Abstract
Background
Our study aimed to explore the levels of nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF) in healthy participants, type 2 diabetes mellitus (T2DM) patients, and diabetic peripheral neuropathy (DPN) patients in order to find their effects on DPN.
Material/Methods
The clinical data of 110 healthy participants (age: 57.3±8.2 year, height: 165.4±5.5 cm, weight: 64.1±7.5 kg), 83 T2DM patients (age: 56.5±7.9 year, height: 164.8±6.2 cm, and weight: 63.6±6.6 kg), and 65 DPN patients (age: 58.2±7.3 year, height: 166.7±6.7 cm, weight: 63.1±5.8 kg) were observed. ELISA was applied to detect serum NGF and BDNF levels. Receiver operating characteristic (ROC) curve analysis was performed to evaluate diagnostic value of serum NGF and BDNF levels in DPN. Logistic regression analysis was performed to analyze risk factors for DPN.
Results
Serum NGF and BDNF levels decreased most in DPN patients. Subsequently, we determined that serum NGF and BDNF levels were correlated with: the course of disease for patients, fasting C-peptide (FCP), 2-hour postprandial C-peptide level (2-h PCP), glycosylated hemoglobin level (HbAlc), and 24-hour urinary microalbumin excretion (24-h UME). ROC curve analysis identified high sensitivity, specificity, and accuracy of NGF and BDNF levels on DPN. Serum levels of NGF and BDNF, course of disease, 2-h PCP level, and postprandial blood glucose level were determined to be risk factors for DPN.
Conclusions
Our study highlights that serum levels of NGF and BDNF might be associated with the occurrence and development of DPN.
MeSH Keywords: Nerve Growth Factor; Peripheral Nervous System Diseases; Receptor, trkB; Diabetes Mellitus, Type 2
Background
Diabetes mellitus (DM) is a kind of disease with metabolism disturbances in which glucose is underused and overproduced, mainly represented by hyperglycemia [1]. Type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) were the major types of DM [2]. A recent report estimated that over 96 million people have suffered from diabetes in China and the number is predicted to rise to 142.7 million by 2035 without actions taken [3]. Diabetic peripheral neuropathy (DPN) is a form of peripheral neuropathy with peripheral and autonomic nerve injury resulting from DM [4]. DPN, as one of complications of DM, is characterized by paresthesia, sensory loss, hyperalgesia, and lancinating pain or symmetrical burning [5]. About 50% of patients with DM develop DPN, which is a leading cause of morbidity and increased mortality [6]. Moreover, about one-quarter of T1DM patients developed DPN with age, duration of diabetes, and poor glycemic control as leading risk factors [7]. Recently, accumulating studies have disclosed that some serum biomarkers, including uric acid and vitamin D, are associated with progression of DPN, the initial manifestation of T2DM [8,9]. Therefore, we hypothesized that identifying crucial cytokines in DPN development and their association with DPN is beneficial to the early diagnosis and personalized treatment for DPN patients.
Nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF) are members of the neurotrophins family, a type of brain signaling molecules that are essential in maturation of synapses, axon targeting, synaptic plasticity, as well as neuron growth [10]. NGF is significant in the nociception of adult injured tissues, and the altered gene expression induced by NGF may have an influence on the phenotypic alteration [11]. It has been found that NGF plays a key role in the reduction of diabetic neuropathy morbidity, thus exogenous NGF might contribute to the diabetic neuropathy treatment [12]. BDNF participates in the plasticity and development of peripheral nervous system (PNS) as well as central nervous system (CNS), as it affects not only differentiation and proliferation but also synaptic activity and neurotransmission [13]. In addition, it has been proven that the serum level of BNDF is reduced in Chinese patients with T2DM [14–18]. Zhen et al. also have shown that serum levels of BNDF decreased in T2DM patients and poorly expressed BDNF contributes to the cognitive deficits in patients with T2DM [19]. In the current study, we focused on the expression of serum NGF and BDNF level in healthy participants, T2DM patients, and DPN patients, and aimed to find the relevance of these two factors to DPN and the potential influence factors.
Material and Methods
Ethics statement
This study was approved by the Ethical Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital and informed consents were obtained from patients’ family or guardians.
