Abstract
Background
This study explored the diagnostic value of a combined modality of Superb Microvascular Imaging (SMI) and shear-wave elastography in differentiating malignant and benign breast lesions.
Material/Methods
A total of 121 patients with 123 breast lesions enrolled underwent conventional ultrasound exam (US), Color Doppler Flow Imaging (CDFI), SMI examination, and Virtual Touch Tissue Quantification (VTQ) measurement between May 2016 and October 2017. Vessels were detected by both CDFI and SMI in a quantitative manner. The stiffness of all the breast tissues was evaluated by VTQ method. We further assessed the diagnostic performances of CDFI, SMI, VTQ, CDFI+VTQ, and SMI+VTQ.
Results
Both CDFI and SMI exhibited significant differences between malignant and benign masses (p<0.001) in terms of Adler classification. The mean shear-wave velocity (SWV) of malignant neoplasms was 5.28 m/s, with interquartile range (IQR) 4.01–6.39 m/s (p<0.001). The mean SWV of benign lesions was 2.64 m/s, with IQR 2.30–5.01 m/s (p<0.001). No significant difference was found for the area under the receiver operating characteristic curve (AUC) for CDFI, SMI, and VTQ (χ2=2.29, P=0.3715). The sensitivity was the highest on SMI+VTQ (85.42%) and the lowest on CDFI (58.33%). CDFI+VTQ (85.33%) had a slightly higher specificity than SMI+VTQ (84.00%). The accuracy rate of these 2 modalities remained the same (84.55%).
Conclusions
Superb Microvascular Imaging yields more detailed vascular information in the bloodstream in benign and malignant breast masses compared with conventional ultrasonography. VTQ provides standardized quantified results in assessing tissue stiffness. The combined modality of SMI+VTQ added to conventional ultrasonography presented a better diagnostic performance in differentiating malignant breast neoplasms.
MeSH Keywords: Breast Neoplasms, Elasticity Imaging Techniques, Microvessels, Ultrasonography
Background
Although the morbidity and mortality of breast carcinoma is relatively low among Chinese women, the incidence rate in China has been increasing in recent decades [1]. Ultrasonography is a primary imaging method for examining breast lesions in dense breast tissue, providing ultrasonic features to differentiate breast lesion malignancy, such as mass shape, margin, echo pattern, architecture distortion, and micro-calcification [2,3]. However, none of these features are sufficient to use individually to characterize lesion malignancy. Angiogenesis, an essential event in oncology, is pivotal in local growth, invasion, and distant metastasis of cancer [4]. Thus, the significant distinguishing feature of tumor microvessels between malignant and benign breast lesions suggests a high preference of imaging tumor angiogenesis for breast neoplasm differentiation.
Currently, 2 non-invasive methods are widely used to assess breast tumor vascularity: color Doppler flow imaging (CDFI) and power Doppler flow imaging. However, a previous study has reported that these forms of conventional ultrasonography have low sensitivity in microvascularity patterns [5]. Due to the intimated association between microvascularity and malignancy, an emerging form of Doppler ultrasonography called superb microvascular imaging (SMI) has been applied to determine microvascular blood flow. SMI technology is capable of detecting both low-velocity and high-velocity flows, while CDFI cannot image very low flow states.
In addition, another feature with high potential for use in distinguishing between malignant and benign masses is tissue stiffness. In other words, benign lesions tend to be soft while malignant ones tend to be harder. Tissue stiffness is closely related to shear-wave velocity. Therefore, shear-wave elastography, including a point shear-wave speed (SWS) measurement called virtual touch tissue quantification (VTQ), has gradually attracted increased attention in the light of progress in multiple-point measurement and shear-wave quality mapping. However, to the best of our knowledge, few studies have reported the additional application of SMI and VTQ to conventional ultrasound (US) for the purpose of differentiating benign and malignant breast lesions. Therefore, the present study was designed to demonstrate the combined application of SMI in exploring breast mass vascularity and VTQ in determining tissue stiffness to distinguish breast lesion malignancy. Furthermore, this study aimed to investigate the diagnostic value for integrating VTQ and SMI to US in the diagnosis of malignant and benign breast tumors.
Material and Methods
Patients and breast lesions
This prospective study was approved by the Ethics Committee of Shanghai Pudong New Area People’s Hospital. All enrolled patients were notified of the examinations and procedures and all patients provided informed consent.
