Abstract
Epithelial-mesenchymal transition (EMT) occurs in the development of fibrosis and carcinogenesis. EMT is associated with chronic liver injury. Evidence shows that hepatocytes undergo EMT in the adult liver. The Qinggan Huoxue Recipe (QGHXR), a Traditional Chinese Medicinal formula, shows a range of pharmacological effects in treating alcoholic liver disease. The present study aimed to investigate the effect of four major components of QGHXR, baicalin, salvianic acid, puerarin and saikosaponin, on EMT in vitro, and to elucidate the potential mechanism of QGHXR against EMT via the transforming growth factor-β1 (TGF-β1)/Smads signaling pathway. EMT models were established using LO2 hepatocytes and HepG2 cells treated with acetaldehyde in vitro. Acetaldehyde presented a mesenchymal cell characteristic in hepatocytes, accompanied by an increased expression of mesenchymal markers, including vimentin and fibronectin, and decreased E-cadherin. Baicalin and puerarin abrogated the increased expression of vimentin and fibronectin, and rescued E-cadherin expression in acetaldehyde-treated hepatocytes. It was further demonstrated that baicalin and puerarin reduced the gene expression of snail, TGF-β1 and Smad3. A decreased expression of tight function markers, including ZO-1, occludin and claudin, were also found in the acetaldehyde-treated hepatocytes. Barcacin regulated the mRNA level of TGF-βl and snail, and then suppressed the EMT process. This was accompanied by an increased mRNA level of E-cadherin and decreased levels of vimentin and fibronectin, but no significant differences in of Smad3, occludin, ZO-1 and claudin were observed. Puerarin regulated the mRNA level of TGF-βl, Smad3 and snail, suppresing the EMT process, which was accompanied by an increased mRNA level of E-cadherin and decreased levels of vimentin and fibronectin, along with increased levels of occludin, ZO-1 and claudin. When the snail gene was silent, barcacin and puerarin did not show significant effects in the acetaldehyde-treated cells. The results presented a novel mechanism through which baicalin and puerarin modulated hepatocyte EMT to improve liver fibrosis.
Keywords: epithelial-to-mesenchymal transition, baicalin, puerarin, TGF-β1, Smad3
Introduction
Excessive alcohol consumption is a global problem that accounted for 3.3 million deaths in 2012 (World Health Organization 2014). Alcoholic liver disease (ALD) starts with a fatty liver, fibrosis and cirrhosis, as well as a severe form of ALD known as alcoholic hepatitis, and/or eventually hepatocellular carcinoma (HCC) (1). There were approximately 75,766 cases of alcohol-related death in 2001 in the United States according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (2).
Epithelial-mesenchymal transition (EMT) is involved in chronic liver injury; evidence shows that hepatocytes in the adult liver undergo EMT. EMT is a complicated process, with the loss of cell-cell junctions and polarity, and results in the formation of migratory mesenchymal cells with invasive characteristics (3). Transforming growth factor-beta (TGF-β) initiates intracellular signals that bind to its receptors through the activation of downstream mediator Mothers Against DPP Homolog (Smad) proteins (4). Smads are important intracellular effectors of the TGF-β1 signaling superfamily (5). Generally, Smad2 and Smad3 are phosphorylated by TGF-β receptors and are then translocated to the nucleus after forming a complex with Smad4 (6). TGF-β/Smad3 plays a critical role in EMT during fibrosis, and therefore, a potential therapeutic approach for preventing and treating fibrosis is to develop TGF-β/Smad3 signaling inhibitors by targeting EMT.
Qinggan huoxue recipe (QGHXR), a traditional Chinese medicinal (TCM) formula, consists of bupleurum, scutellaria, red sage, carapax trionycis and radix puerariae, and shows pharmacological effects in treating ALD, including reversible steatosis, improving lipid peroxidation and inflammatory cytokines (7,8). A recent study showed that QGHXR improved the pathological changes of ALD rats through the Lipopolysaccharides (LPS)-Kupffer cells signal pathway (9). Furthermore, an in vivo study detected that QGHXR inhibited EMT in ALD rats by regulating the TGF-β1/Smad pathway (10).
