Abstract
Introduction:
Environmental noise is associated with negative developmental outcomes for infants treated in the neonatal intensive care unit (NICU). The existing noise level recommendations are outdated, with current studies showing that these standards are universally unattainable in the modern NICU environment.
Study Aim:
This study sought to identify the types, rate, and levels of acoustic events that occur in the NICU and their potential effects on infant physiologic state.
Materials and Methods:
Dosimeters were used to record the acoustic environment in open and private room settings of a large hospital NICU. Heart and respiratory rate data of three infants located near the dosimeters were obtained. Infant physiologic data measured at time points when there was a marked increase in sound levels were compared to data measured at time points when the acoustic levels were steady.
Results:
All recorded sound levels exceeded the recommended noise level of 45 decibels, A-weighted (dBA). The 4-h Leq of the open-pod environment was 58.1 dBA, while the private room was 54.7 dBA. The average level of acoustic events was 11–14 dB higher than the background noise. The occurrence of transient events was 600% greater in the open room when compared to the private room. While correlations between acoustic events and infant physiologic state could not be established due to the extreme variability of infant state, a few trends were visible. Increasing the number of data points to overcome the extreme physiologic variability of medically fragile neonates would not be feasible or cost-effective in this environment.
Conclusion:
NICU noise level recommendations need to be modified with an emphasis placed on reducing acoustic events that disrupt infant state. The goal of all future standards should be to optimize infant neurodevelopmental outcomes.
Keywords: acoustic environment, hospital noise, infant development, neonatal intensive care unit, physiologic monitoring
Introduction
The neonatal intensive care unit (NICU) is a specialized care unit that treats premature and medically fragile newborns and infants. Advancements in medical technology, focused-training of skilled medical personnel, and the establishment of critical care protocols have resulted in greater neonatal survival in the NICU. In the United States, although the number of babies admitted to the NICU has increased over the years to 77.9 per 1000 births,[1] the infant mortality rate is at an all-time low with only 5.98 infant deaths per 1000 live births.[2] Because of the growth in infant survival rates and NICU admission rates, a great deal of research has focused on identifying and creating the ideal NICU environment for both the neonate and the staff. Specifically, attention has been dedicated to monitoring and regulating the acoustic environment in the NICU.
The current noise standards for the NICU environment were established decades ago and have not been modified. In 1974, the U.S. Environmental Protection Agency (EPA) recommended that indoor hospital areas maintain an average sound level of less than or equal to 45 decibels, A-weighted (dBA) during the day, and 35 dBA at night to maximize opportunity for patient recovery.[3] In a 1997 Position Statement summarizing the effects of noise upon the developing fetus and the infant, the American Academy of Pediatrics (AAP) cited EPA standards and applied this recommendation to the NICU environment, stating that average sound levels in the NICU should not exceed 45 dBA.[4] Over the following decades, several research groups have made additional or updated recommendations in an effort to re-evaluate the EPA and AAP standards. While the recommendations for the average sound levels in the NICU environment did not drastically change, new recommendations regarding the presence of transient sounds in the environment (i.e., doors slamming) were issued, with the maximum level for transient sounds (L max averaged over 1 s) being either 65 or 70 dBA.[5,6]
Despite the presence of these recommendations, existing analyses of the acoustic environment in the NICU have indicated that these noise standards are being exceeded regularly. Studies from separate NICU environments have demonstrated that average noise levels range from 48 to 55 dBA[7] and 53.9 to 60.6 dBA.[8] An additional study found that sound levels exceeded the recommended standard more than 70% of the time,[9] while another found that noise levels only met recommendations 5.51% of the time.[10] Furthermore, it appears that NICU incubators also serve as a potential source of noise. Sound levels measured inside enclosed and activated incubators were never measured below recommended noise standards.[7]
In an effort to decrease noise levels and comply with recommendations, numerous noise abatement programs have been developed and implemented; however, none have been successful in creating an environment that effectively maintains current standards. Most programs have focused on a combination of staff training programs and minor structural modifications, but results have demonstrated that these adjustments did not result in any significant reduction in noise levels.[11,12,13] Because acoustic noise cannot be avoided in the NICU, it is important to know the effect of noise on the neonate.
A large contrast exists between the intrauterine and NICU acoustic environments. While the fetal peripheral auditory system is fully developed by approximately the 24th week of gestation,[14] responses to sound emerge earlier. Fetal responses to low frequency sounds emerge approximately six to eight weeks earlier than responses to high frequency sounds.[15] This evolution of responses may reflect the natural maturation of the fetal central auditory system and have major implications for auditory brain development in infants born prematurely. Studies have demonstrated that exposure to sounds containing frequencies above 500 Hz is atypical for the developing fetus.[16] Environmental sound is attenuated 40–50 dB at frequencies above 500 Hz by the body barrier of the womb and the impedance mismatch between air and embryonic fluid. In contrast, in the NICU environment, infants are exposed to acoustic frequencies higher than 500 Hz 57% of the time.[17] Alarms and sounds emitted from necessary life support equipment such as extracorporeal membrane oxygenation machines may contain frequencies as high as 16,000 Hz.[18] Therefore, infants who are born prematurely and cared for in the NICU are routinely exposed to sound levels and frequencies they are not developmentally prepared to handle.[19]