From May 2016 to October 2017, 128 patients (age range, 16–64 years; mean age, 52.61±7.93 years) were recruited into our study. Three women were excluded due to having breast implants and 4 women were excluded for having treatment of the same lesions previously. Finally, 123 lesions in 121 patients were recruited into this study. The mean diameter of the breast lesions was 18.7±14.8 mm (range 5.7–52.1 mm) while the mean depth was 26.4±11.2 mm (range 8.1–47.3 mm). All the breast lesions were pathologically confirmed via ultrasound-guided core-needle biopsy and/or surgery in terms of standard clinical protocols.
Breast ultrasound examination and imaging interpretation
The ultrasound examination was conducted using a TOSHIBA Aplio 500 (Toshiba Medical System Corporation, Tokyo, Japan) with high-frequency (14 Mhz) line array transducers. First, the patients underwent a B-mode US exam. Once a lesion was detected, the following information were recorded in our study: lesion size in diameter, lesion depth (vertical distance from the skin to the bottom of the lesion), lesion number, lesion position and shape, and other ultrasonic features such as margin and echo pattern. The lesion was further rated by using the latest US BI-RADS of the American College of Radiology (ACR) [6]. Thereafter, we used CDFI (Frame Rate 10–15 Hz) and SMI (Frame Rate >50 Hz) technologies. The velocity scope of SMI was adapted to 1.0 to 2.0 centimeters per second. During the whole examination process, we asked the patients to breathe quietly. Regarding the risk of vessel collapse, there was little pressure applied through the transducer. Consequently, the evaluation of vascularity in and around the lesions was performed.
After the aforementioned examinations, the patients then received an ultrasound elastography technique called VTQ diagnosis. We used the Siemens Acuson S2000™ ultrasound system (Siemens Medical Solutions, Inc.) equipped with a 9L4 linear transducer (4–9 MHz, 4.0 cm) for VTQ measurement. The region of interest (ROI) box on the SWS image includes the mass and surrounding breast tissue, without the skin and chest wall. In addition, using acoustic radiation force impulse technology, the advanced VTQ could directly denotes the velocities of transverse waves mechanically, indicating quantitative information on tissue deformations. Absolute measurements of SWV displayed in m/s were generated by evaluating the peak displacement at each transverse.
The same radiologist recorded traditional ultrasonic features by US and observed the vasculature of the breast lesions using SMI and CDFI. Adler’s classification was used to assess the grades of the detected vascularity (Figure 1). Vascularity was categorized from Grade 0 to Grade 3, depending on the amount of vascularity. Determination blood flow was based on ROI box. Grade 0 is regarded as absent. Minimal (Grade 1) flow is generally regarded as 1 or 2 pixels containing flow (<0.1 cm in diameter). When a certain number of small vessels and/or a main vessel was seen, clinicians graded it as moderate (Grade 2). Marked (Grade 3) vascularity was rated when 4 or more vessels were visualized [7].
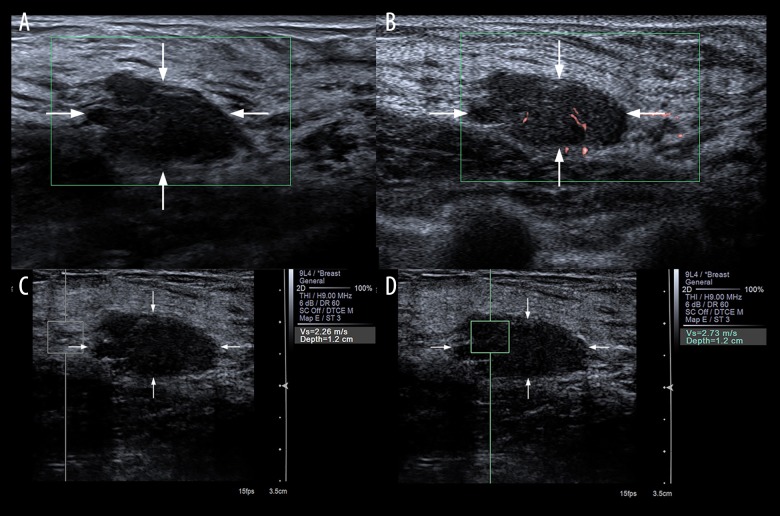
Figure 1.