In the present study, we detected the expression of molecules related to the TGF-β/Smad3 signaling and EMT in acetaldehyde-treated L02 cells. Baicalin, salvianic acid, puerarin, and saikosaponin are the major components from the QGHXR formula, and recent studies show their roles in improving liver injury. For example, baicalin exerts a hepatoprotective role in alcohol-induced liver injury by inhibiting oxidative stress, the inflammatory response, and the regulation of the sonic hedgehog pathway (11). Salvianic acid produces a protective role on acute hepatic injury induced by CCl4 via an antioxidative mechanism (12). Puerarin improves chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms (13). Saikosaponin exerts a protective role in the septic process by suppressing TNF-α and IL-6 concentrations in the intestines of septic rats via inhibiting the NOD2/NF-κB signaling pathway (14). However, it is unclear whether those components improve alcoholic liver injury via EMT or not. Therefore, we analyzed the effect of the four major components of QGHXR on TGF-β/Smad3 proteins and EMT proteins. Furthermore, snail siRNA was transferred into acetaldehyde-treated HepG2 cells to illustrate the changes to the TGF-β/Smad3 pathway by the four components.
Materials and methods
Cell culture and treatment
The cells were purchased from Cell Resource Center, Shanghai Science Research Center, Chinese Academy of Sciences (Shanghai, China). L02 and HepG2 (human liver cancer cells) cells were seeded in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and 100 units/ml penicillin/streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were incubated at 37°C in 5% CO2.
Cell proliferation
The proliferation of the L02 s cells was evaluated using the 3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) method. The cells were seeded at 2 × 105 cells per well in 200 µl of DMEM into 96-well culture plates and were cultured overnight. The medium was replaced with 20 µl of fresh medium containing 5 mg/ml MTT (Sigma-Aldrich; Merck KGaA) at different time points. The supernatants were removed four h later. Each well was supplemented with 100 µl of dimethylsulfoxide to dissolve the crystals. Cell viability was determined at 490 and 630 nm. All the experiments were performed in triplicate. Therapeutic agents clinically used against acetaldehyde were examined for efficacy by determining the 50% inhibitory concentration (IC50) values.
RNA preparation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the cells using the Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and was diluted to 200 ng/ml. Then, the PCR amplification was performed using the SYBR® PrimeScript™ RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China) and was conducted using the Applied Biosystems real-time PCR machine (ABI7500; Applied Biosystems; Thermo Fisher Scientific, Inc.). The reactions were performed as follows: 95°C for 3 min, with 34 cycles of 95°C for 30 sec; 65°C for 40 sec; and 72°C for 40 sec. The results were analyzed using the comparative threshold cycle value method (2−ΔΔCq) (15). The primer sequences are listed in Table I. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control.
Table I.
Sequence of primers.
| Genes | Sequence | Bp |
|---|---|---|
| E-cadherin | ||
| F | 5′-TGAGAACGAGGCTAACG-3′ | 358 |
| R | 5′-GCTGTGGAGGTGGTGAG-3′ | |
| Vimentin | ||
| F | 5′-CGCCAGATGCGTGAAAT-3′ | 119 |
| R | 5′-CGAAGGTGACGAGCCATT-3′ | |
| Fibronectin | ||
| F | 5′-CAGGACGGACATCTTTG-3′ | 232 |
| R | 5′-TCTGGTCGGCATCATAG-3′ | |
| TGF-β1 | ||
| F | 5′-GCGATACCTCAGCAACCG-3′ | 121 |
| R | 5′-AAGGCGAAAGCCCTCAAT-3′ | |
| Smad3 | ||
| F | 5′-CCTTTCAGGTAACCGTCTT-3′ | 168 |
| R | 5′-TTAGCCCATCATCTCCC-3′ | |
| Snail | ||
| F | 5′-CCCCACAGGACTTTGATG-3′ | 212 |
| R | 5′-GTGAGTCTGTCAGCCTTTGTC-3′ | |
| Occludin | ||
| F | 5′-TCAGGGTGTTTCTGTTGG-3′ | 123 |
| R | 5′-GAAATGGAAGGGATGTCG-3′ | |
| ZO-1 | ||
| F | 5′-AGATGAACGGGCTACGC-3′ | 173 |
| R | 5′-ACCGCTGGTCAGGAGAT-3′ | |
| Claudin-1 | ||
| F | 5′-CAGTTAGGAGCCTTGATGC-3′ | 128 |
| R | 5′-CGGCACAGGGAGTAGGA-3′ |
TGF-β1, tumor growth factor-β1; ZO-1, zona occludens-1; F, forward; R, reverse.