Participants
From February 2014 to June 2017, 65 DPN patients and 83 T2DM patients underwent treatment, and 110 healthy individuals who underwent physical examination in Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital were selected for our research. The DPN group included 30 males and 35 females with the mean age of 58.2±7.3 years and the mean weight of 63.1±5.8 kg. The T2DM group included 41 males and 42 females with the mean age of 56.5±7.9 years and the mean weight of 63.6±6.6 k. The control group included 47 males and 63 females with the mean age at 57.3±8.2 years and the mean weight at 64.1±7.5 kg. Clinical data were recorded for all participants including gender, age, height, weight, waist-to-hip ratio, blood pressure, body mass index (BMI), cholesterol, low and high density lipoproteins, triglycerides (TG), fasting C-peptide (FCP), 2-h postprandial C-peptide (2-h PCP), blood glucose, and 24-hour urinary microalbumin excretion (24-h UME). Inclusion criteria for DPN were as follows: meeting diagnostic criteria for T2DM; spontaneous pain in arms and legs, unilateral or symmetrical limb numbness, feeling of dullness and body tension; weakened muscle strength, diminished or lacking tendon reflexes, significantly decreased sensory nerve conduction velocity and motor nerve conduction velocity shown in electromyogram, positive involvement indicated in 2 or more nerves, and normal arterial pulse in foot and back. Inclusion criteria for T2DM were as follows: diagnosed with T2D according to the diagnostic criteria for T2DM from the American Diabetes Association in 2003 and World Health Organization in 1999, a normal result of nerve electromyogram test and without DPN-related symptoms; urinary albumin within 24 hours less than 30 mg. Exclusion criteria: patients who were ranked in 3–5 grade in conformity to Wagner classification of diabetic foot [16]; accompanied by alcoholism, skin lesions, psychiatric or psychological diseases or central and peripheral nervous lesions due to other reasons; other types of diabetes; coupled with acute inflammation or tumors.
Parameter detection
All blood specimens were detected using Beckman Coulter UniCel DxC 800 automatic biochemical analyzer. Continuous monitoring was applied to monitor total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). In oral glucose tolerance test, glucose biosensor based on an oxygen electrode was used to measure fasting blood glucose (FBG) and 2-hour postprandial blood glucose (2-h PBG) and chemiluminescent immunoassay was utilized to examine FCP level and 2-h PCP. TOSOH G7 automatic analyzer and high-performance liquid chromatography (HPLC) was performed to detect glycosylated hemoglobin (HbAlc). And immunoturbidimetry (ITM, Siemens DCA Vantage Analyzer, USA) was conducted to evaluate the 24-h UME.
Enzyme-linked immunosorbent assay (ELISA)
On admission day or in the morning of the next day, 5 mL peripheral venous blood of each study participant was collected in the fasting state, and then placed in acid-citrate dextrose anticoagulant tube (Shanghai haling Biological Technology Co., Ltd., Shanghai, China) at room temperature. After 2 hours, the blood specimens were centrifuged for 10 min at a rate of 3000 rpm. Then the serum was collected and preserved at −80°C for later use. Serum NGF and BDNF levels were determined using ELISA (reagent kits from R&D Systems Inc., Minneapolis, MN, USA). Each sample of serum was tested 2 times and the average data were collected as experimental reference data. Likewise, blood of cases in control group was tested 2 times when enrolled.
Receiver operating characteristic (ROC) curve
The receiver operating characteristic (ROC) curve was established with sensitivity as ordinate and (1-specificity) as horizontal ordinate. The cutoff point was determined according to the critical point of Youden index. And then the diagnostic values of serum levels of NGF and BDNF on DPN were analyzed and compared.
Statistical analysis
Statistical analysis was performed using the SPSS22.0 software (IBM, Armonk, NY, USA). Enumeration data was presented in percentage or rate with chi-square test applied for comparison between groups. Measurement data were expressed in mean ± standard deviation with t-test used for comparisons between 2 groups and One-way analysis of variance (ANOVA) employed for comparisons of mean among three groups. Serum NGF and BDNF levels and other indicators were analyzed using Spearman rank correlation test and the diagnostic values of NGF and BDNF were measured applying ROC curve analysis. Risk factors correlated with DPN were explored using logistic regression analysis. The 2-tailed test was used in all tests in the procedures of experiments. P<0.05 was considered significantly different.