Evaluation of blood build-up in a lesion of the right breast with CDFI (A) and SMI (B) in a 34-year-old female. According to Adler’s classification, microvascular imaging was rated as Grade 0 with CDFI and Grade 1 with SMI. At VTQ examination, the SWV of the adjacent breast tissue (C) was 2.26 m/s while the SWV of the mass (D) revealed 2.73 m/s. The lesion was classified as US BI-RADS category 4a and pathologically proved as fibroadenoma.
In VTQ mode, the mean SWV values and the percentage of successful measurements were recorded and examined. The radiologist initiated the VTQ measurement after asking patients to hold their breath. The SWV values were obtained from ROI in both the solid portion of the mass and its surrounding breast tissue at the same depth. The operator conducted the same methodology 7 times. It generally took the radiologist 5–10 minutes to perform the entire procedure if there were no problems encountered. The scale for the SWV was 0–9.1 meters per second. A non-applicable situation occurred when the screen displayed “X.XX m/s” (Figure 2). It could be interpreted that the tissue was either extremely soft or hard, exceeding the range for the SWV value. The value was then allotted to be either 9.1 m/s or 0 m/s, standing for solid portion and cystic portion, respectively, under the condition of ruling out technical failures or other potential factors such as patient breathing.
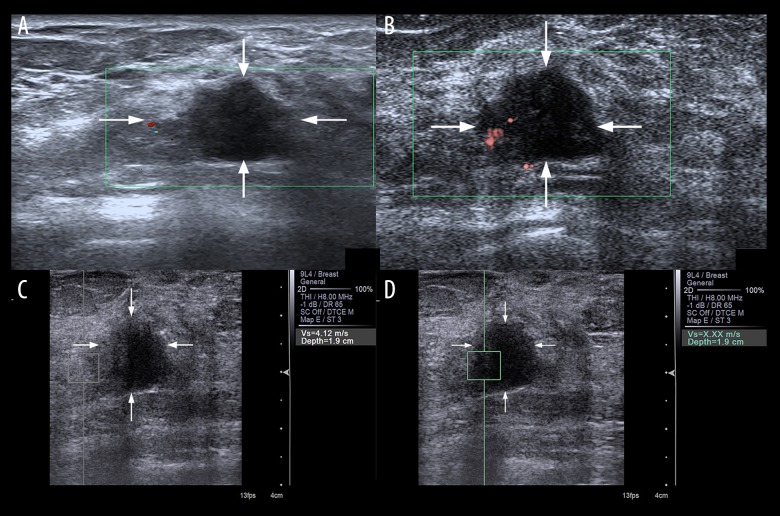
Figure 2.
Evaluation of blood build-up in a lesion of the right breast with CDFI (A) and SMI (B) in a 59-year-old female. According to Adler’s classification, microvascular imaging was rated as Grade 0 with CDFI and Grade 2 with SMI. At VTQ examination, the SWV of the surrounding breast tissue (C) was 4.12 m/s while the SWV of the mass (D) displayed X.XX m/s. The lesion was classified as US BI-RADS category 4b and pathologically proved as invasive ductal carcinoma.
The same radiologist conducted the entire examination. This radiologist had more than 5 years of experience in US, CDFI, and breast sonography and had 3 months of experience in SMI and VTQ measurements. The same imaging area of US, CDFI, SMI, and VTQ were acquired as the reference area for the breast tissue. The images were thereafter evaluated by 2 radiologists with consensus, who had 10 years of experience in US, CDFI, and breast ultrasound and 4 months of experience in SMI and VTQ. These 2 radiologists were blind to the pathological findings. The 2 specialists first evaluated lesion vascularity by CDFI and SMI in terms of Adler’s classification. Thereafter, they rated each breast lesion according to BI-RADS based on ultra-sonographic features.
Statistical analysis
Adler’s method was used to Grade the vascularity difference between CDFI and SMI. VTQ, CDFI, and SMI were compared between malignant and benign lesions using the Wilcoxon rank-sum test. We formulated a receiver operating characteristic (ROC) curve to observe the optimum threshold value of VTQ, CDFI, SMI, CDFI+VTQ, and SMI+VTQ. Sensitivity, specificity, and correct rate were calculated based on the cut-off value. Areas under the ROC (AUCs) of different diagnostic modalities were compared using the chi-squared test. The statistical analysis was done with pathological results as the criterion standard. The statistical significance level was defined as 0.05. All data were analyzed by STATA software package (Version 14.0; StataCorp, Texas, USA).
