11.136 | 216.667±8.737a | 206.333±7.506a,c | 168.667±12.583a,c |
| 2 MIC BN | 196.333±16.503a,c | 194.000±31.241a,c | 180.333±14.048a–c | 116.000±14.799a–c |
| 1/2 MIC VCM+1/2 MIC BN | 200.667±2.517a,c | 192.000±17.692a,c | 182.000±19.313a–c | 113.000±17.692a–c |
| 1/2 MIC VCM+1 MIC BN | 184.667±26.764a–c | 170.000±33.151a–c | 154.000±13.454a–c | 109.667±9.609a–c |
| 1/2 MIC VCM+2 MIC BN | 176.667±15.011a–c | 163.000±26.665a–c | 144.667±13.577a–c | 100.667±13.317a–c |
| 1 MIC VCM+1/2 MIC BN | 177.000±10.583a–c | 156.000±12.530a–c | 137.000±13.454a–c | 95.667±9.074a–c |
| 1 MIC VCM+1 MIC BN | 168.667±20.550a–c | 149.667±17.474a–c | 128.667±16.258a–c | 84.333±10.408a–c |
| 1 MIC VCM+2 MIC BN | 146.333±23.029a–c | 143.000±23.516a–c | 111.333±15.275a–c | 70.333±15.308a–c |
| 2 MIC VCM+1/2 MIC BN | 141.667±35.726a–c | 135.667±11.930a–c | 104.333±13.868a–c | 61.000±10.536a–c |
| 2 MIC VCM+1 MIC BN | 140.333±23.116a–c | 127.000±5.292a–c | 96.000±7.937a–c | 56.33±9.866a–c |
| 2 MIC VCM+2 MIC BN | 135.667±29.143a–c | 119.000±17.578a–c | 83.000±16.371a–c | 47.333±11.604a–c |
P<0.05 vs. control
P<0.05 vs. 1/2 and 1 MIC BN
P<0.05 vs. VCM. BN, brazilin; VCM, vancomycin; MIC, minimum inhibitory concentration.
Biofilm analysis using CLSM
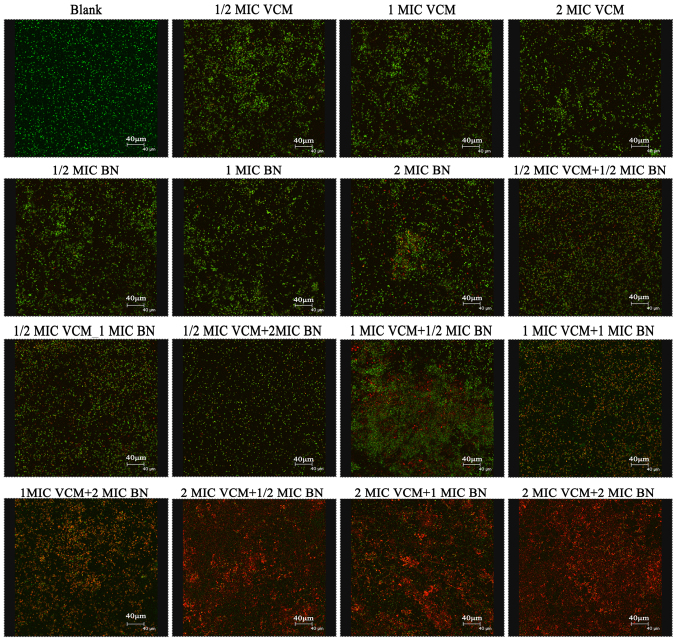
SYTO9 stain labels live bacteria, whereas PI penetrates bacteria with damaged membranes. The results demonstrated that VCM was unable to damage the biofilm (Fig. 3). VCM groups exhibited mainly green fluorescence, representing live cells. Increasing concentrations of BN demonstrated an effect on the biofilm, exhibiting increasing red fluorescence, representing dead cells. BN + VCM groups exhibited increased levels of dead cells, represented by red fluorescence.
Figure 3.
Biofilm assessment using confocal laser scanning microscopy (magnification, ×400). Mature biofilms were stained with SYTO9/propidium iodide. Effects of varying concentrations of BN (1/2, 1 and 2 MIC) in the presence and absence of VCM (1/2, 1 and 2 MIC) on mature biofilms were evaluated following 48 h treatment. Green fluorescence represents live and red fluorescence represents dead cells. Scale bars, 40 µm. BN, brazilin; VCM, vancomycin; MIC, minimum inhibitory concentration.