Results
FBG, 2-h PBG, HbAlc, FCP, 2-h PCP, and 24-h UME were associated with DPN
Clinical characteristics of patients were compared among the 3 groups. Gender, mean age, height, weight, and BMI of study participants showed no significant difference among groups (P>0.05). The clinical parameters of the 3 groups in pairwise comparison illustrated FBG, 2-h PBG, HbAlc, and 24-h UME in the T2DM group and the DPN group were much higher than that of the control group (both P<0.05), while for the course of disease for patients, FBG, 2-h PBG, HbAlc, FCP and 2-h PCP and 24-h UME in the DPN group were obviously higher than those of the T2DM group (all P<0.05). However, the TG and TC levels, low- and high-density lipoproteins showed no difference among the 3 groups (all P>0.05) (Table 1). The results indicated that high levels of FBG, 2-h PBG, HbAlc, FCP and 2-h PCP and 24-h UME had associations with the development of DPN.
Table 1.
Baseline characteristics and clinical parameters of control, T2DM and DPN groups.
| General characteristic | Control group | T2DM group | DPN group | p* | p# | p& |
|---|---|---|---|---|---|---|
| Number of cases | 110 | 83 | 65 | |||
| Gender (Male/Female) | 47/63 | 41/42 | 30/35 | 0.357 | 0.659 | 0.695 |
| Average age (years) | 57.3±8.2 | 56.5±7.9 | 58.2±7.3 | 0.496 | 0.466 | 0.181 |
| Height (cm) | 165.4±5.5 | 164.8±6.2 | 166.7±6.7 | 0.479 | 0.166 | 0.076 |
| Weight (kg) | 64.1±7.5 | 63.6±6.6 | 63.1±5.8 | 0.630 | 0.357 | 0.631 |
| BMI (kg/m2) | 23.4±2.5 | 23.5±3.2 | 22.8±2.7 | 0.808 | 0.138 | 0.159 |
| Clinical parameters | ||||||
| Course of disease | 8.4±2.6 | 12.7±4.0 | / | / | <0.001 | |
| FBG (mmol/L) | 4.9±0.4 | 7.1±1.7 | 9.2±1.4 | <0.001 | <0.001 | <0.001 |
| PGL (mmol/L) | 6.8±0.7 | 12.7±2.3 | 16.1±3.1 | <0.001 | <0.001 | <0.001 |
| HbAlc | 4.2±0.5 | 7.0±0.4 | 8.7±1.5 | <0.001 | <0.001 | <0.001 |
| 2-h FCP (ng/ml) | 4.3±0.3 | 5.8±0.5 | 9.2±2.0 | <0.001 | <0.001 | <0.001 |
| FCP (ng/ml) | 1.1±0.1 | 2.1±0.2 | 5.8±1.5 | <0.001 | <0.001 | <0.001 |
| TG (mmol/L) | 1.7±0.2 | 1.7±0.3 | 1.8±0.5 | 0.999 | 0.064 | 0.134 |
| Cholestenone (mmol/L) | 4.9±0.7 | 4.7±0.8 | 4.8±0.5 | 0.066 | 0.314 | 0.379 |
| HDL (mmol/L) | 1.4±0.5 | 1.3±0.2 | 1.3±0.4 | 0.087 | 0.172 | 0.999 |
| LDL (mmol/L) | 2.4±0.3 | 2.4±0.4 | 2.5±0.4 | 0.999 | 0.062 | 0.133 |
| 24 h UME (mg/d)*# | 8.2±1.1 | 73.3±9.6 | 120.8±14.3 | <0.001 | <0.001 | <0.001 |
T2DM – type 2 diabetes mellitus; DPN – diabetic peripheral neuropathy; FBG – fasting blood glucose; PGL – postprandial glucose level; HbAlc – glycosylated hemoglobin; 2-h FCP – 2 h fasting C-peptide; FCP – fasting C-peptide; TG – triglycerides; HDL – high-density lipoprotein; LDL – dow-density lipoprotein; UME – urinary microalbumin excretion; BMI – body mass index;
p<0.05, control group vs. T2DM group;
control group vs. DPN group;
T2DM group vs. DPN group.
The independent samples t-test was used to analyze data.
