Abstract
Background
Acute graft-versus-host disease (aGVHD) limits the wider application of hematopoietic stem cell transplantation (HSCT). We explored the relationship between the Nrf2-ARE signaling pathway and aGVHD and identified effective and efficient therapeutic targets for the prevention and management of aGVHD following HSCT.
Material/Methods
C57BL/6 and BALB/c mice were used to establish the aGVHD model. The bone marrow and spleen mononuclear cells were separated from the donor mice and injected into the caudal vein of recipient mice that had undergone total body irradiation (TBI, 8 Gy). Sulforaphane (SFN) was used to activate the Nrf2-ARE signaling pathway.
Results
The long-term survival rate of the SFN group was higher than that of the control group (40% vs. 0%, p<0.05, n=10). There were worse pathological changes and a greater infiltration of inflammatory cells in the liver, small intestine, and lung tissues of the control group. Furthermore, the Nrf2, NQO1, and HO-1 mRNA and protein levels were higher in the small intestines of the SFN group than in the control group (p<0.05, n=4).
Conclusions
The Nrf2-ARE signaling pathway plays a vital role in preventing aGVHD in an HSCT mouse model by regulating the expression of the downstream antioxidant genes NQO1 and HO-1 and by inhibiting the local inflammatory reaction.
MeSH Keywords: Graft vs Host Disease, Hematopoietic Stem Cell Transplantation, NF-E2-Related Factor 1
Background
Hematopoietic stem cell transplantation is increasingly used as a standard treatment for many hematological malignancies. The main complication of HSCT is GVHD, an immunological disorder that affects many tissues and organs, including the gastrointestinal system, skin, and lungs [1–3]. So far, no single drug can completely inhibit GVHD. Once patients who underwent HSCT develop GVHD, the transplantation success rate significantly decreases and the patients have a worse prognosis than those without GVHD. Therefore, reducing the incidence of GVHD and improving the survival rate of GVHD patients is a tremendous clinical challenge [4,5].
In recent years, many investigations [6–8] reported that oxidative stress products have a crucial influence on GVHD, especially during the development and progression of complications following HSCT. Therefore, reducing oxidative stress is thought to be a beneficial therapy for patients with GVHD following HSCT. However, it is difficult to manage the balance between oxidants and antioxidants in the pathological state and further research needs to be conducted on the mechanism.
Nuclear factor-erythroid-related factor 2 (Nrf2) is a crucial transcription factor that plays a vital role in regulating the expression of oxidative stress-response proteins [9]. Studies have found that sulforaphane (SFN) is one of the most fascinating phytochemicals because it protects aerobic cells against carcinogens, can activate the Nrf2-ARE signaling pathway, and protects neurons by inducing the expression of downstream antioxidant genes such as HO-1 [10,11]. However, whether SFN can activate Nrf2 and protect organs against aGVHD after hematopoietic stem cell transplantation is unknown.
In this study, we sought to: (1) establish a successful GVHD mouse model, (2) determine whether the Nrf2-ARE signaling pathway participates in aGVHD injury by reducing oxidative stress, and (3) investigate whether activation of the Nrf2-ARE signaling pathway affects inflammation in the tissues of transplanted mice.
Material and Methods
Experimental animal groups
The C57BL/6 mice (male, 8–12 weeks old, 18–22 g) and BALB/c mice (female, 8–12 weeks old, 18–22 g) were purchased from the Animal Experimental Center of Central South University. The C57BL/6 mice provided the bone marrow cells (BMCs) and spleen mononuclear cells (SMCs). The BALB/c mice were randomly divided into 4 groups consisting of 10 mice each. All of the mice were fed a standard diet and acclimatized for 10 days before transplantation. The animal protocol was reviewed and approved by the Ethics Committee of the Third Xiangya Hospital of Central South University.
Four experimental groups were created. The TBI group was irradiated and injected with 0.5 ml of 1× phosphate-buffered saline (1×PBS); the BM group was irradiated and injected with 0.5 ml of cell suspension (5×106 BMCs); the GVHD-SFN group was irradiated, injected with 0.5 ml of cell suspension (5×106 BMCs+0.5×106 SMCs), and treated with sulforaphane (10 mg/kg, once every 2 days, LKT Labs, USA) by intraperitoneal injection after HSCT; and the GVHD-NS group was treated in the same manner as the GVHD-SFN group except that 0.9% normal saline (NS) was used instead of sulforaphane.