Biofilm damage assay by SEM
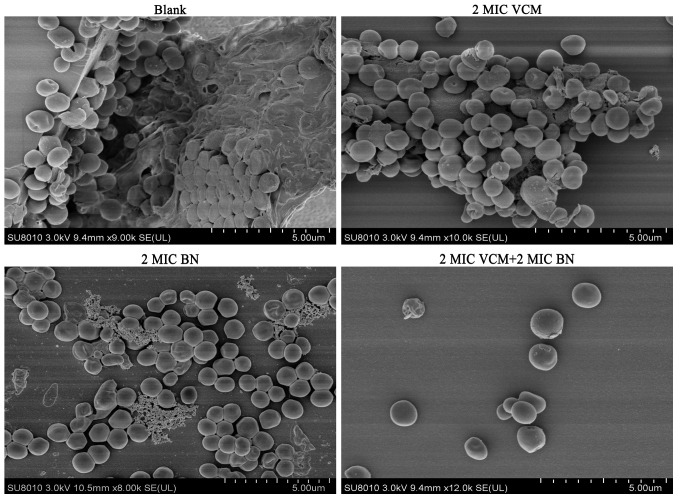
It was observed that biofilms treated with VCM exhibited large extracellular matrices and a large biofilm mass compared with the control group. BN was able to reduce the extracellular matrix and BN + VCM groups exhibited increased effectiveness, illustrated by damaged biofilms and individual bacteria (Fig. 4). These findings indicate that BN may be able to penetrate biofilms.
Figure 4.
Biofilm topology by scanning electron microscopy (magnification, ×8,000). Mature biofilms treated with BN (2 MIC) with or without VCM (2 MIC) and were cultivated at 37°C for 48 h. Biofilms were gold coated prior to analysis. Scale bars, 5 µm. BN, brazilin; VCM, vancomycin; MIC, minimum inhibitory concentration.
Gene expression analysis
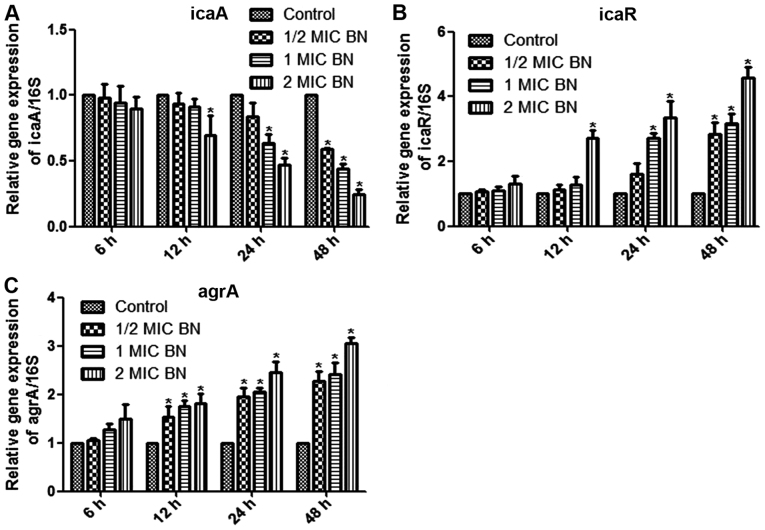
The current study aimed to measure mRNA levels in different samples using RT-qPCR, with 16SrRNA as internal control. Mature biofilms were treated with 1/2, 1 and 2 MIC BN for 6, 12, 24 and 48 h. Treatment with 2 MIC BN for 12 h, with 1 or 2 MIC BN for 24 h or with 1/2, 1 or 2 MIC BN for 48 h significantly lowered icaA expression levels compared with the untreated control (P<0.05; Fig 5A). Transcription levels of icaR and agrA were significantly upregulated in BN treatment groups, compared with the control (P<0.05; Fig. 5B and C). IcaR expression significantly increased compared with the control using 2 MIC BN for 12 h, 1 or 2 MIC BN for 24 h or 1/2, 1 or 2 MIC BN for 48 h (P<0.05; Fig. 5B). BN treatment significantly upregulated transcription levels of agrA following treatment times ≥12 h (Fig. 5C). No significant difference was observed following BN treatment for 6 h compared with the control.