Results
Reproducibility
The Adler’s grading on CDFI and SMI showed good inter-observer agreement, at 0.893 and 0.876, respectively. The correlation coefficient was 0.902 for intra-observer difference in measuring SWV.
Lesion diagnostic results
Of all 123 breast lesions, 48 (39.02%) lesions were pathologically confirmed as malignant. The malignant lesions were further categorized as invasive ductal carcinomas (n=40), ductal carcinoma in situ (DCIS) (n=4), solid papillary carcinomas (n=2), an intraductal papillary carcinoma (n=1), and an invasive micropapillary carcinoma (n=1). The remaining 75 benign lesions included mammary adenosis (n=12), fibroadenomas (n=49), complex sclerosing lesions (n=2), inflammation (n=10), an intraductal papilloma (n=1), and a mastitis (n=1). Table 1 presents the pathological results of all the breast masses. In addition, conventional ultrasonic features such as irregular shape and ill-defined margins were commonly found in malignant breast lesions. All the breast lesions were determined in terms of BI-RADS as follows: category 3 for 43 lesions, category 4 for 61 lesions, and category 5 for 19 lesions (Table 2).
Table 1.
Pathologic diagnosis.
| Malignant masses | No (%) | Benign masses | No (%) |
|---|---|---|---|
| Invasive ductal carcinomas | 40 (83.34%) | Mammary adenosis | 12 (16.00%) |
| Ductal carcinoma in situ (DCIS) | 4 (8.34%) | Fibroadenomas | 49 (65.34%) |
| Solid papillary carcinoma | 2 (4.17%) | Complex sclerosing lesion | 2 (2.67%) |
| Intraductal papillary carcinoma | 1 (2.08%) | Inflammation | 10 (13.34%) |
| Invasive micropapillary carcinoma | 1 (2.08%) | Intraductal papilloma | 1 (1.34%) |
| Mastitis | 1 (1.34%) |
Table 2.
Conventional ultrasonic features and BI-RADS stratification.
| Characteristic | Overall | Malignant | Benign | P value |
|---|---|---|---|---|
| Mean age (yrs) | 52.61±7.93 (16–64) | 51.88±7.53 (29–64) | 43.61±8.5 (16–59) | <0.001 |
| Lesion number | 0.748 | |||
| Solitary | 121 (98.4%) | 47 (97.9%) | 74 (98.7%) | |
| Multiple | 2 (1.6%) | 1 (2.1%) | 1 (1.3%) | |
| Lesion position | 0.523 | |||
| Right | 52 (42.3%) | 22 (45.8%) | 30 (40.0%) | |
| Left | 71 (57.7%) | 26 (54.2%) | 45 (60.0%) | |
| Diameter (mm) | 18.71±14.81 | 22.5±18.8 | 16.3±11.1 | <0.001 |
| Maximum depth (mm) | 26.4±11.2 | 28.1±11.6 | 25.3±10.8 | <0.001 |
| Shape | <0.001 | |||
| Oval/Round | 59 (48.0%) | 10 (20.8%) | 49 (65.3%) | |
| Irregular | 64 (52.0%) | 38 (79.2%) | 26 (40.6%) | |
| Orientation | <0.001 | |||
| Parallel | 90 (73.2%) | 26 (54.2%) | 64 (85.3%) | |
| Non-parallel | 33 (26.8%) | 22 (45.8%) | 11 (14.7%) | |
| Margin | <0.001 | |||