Establishment of the aGVHD model
To prepare the host mice, all of the BALB/c mice were given 8 Gy of total body irradiation by linear accelerator before transplantation. To obtain the BMC and SMC suspensions, the C57BL/6 mice were killed by cervical dislocation and subsequently put into a 75% alcohol solution for 15 min. The bilateral femur, tibia, and spleen were harvested from the mice in an aseptic environment. The epiphyseal sides of the bones were cut off and the bone marrow was flushed out with RPMI 1640 medium (Gibco, Shanghai, China). RBC pyrolysis liquid (CW-Biotech, Beijing, China) was used to remove the erythrocytes from the cell suspension. For the most important step – removing the T lymphocyte cells – anti-Thy1.2 (Abcam, Shanghai, China) was used for 30 min at 4°C. The cell suspension was washed once with PBS and then treated with 10% immature rabbit complement (AbD Serotec, Beijing, China) and 2% DNase (CW-Biotech, Beijing, China) for 45 min at 37°C. Finally, the cell suspension was diluted to 2.0×107 cells/ml with 1×PBS and stored at 4°C. The spleens were carefully milled through a 200-mesh cell sieve, and after lymphocyte cells separation medium (MPBio, Beijing, China) was added, the mononuclear cells were separated by density-gradient centrifugation. Finally, the cell suspension was diluted to 2.0×106 cells/ml with 1×PBS and stored at 4°C.
Assessment of aGVHD
The severity of GVHD was assessed by weight loss in the host mice [12, 13]. A weight loss of more than 10% indicated the occurrence of GVHD. In addition to weight loss, hunching, activity, fur texture, and skin injury were recorded. The degree of GVHD was mainly assessed using the clinical scoring system [14] (described in Table 1). All of the host mice were assessed twice a week after transplantation.
Table 1.
Assessment of aGVHD after transplantation [11].
| Criteria | Grade 0 | Grade 1 | Grade 2 |
|---|---|---|---|
| Weight loss | <10% | 10–25% | >25% |
| Posture | Normal | Hunching noted only at rest | Severe hunching impairs movement |
| Activity | Normal | Mild to moderately decreased | Stationary unless stimulated |
| Fur texture | Normal | Mild to moderately ruffling | Severe ruffling/poor grooming |
| Skin integrity | Normal | Scaling of paws/tail | Obvious areas of denuded skin |
Detection of Nrf2, NQO1, and HO-1 expression in the small intestine by real-time PCR and western blotting
All of the genes were amplified by real-time PCR using the following primers (GenScript, Nanjing, China):
Actin: F: 5′-CATCCTGCGTCTGGACCTGG-3;
R: 5′-TAATGTCACGCACGATTTCC-3′;
Nrf2: F: 5′-CTTTCAACCCGAAGCACG-3′;
R: 5′-GCGGTGGGTCTCCGTAA -3′;
NQO1: F: 5′-ATCCTGCGTTTCTGTGGCT-3′;
R: 5′-TCCTCCCAGACGGTTTCC-3′;
HO-1: F: 5′-CTCACAGATGGCGTCACTTCG-3′;
R: 5′-TGAGGACCCACTGGAGGAGC-3′
Western blotting was performed to detect the expression of the Nrf2, NQO1, and HO-1 proteins in the tissues of the small intestine. Monoclonal antibodies against Nrf2, NQO1, and HO-1 were used as the primary antibodies (ProteinTech, Chicago, USA), and HRP-labeled goat-anti-rabbit IgG (CW-Biotech, Beijing, China) was used as the secondary antibody.
Observation of histopathological changes in the liver, lungs, and small intestine of transplanted mice
The liver, lungs, and small intestine were harvested from the mice on day 14 after transplantation. Paraffin sections of these tissues were made and analyzed by HE staining to determine the changes in tissue morphology and to assess the severity of target organ GVHD-specific pathological damage.