Western blot analysis
The cells were collected and homogenized in cell lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). The supernatants were collected after centrifugation at 12,000 × g for 30 min at 4°C. The bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology) was used to measure the concentration of the protein. A total of 60 µg of the protein samples was separated electrophoretically by 10% SDS-PAGE and was then transferred onto polyvinylidene difluoride membranes. Non-specific binding was blocked with 5% nonfat milk in Tris-buffered saline containing Tween 20 (TBST) for 2 h at room temperature, and then, the membranes were incubated with a primary antibody against snail (1:1,000; Abcam, Cambridge, MA, USA) at 4°C overnight. The membranes were then incubated with a secondary antibody. Finally, the blots were visualized with an enhanced chemical luminescence (ECL) system (GE Healthcare, Chicago, IL, USA).
Transfection of small interfering RNA (siRNA)
To further analyze the role of snail in EMT, the siRNAs targeting snail was obtained from Shanghai GenePharma Co., Ltd, (Shanghai, China). When the HepG2 cells were grown to almost 30% confluency, the siRNAs were transfected using lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). To verify the transfection efficiency, RT-qPCR and western blotting were performed.
Statistical analysis
The group values are shown as the mean ± standard deviation (SD). A one-way analysis of variance with post hoc tests by Student-Newman-Keuls test was used to evaluate the significance of the differences with SPSS v.17 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference. The histograms were generated with GraphPad Prism v.5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Acetaldehyde-induced EMT in human L02 cells
To establish an EMT model, different concentrations of acetaldehyde, including 0.0, 0.5, 1.0, 2.0, and 4.0 mmol/l were added into the L02 cells. Cell proliferation was evaluated by an MTT assay. We found that 2.127 mmol/l was the IC50 concentration of the acetaldehyde as shown in Fig. 1. Therefore, we used ¼ of the IC50, i.e., 0.532 mmol/l acetaldehyde, for the subsequent experiment of the EMT model of L02.
Figure 1.
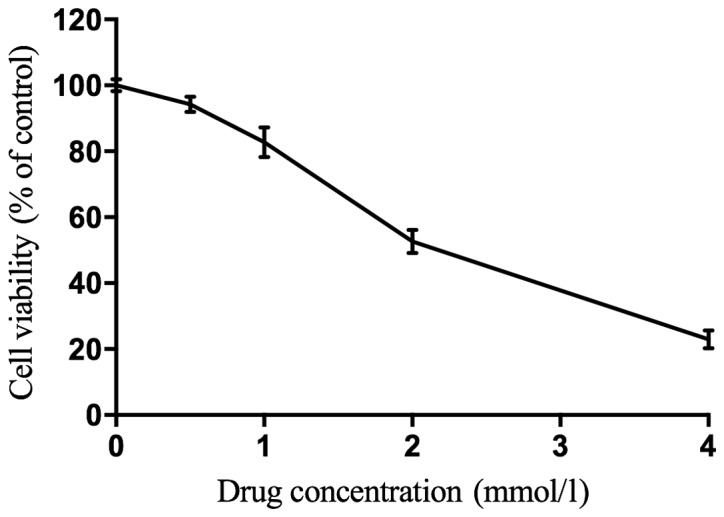
Concentration selection of acetaldehyde in human L02 cells. The MTT analysis showed that 2.127 mmol/l was the IC50 concentration of acetaldehyde.