| Circumscribed | 21 (17.1%) | 2 (4.2%) | 19 (25.3%) | |
| Indistinct | 28 (22.8%) | 2 (4.2%) | 26 (34.7%) | |
| Angular | 9 (7.3%) | 4 (8.3%) | 5 (6.7%) | |
| Microlobulated | 56 (45.5%) | 31 (64.6%) | 25 (33.3%) | |
| Spiculated | 9 (7.3%) | 9 (18.8%) | 0 (0.0%) | |
| Posterior acoustic feature | <0.001 | |||
| Enhancement | 15 (12.2%) | 9 (18.8%) | 6 (8.0%) | |
| Shadowing | 31 (25.2%) | 19 (39.6%) | 12 (16.0%) | |
| Combined pattern | 11 (8.9%) | 11 (22.9%) | 0 (0.0%) | |
| No posterior acoustic features | 66 (53.7%) | 9 (18.8%) | 57 (76.0%) | |
| Echo pattern | <0.001 | |||
| Hypoechoic | 79 (64.2%) | 25 (52.1%) | 54 (72.0%) | |
| Hyperechoic | 6 (4.9%) | 6 (12.5%) | 0 (0.0%) | |
| Isoechoic | 20 (16.3%) | 4 (8.3%) | 16 (21.3%) | |
| Complex | 18 (14.6%) | 13 (27.1%) | 5 (6.7%) | |
| BI-RADS | <0.001 | |||
| 3 | 43 (40.0%) | 0 (0.0%) | 43 (57.8%) | |
| 4a | 12 (9.8%) | 1 (2.4%) | 11 (14.8%) | |
| 4b | 23 (18.7%) | 6 (12.2%) | 17 (22.7%) | |
| 4c | 26 (21.1%) | 22 (45.1%) | 4 (4.7%) | |
| 5 | 19 (15.4%) | 19 (40.2%) | 0 (0.0%) |
Vascularity findings
The amounts of vascularity were examined on the foundation of Adler classification as shown in Table 3. Both CDFI and SMI exhibited noticeable variance between malignant and benign masses (p<0.001). SMI showed that 81.25% of the malignant lesions contained 4 or more vessels, while CDFI only revealed 58.33% of them.
Table 3.
The degree of vascularity according to Adler classification, [n (%)].
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | z | P | ||
|---|---|---|---|---|---|---|---|
| CDFI | Malignant | 2 (4.17) | 8 (16.67) | 10 (20.83) | 28 (58.33) | 4.76 | <0.001 |
| Benign | 25 (33.33) | 18 (24.00) | 16 (21.33) | 16 (21.33) | |||
| SMI | Malignant | 1 (2.08) | 3 (6.25) | 5 (10.42) | 39 (81.25) | 5.51 | <0.001 |
| Benign | 14 (18.67) | 13 (17.33) | 27 (36.00) | 21 (28.00) |
Lesion stiffness findings
The mean SWV of malignant neoplasms was 5.28 m/s, with interquartile range (IQR) 4.01–6.39 m/s. The mean SWV of benign lesions was 2.64 m/s, with IQR 2.30–5.01 (Table 4). Both the z values were 3.66 and p values were less than 0.001. The SWV values of malignancy were nearly double those of benign lesions (Figure 3).
Table 4.
Descriptions and comparisons of SWVs in malignant and benign lesions.
| Q50 (Q25~Q75) | |||
|---|---|---|---|
| SWV (Min) | SWV (Mean) | SWV (Max) | |
| Malignant | 4.53 (3.40~5.59) | 5.28 (4.01~6.39) | 6.03 (4.57~7.19) |
| Benign | 2.47 (2.13~4.76) | 2.64 (2.30~5.01) | 2.79 (2.49~5.34) |
| z | 3.42 | 3.66 | 3.82 |
| P | 0.001 | <0.001 | <0.001 |
Figure 3.
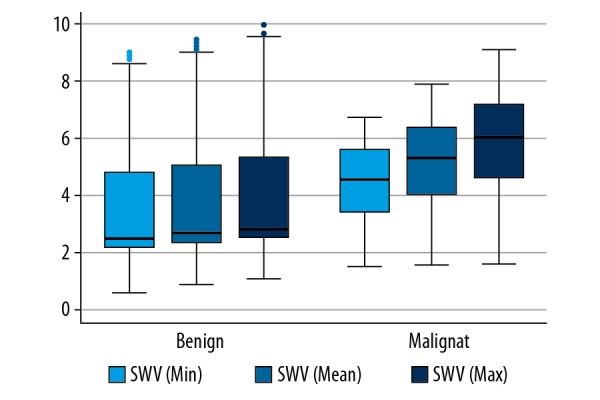
Boxplot of SWV values for malignant and benign breast lesions.