Determination of the concentration of TNF-α and IFN-γ in the plasma by enzyme-linked immunosorbent assay
Blood (0.1 ml) was taken from the angular vein of all of the host mice on day 14 after transplantation and anti-coagulated with EDTA. The concentration of TNF-α and IFN-γ in the resulting supernatant was measured by ELISA (CUSABIO, Wuhan, China).
Statistical analyses
The data were analyzed with SPSS version 21.0 and are expressed throughout the text as the mean ±SEM. We used the t test to compare the means between 2 groups, and one-way ANOVAs with S-N-K multiple comparison tests were used to compare multiple groups. The Kaplan-Meier method was used to analyze the survival time of the transplanted mice, and the log-rank test was used to compare the survival rates between 2 groups. In all analyses, P<0.05 was taken to indicate significance. All of the figures were made using GraphPad Prism version 6.01.
Results
The clinical scores of transplanted mice
All of the mice were assessed according to the standard clinical score system, and the following clinical symptoms or features were recorded: weight loss, hunching, activity, fur texture, and skin injury. The results are shown in Figure 1.
Figure 1.
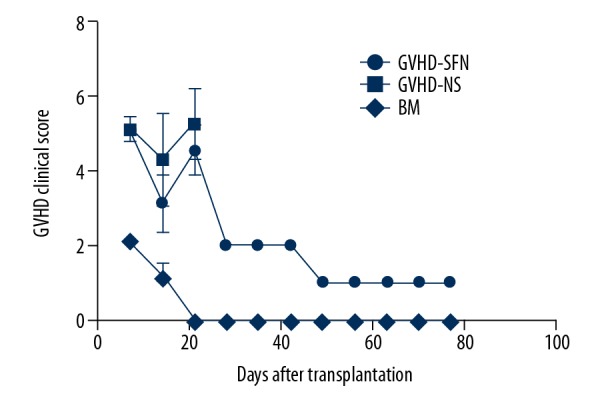
Clinical score of transplanted mice. Both the GVHD-SFN group and the GVHD-NS group displayed similar changes within 3 weeks after transplantation. Treatment with sulforaphane significantly reduced the clinical scores. Mice in the BM group survived for the entire observation period (80 days) and some displayed mild hunching and ruffling within 3 weeks after transplantation.
Survival times of transplanted mice
All of the mice in the TBI group were discontinuously killed within 5 days after transplantation. All of the mice in the BM group survived for the entire observation period (80 days), although some displayed mild hunching and ruffling. The survival curves are shown in Figure 2.
Figure 2.
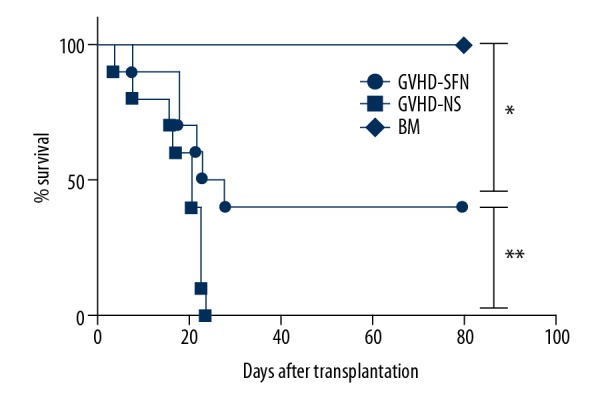
The survival rate of transplanted mice. The mice in the GVHD-NS group were discontinuously killed within 3 weeks following transplantation. In the GVHD-SFN group, all of the mice were still alive by day 8, and the long-term survival rate was 40%. There were statistically significant differences between each group (* P<0.01, ** P<0.05), and the survival rate of the GVHD-SFN group was higher than that of the GVHD-NS group (40% vs. 0%, ** P<0.05).