Serum levels of NGF and BDNF decreased in the DPN group
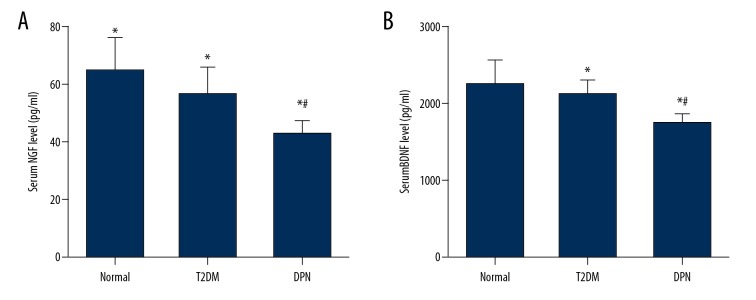
The ELISA was adopted to determine serum levels of NGF and BDNF. As shown in Figure 1, at serum NGF level (42.7±4.9 pg/mL) and BDNF level (1739.8±132.9 pg/mL) in the DPN group decreased when compared with the T2DM group (56.5±9.2 pg/mL, 2126.8±171.1 pg/mL, respectively) and the control group (64.3±11.7 pg/mL and 2246.7±331.5 pg/mL, respectively) (P<0.05). Serum NGF and BDNF levels in the T2DM group also decreased in comparison to the control group (P<0.05). This suggested that serum NGF and BDNF levels were related to the incidence of DPN.
Figure 1.
ELISA shows that serum levels of NGF and BDNF decrease in DPN group. * P<0.05 compared with control group; # P<0.05 compared with T2DM group. NGF – nerve growth factor; BDNF – brain derived neurotrophic factor; ELISA – enzyme-linked immunosorbent assay.
Serum levels of NGF and BDNF had high sensitivity, specificity and accuracy in the diagnosis of DPN
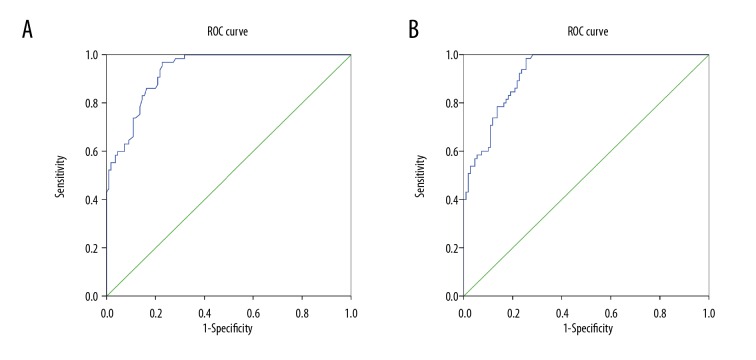
ROC curve analysis was used to evaluate the diagnostic efficacy of DPN by serum levels of NGF and BDNF. The optimal level of serum NGF was 50.25 pg/mL. The corresponding sensitivity, specificity, and accuracy of DPN were 96.9%, 77.3%, 84.6%, respectively, and the area under curve (AUC) was 0.933 (Figure 2A). And the optimal level of serum BNDF was 1981.05 pg/mL. The corresponding sensitivity, specificity, and accuracy of DPN were 98.5%, 74.5%, 83.4%, respectively, and the AUC was 0.925 (Figure 2B).
Figure 2.
Serum levels of NGF and BDNF display the important diagnostic value for DPN by ROC curve analysis: (A) serum NGF, (B) serum BDNF. ROC curve – receiver operating characteristic curve; DPN – diabetic peripheral neuropathy; NGF – nerve growth factor; BDNF – brain derived neurotrophic factor.
Serum levels of NGF and BDNF were associated with the course of disease, HbAlc, FCP, 2-h PCP, and 24-h UME
The correlations of serum NGF and BDNF levels with clinical parameters in DPN patients were detected. As shown in Table 2, the data showed that serum NGF and BDNF levels were correlated with: the course of disease for patients, HbAlc, FCP, 2-h PCP, and 24-h UME (all P<0.05). In addition, by comparing and analyzing the serum NGF and BDNF levels of patients, we found that the effects of serum NGF and BDNF levels on other clinical parameters showed no significant difference (P>0.05). These findings revealed that serum NGF and BDNF levels are involved in the pathogenesis of DPN by affecting these clinical parameters.