Diagnostic performance of 5 modalities
In ROC analysis, the top AUC was on SMI+VTQ, followed by CDFI+VTQ, SMI, CDFI, and VTQ. The AUC values were 0.8969 (95%CI: 0.8374–0.9565, z=29.53), 0.8633 (95%CI: 0.7964–0.9329, z=24.63), 0.7740 (95%CI: 0.6974–0.8506, z=19.80), 0.7453 (95%CI: 0.6623–0.8283, z=17.60), and 0.6962 (95%CI: 0.6021–0.7904, z=14.50), respectively. All p values were lower than 0.001. There was no significant difference in AUC for CDFI, SMI, and VTQ (χ2=2.29, p=0.3715). A significant difference of AUC was found for CDFI and CDFI with VTQ (χ2=11.14, p=0.0008) as well as SMI and SMI with VTQ (χ2=18.35, p<0.0001). Sensitivity was highest on SMI+VTQ (85.42%), followed by CDFI+VTQ (83.33%), SMI (81.25%), VTQ (79.17%), and CDFI (58.33%). SMI+VTQ (84.00%) did not sacrifice too much in terms of specificity compared with CDFI+VTQ (85.33%). Both SMI+VTQ and CDFI+VTQ achieved correct rates of 84.55%, followed by SMI (75.61%), VTQ (71.54%), and CDFI (70.73%) (Table 5).
Table 5.
Diagnostic performances of five modalities.
| Cut-off | Sensitivity | Specificity | Correct rate | AUC | 95%CI | z | P | |
|---|---|---|---|---|---|---|---|---|
| VTQ | ≥3.76 | 79.17% | 66.67% | 71.54% | 0.6962 | 0.6021~0.7904 | 14.50 | <0.001 |
| CDFI | ≥3 | 58.33% | 78.67% | 70.73% | 0.7453 | 0.6623~0.8283 | 17.60 | <0.001 |
| SMI | ≥3 | 81.25% | 72.00% | 75.61% | 0.7740 | 0.6974~0.8506 | 19.80 | <0.001 |
| CDFI+VTQ | P(ϖ) ≥0.4815 | 83.33% | 85.33% | 84.55% | 0.8633 | 0.7946~0.9320 | 24.63 | <0.001 |
| SMI+VTQ | P(ϖ) ≥0.4200 | 85.42% | 84.00% | 84.55% | 0.8969 | 0.8374~0.9565 | 29.53 | <0.001 |
Discussion
Ultrasound has been widely accepted as a readily available and non-invasive imaging technique. Therefore, it has been frequently applied as a first-line diagnostic exam. The ability to detect vascularization in the lesions enables clinicians to differentiate benign and malignant tumors thanks to the significant association between microvascularity and malignancy. One of the universally used ultrasound techniques, CDFI, provides valuable data for evaluating blood flow, but is limited in providing low-velocity blood flow data. In other words, CDFI is generally associated with data loss due to movement artifacts. With the progress in ultrasonography, SMI as a pioneering technique shows the ability to visualize lower-peed bloodstreams without motion artifacts. Such an advantage has been widely proved in examining microvascular flow in thyroid nodules [8], renal tumor vascularity [9], testicle blood flow of small children [10], and focal liver lesion vascularity [11]. Therefore, various studies supported the performance of SMI in depicting the minute vessels and slow-speed blood flows with high resolution and fewer motion artifacts. In our study, SMI yielded more data than CDFI with regard to evaluating vascularity. Better visualization of blood flow was achieved via SMI compared with CDFI in both benign and malignant lesions. SMI displayed more microvascular flow signals than did CDFI. According to Adler’s classification, our study indicated the optimal diagnostic threshold was in Grade 3 malignant lesions for SMI. In terms of benign lesions, CDFI’s optimal diagnostic threshold was in Grade 0 and Grade 1, while SMI performed better in Grade 2 and Grade 3.