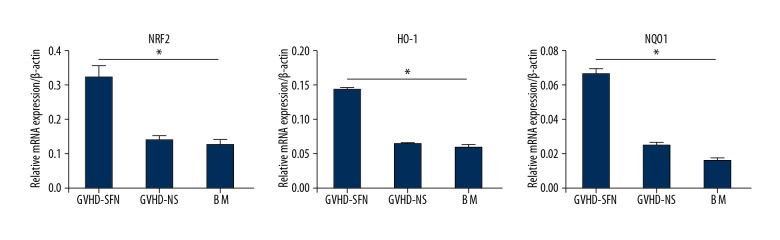
mRNA expression of Nrf2, NQO1, and HO-1 in the small intestine of transplanted mice
We repeated our experiments using the method described above and harvested the small intestines on the 14th day after transplantation. We then detected the mRNA expression of Nrf2, NQO1, and HO-1 in the small intestines (Figure 3).
Figure 3.
mRNA levels of Nrf2, NQO1, and HO-1. The GVHD-SFN group had a higher expression of Nrf2, NQO1, and HO-1 mRNA compared with the GVHD-NS group. The upregulation of these genes was 2.46-, 1.95-, and 2.2-fold, respectively. There was no statistically significant difference between the BM and GVHD-NS groups (P>0.05, n=4).
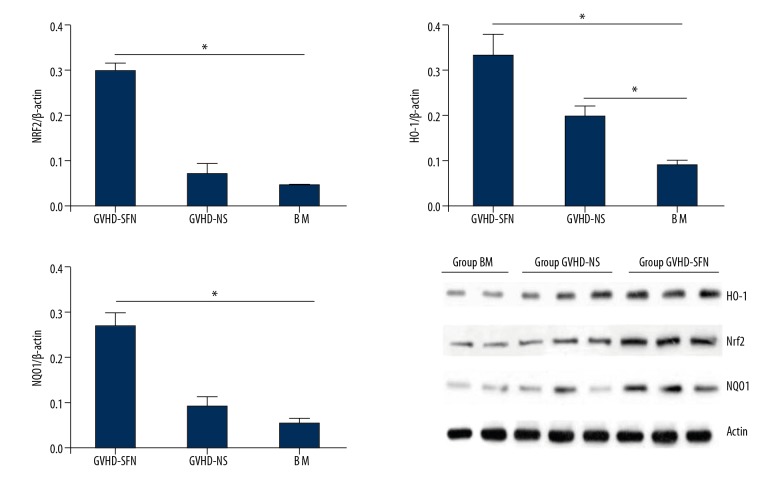
Nrf2, NQO1, and HO-1 protein expression in the small intestine of transplanted mice
Figure 4 shows the expression of Nrf2, NQO1, and HO-1 protein in the small intestines of transplanted mice. The GVHD-SFN group had a higher expression of Nrf2, NQO1, and HO-1 protein compared with the GVHD-NS group, indicating that SFN can significantly activate Nrf2-ARE-related genes in the small intestines.
Figure 4.
Expression of Nrf2, NQO1, and HO-1 protein in the small intestines of transplanted mice. The upregulation of these proteins was 3.11-, 1.25-, and 2.34-fold, respectively. There was no statistically significant difference in the protein of Nrf2 and NQO1 between the BM and GVHD-NS groups (P>0.05, n=4).
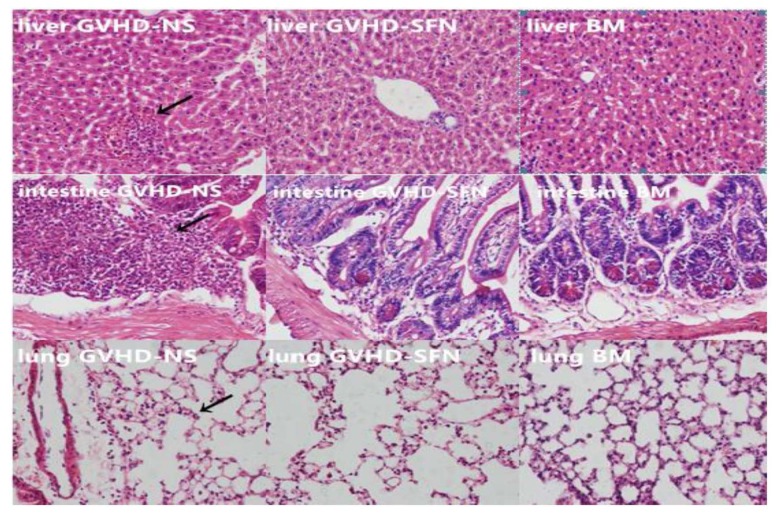
Pathological damage to target organs in transplanted mice
The liver, lung, and small intestine tissues were made into paraffin sections and stained with HE. Figure 5 shows these histopathological changes.