Table 2.
Correlation analysis of serum NGF and BDNF levels with clinical parameters.
| Clinical parameters | Serum NGF level (pg/ml) | Serum BDNF level (pg/ml) | ||||
|---|---|---|---|---|---|---|
| >42.7 | ≤42.7 | p* | >1739.8 | ≤1739.8 | p# | |
| Gender (Male/Female) | 19/20 | 11/15 | 0.612 | 19/19 | 11/16 | 0.461 |
| Average age (years) | 57.7±8.0 | 8.8±6.2 | 0.383 | 58.7±7.1 | 57.3±7.6 | 0.279 |
| Height (cm) | 166.3±7.4 | 167.3±5.7 | 0.389 | 167.2±6.5 | 166.0±7.0 | 0.313 |
| Weight (kg) | 61.7±6.4 | 62.7±4.9 | 0.319 | 62.5±5.6 | 61.5±6.1 | 0.332 |
| Course of disease | 14.0±3.6 | 10.7±3.8 | <0.001 | 14.1±3.8 | 10.6±3.4 | <0.001 |
| FBG (mmol/L) | 9.1±1.5 | 9.3±1.2 | 0.403 | 9.3±1.4 | 9.1±1.5 | 0.433 |
| PGL (mmol/L) | 15.9±3.4 | 16.4±2.6 | 0.348 | 16.3±3.0 | 15.8±3.2 | 0.359 |
| FCP (ng/ml) | 6.6±1.2 | 4.7±1.1 | <0.001 | 6.7±1.1 | 4.7±1.1 | <0.001 |
| 2-h FCP (ng/ml) | 10.2±1.7 | 7.7±1.5 | <0.001 | 10.0±1.9 | 8.1±1.6 | <0.001 |
| HbAlc | 9.5±1.2 | 7.5±1.1 | <0.001 | 9.5±1.2 | 7.6±1.2 | <0.001 |
| TG (mmol/L) | 1.8±0.4 | 1.9±0.4 | 0.157 | 1.8±0.4 | 1.7±0.5 | 0.21 |
| Cholestenone (mmol/L) | 4.8±0.5 | 4.9±0.5 | 0.256 | 4.9±0.4 | 4.8±0.5 | 0.21 |
| HDL (mmol/L) | 1.3±0.4 | 1.4±0.4 | 0.157 | 1.4±0.4 | 1.3±0.4 | 0.157 |
| LDL (mmol/L) | 2.5±0.4 | 2.4±0.4 | 0.157 | 2.5±0.4 | 2.4±0.4 | 0.157 |
| 24 h UME (mg/d) | 127.5±13.2 | 110.8±8.9 | <0.001 | 126.4±15.5 | 113.1±7.4 | <0.001 |
NGF – nerve growth factor; BDNF – brain derived neurotrophic factor; FBG – fasting blood glucose; PGL – postprandial glucose level; FCP – fasting C-peptide; 2-h FCP – 2 h fasting C-peptide; HbAlc – glycosylated hemoglobin; TG – triglycerides; HDL – high-density lipoprotein; LDL – low-density lipoprotein; UME – urinary microalbumin excretion;
NGF ≤42.7 pg/ml vs. NGF ≤42.7pg/ml;
BDNF ≤1739.8 pg/ml vs. BDNF ≤1739.8.
The independent samples t-test was used to analyze data.
Serum levels of NGF and BDNF, course of disease, 2-h PBG, and 2-h PCP were related to the incidence of DPN
Multivariate logistic regression analysis was employed to analyze the risk factors for DPN. The potential risk factors analyzed included: course of disease, FBG, 2-h PBG, FCP, 2-h PCP, 24-h UCM, serum NGF level, and serum BDNF level. The results illustrated that the course of disease, 2-h PBG, 2-h PCP, serum levels of NGF and serum levels of BDNF were all risk factors for DPN (all P<0.05), while FBG, FCP, and 24-h UCM were not considered as risk factors (Table 3). These findings indicated that course of disease and the aberrant levels of NGF and BDNF, 2-h PBG, and 2-h PCP are related to the incidence of DPN.
Table 3.
