Abstract
Pneumomediastinum is a rare, potentially life-threatening complication of PCP that occurs in HIV-positive and HIV-negative patients. We are presenting a rare case pneumomediastinum caused by pneumocystis Jirovecii pneumonia in a HIV-negative patient with history of diffuse B-cell lymphoma on R CHOP chemotherapy. What is unique about our case is that the patient developed pneumomediastinum while in the hospital, on atovaquone that improved when switched to clindamycin and primaquine with improvement in her respiratory status. Another interesting point is that diagnosis was entertained due to the characteristic CT scan finding of ground glass opacities with cystic lung lesions and pneumomediastinum in an immunocompromised patient who was started on empirical treatment for PCP. The diagnosis was eventually confirmed with PCP PCR.
Keywords: Pneumomediastinum, Pneumocystis Jirovecii (carinii), Lung cystic lesions
1. Introduction
Pneumomediastinum is the presence of free air in mediastinum [1], which is thought to arise from free air leaking from ruptured alveoli [2]. Pneumomediastinum can be potentially catastrophic complication [1]. It Can be divided into two groups, spontaneous pneumomediastinum without any obvious primary cause, or secondary pneumomediastinum with a specific responsible pathologic event such as pulmonary or mediastinal infection by gas forming organisms, trauma or esophageal rupture [[1], [3], [4]]. Pneumocystis Jirovecii pneumonia is a rare cause of pneumomediastinum that has been reported in HIV-positive [[3], [4], [5]] and HIV-negative patients [6,2,5]. The most frequently reported symptoms in patients with pneumomediastinum are chest pain, followed by shortness of breath and cough [4]. On physical exam patients can have subcutaneous emphysema and neck swelling. Patients can also have Hamman crunch, which is crepitus heard with the heart beat on chest auscultation [1,4]. Rarely surgical intervention is needed as in most instances the pneumomediastinum resolves without treatment [4].
2. Case report
A 64-year-old female with past medical history of diffuse B-cell lymphoma, non-germinal subtype with T9 epidural extension status post laminectomy at time of diagnosis, 2 months before admission, and status post R CHOP chemotherapy. Last cycle was 15 days before admission. The patient was admitted with worsening shortness of breath for 2 days, associated with productive cough of rusty sputum. On examination, patient was tachypneic with respiratory rate of 33, hypoxic and requiring non rebreather to keep oxygen saturation more than 90%.
Laboratory workup on admission ABG PH 7.46/PCO2 30.5/PaO2 45 on O2 4l/min per nasal cannula, WBC 9 × 109/L, LDH 696 U/L, BNP 1307 pg/ml. Respiratory viral panel is negative, Legionella urine antigen and strep pneumoniae urine antigen are negative. HIV test was negative.
Chest x-ray showed moderate bilateral interstitial changes.
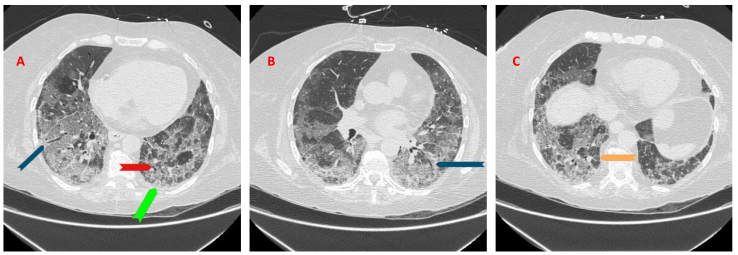
A high resolution CT chest without contrast showed bilateral ground glass opacities (GGOs) (orange arrow) with apical basilar gradient, superimposed interlobular septal thickening, mild bilateral lower lobe traction bronchiectasis (blue arrow) and cystic lesions (green arrow) (Fig. 1A–C).
Fig. 1.
A–C: CT chest high resolution without contrast showed bilateral ground glass opacities (GGOs) (orange arrow) with an apical basilar gradient with superimposed interlobular septal thickening and mild bilateral lower lobe traction bronchiectasis (blue arrow) and cystic lesions (red arrow).
Patient was admitted to the ICU and was started on IV Solumedrol and CPAP then switched to high flow nasal cannula 15 L of oxygen, felt slightly better and transferred to the floor.
Pulmonary service was consulted, and given the patient's immune compromised status, as well as the finding on CT scan, she was started on empirical treatment of PCP with atovaquone as the patient is allergic to sulfa.
Serum Fungitell was 244 (NR < 33), Aspergillus antigen 0.05 (negative), the sputum was sent for PCP silver stain and PCR, PCP silver stain was negative and PCP PCR was positive.