Figure 5.
Pathological damage in the liver, lungs, and small intestines after transplantation (HE staining, 200×). The BM group displayed normal morphology in the liver, lungs, and intestines. However, the GVHD-NS and GVHD-SFN groups showed morphological abnormities. The GVHD-NS group showed an infiltration of inflammatory cells in the parenchyma area of the liver, which was accompanied by bleeding and structural damage. Treatment with SFN reduced the number of inflammatory cells. The small intestines and lungs displayed the same changes as the livers.
Detecting IFN-γ and TNF-α levels in the plasma of transplanted mice
We harvested the plasma of the transplanted groups on the 14th day. The results showed a significant difference. Figure 6 shows the changes in plasma cytokines in the transplanted mice.
Figure 6.
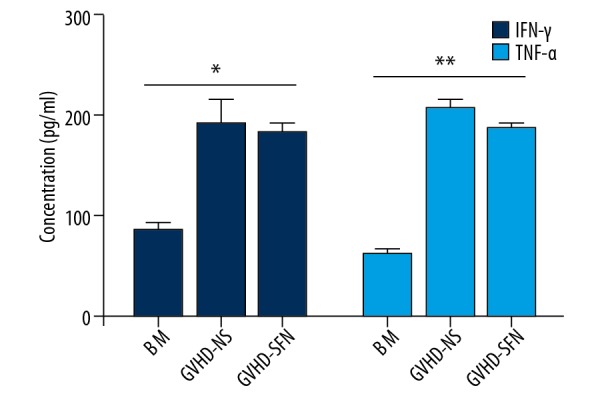
Plasma concentrations of IFN-γ and TNF-α after transplantation (pg/ml). The changes in IFN-γ and TNF-α had the same trend among the 3 groups. The BM group released the lowest concentration of cytokines after transplantation compared with the other 2 groups (P<0.05, n=4).
Discussion
Allogeneic hematopoietic stem cell transplantation is an effective treatment for some malignant hematopoietic diseases, and an increasing number of patients now have hope for survival because of HSCT. Although many scientists are devoted to identifying methods to reduce the complications from HCST and optimize the procedure, the occurrence of GVHD still limits the application of HSCT. In a 2012 study, investigators [15] reported that the cumulative incidence of grade B-D aGVHD was 39% in a sibling donor cohort and more than 59% in an unrelated donor cohort. Therefore, effectively preventing the occurrence and development of GVHD is still an imperative problem.
In this study, we successfully established an HSCT mouse model using C57BL/6 and BABL/C mice, and we optimized the model by referring to the experiments of other researchers [16,17]. Through pre-experiments, we ultimately determined that 8 Gy of total radiation, 5×106 bone marrow cells, and 0.5×106 spleen cells were optimal for our experiments. The survival rate of the transplanted mice in the BM group (Figure 1) indicates that our model is stable and can be used successfully in further research.
The components of the pathway are expressed in a variety of organs, including the small intestines, liver, and lungs. Our data indicate that Nrf2 and its related downstream genes are highly expressed in the small intestines of transplanted mice. Clarke et al. [18] found that in mice, the small intestine, among all the organs, expressed the highest levels of proteins in the Nrf2-ARE pathway following gavage with sulforaphane, which is why our experiment mainly detected the expression of Nrf2 and its downstream genes in transplanted mice. Because diarrhea is the most common clinical adverse effect of HSCT, we speculated that the small intestines had the earliest and most serious damage. The activators of the Nrf2-ARE pathway include tert-butyl hydroquinone (TBHQ), sulforaphane (SFN), and curcumin. Cui et al. [19] reported that SFN can prevent diabetic nephropathy in a type 1 diabetic mouse model via activation of the Nrf2 pathway, and other researchers also found that SFN activated the Nrf2-ARE signaling pathway in models of intestinal injury [20] or lung damage [21]. Wu et al. [22] used Nrf2+/+ and Nrf2−/− C57BL/6 mice as recipient mice to explore the mechanism of GVHD and found that Nrf2 is important for graft survival in a mouse model of allogeneic heart transplant. Ultimately, we concluded that SFN can protect the liver, lungs, and small intestines against aGVHD-induced injury in transplanted mice. Our experimental results (Figures 4, 5) also provide evidence that the antioxidant SFN can significantly activate Nrf2-ARE-related genes and alleviate aGVHD damage in the small intestines, liver, and lungs of transplanted mice. The potential mechanism involves Nrf2-ARE and its downstream antioxidant genes (NQO1 and HO-1), which play a crucial role in the development of aGVHD injury in the HSCT mouse model.