In a quantitative manner, VTQ enables the measurement of SWV in m/s. Different researches have been conducted to examine the quantitative elasticity values in the diagnostic performance of thyroid nodules [12], metastatic and nonmetastatic cervical lymph nodes [13], and acute pancreatitis [14]. In differentiating benign and malignant breast masses, there is an increasing trend to use VTQ measurement [15,16]. The mean SWV of the malignant neoplasms analyzed by Tozaki et al. [17] was 1.81 m/s faster than that of benign masses (P<0.01). Our findings are highly consistent with that finding (Table 4). The failure rate in malignant and benign lesions was assessed in previous studies. In contrast to the unsuccessful percentage of 20.57% for 141 breast lesions examined by Teke et al. [18], our study reached a relatively higher successful ratio of 95%. Both failure rates were calculated based on the rate of obtaining X.XX m/s. We agreed with the interpretation of non-numeric statistic by Bai et al. [19], which implied that shear-wave speed exceeded the highest limit that could be measured. However, there remained a relatively low possibility of technical failure in VTQ module. In our study, 6 out of 123 lesions were read as X.XX m/s, including 5 neoplasms confirmed by histopathology. Consequently, the percentage of technical failure in obtaining SWV was estimated as lower than 5%. The examination in 121 breast tumors by Kang et al. [20] confirmed the capability of VTQ in representing the stiffness of tumors in both three-dimensional and two-dimensional ways. Golatta et al. [21] examined 104 breast lesions in 103 patients via VTQ module, also concluding that malignant lesions tended to be stiffer in terms of the mean maximum velocity of malignant and benign masses. Our study proves that stiffer tissue is more likely to be malignant.
However, we noticed limitations in applying CDFI or SMI or VTQ alone with US in differentiating malignant and benign breast lesions via ROC curve. The standalone application of these 3 technologies showed relatively low sensitivity, specificity, and accuracy (Figure 4). With the combination of 2 methods, the proposed combination of SMI and VTQ suggested a useful modality in making a distinction between benign masses and malignant breast neoplasms. The combined modality distinguished breast lesion malignancy based on the evaluation of vascularization and tissue stiffness. On the basis of US, the combined modality reached the highest sensitivity (85.42%) while not sacrificing specificity (84.00%), maintaining the same accuracy rate (84.55%) in comparison to CDFI with VTQ.
Figure 4.
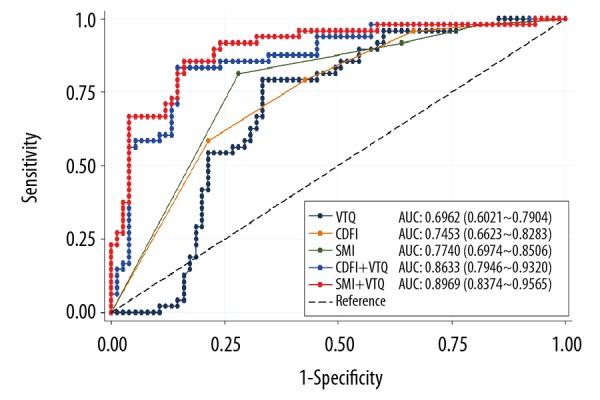
ROCs and AUCs for 5 diagnostic modalities.
Our study has certain limitations. First of all, it was a single-center study with a relatively small sample size. Secondly, all the examinations were conducted by the same radiologist, so the interpretation of inter-observer differences was missed. Thirdly, although the majority of the upper limit of SWV was found in malignant lesions, it still cannot be ignored that there was 1 benign breast mass, which might cause further research to disregard the low failure rate in our study.
Conclusions
In summary, SMI is a non-invasive method without use of any contrast agents in differentiating benign and malignant breast masses. SMI provided more details on tumor vascularity, which was closely linked with tumor angiogenesis. Furthermore, SMI was able to cover the shortage of CDFI in divulging low-velocity bloodstream due to motion artifacts. VTQ was excellent in measuring SWV and thus evaluating the tissue stiffness. Therefore, the combined modality of SMI and VTQ added to US proposed by our study presents a better diagnostic performance than that of SMI or VTQ alone.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Pu Dong New Area Health and Family Planning Commission Important Vulnerable Course Project (no. PWzbr 2017-10) and the Pu Dong New Area Health and Family Planning Commission Subject Leader Course Project (no. PWRd 2017-06)
References
- 1.Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: Incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159:395–406. doi: 10.1007/s10549-016-3947-0. [DOI] [PubMed] [Google Scholar]
- 2.Spick C, Bickel H, Polanec SH, Baltzer PA. Breast lesions classified as probably benign (BI-RADS 3) on magnetic resonance imaging: A systematic review and meta-analysis. Eur Radiol. 2018;28(5):1919–28. doi: 10.1007/s00330-017-5127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo J, Cao Y, Nian W, et al. Benefit of Shear-wave Elastography in the differential diagnosis of breast lesion: A diagnostic meta-analysis. Med Ultrason. 2018;20:43–49. doi: 10.11152/mu-1209. [DOI] [PubMed] [Google Scholar]