Numerous studies have demonstrated the adverse effects of the acoustic environment in the NICU by examining the relationship between acoustic events and alterations in infant physiologic state. Studies have demonstrated that high intensity, transient noises are associated with behavioral disturbances and increases in infant muscle tension.[20] Other studies have documented a relationship between acoustic noise and changes in infant vital signs including heart rate, respiratory rate, O2 saturation, blood pressure, and intracranial pressure.[21,22,23,24,25]
Though the presence of a relationship between noise and infant state is well documented, it is important to consider how the effects of noise may be unique to the infant who is born prematurely. The preterm infant is physiologically immature in all of its major systems, including the central nervous system. As a result of global systemic immaturity and the lack of developed homeostatic mechanisms, preterm infants demonstrate distinctive responses to environmental stress in the NICU.[26] Multiple studies have demonstrated that the effects of noise upon heart rate, respiratory rate, and oxygen saturation were more pronounced in preterm infants than in full-term infants.[21,27] Moreover, in a study that compared term and preterm infant reactions to acoustic stimuli, researchers found that preterm infants, unlike term infants, did not demonstrate the ability to habituate to the stimuli after repeated exposures.[28]
In addition to instantaneous changes in infant state, the stressful stimuli present in the NICU environment have also been associated with long-term effects on infant development. Physiologic stress responses to acoustic events such as changes in heart rate, intracranial pressure, and oxygen saturation may have a significant impact on the preterm infant’s future neurologic development due to altered perfusion and oxygenation of the brain tissue.[26] This effect is likely intensified by the preterm infant’s inability to regulate these systems.[29] Studies have also suggested that the stressful stimuli present in the NICU environment may be associated with an increased risk for future attention, language, and hearing disorders, which may be partially attributed to the immature auditory system’s exposure to certain types of noise in the NICU and limited exposure to speech and language.[30,31]
Because of the negative outcomes associated with NICU stay, researchers agree that the primary purpose for establishing and maintaining noise standards in the NICU should be to create the most ideal environment for the fragile neonate to grow, heal, and thrive. The NICU environment is full of stressful events, and premature infants are forced to expend a significant amount of energy mediating these stressful stimuli. The goal should be to remove as many of these stressors as possible, so that neonates can reserve their energy for healing, which may result in a reduction in their NICU stay and earlier release to their families.[32] To accomplish this, attention must be given to the infant’s sensory systems, and recommendations for all exposures in the NICU must yield a supportive, nurturing setting that optimizes the neurodevelopmental outcomes of preterm infants.[10,33,34,35]
Study Aim
Given the evidence demonstrating an inability for NICUs to comply with current noise level recommendations, the known effects of noise on the physiologic state of the infant, and the long-term impact of certain types of acoustic stimuli on the developmentally immature preterm infant, the aim of the present study is twofold: first, to identify the type, rate, and levels of acoustic events that occur in the NICU, as well as the signal-to-noise ratios (SNRs) corresponding to those events; and second, to identify how these events may affect infant physiologic state as measured by changes in infant heart rate and respiratory rate. This information will lead to a more precise understanding of the characteristics of acoustic events that may impact infant physiologic state and could lead to the development of more practical NICU noise standards that allow for both protection of the infant and reasonable implementation by hospital staff.
Materials and Methods
Sound level recordings
Larson Davis Spark 706RC, 705+, and 703+ Type II Noise Dosimeters (Larson Davis Laboratories, Provo, Utah) were used to record the acoustic environment in both the open and private room settings in the Level IV NICU at St. Louis Children’s Hospital. Recordings took place during the morning and afternoon hours of February 23, 2017. In the private room, dosimeters were placed on a narrow counter behind the infant isolette, and microphones were taped near the level of the infant’s head. In the open room, dosimeters were placed on a counter with microphones taped to a post located between two isolettes. Figure 1 shows the sound recording diagram for both the open (a) and private (b) room. All dosimeters were set to A-weighted, fast detector setting (0.125-s interval), 1-s sample interval, 30 dB gain, and 3 dB exchange rate. Each dosimeter was calibrated before and after the recording session and was found to be following appropriate American National Standards Institute standards.[36] Data were downloaded and exported via Blaze v 6.1.1 software (Larson Davis Laboratories, Provo, Utah).
Figure 1.
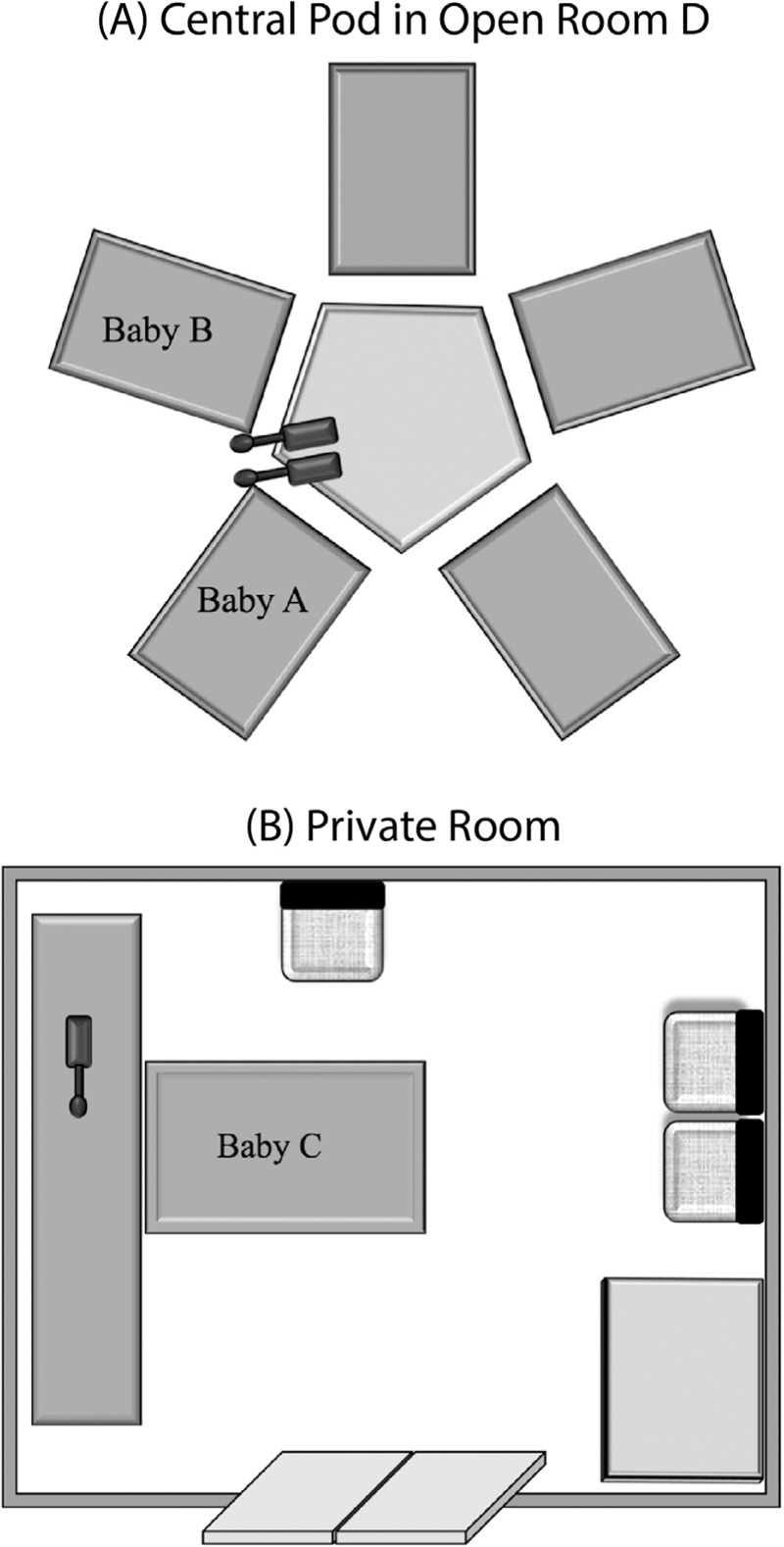
Panel (A) displays the dosimeter placement in the NICU open pod area. Baby A was located in the isolette to the right of the microphone, while Baby B was located immediately to the left. Panel (B) displays the dosimeter placement for the private room.