Figure 5.
Gene expression using reverse transcription-quantitative polymerase chain reaction analysis. Relative gene expression of (A) icaA, (B) icaR and (C) argA in the presence of varying concentrations of BN (1/2, 1 and 2 MIC). 16srRNA was used as internal control. *P<0.05 vs. control. Ica, intercellular adhesion; arg, accessory gene regulator; BN, brazilin; MIC, minimum inhibitory concentration.
Discussion
S. aureus biofilm development primarily includes four stages: Attachment, multiplication, maturation and dispersal (27). The principal mechanism of biofilm formation relies on secretion of extracellular polysaccharides (28). It was suggested that the matrix of biofilms may be responsible for increased resistance to antibiotics by acting as a diffusion barrier, causing chronic persistent infections that are difficult to cure (29,30). At present, it is of great importance to identify methods for prevention and treatment of biofilm-associated infections. Studies have demonstrated that traditional herbal medicines were widely-sourced, had minimal side effects, strong pharmacological action and did not lead to bacterial drug resistance (15–17). The use of herbal medicines in the prevention and treatment of biofilm infections has a particular advantage. Traditional herbal medicine may not only inhibit the formation and destroy biofilms, but may enhance resistance to infections when combined with antibiotics (18).
The aim of the current study was to inhibit S. aureus biofilm formation, as assessed using crystal violet staining and FITC-ConA fluorescence microscopy. VCM was used as positive control. The results of the two experiments were similar: BN inhibited biofilm formation in vitro in a dose-dependent manner and the greatest effect was obtained with 2 MIC BN following 24 and 48 h. The results were similar to those reported by Lázaro-Díez et al (6).
At present, VCM is the most commonly administered drug for S. aureus biofilm-associated infections (31). However, there is cause for concern due to recent developments of VCM-intermediate S. aureus and VRSA strains (10). The question of how to reduce the generation of drug-resistant strains and improve the effectiveness of VCM in biofilm infections requires further research. Combining Chinese herbal medicines with VCM is a possible novel approach to treating biofilm-associated infections.
In the current study, to identify synergistic effects of BN combined with VCM on S. aureus biofilms, crystal violet staining, fluorescence microscopy, CFU counting, CLSM and SEM were used. This array of methods demonstrated that BN and BN + VCM generally enhanced the destruction of biofilms in a concentration-dependent manner; 2 MIC BN + 2 MIC VCM exhibited the most notable effects, whereas VCM alone was ineffective. Chen et al (32) studied inhibitory effects of baicalein on S. aureus biofilm formation using these methods, achieving outcomes similar to experimental results presented here.
In summary, VCM may not disrupt a biofilm when used alone, whereas BN may be able to do so. BN may reduce the production of the extracellular polymeric matrix or it may penetrate the biofilm to increase its permeability to VCM. In the current study, BN and VCM exhibited a synergy in eradicating the biofilm.
S. aureus biofilm formation is a complex dynamic process, involving numerous regulatory mechanisms, including polysaccharide intercellular adhesion (PIA), extracellular DNA, global regulatory factors and the QS system (28). PIA is synthesized by the ica locus and was first identified in the epidermis S. aureus biofilm (33). The ica locus includes icaR (regulatory) and icaADBC (biosynthetic), which affect biofilm formation (34). PIA is the primary component of the Gram-positive bacterial biofilm matrix, has the largest influence on biofilm formation and is a well-studied component (35). Studies have demonstrated that icaR acts as a repressor gene and PIA generation increases following knockout of icaR (34,36). In addition, PIA expression is repressed by tcaR, a transcriptional regulator of the teicoplanin-associated locus. The protein regulator of biofilm formation augments ica gene expression, PIA production and biofilm formation (37–39). In addition, Pamp et al (40) demonstrated that Spx, a global regulator of stress response genes, inhibits biofilm formation, potentially by modulating icaR. When strains were cultured in anaerobic environments, the S. aureus respiratory response regulator SrrAB was responsible for PIA, promoting biofilm formation (41). Regulation of the ica locus influences production of PIA and formation of biofilms, making it a novel potential therapeutic target.