More recently, investigations into innate immunity, the interaction with subsequent downstream cytokines, and ultimate tissue damage have been conducted in regard to HSCT [23]. The donor T-helper cells are particularly crucial in the onset of GVHD because they can reciprocally differentiate into Th1, Th2, and Th17 cells that mediate organ-specific GVHD [24,25]. Although there is no perfect drug to prevent GVHD by inhibiting immunoreactions following HSCT, our results reveal that the reduction of oxidative stress may be an effective and efficient therapeutic method that can be used to improve HSCT. In addition, we observed the infiltration of inflammatory cells in histopathologic sections from transplanted mice and measured the plasma concentration of cytokines (IFN-γ and TNF-α) that are typically associated with tissue damage. The changes in cytokine concentration are associated with the survival time of the mice and the degree of organ damage. SFN treatment reduced the inflammatory cells in the liver, lungs, and small intestines (Figure 5) but did not affect the plasma levels of IFN-γ and TNF-α (Figure 6). Morzadec et al. [26] also found that the modulation of Nrf2 levels does not modify the secretion of inflammatory cytokines from T lymphocytes. This indicates that the Nrf2-ARE pathway has a partial role in the local inflammatory reaction. On the other hand, high levels of cytokines have a detrimental effect on aGVHD in transplanted mice. Both the reduction of oxidative stress and the inhibition of the inflammatory reaction are effective therapeutic measures for the prevention of GVHD after transplantation. In addition, SFN had been verified to activate the Nrf2-ARE signaling pathway [37–30]. Although our experimental results do not exclude the possibility that SFN alleviates aGVHD through other pathways, we found that SFN reduced aGVHD, possibly through the Nrf2-ARE pathway. In future research, Nrf2−/− mice or Nrf2-ARE inhibitor may be best to use.
We showed that the Nrf2-ARE pathway protects HSCT mice from aGVHD-induced damage by regulating oxidative stress and the local inflammatory reaction. However, to the best way to use antioxidant drugs to prevent or remedy aGVHD in the clinic still needs to be explored in future clinical studies.
Conclusions
The Nrf2-ARE signaling pathway plays a vital role in alleviating aGVHD-induced damage in an HSCT mouse model by regulating the expression of the downstream antioxidant genes NQO1 and HO-1 and by inhibiting the local inflammatory reaction.
Footnotes
Conflicts of interests.
None.