The descriptions of acoustic events were documented by an observer, who was physically present in the room during recording periods. The observer categorized perceived events into four main groups: alarm noise, infant-generated noises such as crying, staff and/or family conversations, and transient events such as a cabinet door closing. A clock synced to Greenwich Mean Time (GMT) was used to record the onset time and duration of acoustic events. In addition to tracking acoustic events, the observer also documented perceived infant state throughout the course of the recordings. If the infant was being handled, was moving or crying, or was receiving any type of intervention, the infant’s state was determined to be “active.” For all other times, the infant was considered to be in a “passive” state. The data recorded during times when the infant was active were removed prior to analysis to prevent potentially confounding changes in physiologic state that may have been explained by handling of the infant.
Average equivalent continuous sound level (L eq) and the A-weighted sound levels exceeding 10, 50, and 90% of the time (L 10, L 50, and L 90, respectively) were calculated for the duration of the recording period in each room. Acoustic events were defined as the occurrences of levels that exceeded the L 10 for that room plus 2 dB, which was added to eliminate spurious events that resulted from minor perturbations in the sound level. This addition of 2 dB also accounts for the accuracy of the dosimeter microphones, which is ±2 dB, as well as the difference limen for intensity in humans, which is about 1 dB for average healthy adult ears.[37] To include transient events, time points when the L 10 of the peak levels for that room plus 2 dB was exceeded were also included. The levels of acoustic events were compared to the acoustic level that was exceeded 50% of the time (L 50) for each room, and an SNR L 50 was calculated (level of acoustic event−L 50 = SNR L 50). Average L eq and average peak level were also compared between time points categorized as events and those classified as nonevents for each room type.
The number and description of perceived acoustic events documented by the observer was compared to the number of events recorded in the acoustic log. The average L eq, average peak, and average SNR L 50 were calculated and compared between rooms. The samples of documented alarm noises were examined and determined to have peak levels that ranged between 82 and 86 dBA. To efficiently and accurately compare the number of events that occurred in each room, all 1-s sampling intervals containing peaks exceeding 84 dBA (median peak level of alarms) were counted for each room and averaged over the duration of the recording, yielding an average number of seconds per hour during which the chosen peak was exceeded for each room.
Infant physiologic recordings
Physiologic data were collected from three infants, two in the open room (Babies A and B) and one in the private room (Baby C), using the BedMaster system.[38] BedMaster data were collected for most infants in the NICU at St. Louis Children’s Hospital. It is an automated system that records and stores patient vital sign data and alarms processed by patient monitors. The data extracted for comparison in this study included heart rate and respiratory rate, which have been reported to be good physiologic indicators of stress in the premature infant,[39] and have been associated with infant stress responses to adverse environmental stimuli in the NICU.[26]
The relationship between acoustic events and alterations in infant physiologic state was examined by comparing the physiologic data measured at time points relative to GMT when there was a marked increase in the sound levels to the physiologic data measured at time points when the acoustic levels were steady. To analyze and account for the latency of physiologic responses relative to acoustic events, infant state was offset from acoustic events by 2, 4, 8, and 16 s. Each infant served as his or her own control for baseline state, as physiologic norms are not well established in this population of critically ill infants.[40]
Results
Sound levels and acoustic events
Acoustic data for the open room and private rooms are shown in Tables 1 and 2. A summary table with comparisons of sound levels between room types is located in Table 3. The L eq for the duration of the 4-h recording period in the open room was 58.1 dBA, while the L eq for private room was 54.7 dBA. The noise levels in both environments exceeded the AAP recommended levels, further confirming that noise standards are not being met in the modern NICU. In fact, during the hours of acoustic recordings collected for this study, the 1-s L eq was never measured at or below 45 dBA in either room. The time waveforms of the L eq and peak levels for open (left) and private rooms (right) are shown in Figure 2. Both the absolute levels and the number of acoustic spikes reveal the differing acoustic signatures of the two environments.
Table 1.
Open room acoustic data
| Time of recording | 10:25–14:30 | |
| Overall L eq | 58.1 dBA | |
| Events | Nonevents | |
| Overall L eq | 65.8 dBA | 56.2 dBA |
| SNR L 50 | 11.2 dB | 1.6 dB |
| Average peak | 88.5 dBA | 82.3 dBA |
Table 2.