Logistic regression analysis for risk factors correlated with DPN.
| Factor | p | OR | 95% CI | WALD |
|---|---|---|---|---|
| Serum NGF level | <0.001 | 5.055 | 2.116–12.077 | 13.298 |
| Serum BDNF level | 0.007 | 3.132 | 1.359–7.215 | 7.184 |
| Couse of disease | 0.004 | 3.846 | 1.556–9.506 | 8.509 |
| FBG (mmol/L) | 0.270 | 0.626 | 0.272–1.440 | 1.215 |
| PGL (mmol/L) | 0.009 | 3.182 | 1.339–7.562 | 6.870 |
| FCP (ng/ml) | 0.283 | 0.619 | 0.257–1.487 | 1.152 |
| 2-h FCP (ng/ml) | 0.037 | 0.380 | 0.153–0.945 | 4.336 |
| 24-h UME (mg/d) | 0.181 | 0.571 | 0.252–1.297 | 1.790 |
DPN – diabetic peripheral neuropathy; OR – odds ratio; NGF – nerve growth factor; BDNF – brain derived neurotrophic factor; FBG – fasting blood glucose; PGL – postprandial glucose level; FCP – fasting C-peptide; 2-h FCP – 2 h fasting C-peptide; UME – urinary microalbumin excretion.
Discussion
Defined as the only cause of peripheral, somatic, or autonomic impairment, DPN is considered the most damaging type of peripheral neuropathy, which affects nearly one-third of diabetic patients with considerable morbidity, mortality and poor quality of life [4,20]. As DPN cannot be cured and nerve damage cannot be repaired, the current treatment for DPN, including medications and acupuncture, can only help minimize pain and alleviate some other symptoms [21]. Kim et al. reported that serum total bilirubin level was conversely linked with DPN [22]. Zheng et al. showed that enhanced serum levels of homocysteine increased the risk of DPN development in patients [23]. Therefore, in the current study, we hypothesized that the serum NGF and BDNF levels would be beneficial to understanding the development mechanism and finding new therapeutic targets for DPN detection and treatment.
Initially, our results implied that decreased serum NGF level might be associated with the incidence to DPN. NGF was a neurotrophic factor, which played a significant role in the adult peripheral nervous system and has been studied as a potential therapy for DPN [24]. NGF is known for affecting the phenotype of mature nociceptors receptors as well as normal development of the embryonic nervous system, and changes in NGF levels are involved in the pathophysiology of chronic pain conditions like neuropathic pain [25]. Decreased expression of NGF protein and its 2 receptors, TrkA and the neurotrophin receptor p75NTR, are positively correlated with the death of neurons in clinical diabetes, which further confirms that it is associated with the occurrence and development of DPN [24,26]. Moreover, the results of our study showed that increasing NGF level could protect against DPN. Similarly, recent research has suggested that vitamin A may reverse injuries in nerve and restore functional changes caused by DPN upon inducing the expression of NGF [27]. In addition, Li et al. have suggested that NGF could alleviate high glucose-induced endoplasmic reticulum (ER) stress which triggers progression of DPN, and that treatment with exogenous NGF could repair the structure of the sciatic nerve in DPN rats [28]. Furthermore, KANG TH et al. found that through upregulation of NFG, diosgenin could release its neuroprotective effect on diabetic peripheral neuropathy in diabetic rodent model [29], which is coincident with the findings of our study.
In addition, our study attested that lower serum BDNF level was also associated with the incidence to DPN. BDNF is a neurotrophin that modulates neuronal growth, differentiation, and survival during brain development, with prominent influence on neurogenesis and neuroplasticity [30]. BDNF is widely expressed in the adult mammalian brain and acts in a paracrine and autocrine manner to control a variety of brain processes by binding specific receptors tyrosine receptor kinase B (TrkB) and p75 neurotrophin receptor (p75NTR), including maintenance of neuronal systems, neuronal plasticity [31]. Since BDNF, in neural development and activity, played a crucial role, previous research also demonstrated that it is associated with a variety of neurological disorders such as depression, Alzheimer disease, Parkinson syndrome [10]. It has been demonstrated that serum BDNF level dramatically decreases in patients with T2DM, and that balanced BDNF contributes to glucose metabolism [32]. Li et al. also clarified that exogenous BDNF could alleviate pain symptoms of diabetic rats through decreasing excitability of dorsal root ganglion neurons and might be the potential target for painful diabetic neuropathy treatment [33]. In addition, a previous study illustrated that the reduced BDNF expression in diabetic rat brain endothelium in vivo is regarded as an increased risk of neuronal injury, and also pointed out the correlation of suppressed BDNF expression in diabetes with neuron injury [34], just as in our study. Furthermore, it has been demonstrated that BDNF protects enteric glia cells from apoptosis during inflammation in Crohn’s disease [35]. All of these findings revealed that an elevated BDNF level plays a positive role in DPN treatment.