- 4.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 5.Xiao XY, Chen X, Guan XF, et al. Superb microvascular imaging in diagnosis of breast lesions: A comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol. 2016;89:20160546. doi: 10.1259/bjr.20160546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercado CL. BI-RADS update. Radiol Clin North Am. 2014;52:481–87. doi: 10.1016/j.rcl.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Adler DD, Carson PL, Rubin JM, Quinn-Reid D. Doppler ultrasound color flow imaging in the study of breast cancer: Preliminary findings. Ultrasound Med Biol. 1990;16:553–59. doi: 10.1016/0301-5629(90)90020-d. [DOI] [PubMed] [Google Scholar]
- 8.MacHado P, Segal S, Lyshchik A, Forsberg F. A novel microvascular flow technique: Initial results in thyroids. Ultrasound Q. 2016;32:67–74. doi: 10.1097/RUQ.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 9.Mao Y, Mu J, Zhao J, et al. The value of superb microvascular imaging in differentiating benign renal mass from malignant renal tumor: A retrospective study. Br J Radiol. 2018;91:20170601. doi: 10.1259/bjr.20170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karaca L, Oral A, Kantarci M, et al. Comparison of the superb microvascular imaging technique and the color Doppler techniques for evaluating children’s testicular blood flow. Eur Rev Med Pharmacol Sci. 2016;20:1947–53. [PubMed] [Google Scholar]
- 11.He M-N, Lv K, Jiang Y-X, Jiang T-A. Application of superb microvascular imaging in focal liver lesions. World J Gastroenterol. 2017;23:7765–75. doi: 10.3748/wjg.v23.i43.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F-J, Han R-L, Zhao X-M. The value of virtual touch tissue image (VTI) and virtual touch tissue quantification (VTQ) in the differential diagnosis of thyroid nodules. Eur J Radiol. 2018;83:2033–40. doi: 10.1016/j.ejrad.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Xi J, Zhao B, et al. Preliminary evaluation of virtual touch tissue imaging quantification for differential diagnosis of metastatic and nonmetastatic cervical lymph nodes. J Ultrasound Med. 2017;36:557–63. doi: 10.7863/ultra.16.03077. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Zou L, Yao M, et al. A Prelimiany investigation of normal pancreas and acute pancreatitis elasticity using virtual touch tissue quantification (VTQ) imaging. Med Sci Monit. 2015;21:639–99. doi: 10.12659/MSM.892239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin ZQ, Li XR, Zhou HL, et al. Acoustic radiation force impulse elastography of breast imaging reporting and data system category 4 breast lesions. Clin Breast Cancer. 2012;12:420–27. doi: 10.1016/j.clbc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhou BG, Wang D, Ren WW, et al. Value of shear wave arrival time contour display in shear wave elastography for breast masses diagnosis. Sci Rep. 2017;7:7036. doi: 10.1038/s41598-017-07389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tozaki M, Isobe S, Fukuma E. Preliminary study of ultrasonographic tissue quantification of the breast using the acoustic radiation force impulse (ARFI) technology. Eur J Radiol. 2011;80:e182–87. doi: 10.1016/j.ejrad.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Memik T, Cemil G, Fatma T, et al. Combination of virtual touch tissue imaging and virtual touch tissue quantification for differential diagnosis of breast lesions. J Ultrasound Med. 2015;34:1201–8. doi: 10.7863/ultra.34.7.1201. [DOI] [PubMed] [Google Scholar]
- 19.Min B, Lianfang D, Jiying G, et al. Virtual touch tissue quantification using acoustic radiation force impulse technology. J Ultrasound Med. 2012;31:289–94. doi: 10.7863/jum.2012.31.2.289. [DOI] [PubMed] [Google Scholar]
- 20.Kang HJ, Kim JY, Lee NK, et al. Three-dimensional versus two-dimensional shear-wave elastography: Associations of mean elasticity values with prognostic factors and tumor subtypes of breast cancer. Clin Imaging. 2018;48:79–85. doi: 10.1016/j.clinimag.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Golatta M, Schweitzer-Martin M, Harcos A, et al. Evaluation of virtual touch tissue imaging quantification, a new shear wave velocity imaging method, for breast lesion assessment by ultrasound. Biomed Res Int. 2014;2014 doi: 10.1155/2014/960262. 960262. [DOI] [PMC free article] [PubMed] [Google Scholar]