Private room acoustic data
| Time of recording | 10:15–14:30 | |
| Overall Leq | 54.7 dBA | |
| Events | Nonevents | |
|---|---|---|
| Overall L eq | 63.8 dBA | 50.5 dBA |
| SNR L 50 | 14.1 dB | 0.8 dB |
| Average peak | 90.0 dBA | 76.8 dBA |
Table 3.
Acoustic data by room type
| Open room | Private room | |
|---|---|---|
| Overall L eq | 58.1 dBA | 54.7 dBA |
| L 90 | 51.1 dBA | 48.3 dBA |
| L 50 | 54.6 dBA | 49.7 dBA |
| L 10 | 61 dBA | 53.9 dBA |
| Average level of acoustic events | 65.8 dBA | 63.8 dBA |
| Average SNR L 50 of events | 11.2 dB | 14.1 dB |
| Occurrences (s) of peaks ≥84 dBA/h | 579 s | 94 s |
Figure 2.
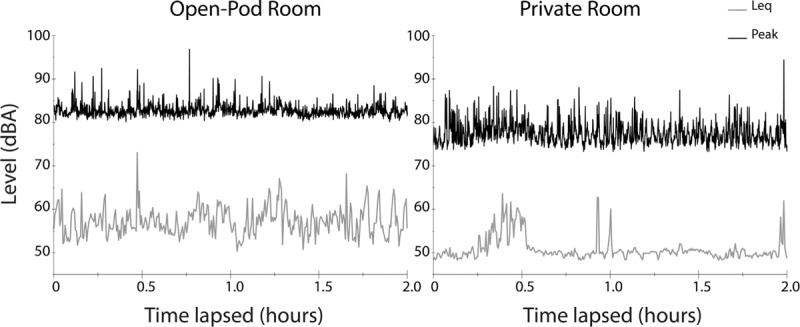
The time waveforms of the L eq and peak levels for open (left) and private rooms (right) are shown. For each figure the recorded, 1-s L eq was logarithmically averaged over a 20-s period while the 1-s peak levels were averaged over a 5-s period for greater resolution of the brief transients. These 2-h samples are a good representation of the overall acoustic environment described in Tables 1–3.
The occurrences of peak levels exceeding 84 dBA were more frequent in the open room than in the private room. Peak levels were exceeded an average of 579 s per hour in the open room compared to an average of 94 s per hour in the private room. The average level of acoustic events in the open room was 65.8 dBA with an SNR L 50 of 11.2 dB, while the average level and SNR L 50 of nonevents in the open room were 52.6 dBA and 1.6 dB, respectively. In contrast, the average level of acoustic events in the private room was 63.8 dBA with an average SNR L 50 of 14.1 dB during acoustic events and 50.5 dBA with an SNR L 50 of 0.8 dB during nonevents.
Infant physiologic responses
The infants in this study were extremely variable in their physiologic state, which is likely a direct result of their medically fragile condition. Figure 3 displays the intersubject variability of infant heart and respiratory rate for all three infants. Each panel displays 1 h of the total recording of the heart rate (black) and respiratory rate (gray) with a 1-s resolution for each infant. While Babies A and B (left) were next to each other in the open room setting with the same acoustic environment, the heart and respiratory rate measures were more variable in Baby B. Consequently, correlations could not be established between acoustic events and alterations in infant physiologic state. Preliminary data analysis showed no difference between mean heart rate or mean respiratory rate during the periods of acoustic events when compared to periods of nonevents due to extreme infant variability (P = 0.42 and 0.43, respectively). Furthermore, as shown in Figure 4, no significant difference was found between mean infant heart rate during the acoustic event (0 s) or at delays of 2, 4, 8, and 16 s from the onset of the acoustic event (F = 0.70, P = 0.53).
Figure 3.
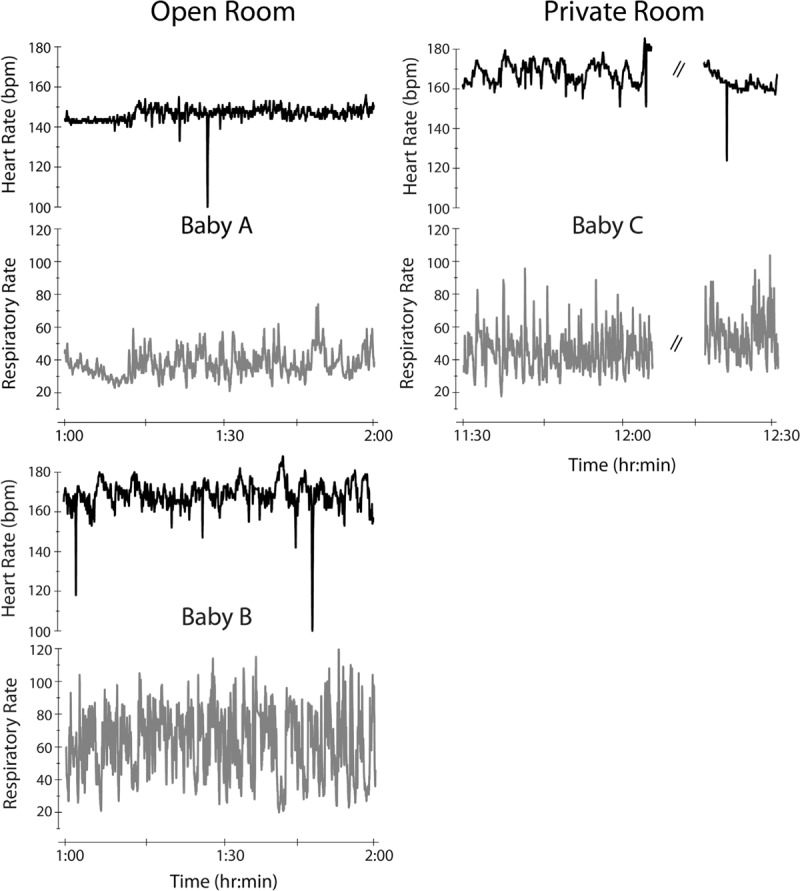
The intersubject variability of infant heart and respiratory rate is shown. Each panel displays 1 h of the total recording of the heart rate (black) and respiratory rate (gray) with a 1-s resolution for each infant. Babies A and B (left) were in the open room environment while Baby C (right) was in the private room environment. Note that while Babies A and B were in the same acoustic environment, the heart and respiratory rate were more variable in Baby B. Dashed lines indicate extraction of physiologic data during infant handling.