Conclusions
Since decreased serum NGF and BDNF levels are involved in progression of DPN, upregulation of them could protect against DPN. Therefore, detection of serum levels of NGF and BDNF may aid in facilitating the existing understanding of the mechanisms of DPN, with high sensitivity, specificity, and accuracy in the diagnosis of DPN. However, due to the study limitation of sample size, further studies are needed to discuss the specific mechanisms of NGF and BDNF levels on DPN. Additionally, in order to verify whether serum NGF and BDNF levels are risk factors of DPN, further studies are needed to be repeated across multiple platforms, universities, groups and in larger settings.
Acknowledgments
We are particularly grateful to all the people who had given help for our article.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61–99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerner W, Bruckel J German Diabetes Association. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122(7):384–86. doi: 10.1055/s-0034-1366278. [DOI] [PubMed] [Google Scholar]
- 3.Chapman A, Liu S, Merkouris S, et al. Psychological interventions for the management of glycemic and psychological outcomes of type 2 diabetes mellitus in China: A systematic review and meta-analyses of randomized controlled trials. Front Public Health. 2015;3:252. doi: 10.3389/fpubh.2015.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinzur MS. Diabetic peripheral neuropathy. Foot Ankle Clin. 2011;16(2):345–49. doi: 10.1016/j.fcl.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Janahi NM, Santos D, Blyth C, et al. Diabetic peripheral neuropathy, is it an autoimmune disease? Immunol Lett. 2015;168(1):73–79. doi: 10.1016/j.imlet.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 7.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–50. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 8.Celikbilek A, Gocmen AY, Tanik N, et al. Decreased serum vitamin D levels are associated with diabetic peripheral neuropathy in a rural area of Turkey. Acta Neurol Belg. 2015;115(1):47–52. doi: 10.1007/s13760-014-0304-0. [DOI] [PubMed] [Google Scholar]
- 9.Kiani J, Habibi Z, Tajziehchi A, et al. Association between serum uric acid level and diabetic peripheral neuropathy (A case control study) Caspian J Intern Med. 2014;5(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–58. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantyh PW, Koltzenburg M, Mendell LM, et al. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115(1):189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu SX, Huang SY, Su YM, et al. Growth and expression of rat bone marrow mesenchymal stem cells modified by nerve growth factor in diabetic rat bladders. Mol Med Rep. 2013;7(6):1791–99. doi: 10.3892/mmr.2013.1425. [DOI] [PubMed] [Google Scholar]
- 13.Rosas-Vargas H, Martinez-Ezquerro JD, Bienvenu T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch Med Res. 2011;42(6):482–94. doi: 10.1016/j.arcmed.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 14.He M, Wang J. Decreased serum brain-derived neurotrophic factor in Chinese patients with Type 2 diabetes mellitus. Acta Biochim Biophys Sin (Shanghai) 2014;46(5):426–57. doi: 10.1093/abbs/gmu008. [DOI] [PubMed] [Google Scholar]
- 15.Lambert GW, Schlaich MP, Esler MD. Brain derived neurotrophic factor (BDNF) release from the human brain in patients with type 2 diabetes – possible influence of venous anatomy and comorbid major depressive disorder. Diabetologia. 2007;50(9):2027–28. doi: 10.1007/s00125-007-0756-3. author reply 2029–30. [DOI] [PubMed] [Google Scholar]