Bronchoscopy for the confirmation of the diagnosis was not done as the patient was in acute hypoxic respiratory failure.
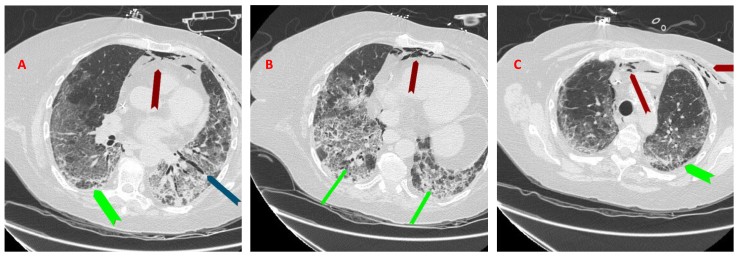
Repeat CT scan of the chest on day 12 showed pneumomediastinum (red arrow), which is new compared to the previous study, stable GGOs and with apical basilar gradient, superimposed interlobular septal thickening, mild bilateral lower lobe traction bronchiectasis (blue arrow) and more prominent cystic lung lesions (green arrow) (Fig. 2A–C). Her respiratory status stayed stable.
Fig. 2.
A-C: Repeat CT scan of the chest on day 12 showing pneumomediastinum (red arrows) which is new from previous study, stable GGOs and with an apical basilar gradient with superimposed interlobular septal thickening and mild bilateral lower lobe traction bronchiectasis (blue arrow) and more prominent cystic lung lesions (blue arrows).
Infectious disease service was consulted, the patient was switched to clindamycin and primaquine, and IV steroids were switched to oral prednisone that was tapered off slowly. The patient's respiratory symptoms improved significantly. She was weaned from 15 L of oxygen by high flow nasal canula to 5 L by regular nasal canula, and was discharged to a skilled nursing facility.
3. Discussion
Pneumocystis Jirovecii (carinii) is a ubiquitous unicellular fungus [7] that is found in the respiratory tracts of many mammals, including humans [2], and can be potentially life threatening opportunistic infection, that can occur in HIV-positive and HIV negative patients [6].
Patients with Pneumocystis Jirovecii pneumonia (PCP) may develop spontaneous pneumothorax as an infrequent complication that was described for the first time in 1984 [5,8], and is an uncommon complication of PCP in patient with HIV-positive [5,4,3] and HIV-negative patients [6,2,5].
PCP infection in HIV-negative patients occurs predominantly in patients on immunosuppression or chemotherapy medications [6]. These patients have a decrease in their cell mediated immunity [6].
The mechanism by which PCP causes pulmonary cysts and subsequent pneumothorax or pneumomediastinum is not yet known. However, various mechanisms have been described, including direct destruction of the lung by PCP, over distention of the lungs secondary to obstructive bronchiolitis which act as ball-valve, interstitial emphysema and interstitial fibrosis secondary to abnormal lung remodeling and release of elastase and other proteolytic enzymes (proteases released by activated macrophages) [5,3]. In most cases of pneumomediastinum, air enters through alveolar or pneumatocele rupture [4], then it tracks along the pulmonary vessels and interstitium to the hilum [3,4].
HIV-negative patients with PCP typically presents with abrupt onset of respiratory distress; they have more neutrophils and fewer pneumocystis organisms in their lungs during an episode of pneumonia caused by PCP than do HIV-negative patients with PCP pneumonia [9].
The most common radiographic finding in patients with PCP is diffuse, ground glass opacities (GGO) and bilateral perihilar interstitial infiltrates. A typical radiographic findings includes cystic spaces, bullae, hilar and mediastinal adenopathy, pleural effusion and pneumothorax [5,9]. It is rare to find pneumomediastinum in patients with PCP [5].
The diagnosis of PCP requires microbiological examination in order to identify pneumocystis from sputum, bronchoalveolar lavage (BAL) fluid or lung tissue biopsy as PCP cannot be cultured [9].
The initial procedure should be sputum induction; if it is negative then bronchoscopy with BAL should be performed. Transbronchial or surgical biopsy is rarely needed [9].
In our case the patient was suspected to have PCP. Given the fact that she was immunocompromised, the history of diffuse B-cell lymphoma on R CHOP treatment, the characteristic finding on CT scan of ground glass opacities with cystic lung lesion and pneumomediastinum that is suggestive of PCP.
Sputum induction was done and was negative for PCP. The patient was not stable for bronchoscopy or lung biopsy. PCP PCR was eventually done, and it was positive.
When PCR primers for gene for pneumocystis mitochondrial large subunit ribosomal RNA is used, PCR has been shown to have greater sensitivity and specificity for diagnosis of PCP form specimens of induced sputum or BAL [9,10].