Source of support: The study was supported by the Science and Technology Agency of Hunan Province (2011FJ6091 and 2013WK3016)
References
- 1.Shono Y, van den Brink M. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18(5):283–95. doi: 10.1038/nrc.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheuk DK. Optimal stem cell source for allogeneic stem cell transplantation for hematological malignancies. World J Transplant. 2013;3(4):99–112. doi: 10.5500/wjt.v3.i4.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpdogan O. Advances in immune regulation in transplantation. Discov Med. 2013;15(82):150–59. [PubMed] [Google Scholar]
- 5.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324(10):667–74. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 6.Yuan L, Chen X, Qian L, et al. Administration of hydrogen-rich saline in mice with allogeneic hematopoietic stem-cell transplantation. Med Sci Monit. 2015;21:749–54. doi: 10.12659/MSM.891338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Wang Y, Toubai T, et al. BET bromodomain inhibition suppresses graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood. 2015;125(17):2724–28. doi: 10.1182/blood-2014-08-598037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im KI, Kim N, Lim JY, et al. The free radical scavenger NecroX-7 attenuates acute graft-versus-host disease via reciprocal regulation of Th1/regulatory T cells and inhibition of HMGB1 release. J Immunol. 2015;194(11):5223–32. doi: 10.4049/jimmunol.1402609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690(1–2):12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Alfieri A, Srivastava S, Siow RC, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–22. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 11.Yin X, Chen Z, Zhou J, et al. Mechanisms underlying the perifocal neuroprotective effect of the Nrf2-ARE signaling pathway after intracranial hemorrhage. Drug Des Devel Ther. 2015;9:5973–86. doi: 10.2147/DDDT.S79399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikstrom ME, Fleming P, Kuns RD, et al. Acute GVHD results in a severe DC defect that prevents T-cell priming and leads to fulminant cytomegalovirus disease in mice. Blood. 2015;126(12):1503–14. doi: 10.1182/blood-2015-01-622837. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Semple K, Suh WK, et al. Roles of CD28, CTLA4, and inducible costimulator in acute graft-versus-host disease in mice. Biol Blood Marrow Transplant. 2011;17(7):962–69. doi: 10.1016/j.bbmt.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–39. [PubMed] [Google Scholar]
- 15.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song EK, Yim JM, Yim JY, et al. Glutamine protects mice from acute graft-versus-host disease (aGVHD) Biochem Biophys Res Commun. 2013;435(1):94–99. doi: 10.1016/j.bbrc.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Taylor PA, Ehrhardt MJ, Lees CJ, et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD) Blood. 2007;110(9):3480–88. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke JD, Hsu A, Williams DE, et al. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2011;28(12):3171–79. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui W, Bai Y, Miao X, et al. Prevention of diabetic nephropathy by sulforaphane: possible role of Nrf2 upregulation and activation. Oxid Med Cell Longev. 2012;2012 doi: 10.1155/2012/821936. 821936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao HD, Zhang F, Shen G, et al. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16(24):3002–10. doi: 10.3748/wjg.v16.i24.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y, Tao S, Lian F, et al. Sulforaphane prevents pulmonary damage in response to inhaled arsenic by activating the Nrf2-defense response. Toxicol Appl Pharmacol. 2012;265(3):292–99. doi: 10.1016/j.taap.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu W, Qiu Q, Wang H, et al. Nrf2 is crucial to graft survival in a rodent model of heart transplantation. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/919313. 919313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson AM, Holler E. Polymorphisms of cytokine and innate immunity genes and GVHD. Best Pract Res Clin Haematol. 2008;21(2):149–64. doi: 10.1016/j.beha.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–58. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon B. Intervention with costimulatory pathways as a therapeutic approach for graft-versus-host disease. Exp Mol Med. 2010;42(10):675–83. doi: 10.3858/emm.2010.42.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morzadec C, Macoch M, Sparfel L, et al. Nrf2 expression and activity in human T lymphocytes: stimulation by T cell receptor activation and priming by inorganic arsenic and tert-butylhydroquinone. Free Radic Biol Med. 2014;71:133–45. doi: 10.1016/j.freeradbiomed.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Xu C, Huang MT, Shen G, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66(16):8293–96. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 28.Kensler TW, Egner PA, Agyeman AS, et al. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem. 2013;329:163–77. doi: 10.1007/128_2012_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dwivedi S, Rajasekar N, Hanif K, et al. Sulforaphane ameliorates okadaic acid-induced memory impairment in rats by activating the Nrf2/HO-1 antioxidant pathway. Mol Neurobiol. 2016;53(8):5310–23. doi: 10.1007/s12035-015-9451-4. [DOI] [PubMed] [Google Scholar]
- 30.Feng S, Xu Z, Wang F, et al. Sulforaphane prevents methylmercury-induced oxidative damage and excitotoxicity through activation of the Nrf2-ARE pathway. Mol Neurobiol. 2017;54(1):375–91. doi: 10.1007/s12035-015-9643-y. [DOI] [PubMed] [Google Scholar]