Figure 4.
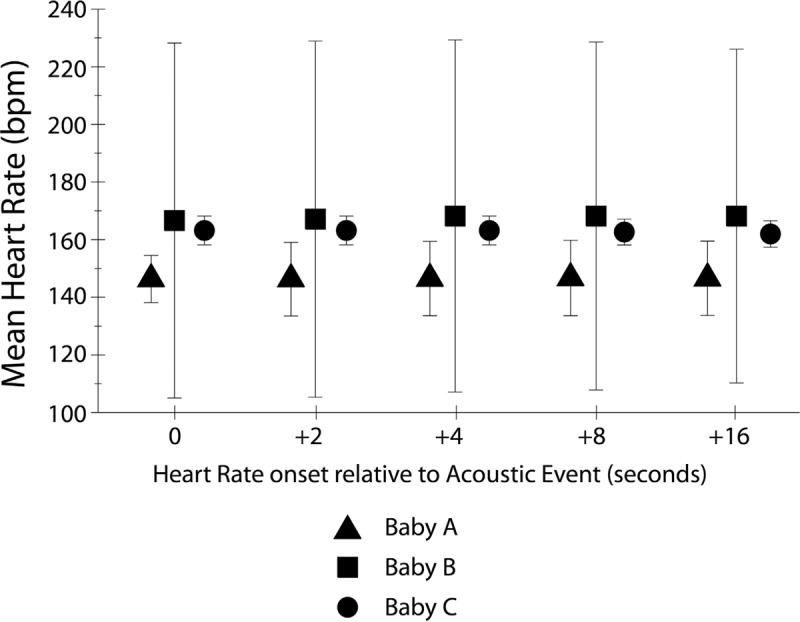
The mean infant heart rates following acoustic events are shown. For each infant (Baby A = triangle, B = square, and C = circle), the mean heart rate during the acoustic event was calculated and shown at 0 s on the graph. Then, mean heart rate was calculated at delays of 2, 4, 8, and 16 s from the onset of the acoustic event. There was no significant effect of delay on heart rate. The bars indicate one standard deviation.
It is worth noting that several instances of change in infant heart rate were identified at specific time points when a documented acoustic event occurred. However, infants were inconsistent in their responses, and they also displayed large disturbances even when the acoustic environment was relatively stable. Figure 5 demonstrates an example of multiple acoustic events that occurred in the open room while both Babies A and B were passive. The observer noted that these events were the result of alarms that were unrelated to either infant. Baby B’s heart rate appeared to fluctuate in concordance with the onset of these events, yet, the heart rate for Baby A in the same environment showed little to no variation. It is possible that Baby A has some other comorbidity such as hearing impairment that could explain this lack of response to the acoustic events. It is also possible that the changes observed in Baby B are coincidental and have more to do with his or her fragile state than with the occurrence of the alarm peaks. Because of the variability in the physiologic state of infants included in this study, the presence or absence of a relationship could not be determined. Given the extreme physiologic variability of these medically fragile neonates, increasing the number of infants observed would not be feasible or cost-effective in this environment as we would need several hundreds of observations to overcome this variability.
Figure 5.
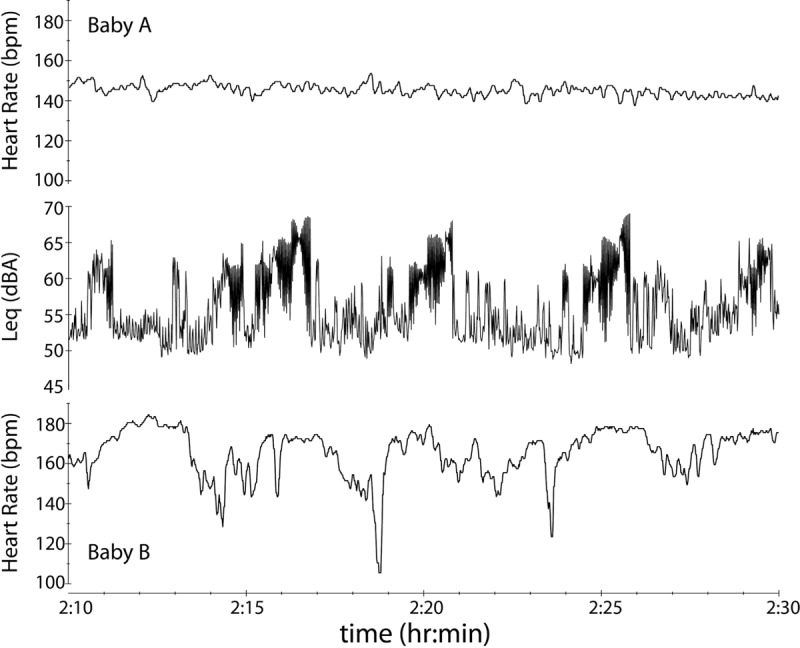
The time waveform of the L eq in the open room is shown over a 20-min period with corresponding heart rate data for Babies A and B. Multiple acoustic events, which were identified by the observer to be alarms that were unrelated to either infant, took place during this time window. Visual examination reveals that Baby B’s heart rate may be fluctuating with the onset of acoustic events; however, Baby A’s heart rate remains stable. Overall variability of infants precluded measurable correlation between events and physiologic changes.