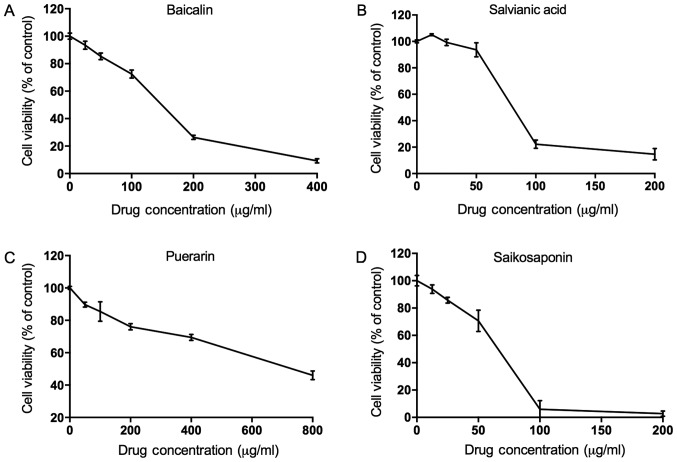
Concentration selection of the four medicines
Furthermore, in order to select the best concentration of the four medicines, cell proliferation was analyzed by an MTT assay after treatments with different concentrations. As shown in Fig. 2, the IC50 concentration of baicalin, salvianic acid, puerarin and saikosaponin, was 150, 79, 695, and 66.3 µg/ml respectively. Thus, ½ of the IC50 concentration of the four medicines, i.e., 75, 39.5, 347.5, and 33.15 µg/ml, was added into the acetaldehyde-treated L02 cells for 3, 6, 12, 24, and 48 h. Inhibition of cell proliferation was found at 48 h, and thus, cells in the 48-h treatment were collected for further experiments.
Figure 2.
Concentration selection of the four medicines. The MTT test showed that the IC50 concentrations of baicalin, salvianic acid, puerarin and saikosaponin were 150, 79, 695, and 66.3 µg/ml, respectively. Cell viability curves for (A) baicalin; (B) salvianic acid; (C) puerarin; (D) saikosaponin.
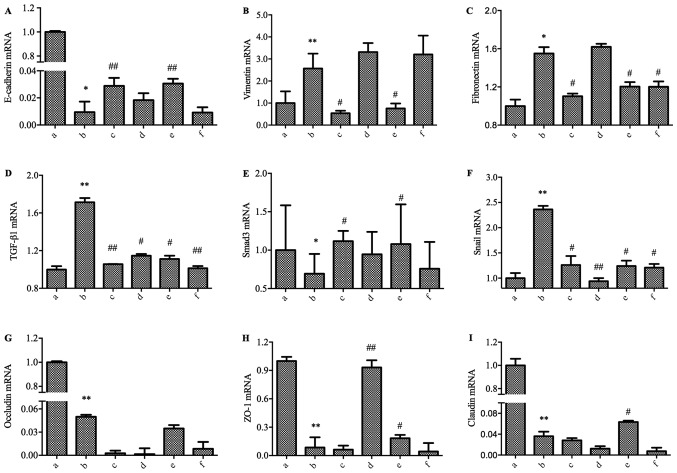
Baicalin and puerarin counteract acetaldehyde-induced EMT in L02 cells
We treated the L02 cells with acetaldehyde for 48 h in culture. We then detected the expressions of E-cadherin, vimentin and fibronectin using RT-qPCR. The gene expression of E-cadherin was significantly downregulated, and the expression of vimentin and fibronectin were significantly increased in the L02 cells with acetaldehyde treatment (Fig. 3A-C). These data indicated that EMT occurred in the L02 cells after the treatment acetaldehyde. To further understand the involvement of the four medicines in improving acetaldehyde-induced EMT in L02 cells, the related molecules involved in TGF-β1/Smads signaling were examined. As shown in Fig. 3D-F, the RT-qPCR experiment indicated that the mRNA levels of TGF-β1 and Snail were significantly increased in the acetaldehyde-treated cells, while the level of Smad3 decreased significantly. These data hinted that TGF-β1/Smad3 signaling might play an important role in the development of EMT. At the same time, the mRNA expression of the markers of tight junctions, including occludin, ZO-1 and claudin, decreased significantly (Fig. 3G-I).
Figure 3.