In the current study, mature biofilms were treated with BN for 6, 12, 24 and 48 h. It was observed that BN was able to downregulate icaA expression. Transcription levels of icaR were significantly upregulated. BN may regulate icaA and icaR expression in order to inhibit the synthesis of PIA and to reduce the secretion of extracellular polysaccharides.
The S. aureus biofilm QS system includes the agr system and luxS/AI-2 (42). The agr QS system has been well-researched and includes agrBDCA and RNAIII (43). The autoinducer aryl hydrocarbon receptor-interacting protein (AIP) is generated from ArgD precursors and is secreted via the AgrB membrane protein (43). When the extracellular AIP concentration reaches a threshold, agrC is phosphorylated. AgrC phosphorylation activates molecular agrA, which in turn activates RNAIII (44). A previous study demonstrated that when the agr system is mutated, biofilm formation is enhanced (45). The agr system promotes biofilm maturation. The addition of AIP to the mature biofilm may additionally promote biofilm dispersal (45).
The current study demonstrated that BN was able to upregulate transcription levels of agrA. BN may inhibit gene expression of the QS signaling molecule agrA and block RNAIII to prevent biofilm formation. However, the question of whether BN inhibits the QS system requires further analysis.
In conclusion, BN was able to damage mature biofilms potentially by enhancing VCM permeability and through potential inhibition of the secretion of extracellular polysaccharides and the QS system. BN is a potential novel treatment against S. aureus biofilm-associated infections. The mechanisms involved require further analysis and experimentation in vivo.
Acknowledgements
The authors would like to thank Dr Xun Min, Dr Tao Zhang and Dr Yingbiao Tian from the Zunyi Medical University (Zunyi, China) for their expert advice and technical assistance throughout the study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZHC, XM, YBT and TZ conceived and designed the study. DP, ALC, XLC and BS performed the experiments. DP, ZLD, ALC, XLC and HY analyzed the data. DP, ZLD, XLC and YBT contributed reagents, materials and analysis tools. DP and ZHC wrote the paper. TZ revised the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University and written informed consent was obtained prior to the collection of all samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yu L, Hisatsune J, Hayashi I, Tatsukawa N, Sato'o Y, Mizumachi E, Kato F, Hirakawa H, Pier GB, Sugaia M. A Novel Repressor of the ica locus discovered in clinically isolated super-biofilm-elaborating Staphylococcus aureus. MBio. 2017;8 doi: 10.1128/mBio.02282-16. pii:e02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshii Y, Okuda KI, Yamada S, Nagakura M, Sugimoto S, Nagano T, Okabe T, Kojima H, Iwamoto T, Kuwano K, Mizunoe Y. Norgestimate inhibits staphylococcal biofilm formation and resensitizes methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. NPJ Biofilms Microbiomes. 2017;3:18. doi: 10.1038/s41522-017-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sollid JU, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: Determinants of human carriage. Infect Genet Evol. 2014;21:531–541. doi: 10.1016/j.meegid.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Kong C, Chee CF, Richter K, Thomas N, Rahman Abd N, Nathan S. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci Rep. 2018;8:2758. doi: 10.1038/s41598-018-21141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waryah CB, Wells K, Ulluwishewa D, Chen-Tan N, Gogoi-Tiwari J, Ravensdale J, Costantino P, Gökçen A, Vilcinskas A, Wiesner J, Mukkur T. In vitro antimicrobial efficacy of tobramycin against Staphylococcus aureus biofilms in combination with or without DNase I and/or Dispersin B: A preliminary investigation. Microb Drug Resist. 2017;23:384–390. doi: 10.1089/mdr.2016.0100. [DOI] [PubMed] [Google Scholar]