- 16.Bravo-Molina A, Linares-Palomino JP, Lozano-Alonso S, et al. Influence of wound scores and microbiology on the outcome of the diabetic foot syndrome. J Diabetes Complications. 2016;30(2):329–34. doi: 10.1016/j.jdiacomp.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Sakashita Y, Nakanishi S, Yoneda M, et al. Regardless of central obesity, metabolic syndrome is a significant predictor of type 2 diabetes in Japanese Americans. J Diabetes Investig. 2015;6(5):527–32. doi: 10.1111/jdi.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyuk B, Degirmencioglu S, Atalay H, et al. Relationship between levels of brain-derived neurotrophic factor and metabolic parameters in patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014 doi: 10.1155/2014/978143. 978143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhen YF, Zhang J, Liu XY, et al. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology (Berl) 2013;227(1):93–100. doi: 10.1007/s00213-012-2942-3. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: Current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Bo C, Xue Z, Yi G, et al. Assessing the quality of reports about randomized controlled trials of acupuncture treatment on Diabetic Peripheral Neuropathy. PLoS One. 2012;7(7):e38461. doi: 10.1371/journal.pone.0038461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim ES, Lee SW, Mo EY, et al. Inverse association between serum total bilirubin levels and diabetic peripheral neuropathy in patients with type 2 diabetes. Endocrine. 2015;50(2):405–12. doi: 10.1007/s12020-015-0583-0. [DOI] [PubMed] [Google Scholar]
- 23.Zheng LQ, Zhang HL, Guan ZH, et al. Elevated serum homocysteine level in the development of diabetic peripheral neuropathy. Genet Mol Res. 2015;14(4):15365–75. doi: 10.4238/2015.November.30.14. [DOI] [PubMed] [Google Scholar]
- 24.Kim HC, Cho YJ, Ahn CW, et al. Nerve growth factor and expression of its receptors in patients with diabetic neuropathy. Diabet Med. 2009;26(12):1228–34. doi: 10.1111/j.1464-5491.2009.02856.x. [DOI] [PubMed] [Google Scholar]
- 25.Evans LJ, Loescher AR, Boissonade FM, et al. Temporal mismatch between pain behaviour, skin Nerve Growth factor and intra-epidermal nerve fibre density in trigeminal neuropathic pain. BMC Neurosci. 2014;15:1. doi: 10.1186/1471-2202-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romon R, Adriaenssens E, Lagadec C, et al. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol Cancer. 2010;9:157. doi: 10.1186/1476-4598-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez-Pedro N, Granados-Soto V, Ordonez G, et al. Vitamin A increases nerve growth factor and retinoic acid receptor beta and improves diabetic neuropathy in rats. Transl Res. 2014;164(3):196–201. doi: 10.1016/j.trsl.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Wu Y, Zou S, et al. NGF attenuates high glucose-induced ER stress, preventing schwann cell apoptosis by activating the PI3K/Akt/GSK3beta and ERK1/2 pathways. Neurochem Res. 2017;42(11):3005–18. doi: 10.1007/s11064-017-2333-6. [DOI] [PubMed] [Google Scholar]
- 29.Kang TH, Moon E, Hong BN, et al. Diosgenin from Dioscorea nipponica ameliorates diabetic neuropathy by inducing nerve growth factor. Biol Pharm Bull. 2011;34(9):1493–98. doi: 10.1248/bpb.34.1493. [DOI] [PubMed] [Google Scholar]
- 30.Green MJ, Matheson SL, Shepherd A, et al. Brain-derived neurotrophic factor levels in schizophrenia: A systematic review with meta-analysis. Mol Psychiatry. 2011;16(9):960–72. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 31.Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81(5–6):294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Fujinami A, Ohta K, Obayashi H, et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem. 2008;41(10–11):812–17. doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Yu T, Yu L, et al. Exogenous brain-derived neurotrophic factor relieves pain symptoms of diabetic rats by reducing excitability of dorsal root ganglion neurons. Int J Neurosci. 2016;126(8):749–58. doi: 10.3109/00207454.2015.1057725. [DOI] [PubMed] [Google Scholar]
- 34.Navaratna D, Guo SZ, Hayakawa K, et al. Decreased cerebrovascular brain-derived neurotrophic factor-mediated neuroprotection in the diabetic brain. Diabetes. 2011;60(6):1789–96. doi: 10.2337/db10-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinkamp M, Schulte N, Spaniol U, et al. Brain derived neurotrophic factor inhibits apoptosis in enteric glia during gut inflammation. Med Sci Monit. 2012;18(4):BR117–22. doi: 10.12659/MSM.882612. [DOI] [PMC free article] [PubMed] [Google Scholar]