Effect of the four medicines in the TGF-βl-Snail-EMT signal pathway in acetaldehyde-treated L02 cells by RT-qPCR. The cells were treated with 0.064 mmol/l of acetaldehyde and were then treated with the four medicines for 48 h. (A) E-cadherin; (B) vimentin; (C) fibronectin; (D) TGF-βl; (E) Smad3; (F) snail; (G) occludin; (H) ZO-1; (I) claudin. Groups: a, Normal; b, Model; c, Baicalin; d, Salvianic acid; e, Puerarin; f, Saikosaponin. *P<0.05, **P<0.01, when compared with the Normal group; #P<0.05, ##P<0.01, when compared with the Model group. EMT, epithelial-mesenchymal transition, RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
After treatment with the four medicines in the high acetaldehyde-treated cells, the related molecules involved in the TGF-β1/Smad3 pathway were regulated differently (Fig. 3). Barcacin regulated the mRNA level of TGF-βl, Smad3, and Snail and then suppressed the EMT process, which was accompanied by the upregulated mRNA level of E-cadherin and the downregulation of fibronectin and vimentin, but with no significant regulation of occludin, ZO-1 and claudin. Salvianic acid did not show an obvious effect on the EMT marker expression, including E-cadherin, vimentin, and fibronectin. However, salvianic acid did regulate the mRNA of TGF-βl, Snail, and ZO-1. Puerarin regulated the mRNA level of TGF-βl, Smad3, and Snail and then suppressed the EMT process, which was accompanied by an increased mRNA level of E-cadherin and decreased levels of vimentin and fibronectin, along with an increased level of ZO-1 and claudin. Saikosaponin did not show an obvious effect on E-cadherin and vimentin, but it regulated the mRNA of fibronectin, TGF-βl, and Snail. None of the treatments regulated the expression of occludin.
Selection of the snail siRNA
To sift out the best snail siRNA, we verified the effect of silencing snail using RT-qPCR and western blot methods (Fig. 4). Then, we transferred six siRNAs into the cells at a 50 nM or 100 nM concentration. Twenty-four h later, the mRNA expression of snail was detected by RT-qPCR, and siRNA6 was the best silencing gene at 100 nM (Fig. 4A). Furthermore, we transferred the six siRNAs into the cells at a 100 nM concentration, and 48 h later, a western blot was used to detect the protein expression of snail. Similar to the PCR result, siRNA6 was the best (Fig. 4B). Then, we used siRNA6 to silence snail, and the sequence was forward: 5′-GCCUUCAACUGCAAAUACUdTdT-3′ and reverse: 5′-AGUAUUUGCAGUUGAAGGCdTdT-3′.
Figure 4.
Selection of snail siRNA. (A) The mRNA expression of snail siRNA using RT-qPCR; (B) The protein expression of snail siRNA using a western blot.
Baicalin and puerarin counteract aldehyde-induced EMT in HepG2 cells via snail
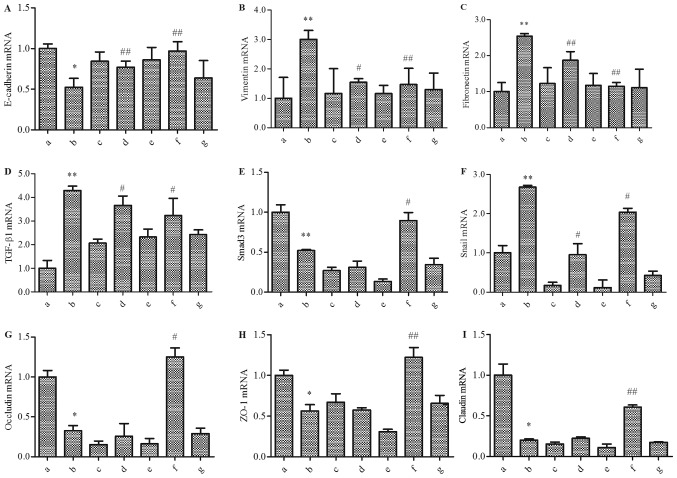
To further understand the role of snail, we used RNA interference technology to silence the expression of snail. Six groups were established, including the normal control, model, snail−/−, balcalin, balcalin + snail−/−, puerarin, and puerarin + snail−/−groups. As shown in Fig. 5, the mRNA expression of snail was significantly decreased in the snail−/− group. Similar with the former results, in the model group, E-cadherin mRNA was significantly downregulated, and vimentin and fibronectin mRNA were significantly upregulated in the HepG2 cells with acetaldehyde treatment compared to the normal control group (Fig. 5A-C). As shown in Fig. 5D-F, the RT-qPCR experiment indicated that the levels of TGF-β1 and snail were significantly increased in the acetaldehyde-treated cells, while the level of the Smad3 gene decreased significantly. Furthermore, the mRNA expression of occludin, ZO-1 and claudin decreased significantly (Fig. 5G-I). When the snail gene was silent, different changes were found in the mRNA expression of those related molecules. They did not show results similar to the model group.
Figure 5.
Effect of the four medicines on the TGF-βl-Snail-EMT signal pathway in acetaldehyde-treated snail−/− HepG2 cells by RT-PCR (A) E-cadherin; (B) vimentin; (C) fibronectin; (D) TGF-βl; (E) Smad3; (F) snail; (G) occludin; (H) ZO-1; (I) claudin. Groups: a, Normal; b, Model; c, Snail−/−; d, Baicalin; e, Baicalin+snail−/−; f, Puerarin; g, Puerarin+snail−/−. *P<0.05, **P<0.01, when compared with the Normal group; #P<0.05, ##P<0.01, when compared with the Model group. TGF-β, transforming growth factor-beta.
In summary, barcacin regulated the mRNA level of TGF-βl and snail and then suppressed the EMT process, which was accompanied with an increased mRNA level of E-cadherin and decreased levels of vimentin and fibronectin but with no significant regulation of Smad3, occludin, ZO-1 and claudin. Puerarin regulated the mRNA level of TGF-βl, Smad3, and snail and then suppressed the EMT process, which was accompanied with an increased mRNA level of E-cadherin and decreased levels of vimentin and fibronectin, as well as increased levels of occludin, ZO-1 and claudin. However, when the snail gene was silent, barcacin and puerarin did not show obvious effects in the acetaldehyde-treated cells.
Discussion
In the present study, an in vitro acetaldehyde-induced EMT model was established, and we investigated the effects of four major compounds on hepatocyte EMT and aimed to explore the potential molecular mechanisms.
EMT is a process by which the mesenchymal phenotype is acquired by epithelial cells, with the characteristics of the loss of cell-cell adhesion, remodeling of the cytoskeleton, and increased migratory properties (16). EMT is an important step in the development of hepatic fibrosis. EMT is characterized by the decreased expression of the epithelial marker E-cadherin and the increased expression of mesenchymal markers, such as α-SMA, fibronectin, collagen I and vimentin (17,18). A recent study showed that hepatic stellate cell (HSC) conditioned medium activates c-myc through the ERK1/2 signaling pathway in hepatoma cells, promoting cell proliferation, invasion and migration, and inducing EMT (19). In the present study, the expression of E-cadherin decreased, while vimentin and fibronectin increased in acetaldehyde-treated L02 and HepG2 cells using high concentrations of acetaldehyde. The results indicated that an EMT model was successfully established using acetaldehyde in vitro.
EMT is often associated with increases in the TGF-β signaling pathway, and TGF-β drives EMT, in part, through Smad-mediated reprogramming of gene expression (20–23). In the present study we mainly paid more attention to the role of Snail in the signaling pathway, thus we blocked Snail and further explored its role in the pathway.
First, we detected that acetaldehyde induced a mesenchymal cell characteristic in hepatocytes, which was accompanied by upregulated vimentin, fibronectin and snail and downregulated E-cadherin. At the same time, the TGF-β1 gene expression in the acetaldehyde-treated cells increased. Acetaldehyde promoted the phosphorylation of Smad-3, and the TGF-β1/Smad3 pathway was then activated.
More and more evidence shows that TCM exerts effects in treating fibrosis. For example, curcumin inhibits cobalt chloride-induced EMT by regulating TGF-β/Smad signaling in hepatocytes (24), and salvianolic acid B prevents EMT through the TGF-β 1 pathway in vivo and in vitro (25). Our results showed that baicalin and puerarin improved the mesenchymal characteristics through the TGF-β1/Smad3 pathway. Salvianic acid and saikosaponin did not show obvious effects on EMT.
EMT is regulated by several transcription factors, such as snail1, snail2, Twist and Zeb1/2 (22,23,26,27). Snail serves as is a critical connection in EMT regulation by repressing E-cadherin expression (28). To further illustrate the role of snail in EMT, snail siRNA was used. It is well-known that Hep G2 is a hepatoblastoma-derived cell line but not a HCC (29). The reason why we did not use primary hepatocytes in the present study is that the proliferative activity of primary hepatocytes is poor and it is hard to be generated, thus it is difficult to study the function of related pathways in the cells. To solve this problem, we then used the Hep-G2 cell line due to its proliferative activity as well as its stability. Finally it was shown that when the snail gene was silent, barcacin and puerarin did not demonstrate obvious effects in the acetaldehyde-treated cells. These results characterized a novel mechanism of baicalin and puerarin in the treatment of liver fibrosis.
However, there are some limitations in the present study. First, the morphological changes of the cells were absent. Second, it would be better to evaluate the changes in the protein expression levels of E-cadherin, vimentin, fibronectin, Smad3, occludin, ZO-1 and claudin. In a further study, we will perform related experiments to explore the role of the above components.
In summary, our present results show that high acetaldehyde induces EMT via activating the TGF-β1/Smad3 pathway. Furthermore, baicalin and puerarin reverse the changes. These results provide a novel target for the prevention and treatment of alcoholic liver injury.
Acknowledgements
The authors thank Professor Jianwen Liu (East China University of Science and Technology, Shanghai, China) for help in performing the experiments and Professor Peiyong Zheng (Longhua Hospital, Shanghai University of Traditional Chinese Medicine Shanghai, China) for his help in the writing and revision of the mansucript.
Funding
The present study was funded by National Natural Science Foundation of China (grant no. 81620108030), the 3-year Action Plan of Shanghai Municipal Committee of Health and Family Planning (grant no. ZY3-CCCX-2-1002), the Shanghai Rising-Star Project (grant no. 15QA1403500) and the Shanghai Talents Development Fund Project (grant no. 2017090).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
GJ and TW designed the research. TW and TL performed the in vitro experiment. TW and LJX performed statistical analysis. TW wrote the paper. GJ revised the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study is in vitro study, thus no Ethics approval and consent to participate is provided.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interests.
References
- 1.Morgan TR. Treatment of alcoholic liver disease. Gastroenterol Hepatol (N Y) 2017;13:425–427. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease C and Prevention (CDC): Alcohol-attributable deaths and years of potential life lost-United States, 2001. MMWR Morb Mortal Wkly Rep. 2004;53:866–870. [PubMed] [Google Scholar]
- 3.Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR, Goel A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315–1326. doi: 10.1136/gutjnl-2011-301846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CL, Chen YH, Tai MC, Liang CM, Lu DW, Chen JT. Resveratrol inhibits transforming growth factor-β2-induced epithelial-to-mesenchymal transition in human retinal pigment epithelial cells by suppressing the Smad pathway. Drug Des Devel Ther. 2017;11:163–173. doi: 10.2147/DDDT.S126743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimowska M. Signaling pathways of transforming growth factor beta family members. Postepy Biochem. 2006;52:360–366. (In Polish) [PubMed] [Google Scholar]
- 6.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Chen JM, Wang L, Xing LJ. Regulatory effects of Qinggan Huoxue Recipe on matrix metalloproteinases of alcoholic liver fibrosis rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31:1538–1544. (In Chinese) [PubMed] [Google Scholar]
- 8.Wu T, Liu T, Zheng PY, Xing LJ, Ji G. Effects of Qinggan Huoxue Recipe and its separated recipes on the expression of tumor necrosis factor-alpha in rats with alcoholic liver injury. Zhong Xi Yi Jie He Xue Bao. 2008;6:1145–1151. doi: 10.3736/jcim20081108. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 9.Wu T, Liu T, Zhang L, Xing LJ, Zheng PY, Ji G. Chinese medicinal formula, Qinggan Huoxue Recipe protects rats from alcoholic liver disease via the lipopolysaccharide-Kupffer cell signal conduction pathway. Exp Ther Med. 2014;8:363–370. doi: 10.3892/etm.2014.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu T, Chen JM, Xiao TG, Shu XB, Xu HC, Yang LL, Xing LJ, Zheng PY, Ji G. Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway. World J Gastroenterol. 2016;22:4695–4706. doi: 10.3748/wjg.v22.i19.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Zhang Y, Bai R, Wang M, Du S. Baicalin attenuates alcoholic liver injury through modulation of hepatic oxidative stress, inflammation and sonic hedgehog pathway in rats. Cell Physiol Biochem. 2016;39:1129–1140. doi: 10.1159/000447820. [DOI] [PubMed] [Google Scholar]
- 12.Wang CY, Ma FL, Liu JT, Tian JW, Fu FH. Protective effect of salvianic acid a on acute liver injury induced by carbon tetrachloride in rats. Biol Pharm Bull. 2007;30:44–47. doi: 10.1248/bpb.30.44. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Wang Y, Liu J, Wang K, Guo X, Ji B, Wu W, Zhou F. Correction to protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory and anti-apoptotic mechanisms. J Agric Food Chem. 2016;64:8463. doi: 10.1021/acs.jafc.6b04807. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Li S, Zhang H, Wang G, Xu G, Zhang H. Saikosaponin A protects against experimental sepsis via inhibition of NOD2-mediated NF-κB activation. Exp Ther Med. 2015;10:823–827. doi: 10.3892/etm.2015.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 17.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Zeng L, Zhao Y, Zhu B, Ren W, Wu C. Selenium suppresses lipopolysaccharide-induced fibrosis in peritoneal mesothelial cells through inhibition of epithelial-to-mesenchymal transition. Biol Trace Elem Res. 2014;161:202–209. doi: 10.1007/s12011-014-0091-8. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Liao R, Pan L, Fan K, Peng C, Du C. Hepatic stellate cell conditioned medium induces proliferation and epithelial-mesenchymal transition via activating ERK1/2 signaling pathway in hepatoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:210–214. (In Chinece) [PubMed] [Google Scholar]
- 20.Park JH, Park B, Park KK. Suppression of hepatic epithelial-to-mesenchymal transition by melittin via blocking of TGFβ/Smad and MAPK-JNK signaling pathways. Toxins (Basel) 2017;9 doi: 10.3390/toxins9040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carl C, Flindt A, Hartmann J, Dahlke M, Rades D, Dunst J, Lehnert H, Gieseler F, Ungefroren H. Ionizing radiation induces a motile phenotype in human carcinoma cells in vitro through hyperactivation of the TGF-beta signaling pathway. Cellular and molecular life sciences: CMLS. 2016;73:427–443. doi: 10.1007/s00018-015-2003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.e04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthusamy BP, Budi EH, Katsuno Y, Lee MK, Smith SM, Mirza AM, Akhurst RJ, Derynck R. ShcA protects against epithelial-mesenchymal transition through compartmentalized inhibition of tgf-β-induced smad activation. PLoS Biol. 2015;13:e1002325. doi: 10.1371/journal.pbio.1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong D, Zhang F, Shao J, Wu L, Zhang X, Chen L, Lu Y, Zheng S. Curcumin inhibits cobalt chloride-induced epithelial-to-mesenchymal transition associated with interference with TGF-β/Smad signaling in hepatocytes. Lab Invest. 2015;95:1234–1245. doi: 10.1038/labinvest.2015.107. [DOI] [PubMed] [Google Scholar]
- 25.Wang QL, Tao YY, Yuan JL, Shen L, Liu CH. Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biol. 2010;11:31. doi: 10.1186/1471-2121-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 27.Sciacovelli M, Goncalves E, Johnson TI, Wu L, Zhang X, Chen L, Lu Y, Zheng S. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;537:544–547. doi: 10.1038/nature19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia W, Ma X, Li X, Dong H, Yi J, Zeng W, Yang Z. miR-153 inhibits epithelial-to-mesenchymal transition in hepatocellular carcinoma by targeting Snail. Oncol Rep. 2015;34:655–662. doi: 10.3892/or.2015.4008. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Terrada D, Cheung SW, Finegold MJ, Knowles BB. Hep G2 is a hepatoblastoma-derived cell line. Human Pathol. 2009;40:1512–1515. doi: 10.1016/j.humpath.2009.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.