- 6.Lázaro-Díez M, Remuzgo-Martínez S, Rodríguez-Mirones C, Acosta F, Icardo JM, Martínez-Martínez L, Ramos-Vivas J. Effects of subinhibitory concentrations of ceftaroline on methicillin-resistant Staphylococcus aureus (MRSA) biofilms. PLoS One. 2016;11:e0147569. doi: 10.1371/journal.pone.0147569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuillie C, Formosa-Dague C, Hays LM, Vervaecka O, Derclayea S, Brennanc MP, Fosterb TJ, Geogheganb JA, Dufrêne YF. Molecular interactions and inhibition of the staphylococcal biofilm-forming protein SdrC. Proc Natl Acad Sci USA. 2017;114:3738–3743. doi: 10.1073/pnas.1616805114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS, Stoodley P. Antibiotic-loaded synthetic Calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother. 2015;59:111–120. doi: 10.1128/AAC.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch G, Yepes A, Förstner KU, Wermser C, Stengel ST, Modamio J, Ohlsen K, Foster KR, Lopez D. Evolution of resistance to a last-resort antibiotic in Staphylococcus aureus via bacterial competition. Cell. 2014;158:1060–1071. doi: 10.1016/j.cell.2014.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2016;23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiedrowski MR, Horswill AR. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci. 2011;1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther. 2015;13:1499–1516. doi: 10.1586/14787210.2015.1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem AH, Elkhatib WF, Noreddin AM. Pharmacodynamic assessment of vancomycin-rifampicin combination against methicillin resistant Staphylococcus aureus biofilm: A parametric response surface analysis. J Pharma Pharmacol. 2011;63:73–79. doi: 10.1111/j.2042-7158.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 14.Olson ME, Slater SR, Rupp ME, Fey PD. Rifampicin enhances activity of daptomycin and vancomycin against both a polysaccharide intercellular adhesin (PIA)-dependent and -independent Staphylococcus epidermidis biofilm. J Antimicrob Chemother. 2010;65:2164–2171. doi: 10.1093/jac/dkq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontaine BM, Nelson K, Lyles JT, Jariwala PB, García-Rodriguez JM, Quave CL, Weinert EE. Identification of ellagic acid rhamnoside as a bioactive component of a complex botanical extract with anti-biofilm activity. Front Microbiol. 2017;8:496. doi: 10.3389/fmicb.2017.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu B, Wu Q, Dang M, Bai D, Guo Q, Shen L, Duan K. Inhibition of pseudomonas aeruginosa biofilm formation by traditional chinese medicinal herb herba patriniae. Biomed Res Int. 2017;2017:9584703. doi: 10.1155/2017/9584703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo J, Dong B, Wang K, Cai S, Liu T, Cheng X, Lei D, Chen Y, Li Y, Kong J, Chen Y. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One. 2017;12:e0176883. doi: 10.1371/journal.pone.0176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Heredia A, García S, Merino-Mascorro JÁ, Feng P, Heredia N. Natural plant products inhibits growth and alters the swarming motility, biofilm formation, and expression of virulence genes in enteroaggregative and enterohemorrhagic Escherichia coli. Food Microbiol. 2016;59:124–132. doi: 10.1016/j.fm.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Lee HK, Chang TS, Kang KS, Hwang GS. Inhibitory effect of brazilin on osteoclast differentiation and its mechanism of action. Int Immunopharmacol. 2015;29:628–634. doi: 10.1016/j.intimp.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Gao XJ, Wang TC, Zhang ZC, Cao YG, Zhang NS, Guo MY. Brazilin plays an anti-inflammatory role with regulating Toll-like receptor 2 and TLR 2 downstream pathways in Staphylococcus aureus-induced mastitis in mice. Int Immunopharmacol. 2015;27:130–137. doi: 10.1016/j.intimp.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Dan P, Zhou X, Chen Z. Research progress on mechanism and treatment of Staphylococcus aureus biofilms. Molecular Biomedicine. 2017;23:3745–3749. [Google Scholar]
- 22.Nirmal NP, Panichayupakaranant P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm Biol. 2015;53:1339–1343. doi: 10.3109/13880209.2014.982295. [DOI] [PubMed] [Google Scholar]
- 23.Yan Y, Chen YC, Lin YH, Guo J, Niu ZR, Li L, Wang SB, Fang LH, Du GH. Brazilin isolated from the heartwood of Caesalpinia sappan L induces endothelium-dependent and-independent relaxation of rat aortic rings. Acta Pharmacol Sin. 2015;36:1318–1326. doi: 10.1038/aps.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M7-A9. Clinical and Laboratory Standards Institute; Wayne, PA: 2012. [Google Scholar]
- 25.Shi SF, Jia JF, Guo XK, Zhao YP, Chen DS, Guo YY, Zhang XL. Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles. Int J Nanomedicine. 2016;11:6499–6506. doi: 10.2147/IJN.S41371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian S, Othman EM, Kampik D, Stopper H, Hentschel U, Ziebuhr1 W, Oelschlaeger1 TA, Abdelmohsen UR. Marine sponge-derived Streptomyces sp. SBT343 extract inhibits Staphylococcal biofilm formation. Front Microbiol. 2017;8:236. doi: 10.3389/fmicb.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimoto S, Sato F, Miyakawa R, Chiba A, Onodera S, Hori S, Mizunoe Y. Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and-sensitive strains of Staphylococcus aureus. Sci Rep. 2018;8:2254. doi: 10.1038/s41598-018-20485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AK, Prakash P, Achra A, Singh GP, Das A, Singh RK. Standardization and classification of in vitro biofilm formation by clinical isolates of Staphylococcus aureus. J Glob Infect Dis. 2017;9:93–101. doi: 10.4103/jgid.jgid_91_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks JL, Jefferson KK. Staphylococcal biofilms: Quest for the magic bullet. Adv Appl Microbiol. 2012;81:63–87. doi: 10.1016/B978-0-12-394382-8.00002-2. [DOI] [PubMed] [Google Scholar]
- 31.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: A systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Liu T, Wang K, Hou C, Cai S, Huang Y, Du Z, Huang H, Kong J, Chen Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS One. 2016;11:e0153468. doi: 10.1371/journal.pone.0153468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'gara JP. Ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270:179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 34.Figueiredo AMS, Ferreira FA, Beltrame CO, Côrtes MF. The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit Rev Microbiol. 2017;43:602–620. doi: 10.1080/1040841X.2017.1282941. [DOI] [PubMed] [Google Scholar]
- 35.Ma R, Qiu S, Jiang Q, Sun H, Xue T, Cai G, Sun B. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int J Med Microbiol. 2017;307:257–267. doi: 10.1016/j.ijmm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A, Mishra S, Singh S, Mishra S. Prevention of IcaA regulated poly N-acetyl glucosamine formation in Staphylococcus aureus biofilm through new-drug like inhibitors: In silico approach and MD simulation study. Microb Pathog. 2017;110:659–669. doi: 10.1016/j.micpath.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Skurnik D, Cywes-Bentley C, Pier GB. The exceptionally broad-based potential of active and passive vaccination targeting the conserved microbial surface polysaccharide PNAG. Expert Rev Vaccines. 2016;15:1041–1053. doi: 10.1586/14760584.2016.1159135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Formosa-Dague C, Feuillie C, Beaussart A, Derclaye S, Kucharíková S, Lasa I, Van Dijck P, Dufrêne YF. Sticky matrix: Adhesion mechanism of the staphylococcal polysaccharide intercellular adhesin. ACS Nano. 2016;10:3443–3452. doi: 10.1021/acsnano.5b07515. [DOI] [PubMed] [Google Scholar]
- 39.Doulgeraki AI, Di Ciccio P, Ianieri A, Nychas GE. Methicillin-resistant food-related Staphylococcus aureus: A review of current knowledge and biofilm formation for future studies and applications. Res Microbiol. 2017;168:1–15. doi: 10.1016/j.resmic.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Pamp SJ, Frees D, Engelmann S, Hecker M, Ingmer H. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J Bacteriol. 2006;188:4861–4870. doi: 10.1128/JB.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinkel TL, Roux CM, Dunman PM, Fang FC. The Staphylococcus aureus SrrAB two-component system promotes resistance to nitrosative stress and hypoxia. MBio. 2013;4:e00696–e13. doi: 10.1128/mBio.00696-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coelho LR, Souza RR, Ferreira FA, Guimarães MA, Ferreira-Carvalho BT, Figueiredo AM. Agr RNAIII divergently regulates glucose-induced biofilm formation in clinical isolates of Staphylococcus aureus. Microbiology. 2008;154:3480–3490. doi: 10.1099/mic.0.2007/016014-0. [DOI] [PubMed] [Google Scholar]
- 43.Atwood DN, Beenken KE, Loughran AJ, Meeker DG, Lantz TL, Graham JW, Spencer HJ, Smeltzer MS. XerC contributes to diverse forms of Staphylococcus aureus infection via agr-dependent and agr-independent pathway. Infect Immun. 2016;84:1214–1225. doi: 10.1128/IAI.01462-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le KY, Otto M. Quorum-sensing regulation in staphylococci-an overview. Front Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.