Discussion
The results of this study regarding overall sound levels support and expand upon the findings of earlier research, confirming the need for an improved method of quantifying and regulating the acoustic environment in the NICU. Levels in both the open pod and private rooms consistently exceeded the 45 dBA standard, with sound levels in the open pod room being 3.4 dB higher than levels recorded in the private room. Given the fact that the combined noise emitted from the necessary life support equipment, heating, ventilation, and air conditioning (HVAC) system, lights, and monitors does not meet standards even without additional noise introduced by staff, visitors, or maintenance, it would be futile to continue making efforts to meet the standards as they are written today. Rather than continuing to focus on the overall noise level, an event-based approach may lead to an attainable standard that minimizes stress on the preterm infant and addresses the most critical needs of neonates.
The results of this study indicate that the frequency of acoustic events as well as the levels of these events relative to the background noise level is dependent on NICU room type (open pod vs. private room). The number of events was far greater in the open room due to the combined activities of multiple infants, families, and staff members. The occurrence of events with peak sound levels exceeding 84 dBA was 600% greater in the open room than in the private room. As a result, the L 50, or the sound level of the room exceeded 50% of the time, was 4.9 dB lower in the private room (49.7 dBA) than the open room (54.6 dBA). Therefore, the SNR of the documented acoustic events to the estimated background noise level (L 50) is higher for the private room than the open-pod nursery. This caused acoustic events to be much more salient in the private room.
Despite many documented examples of acoustic events throughout the recording periods, the constant variability in infant physiologic state precluded a qualitative assessment of whether or not correlations between the acoustic environment and physiologic state exist. While visual examination of Figure 5 suggests a trend in heart rate variance with the onset of acoustic events for Baby B, the overall variation of Baby B’s heart rate as shown in Figures 3 and 4 prohibits any meaningful correlation between events and physiologic changes. It may not be helpful or cost-effective to collect more data in this medically fragile population due to the extreme physiologic variability that clouds any relationship between infant state and acoustic events. Future studies should consider analyzing potential correlations between these events and changes in physiologic state for infants who are more stable, such as those in the well-baby nursery. If a relationship between acoustic events and physiologic state is observed in healthier infants, these findings may be used to develop recommendations that can be applied to the NICU environment. Although it is not yet clear which acoustic events in the environment might disturb preterm infants most, the findings in this study suggest a need to decrease both the rate of occurrence and the SNR of events in the NICU. Therefore, rather than continuing to focus efforts solely on gross sound abatement strategies, efforts should be made to reduce the occurrence of unnecessary acoustic events. Many events, such as alarms on feeding pumps or heart rate monitors, cannot be prevented and are essential for the care and safety of infants. However, a portion of the transient acoustic events recorded by the observers in both rooms included supplies being dropped on hard surfaces or cabinet and formula refrigerator doors closing loudly, which could possibly be circumvented with minor modifications in equipment and/or human behavior. It may be feasible to develop a software program that analyzes sound levels in the NICU to track the frequency with which these types of events occur. The digital monitoring of acoustic events will eliminate human observer bias and error. Hospital administrators could then use these data to establish protocols for decreasing the amount of preventable events.
Along with reducing as many unnecessary events as possible, it may be beneficial to decrease the SNR of unavoidable acoustic events, such as alarms. One such method might include the introduction of a developmentally appropriate stimulus into the infant’s environment. While this approach entirely contradicts the recommendation for reducing overall noise level set forth by current NICU noise standards, this added stimulus could serve as an effective masker that would elevate the noise floor to decrease the SNR of acoustic events perceived by the infant. Though correlations between acoustic events and changes in physiologic state could not be established in this study, it is worth noting that several examples of acoustic events that occurred in the open room were documented during the periods of relative infant physiologic stability and did not yield any significant change in infant state. This could potentially be explained by the fact that the infants whose data were extracted for this study were housed in covered isolettes with ports closed, which are known to have an interior running noise of 58 dBA.[7] It is possible that this mechanical noise served as an acoustic masker that elevated the noise floor and decreased the SNR of acoustic events at the infant’s ear. If this masking noise did contribute to a lack of response from the infants, it would be beneficial to explore other types of acoustic maskers that could provide a developmentally supportive stimulus while simultaneously shielding the infant from potentially adverse acoustic events. This would generate a more tranquil environment that promotes long-term recovery and development.
One example of an appropriate stimulus may include an acoustic recording of the infant’s mother. Numerous studies have demonstrated that exposure to recorded maternal sounds can influence the physiologic state of infants in the NICU. In one study, researchers found that infants exposed to maternal sound stimulation experienced fewer cardiorespiratory events compared to infants who were exposed to routine hospital sounds and concluded that maternal sound stimulation may provide short-term improvements in the physiologic stability of preterm infants.[41] Similarly, another study monitored the heart rate of preterm infants in the NICU during the times of exposure to maternal sounds and generic hospital background noise and found that all infants displayed lower heart rate when exposed to the maternal recordings. This led the researchers to conclude that preserving the preterm infant’s natural environment to the greatest extent possible through exposure to maternal sounds may improve the infant’s autonomic stability while creating a more soothing environment, which promotes sleep and recovery.[42]
In addition to providing a more tranquil setting for the infant, some sounds, specifically spoken language, have been shown to augment the infant’s development. The NICU environment has been associated with negative long-term outcomes in language development.[43] Though previous standards have fixated upon the need to create silence in the NICU, this sensory deprivation and elimination of speech and language exposure has been demonstrated to have negative effects on the infant’s developing auditory cortex, which can result in language delays later in life.[44] Additionally, research has shown that language comprises a very small percentage of the sounds to which infants in the NICU are exposed,[45] despite the fact that greater adult language exposure is associated with better infant language and cognitive scores later in life.[46] One study found that preterm infants who were exposed to recorded maternal voice and heartbeat showed significantly larger auditory cortical development compared to preterm infants who experienced routine hospital sounds.[47] Therefore, one reasonable approach could be to incorporate more meaningful sounds, such as maternal speech, into the NICU environment to serve both as acoustic maskers and stimuli that developmentally benefit the infant.The findings of these studies demonstrate that a balance needs to be established between reducing unnecessary stimuli that could cause the infant stress and depriving the infant of meaningful stimuli that could result in impaired development. Thus, future research should focus on the methods of decreasing alarm noise, and other nonhuman noises that can stress the infant,[48] but should also seek to identify the types of stimulation that will generate the most tranquil, developmentally supportive acoustic environment possible for the preterm infant to achieve optimal neurodevelopment.
This study proposes a method for quantifying and describing acoustic events in the NICU. The findings indicate that acoustic events in the NICU occur frequently, and though a relationship between events and changes in infant physiologic state could not be established due to infant variability, it is likely that these events do disturb the infants. Given the results of this study regarding the rate of occurrence and high SNR of acoustic events as well as the documented developmental benefits of introducing the infant to meaningful stimuli, it is concluded that rather than continuing to strive for the diminution of all sound, the goal should be to incorporate developmentally appropriate acoustic stimuli into the infant’s environment. Noise regulations should focus on reducing the number of preventable acoustic events that occur in the NICU, and future studies should aim to identify the characteristics of acoustic stimulation that would decrease the SNR of unavoidable events and result in the most positive developmental outcomes for preterm infants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Harrison W, Goodman D. Epidemiologic trends in neonatal intensive care, 2007–2012. JAMA Pediatr. 2015;169:855–62. doi: 10.1001/jamapediatrics.2015.1305. [DOI] [PubMed] [Google Scholar]
- 2.Deaths: Final Data for2012. (Internet) National Vital Statistics Reports. 2015. [[Last accessed on 2017 Jan 30]]. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr63/nvsr63_09.pdf .
- 3.Environmental Protection Agency, Office of Noise Abatement and Control. Information on Levels of Environmental Noise Requisite to Protect Public Health and Welfare With an Adequate Margin of Safety. Washington, DC: Government Printing Office; 1974. [Google Scholar]
- 4.American Academy of Pediatrics Committee on Environmental Health. Noise: A hazard for the fetus and newborn. Pediatrics. 1997;100:724–7. [PubMed] [Google Scholar]
- 5.White RD, Smith JA, Shepley MM. Recommended standards for newborn ICU design, eighth edition. J Perinatol. 2013;33:2–16. doi: 10.1038/jp.2013.10. [DOI] [PubMed] [Google Scholar]
- 6.Graven SN. Sound and the developing infant in the NICU: Conclusions and recommendations for care. J Perinatol. 2000;20:88–93. doi: 10.1038/sj.jp.7200444. [DOI] [PubMed] [Google Scholar]
- 7.Knutson A. Acceptable Noise Levels for Neonates in the Neonatal Intensive Care Unit. Independent Studies and Capstones. 2013. [[Last accessed on 2017 Jan 9]]. Available from http://digitalcommons.wustl.edu/pacs_capstones/643 .
- 8.Darcy AE, Hancock LE, Ware EJ. A descriptive study of noise in the neonatal intensive care unit: Ambient levels and perceptions of contributing factors. Adv Neonatal Care. 2008;8:165–75. doi: 10.1097/01.ANC.0000324341.24841.6e. [DOI] [PubMed] [Google Scholar]
- 9.Williams A, van Drongelen W, Lasky RE. Noise in contemporary neonatal intensive care. J Acoust Soc Am. 2007;121:2681–90. doi: 10.1121/1.2717500. [DOI] [PubMed] [Google Scholar]
- 10.Lasky RE, Williams AL. Noise and light exposures for extremely low birth weight newborns during their stay in the neonatal intensive care unit. Pediatrics. 2009;123:540–6. doi: 10.1542/peds.2007-3418. [DOI] [PubMed] [Google Scholar]
- 11.Carvalhais C, Santos J, da Silva MV, Xavier A. Is there sufficient training of health care staff on noise reduction in neonatal intensive care units? A pilot study from neonoise project. J Toxicol Environ Health A. 2015;78:897–903. doi: 10.1080/15287394.2015.1051204. [DOI] [PubMed] [Google Scholar]
- 12.Liu WF. The impact of a noise reduction quality improvement project upon sound levels in the open-unit-design neonatal intensive care unit. J Perinatol. 2010;30:489–96. doi: 10.1038/jp.2009.188. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Aubertin C, Barrowman N, Moreau K, Dunn S, Harold J. Examining the effects of a targeted noise reduction program in a neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2014;99:203–6. doi: 10.1136/archdischild-2013-304928. [DOI] [PubMed] [Google Scholar]
- 14.Spence MJ, DeCasper AJ. Prenatal experience with low-frequency maternal-voice sounds influence neonatal perception of maternal voice samples. Infant Behav Dev. 1987;10:133–42. [Google Scholar]
- 15.Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child Fetal Neonatal Ed. 1994;71:81–7. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrams RM, Gerhardt KJ. The acoustic environment and physiological responses of the fetus. J Perinatol. 2000;20:31–6. doi: 10.1038/sj.jp.7200445. [DOI] [PubMed] [Google Scholar]
- 17.Lahav A. Questionable sound exposure outside of the womb: Frequency analysis of environmental noise in the neonatal intensive care unit. Acta Paediatr. 2015;104:14–9. doi: 10.1111/apa.12816. [DOI] [PubMed] [Google Scholar]
- 18.Kellam B, Bhatia J. Sound spectral analysis in the intensive care nursery: Measuring high-frequency sound. J Pediatr Nurs. 2008;23:317–23. doi: 10.1016/j.pedn.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Zahr LK, Balian S. Responses of premature infants to routine nursing interventions and noise in the NICU. Nurs Res. 1995;44:179–85. [PubMed] [Google Scholar]
- 20.Trapanotto M, Benini F, Farina M, Gobber D, Magnavita V, Zacchello F. Behavioural and physiological reactivity to noise in the newborn. J Pediatr Child Health. 2004;40:275–81. doi: 10.1111/j.1440-1754.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso SM, Kozlowski LC, de Lacerda AB, Marques JM, Ribas A. Newborn physiological responses to noise in the neonatal unit. Braz J Otorhinolaryngol. 2015;81:583–8. doi: 10.1016/j.bjorl.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai TT, Bearer CF. Iatrogenic environmental hazards in the neonatal intensive care unit. Clin Perinatol. 2008;35:163–81. doi: 10.1016/j.clp.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long JG, Lucey JF, Philip AG. Noise and hypoxemia in the intensive care nursery. Pediatrics. 1980;65:143–5. [PubMed] [Google Scholar]
- 24.Wachman EM, Lahav A. The effects of noise on preterm infants in the NICU. Arch Dis Child Fetal Neonatal Ed. 2011;96:305–9. doi: 10.1136/adc.2009.182014. [DOI] [PubMed] [Google Scholar]
- 25.Williams AL, Sanderson M, Lai D, Selwyn BJ, Lasky RE. Intensive care noise and mean arterial blood pressure in extremely low-birth-weight neonates. Am J Perinatol. 2009;26:323–9. doi: 10.1055/s-0028-1104741. [DOI] [PubMed] [Google Scholar]
- 26.Peng NH, Bachman J, Jenkins R, Chen CH, Chang YC, Chang YS, et al. Relationships between environmental stressors and stress biobehavioral responses of preterm infants in NICU. Adv Neonatal Care. 2013;13:2–10. doi: 10.1097/ANC.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 27.Hassanein SM, Raggal NM, Shalaby AA. Neonatal nursery noise: Practice-based learning and improvement. J Matern Fetal Neonatal Med. 2013;26:392–5. doi: 10.3109/14767058.2012.733759. [DOI] [PubMed] [Google Scholar]
- 28.Field TM, Dempsey JR, Hatch J, Ting G, Clifton RK. Cardiac and behavioral responses to repeated tactile and auditory stimulation by preterm and term neonates. Dev Psychol. 1979;15:406–16. [Google Scholar]
- 29.Morris BH, Philbin MK, Bose C. Physiological effects of sound on the newborn. J Perinatol. 2000;20:55–60. doi: 10.1038/sj.jp.7200451. [DOI] [PubMed] [Google Scholar]
- 30.Lahav A, Skoe E. An acoustic gap between the NICU and womb: A potential risk for compromised neuroplasticity of the auditory system in preterm infants. Front Neurosci. 2014;8:381. doi: 10.3389/fnins.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lejeune F, Parra J, Berne-Audéoud F, Marcus L, Barisnikov K, Gentaz E, et al. Sound interferes with the early tactile manual abilities of preterm infants. Sci Rep. 2016;6:1–8. doi: 10.1038/srep23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bremmer P, Byers JF, Kiehl E. Noise and the premature infant: Physiological effects and practice implications. J Obstet Gynecol Neonatal Nurs. 2003;32:447–54. doi: 10.1177/0884217503255009. [DOI] [PubMed] [Google Scholar]
- 33.Blackburn S. Environmental impact of the NICU on developmental outcomes. J Pediatr Nurs. 1998;13:279–89. doi: 10.1016/S0882-5963(98)80013-4. [DOI] [PubMed] [Google Scholar]
- 34.Laudert S, Liu WF, Blackington S, Perkins B, Martin S, MacMillan-York E, et al. Implementing potentially better practices to support the neurodevelopment of infants in the NICU. J Perinatol. 2007;27:75–93. doi: 10.1038/sj.jp.7211843. [DOI] [PubMed] [Google Scholar]
- 35.Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am. 2009;56:631–46. doi: 10.1016/j.pcl.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 36.American National Standards Institute. ANSI/ASA S1.25: Specification for Personal Noise Dosimeters. Washington, DC: Office of the Federal Register; 1991. [Google Scholar]
- 37.Mills AW. Lateralization of high frequency tones. J Acoust Soc Am. 1960;32:132–4. [Google Scholar]
- 38.BedmasterEX Medical Device Data Acquisition. [[Last accessed on2017 Jan 30]]. Available from: http://www.anandic.com/en/clinical-information-systems/bedmaster-ex-
- 39.Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: Development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Goel VV, Poole SF, Longhurst CA, Platchek TS, Pageler NM, Sharek PJ, et al. Safety analysis of proposed data-driven physiologic alarm parameters for hospitalized children. J Hosp Med. 2016;11:817–23. doi: 10.1002/jhm.2635. [DOI] [PubMed] [Google Scholar]
- 41.Doheny L, Hurwitz S, Insoft R, Ringer S, Lahav A. Exposure to biological maternal sounds improves cardiorespiratory regulation in extremely preterm infants. J Matern Fetal Neonatal Med. 2012;25:1591–4. doi: 10.3109/14767058.2011.648237. [DOI] [PubMed] [Google Scholar]
- 42.Rand K, Lahav A. Maternal sounds elicit lower heart rate in preterm newborns in the first month of life. Early Hum Dev. 2014;90:679–83. doi: 10.1016/j.earlhumdev.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: A meta-analysis. J Pediatr. 2011;158:766–74. doi: 10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr. 2014;164:52–60. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caskey M, Stephens B, Tucker R, Vohr B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics. 2011;128:910–6. doi: 10.1542/peds.2011-0609. [DOI] [PubMed] [Google Scholar]
- 46.Caskey M, Stephens B, Tucker R, Vohr B. Adult talk in the NICU with preterm infants and developmental outcomes. Pediatrics. 2014;133:578–84. doi: 10.1542/peds.2013-0104. [DOI] [PubMed] [Google Scholar]
- 47.Webb AR, Heller HT, Benson CB, Lahav A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci. 2015;112:3152–7. doi: 10.1073/pnas.1414924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jobe AH. A risk of sensory deprivation in the neonatal intensive care unit. J Pediatr. 2014;164:1265–7. doi: 10.1016/j.jpeds.2014.01.072. [DOI] [PubMed] [Google Scholar]