In our case the serum lactate dehydrogenase (LDH) level was high. It has been noted that serum LDH level is elevated, likely as reflection of the underlying lung injury and inflammation rather than a specific marker for PCP [9].
Trimethoprim-sulfamethoxazole is the most effective treatment for severe PCP infection [9]. Our patient has sulfa allergy, so she was initially treated with atovaquone and then switched to clindamycin and primaquine after infectious disease was consulted.
Adjunctive steroid use in the treatment of PCP has been shown to reduce the mortality rate and incidence of respiratory failure associated with severe infection [2,11].
4. Conclusion
Pneumomediastinum is a rare potentially life-threatening complication of PCP that occurs in HIV-positive and HIV-negative patients. We are presenting this case and are confident that it will be a valuable addition to medical literature in raising awareness about pneumomediastinum as a rare complication of PCP in HIV negative patients. Timely work up and evaluation may be of value for early detection and treatment in order to prevent this serious complication and to potentially improve the patient's outcome. We recommend sending PCP PCR at the same time with sputum induction as PCP PCR has higher sensitivity and specificity, especially in patients who are not stable to undergo invasive diagnostic procedures.
Declarations
Funding
Not applicable.
Availability of data and materials
All data are available in the manuscript.
Authors' contributions
Hazim Bukamur, Emhemmid Karem and Serag Fares had substantial contributions in data collection and interpretation, designing this manuscript, searched for English written literatures and critically revised and written the manuscript. Amro Al-Astal and Mohammed Al-Ourani supervised and helped writing the case report, revised it critically and approved the final version. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable as it is a case report.
Consent for publication
Consent was taken from the patient and available upon request.
Competing interests
Hazim Bukamur, Emhemmid Karem, Serag Fares, Mohammed Al-Ourani and Amro Al-Astal have no competing interests to disclose related to the contents of the manuscript.
Acknowledgements
The authors acknowledge and thank the department of Pathology at VA Medical Center for their help and contribution.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2018.08.025.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Caceres M., Ali S.Z., Braud R., Weiman D., Garrett H.E. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann. Thorac. Surg. 2008;86:962–966. doi: 10.1016/j.athoracsur.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 2.Lee J.C., Bell D.C., Guinness R.M., Ahmad T. Pneumocystis jiroveci pneumonia and pneumomediastinum in an anti-TNFα naive patient with ulcerative colitis. World J. Gastroenterol. WJG. 2009;15:1897. doi: 10.3748/wjg.15.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss S., Carey P.B., Hind C.R. Pneumocystis carinii pneumonia presenting with pneumomediastinum in an HIV-positive patient. Postgrad. Med. 1995;71:96. doi: 10.1136/pgmj.71.832.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen D.J., Blasberg J.D., Ashton C.P., Connery C. Spontaneous pneumomediastinum in pneumocystis pneumonia and acquired immunodeficiency syndrome. Internet J. Pulm. Med. 2009;10 [Google Scholar]
- 5.Cho J.-Y., Kim D.-M., Kwon Y.E., Yoon S.H., Il Lee S. Newly formed cystic lesions for the development of pneumomediastinum in Pneumocystis jirovecii pneumonia. BMC Infect. Dis. 2009;9:171. doi: 10.1186/1471-2334-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandhu G., Dasgupta R., Ranade A., Baskin M. Pneumocystis pneumonia in an HIV-negative patient with no overt risk factors on presentation. Eur. Respir. J. 2010;35:927–929. doi: 10.1183/09031936.00180509. [DOI] [PubMed] [Google Scholar]
- 7.Santamauro J.T., Aurora R.N., Stover D.E. Pneumocystis carinii pneumonia in patients with and without HIV infection. Compr. Ther. 2002;28:96–108. doi: 10.1007/s12019-002-0047-3. [DOI] [PubMed] [Google Scholar]
- 8.Wollschlager C.M., Khan F.A., Chitkara R.K., Shivaram U. Pulmonary manifestations of the acquired immunodeficiency syndrome (AIDS) Chest. 1984;85:197–202. doi: 10.1378/chest.85.2.197. [DOI] [PubMed] [Google Scholar]
- 9.Thomas C.F., Jr., Limper A.H. Pneumocystis pneumonia. N. Engl. J. Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 10.Doyle L., Vogel S., Procop G.W. Open Forum Infectious Diseases. Oxford University Press US; 2017. Pneumocystis PCR: it is time to make PCR the test of choice. ofx193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briel M., Bucher H.C., Boscacci R., Furrer H. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV-infection. Cochrane Database Syst. Rev. 2006;3 doi: 10.1002/14651858.CD006150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript.