Abstract
The current study reveals the clinicopathological association of PD-L1 in Hong Kong esophageal squamous cell carcinoma (ESCC) patients and the differential regulation of PD-L1 by standard first-line chemotherapy in ESCC. Immunohistochemical analysis of tissue microarray data from 84 Hong Kong ESCC patients shows that PD-L1 was expressed in 21% of the tumors. Positive PD-L1 staining was significantly associated with later disease stage (stages III and IV) (P value = .0379) and lymph node metastasis (P value = .0466) in the Hong Kong cohort. Furthermore, PD-L1 expression was significantly induced in ESCC cell lines after standard chemotherapy treatments, along with EGFR and ERK activation in both in vitro studies and the in vivo esophageal orthotopic model. The endogenous expression of PD-L1 was reduced by treatment with an EGFR inhibitor (erlotinib) or by the knockdown of EGFR. Moreover, the upregulation of PD-L1 by chemotherapy was also attenuated by the treatment with erlotinib and a MAPK/MEK inhibitor (AZD6244), suggesting that PD-L1 is regulated by the EGFR/ERK pathway in ESCC. The regulation of PD-L1 by the EGFR pathway was further supported by the correlation of PD-L1 and EGFR expression observed in the commercially available tissue microarray set (P value = .028). Taken together, the current study was the first to demonstrate the upregulation of PD-L1 by chemotherapy in ESCC and its regulation through the EGFR/ERK pathway. The results suggest the potential usefulness of combined conventional chemotherapy together with anti–PD-L1 immunotherapy to achieve better treatment outcome.
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive and lethal cancers with a poor prognosis and a dismal survival rate [1]. Preoperative chemotherapy or chemoradiotherapy followed by surgery is widely utilized for patients with esophageal cancer. Unfortunately, 20% of patients do not respond to the treatment, while 50% of patients do not respond satisfactorily [2]. Resistance of cancer cells to chemotherapy remains a main obstacle for cancer treatment [3]. Development of combined treatment along with conventional chemotherapy may be useful to eradicate cancer cells that are unresponsive to chemotherapy and are expected to enhance the prognosis of patients. In recent years, the advancement in immunotherapy has shed the light on combining chemotherapy and immunotherapy and resulted in successful identification of immune checkpoints, such as cytotoxic T-lymphocyte antigen 4 (CTLA4) and programmed death 1 (PD-1) receptor. PD-1 functions as an immunoinhibitory receptor in T cells and, along with its ligand PD-L1, plays an important role in evasion from the host immunosurveillance of cancer cells. By expressing PD-L1 at the surface, cancer cells engage with tumor-infiltrating lymphocytes and activate PD-1–mediated inhibitory effects on lymphocyte activity [4]. PD-L1 is overexpressed in various solid cancers, including breast, colon, gastric, lung, ovarian, and pancreatic cancers, and is generally categorized as a poor prognostic marker [5], [6]. By exploiting the interaction between PD-1 and PD-L1, antibodies targeting PD-L1 have been developed to interrupt the activation of the inhibitory signal of immune cells, which is triggered by the binding with PD-L1 located on cancer cells. MPDL3280A, which is a human anti–PD-L1 monoclonal antibody, has now been approved by the FDA for treating PD-L1–positive urothelial bladder cancer and non–small cell lung cancer [7].
The possibility of improving treatment outcomes by combining immunotherapy with conventional chemotherapy or radiotherapy is supported by the studies showing that PD-L1 expression is induced by chemotherapy. For example, it is demonstrated that PD-L1 expression increased after the treatment with cisplatin/carboplatin in urothelial carcinoma [8]. Also, paclitaxel induced PD-L1 in the human colon adenocarcinoma cell line SW480 and the hepatocellular carcinoma cell line HepG2 [9]. There are only few studies that investigate the dysregulation of PD-L1 in response to chemotherapy in different cancer types. Thus, the mechanism for how ESCC responds to chemotherapy in terms of PD-L1 expression and, in particular, to see whether the PD-L1 level will increase in response to chemotherapy, as observed in other cancer types, remains to be further elucidated. Whether any genetic alterations in ESCC cause dysregulation of PD-L1 and how this affects PD-L1 expression are still unclear.
To seek the answers to the above questions, we investigated the PD-L1 expression in ESCC patients to evaluate the significance of PD-L1 in ESCC. To scrutinize the differential regulation of PD-L1 expression by chemotherapy agents in ESCC, both an in vitro ESCC cell culture model and in vivo esophageal orthotopic model were utilized. The change in PD-L1 expression level in response to carboplatin plus paclitaxel and 5-FU with cisplatin, which are two regimens that are currently used clinically for ESCC patients, was evaluated. The regulatory mechanism of the endogenous PD-L1, as well as the mechanism that is responsible for the differential regulation of PD-L1 by chemotherapy in ESCC, was studied using pathway inhibitors.
Materials and Methods
Cell Lines and Cell Culture
Two ESCC cell lines, KYSE150, which originated from a Japanese patient [10], and SLMT, which originated from a Hong Kong patient [11], were used in the in vitro studies. For the in vivo drug treatment experiment, KYSE150Luc, which was labeled with firefly luciferase, was used. The cell lines were cultured as described previously and were checked for mycoplasma contamination prior to usage [12].
In Vitro Drug Treatment
The concentrations of drugs used in the in vitro drug experiment were determined by IC50 experiments. The concentration that kills half of the cells was chosen for the subsequent in vitro assays. For the carboplatin with paclitaxel experiment, 350 μM carboplatin with 35 μM paclitaxel was used in KYSE150, and 25 μM carboplatin with 1 μM paclitaxel was used in SLMT. For the 5-FU with cisplatin study, 500 μM 5-FU and 30 μM cisplatin were used for KYSE150, while 25 μM 5-FU and 5 μM cisplatin were used for SLMT. To study the effect on EGFR and ERK activation on PD-L1 expression, 500 μM erlotinib was used to inhibit EGFR activation [13], and 10 μM AZD6244 was used to inhibit the MEK pathway [14] in both cell lines.
Knockdown of EGFR by CRISPR/Cas9 Gene Editing
The lentiCRISPR genome (Addgene plasmid 49535) was used for the knockdown assay of EGFR in cell line as previously described [15]. Target genomic sequences and cloning primers were designed using CRISPR Design (http://crispr.mit.edu). Nontarget scrambled sequence (sequence: GTTCCGCGTTACATAACTTA) was used as negative control. The sequences for sgRNA are listed in Supplementary Table S1.
Tissue Microarray (TMA) Analysis
Commercially available tissue microarrays (US Biomax; HEso-Squ180Sur-01 and HEso-Squ180Sur-02) consisting of specimens from 188 ESCC patients with 180 matched nonneoplastic and tumor samples were used to compare PD-L1 expression between ESCC tissue and the nonneoplastic esophageal tissue. It was also used to investigate the correlation between PD-L1 and EGFR expression. To study the association of PD-L1 expression level with clinicopathological parameters among the Chinese population, an in-house ESCC TMA consisting of 84 tumor samples from Chinese patients recruited in Queen Mary Hospital, Hong Kong was constructed. The clinicopathological information of this TMA is summarized in Table 1. The sample was considered as PD-L1 or EGFR positive when more than 5% of the tumor cells were positively stained.
Table 1.
Correlation of PD-L1 Expression and Clinicopathologic Features
| PD-L1 |
||||
|---|---|---|---|---|
| Negative n=66 |
Positive n=18 |
P Value | ||
| Age (years old) | <=62 | 34 | 8 | .595 |
| >62 | 32 | 10 | ||
| Sex | Male | 55 | 17 | .447 |
| Female | 11 | 1 | ||
| Stage | Early (I, II) | 28 | 3 | .0379 |
| Late (III, IV) | 38 | 15 | ||
| Differentiation | Well | 19 | 6 | .718 |
| Middle | 29 | 6 | ||
| Poor | 18 | 6 | ||
| Location | Cervical | 4 | 0 | .592 |
| Upper | 6 | 3 | ||
| Middle | 28 | 8 | ||
| Lower | 28 | 7 | ||
| LN metastasis | Negative | 33 | 5 | .0466 |
| Positive | 33 | 13 | ||
| Distant metastasis | Negative | 61 | 17 | .6193 |
| Positive | 5 | 1 | ||
Western Blotting
Western blot analyses were performed as described previously [12]. Briefly, cells were seeded and treated with chemotherapeutic drugs or erlotinib. After drug treatment, the cells were lysed with radioimmunoprecipitation assay buffer with protease inhibitors and phosphatase inhibitors added. All the experiments were performed in triplicate, and the representative figures are presented. Antibodies used in this study are summarized in Supplementary Table S2.
Orthotopic In Vivo Mouse Model
The assay was performed as previously described [16]. Briefly, 8×105 cells of luciferase-labeled KYSE150luc cells were injected into the esophagus of BALB/cAnN-nu (nude) mice. The growth of the tumor was monitored by measuring the bioluminescent signal every week using the Xenogen IVIS 100 In vivo Imaging System (PerkinElmer). Mice were randomized to treatment group and control group for each treatment experiment. Drugs were administered to the mice after the tumors reached the signal of 1×105 p/s/cm2/sr. For the carboplatin with paclitaxel study, 25 mg/kg carboplatin with 10 mg/kg paclitaxel was administered to the mice intraperitoneally three times per week. For the 5-FU with cisplatin study, 25 mg/kg 5-FU and 3 mg/kg cisplatin were administered to the mice intraperitoneally three times per week. For the erlotinib study, 200 mg/kg erlotinib (epidermal growth factor receptor inhibitor) was given via the oral route three times a week. The body weight of the mice was measured regularly, and the health of the mice was monitored continuously. Mice were sacrificed and the orthotopic tumors were excised for the evaluation of PD-L1 expression level by Western blotting and immunohistochemical (IHC) staining after one week of drug treatment.
Paraffin Embedding and Immunohistochemical Staining
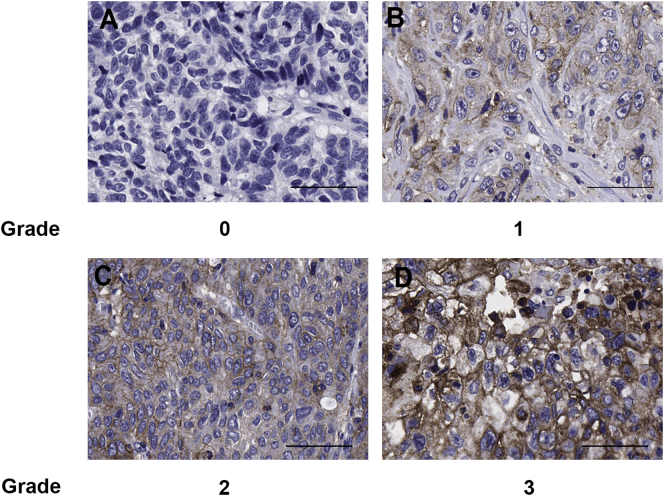
Excised mouse tumors were paraffin embedded as previously described [17]. For immunohistochemical staining, antigen retrieval was performed by heat-induced epitope retrieval with Tris EDTA buffer. PD-L1 staining was performed with anti–PD-L1 antibody at a 1:100 dilution. Detection was performed with SIGMAFAST 3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA). The positivity of the IHC staining was represented by the percentage of positively stained cells using an intensity score with grade 0 <5%, grade 1 5%-≤25%, grade 2 25%-≤50%, and grade 3 >50%, as evaluated by a pathologist as has been previously described [18].
Statistical Analysis
The chi-square or Fisher exact test was used to determine the associations between PD-L1 expression and clinicopathologic parameters. The Student’s t test was used for cell line experiments, and Kaplan-Meier analysis was performed for the survival study. A P value less than .05 was considered as statistically significant. All statistical analyses were carried out using IBM SPSS Statistics 24 (New York, USA).
Results
PD-L1 Is Expressed in ESCC Tumors and Its Expression Was Correlated with Advanced Disease Stage and Lymph Node Metastasis
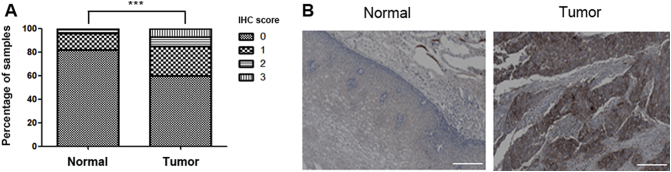
The dysregulation of PD-L1 in ESCC was determined with a set of commercially available ESCC microarray slides containing both the tumor tissues and their matched nonneoplastic esophageal tissue. Remarkably, the expression level of PD-L1 is significantly higher in the tumor compared to the nonneoplastic tissues, which show little or no PD-L1 staining in most of the patients, indicating that PD-L1 was upregulated during tumorigenesis in ESCC (Figure 1; P value = .00001). Since there was insufficient clinicopathological information available with this commercial TMA for further analysis, a set of in-house ESCC TMA slides including 84 tumor samples from Chinese ESCC patients was utilized in order to address the correlation of PD-L1 expression level with clinicopathological parameters among the Chinese population (Figure 2). IHC staining indicates that the PD-L1–positive rate was 21% (18/84). Furthermore, positive PD-L1 staining were associated with advanced disease state (stages III and IV; P value = .0379) and lymph node metastasis (P value = .0466) as determined by chi-square analysis (Table 1).
Figure 1.
PD-L1 was overexpressed in ESCC tumors compared to the matched nonneoplastic esophageal tissue. (A) The expression of PD-L1 in ESCC tumors and their matched nonneoplastic esophageal tissues for 188 patients was detected by staining with anti–PD-L1 antibody, and each sample was scored with the scale of 0, 1, 2, or 3 according to the staining intensity and abundance. The expression of PD-L1 was demonstrated to be significantly higher in the tumors by chi-square test. *** indicates P value = .00001 (B) Representative PD-L1 immunochemical staining in tumor and its adjacent nonneoplastic esophageal mucosa. Scale bar represents 200 μm.
Figure 2.
Representative images of PD-L1 staining of ESCC tumors with (A) grade 0, (B) grade 1, (C) grade 2, and (D) grade 3 in the Hong Kong patients used for TMA analysis. Scale bar represents 50 μm.
Chemotherapy Induces PD-L1 Expression in ESCC Cell Lines in Both In Vitro and In Vivo Orthotopic Models
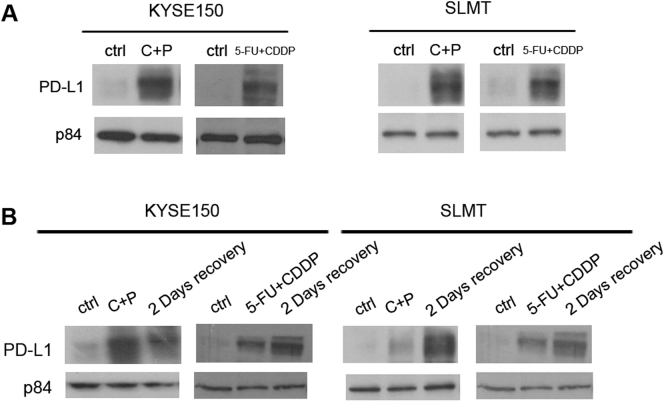
To study the differential expression of PD-L1 induced by standard chemotherapy treatments in ESCC, two ESCC cell lines, KYSE150 and SLMT, were treated with two chemotherapy regimens, 5-FU plus cisplatin and carboplatin plus paclitaxel, which are frequently used as standard chemotherapy for ESCC clinically. After exposing to the chemotherapeutic agents, the expression level of PD-L1 in both cell lines increased drastically for both carboplatin plus paclitaxel and 5-FU plus cisplatin treatment (Figure 3A).
Figure 3.
Chemotherapy induced PD-L1 expression in ESCC cell lines in vitro. (A) Two ESCC cell lines, KYSE150 and SLMT, were treated with carboplatin plus paclitaxel (C+P) or 5-FU plus cisplatin (5FU+CDDP) in vitro. PD-L1 expression increased in both cell lines after the treatment with both chemotherapeutic treatment regimens. (B) Elevated level of PD-L1 induced by chemotherapy was sustained even after drug removal in both KYSE150 and SLMT in vitro. Cells were first treated with chemotherapeutic drugs for 2 days. The drugs were then removed, and the cells were permitted to recover 2 days more prior to lysate preparation. p84 was used as the loading control in all Western blotting experiments.
In clinical settings, patients are given chemotherapy usually in cycles, which may be given on a single day or several consecutive days, rather than a continuous exposure to the chemotherapeutic agents. To study whether the elevated PD-L1 triggered by drug exposure is maintained after the treatment is suspended, ESCC cells were treated with carboplatin plus paclitaxel or 5-FU plus cisplatin for 2 days followed by the removal of drug and a recovery period of 2 days. Surprisingly, instead of a drop, the elevated level of PD-L1 in response to chemotherapy was sustained or even further increased after drug removal (Figure 3B), indicating that the rise in PD-L1 by chemotherapy could be maintained at a high level even after the cells are no longer exposed to the chemotherapy.
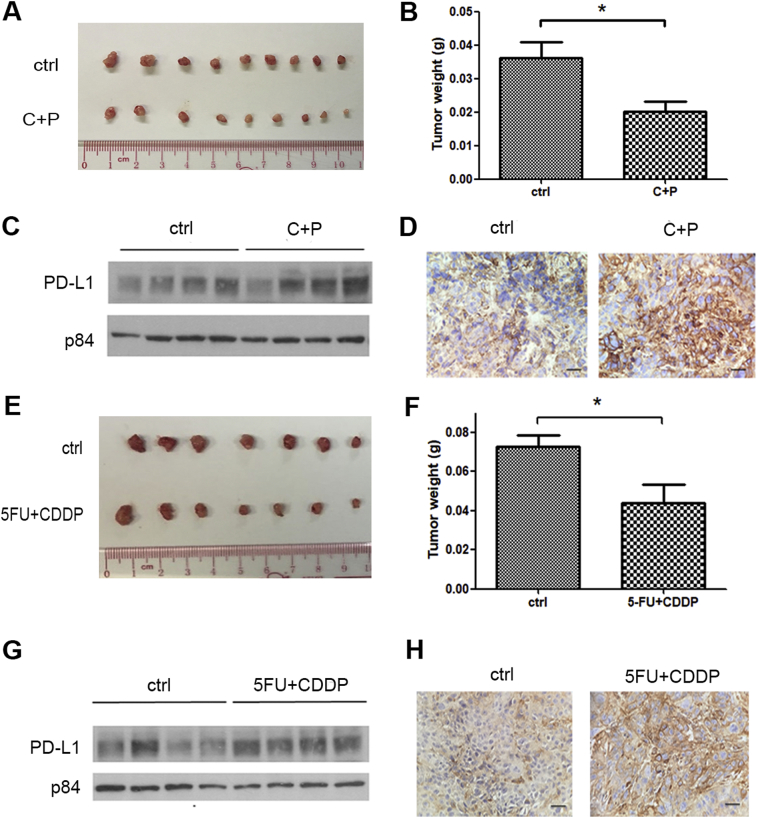
The induction of PD-L1 by chemotherapeutic agents was further tested by the in vivo study. An orthotopic in vivo nude mouse animal model was used to more closely resemble the tumor microenvironment for the study of the response to drug treatment. Carboplatin plus paclitaxel and 5-FU plus cisplatin treatments were administered to the mice after the orthotopic tumors were established. After 1 week of treatment, the mice were sacrificed and the orthotopic tumors were excised for the determination of PD-L1 by Western blotting and IHC staining. Expectedly, there was a statistically significant reduction of tumor size in the treatment group of both carboplatin plus paclitaxel (Figure 4, A and B) and 5-FU plus cisplatin (Figure 4, E and F) compared to the control, which indicates the effectiveness of the drug treatment. In concordance with our results using the in vitro model, the PD-L1 protein level was considerably higher in the treatment group compared to the control group for both treatments (Figure 4, C and D and G and H). The results further support the conclusion that the expression of PD-L1 is induced by chemotherapy in ESCC.
Figure 4.
Chemotherapy induced PD-L1 expression in esophageal orthotopic tumors. KYSE150 cells were injected into the mouse esophagus to form solid tumors, and the mice were randomized to treatment and control groups. After 1 week of treatment, the mice were sacrificed, and PD-L1 expression was evaluated by Western blotting and IHC staining. Carboplatin plus paclitaxel treatment (C+P) reduced (A) tumor size and (B) tumor weight. PD-L1 expression in the KYSE150 orthotopic tumors was higher in the C+P-treated group compared to the control group (ctrl), as demonstrated by (C) Western blotting and (D) IHC staining. Similarly, 5-FU plus cisplatin treatment also reduced (E) tumor size and (F) tumor weight. PD-L1 expression was also induced by 5-FU plus cisplatin treatment as detected in (G) Western blotting and (H) IHC staining. Each lane in C and G represents an individual orthotopic tumor from different mice. The scale bars in D and H represent 25 μm. * indicates a P value < .05.
PD-L1 Expression Was Regulated by EGFR and Its Downstream Modulator ERK
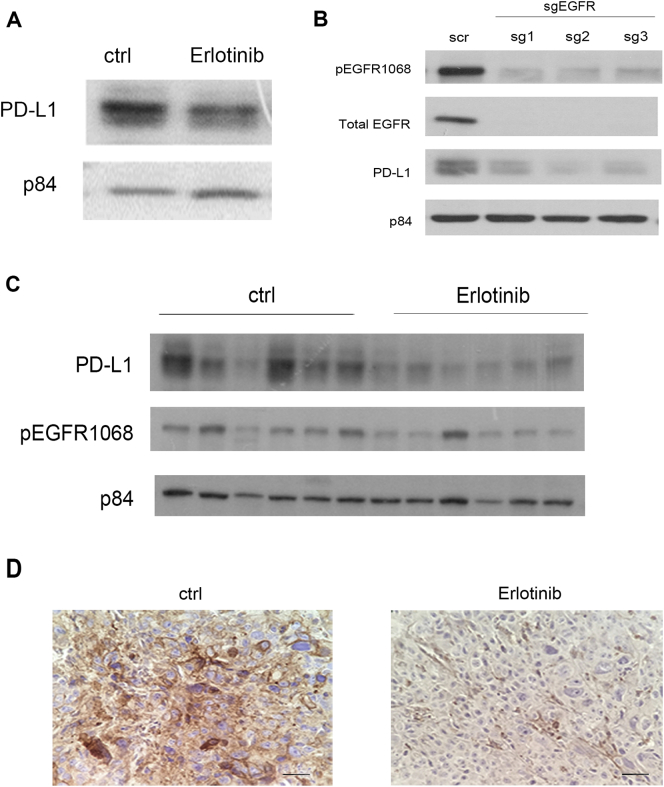
The finding that PD-L1 is upregulated by an activating mutation in EGFR in non–small cell lung cancer (NSCLC) links the association of PD-L1 expression with the EGFR pathway and prompted us to study the regulation of PD-L1 by EGFR signaling pathway in ESCC [19]. Erlotinib, which is an FDA-approved EGFR inhibitor for NSCLC, was used in this study to evaluate the influence of inhibiting EGFR activation on PD-L1 expression level. After KYSE150 cells were treated with erlotinib, the expression of PD-L1 was reduced notably (Figure 5A), suggesting that the expression of PD-L1 is regulated by the EGFR pathway. Moreover, the reduction of PD-L1 expression was also observed when EGFR expression was knocked down by CRISPR-Cas9 gene editing (Figure 5B). To further support this notion, the effect of EGFR inhibitor on PD-L1 expression level was further tested in the in vivo orthotopic mouse model. Erlotinib treatment was administered to the mice, and the change in PD-L1 level in the orthotopic tumors was evaluated. In agreement with the in vitro study, the expression of PD-L1, along with the phosphorylation status of EGFR, was lower in the erlotinib-treated group compared to the control group (Figure 5, C and D), although the treatment did not affect the tumor size (Supplementary Figure 3). The ESCC cell line, SLMT, was not used for this experiment, as the endogenous level of PD-L1 in SLMT cells was only barely detectable by Western blotting and, therefore, SLMT cells were not suitable for the demonstration of the reduction effect of erlotinib on PD-L1 level.
Figure 5.
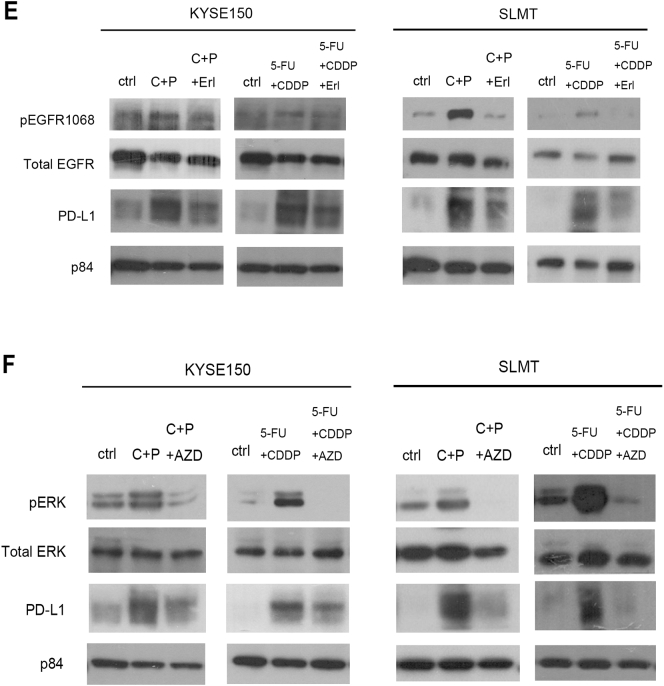
EGFR and its downstream ERK pathway regulate endogenous PD-L1 expression and the upregulation of PD-L1 induced by chemotherapy. (A) Western blotting results showed that the inhibition of the EGFR activation by the treatment of erlotinib reduced endogenous PD-L1 level in KYSE150 in vitro. (B) The expression of PD-L1 was significantly reduced when EGFR was knocked down by CRISPR-Cas9 gene editing. Nontargeting scrambled sequence (scr) was used as control. In vivo orthotopic mouse results also demonstrated that PD-L1 expression was lower in the erlotinib-treated group compared to the control (ctrl) group, as demonstrated by (C) Western blotting and (D) IHC staining. Each lane in C represents an individual orthotopic tumor from different mice. Scale bar in D represents 25 μm. (E) The addition of erlotinib (Erl) attenuated the upregulation of PD-L1 induced by carboplatin plus paclitaxel treatment (C+P) and 5-FU plus cisplatin treatment (5-FU+CDDP) in both KYSE150 and SLMT. (F) Inhibition of ERK by AZD6244 (AZD) reduced the increase in PD-L1 level in response to chemotherapy treatment in KYSE150 and SLMT.
The concurrent upregulation of phospho-EGFR and PD-L1 by chemotherapy further suggests the regulation of PD-L1 by EGFR. To investigate whether the increase in PD-L1 level induced by chemotherapeutic agents is also regulated by the EGFR pathway, erlotinib was used to suppress the upregulation of EGFR activation triggered by chemotherapy. After the cells were exposed to erlotinib, the upregulation in PD-L1 induced by 5-FU plus cisplatin or carboplatin plus paclitaxel treatment was attenuated when the EGFR activation was inhibited in both KYSE150 and SLMT cell lines (Figure 5E). The EGFR pathway impacts several downstream signaling pathways, including PI3K/AKT, MEK/ERK, and Src/STAT effector pathways. The upregulation of phospho-ERK in response to chemotherapy provides us the hint to investigate the effect of ERK activation in PD-L1 expression. AZD6244 (selumetinib), which is a MEK inhibitor, was used to suppress the activation of ERK induced by the chemotherapeutic treatments. The upregulation of PD-L1 by chemotherapy was attenuated when the activation of ERK was inhibited (Figure 5F), indicating that the upregulation of PD-L1 by chemotherapy is regulated by the ERK pathway.
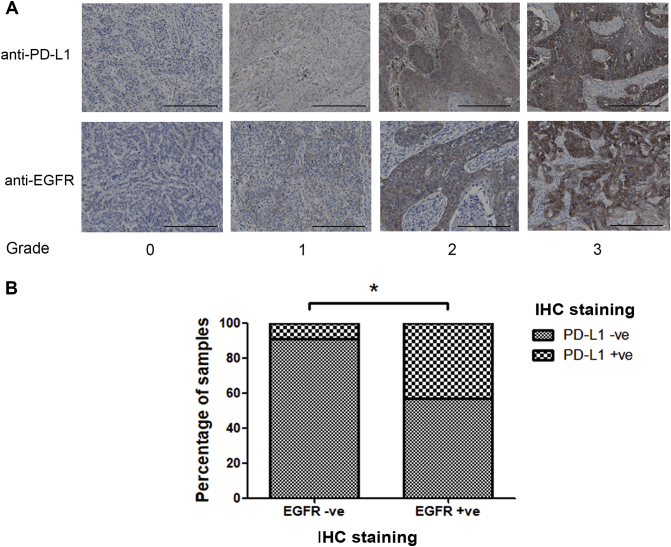
The regulation of PD-L1 by EGFR expression is further supported by the TMA analysis showing that the expression of PD-L1 was positively correlated with EGFR in ESCC patients samples (P value = .028), as 43% of the EGFR-positive samples are PD-L1 positive compared to only 9% of the EGFR-negative PD-L1–positive samples (Figure 6B). Taken together with the in vitro and in vivo results, these observations suggest that PD-L1 expression is controlled by the EGFR pathway in ESCC.
Figure 6.
The expression of PD-L1 was correlated with the expression of EGFR in the commercial TMA set of 188 ESCC samples. The TMA set was stained with anti–PD-L1 and anti-EGFR antibodies. (A) Representative images of different grades anti–PD-L1 and anti-EGFR staining scores in the TMA analysis. The scale bar represents 200 μm. (B) Chi-square analysis shows that the expression of PD-L1 was significantly correlated with the EGFR expression. * indicates P value < .05.
Discussion
Currently, chemotherapy remains as the adjuvant therapy for patients with ESCC. However, the development of chemoresistance remains the main obstacle for successful treatment outcome. Therefore, it is crucial to understand the biological changes in response to chemotherapy that lead to chemoresistance for designing the optimal treatment regimen. To the best of our knowledge, this is the first study that scrutinizes the differential regulation of PD-L1 by chemotherapy and that determines its regulatory mechanism in ESCC. The discovery of PD-L1 as an immune checkpoint and a druggable target is one of the major breakthroughs in oncology in the past decade. Specifically, the anti–PD-1/PD-L1 therapy has generated excitement in a number of clinical trials for various cancers such as melanoma [20]. The importance of PD-L1 in tumor development is demonstrated by its overexpression in a wide range of solid tumors. In ESCC, it has previously been reported that 18.9% to 79.7% of ESCC tumors are PD-L1 positive [21], [22], [23], [24], [25]. In this study, we demonstrate that 21% of the tumors are PD-L1 positive among the patients with ESCC in Hong Kong. The differences observed in the positivity rate of PD-L1 in ESCC may be attributed to the antibodies used in the IHC staining and the scoring criteria for the staining. The positivity rate of PD-L1 is also dependent on whether the patients received any treatment before sample collection since chemotherapy and radiotherapy induce PD-L1 expression, as demonstrated by our current study and other reports [8], [26], [27]. Previous studies demonstrate that high PD-L1 expression is associated with advanced disease and lymph node metastasis in various cancers [28], [29], [30]. It is uncertain whether such association is also observed in ESCC. Our results show that PD-L1 expression is correlated with disease stage and lymph node metastasis, as previously reported [22], [23]. Although other reports suggest that PD-L1 positivity is correlated with poor survival, our results did not show any significant correlation between PD-L1 and survival (Supplementary Figure 1). Interestingly, there is another ESCC study reporting the opposite correlation, as patients with PD-L1 expression had a longer disease-free survival than the patients without PD-1 expression [24]. Further studies are needed to elucidate the correlation between PD-L1 and other clinical parameters in different populations.
Studies illustrate that the upregulation of the PD-1/PD-L1 axis promotes chemoresistance in various cancers, such as gastric cancer and B-cell lymphoma [31], [32], [33], [34]. One possible explanation is that PD-L1–expressing cells have a survival advantage against chemotherapy [35], and this selected population increases after chemotherapy, leading to the development of chemoresistance. Whether PD-L1 is differentially regulated by chemotherapy and facilitates chemoresistance in ESCC remains unclear. To address this, in this study, we investigated the change in PD-L1 level after exposing the cells or animal model to chemotherapy. Two chemotherapy treatment regimens, carboplatin plus paclitaxel and 5-FU plus cisplatin, were used in this study since they are currently the major treatment ESCC regimens in Hong Kong public hospitals. In contrast to previous reports, which mostly consider the effect of a single chemotherapeutic agent, a combination of chemotherapeutic agents was used in this study, which more closely resembles the clinical situation. Our results reveal that PD-L1 expression increases after the cells were exposed to both chemotherapeutic agents for the ESCC KYSE150 and SLMT cell lines, as observed in other cancer types. Intriguingly, although the two cell lines contain a huge difference in the endogenous level of PD-L1, with KYSE150, which contains a significantly higher level of endogenous PD-L1 than SLMT, both cell lines respond similarly to chemotherapy by upregulating the PD-L1 expression. This indicates that the response in the increased level of PD-L1 induced by chemotherapy is independent of the endogenous PD-L1 level and is not constrained in cells with high endogenous PD-L1 level. To validate the results observed in vitro, our esophageal orthotopic mouse model was utilized to study the change of PD-L1 expression by chemotherapy in vivo. To the extent of our knowledge, this is the first study employing such a model to study the change of PD-L1 to drug response. Compared to conventional subcutaneous nude mouse tumorigenicity model, the orthotopic ESCC model recapitulates more closely the microenvironment of the tumor in its organ of origin and is more reflective of the actual response observed in the esophageal tumor. Thus, the ESCC orthotopic model was used as it is a better choice for drug response study due to the unique tumor microenvironment [16], [36]. Consistent with the in vitro study, the in vivo study also shows that the treatment of carboplatin plus paclitaxel and 5-FU plus cisplatin caused an induction of PD-L1 level in the ESCC orthotopic tumors, as demonstrated by the Western blotting and IHC staining results. In a clinical setting, patients received chemotherapy in a cyclical manner rather than by continuous exposure. This study demonstrates that the elevated level of PD-L1 on cancer cells induced by chemotherapy is sustained at a high level even after the removal of the chemotherapeutic agents, indicating that the increase of PD-L1 is long-lasting and may still be present for the action of the immune checkpoint inhibitors. Thus, clearly, the exposure to the inhibitors may remain beneficial to the patient even after termination of the chemotherapy.
There is accumulating knowledge regarding the regulation of PD-L1 by various oncogenic pathways, including the EGFR pathway. Studies demonstrate that the EGFR activation, by EGF stimulation or mutation, upregulates PD-L1 in lung cancer [19], [37]. However, whether PD-L1 is regulated by the EGFR pathway in ESCC, as well as the regulatory mechanism of the change in PD-L1 expression by chemotherapy, is unexplored. The simultaneous upregulation of EGFR phosphorylation with PD-L1 observed in our chemotherapy experiments leads to the postulation that PD-L1 is regulated by the EGFR pathway in ESCC. This postulation was tested by treating KYSE150 cells, having high endogenous PD-L1 levels, with an EGFR inhibitor, erlotinib, to inhibit EGFR activation. As demonstrated in both in vitro and in vivo models, erlotinib treatment reduced PD-L1 expression level, as observed in an earlier study using a lung cancer cell line [38]. The regulation of PD-L1 by EGFR in ESCC is further supported by the reduction of PD-L1 expression observed in EGFR knockdown cells. Apart from the endogenous expression, erlotinib also attenuated the increase in PD-L1 induced by chemotherapy in both KYSE150 and SLMT cell lines. Our finding suggests that with both the endogenous PD-L1 level as well as the induction of PD-L1 by chemotherapy, PD-L1 is regulated by the EGFR activation in ESCC. Recently, various reports have shown that the phospho-MAPK/ERK kinase upregulation along with PD-L1 upregulation after chemotherapy and the inhibition of MEK pathway attenuated PD-L1 upregulation in lung cancer and head and neck cancer [39], [40], [41]. In concordance with this, our results illustrate an increase in the ERK phosphorylation along with the induction of PD-L1, after chemotherapy treatment, and its inhibition by AZD6244 attenuated the upregulation of PD-L1 in ESCC cells, indicating the regulation of PD-L1 expression by the MAPK/ERK pathway, likely as a downstream effector of EGFR activation.
Our study provides novel evidence that links PD-L1 expression with chemotherapy in ESCC. In conclusion, we demonstrate that PD-L1 is induced by chemotherapy and is regulated by the EGFR/ERK pathway in ESCC. Further studies are prompted to gain a better understanding of how PD-L1 regulates chemoresistance in ESCC. For instance, whether the increase in PD-L1 induced by chemotherapy affects the tumor behavior, such as metastasis, or the sensitivity to chemotherapy is of interest, as there are reports showing that PD-L1 affects tumor metastasis and chemosensitivity [32], [42]. The results from both in vitro and in vivo experiments suggest that use of a combination of conventional chemotherapy with immune checkpoint inhibitors may be more beneficial than using a single therapy, as the chemotherapy induces PD-L1 expression, which may render the cancer cells more susceptible to immunotherapy. Accumulating evidence indicates that conventional chemotherapy modulates the composition and functionality of the immune infiltrates and affects its outcome [43]. The upregulation of PD-L1 by chemotherapy may allow the tumor escape from immune surveillance, as tumor PD-L1 plays critical roles in immunosuppression by inhibiting CD8 T-cell cytotoxicity [44]. This may be a contributing factor to the failure of conventional chemotherapy. Furthermore, our results suggest that the induction of PD-L1 by chemotherapy is independent of the endogenous level of PD-L1. Unlike the use of anti–PD-L1 treatment, whose effectiveness depends on the endogenous level of PD-L1 in the tumor [45], the combinational treatment may be applicable to a larger proportion of patients regardless of the initial PD-L1 level before treatment. Moreover, this current study provides greater insight regarding the mechanistic regulation of PD-L1 by the EGFR/ERK pathway in ESCC, which was not reported previously. Further study is needed to demonstrate the effectiveness of combining the use of the anti–PD-L1 with pathway inhibitors in treating ESCC. This current study now provides foundational evidence to support the use of combinational treatment of immunotherapy with conventional chemotherapy or an oncogenic pathway inhibitor to achieve enhanced treatment outcome.
Acknowledgements
This project was funded by the Innovation and Technology Fund from Innovation and Technology Commission and matching fund from Lee’s Pharmaceutical (Hong Kong) Limited. We thank Dr. Johnny Tang, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hong Kong, for providing the SLMT cell line and DSMZ (German Collection of Microorganisms and Cell Culture) for the KYSE150 cell line. We acknowledge the Core Facility of the Li Ka Shing Faculty of Medicine, The University of Hong Kong, for providing the IVIS Spectrum In Vivo Imaging System.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2018.08.005.
Appendix A. Supplementary data
Supplementary material
References
- 1.Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. 2013. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC. CancerBase No. 11. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Plukker JTM, Coppes RP. Cancer stem cells with increased metastatic potential as a therapeutic target for esophageal cancer. Semin Cancer Biol. 2017;44:60–66. doi: 10.1016/j.semcancer.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Lacaci I, García Morales P, Soto JL, Saceda M. Tumour cells resistance in cancer therapy. Clin Transl Oncol. 2007;9(1):13–20. doi: 10.1007/s12094-007-0004-9. [DOI] [PubMed] [Google Scholar]
- 4.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207. doi: 10.1038/nrendo.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P, Wu D, Li L, Chai Y, Huang J, Wang W. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 7.Xue S, Hu M, Iyer V, Yu J. Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol. 2017;10(1):81–90. doi: 10.1186/s13045-017-0455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcdaniel AS, Alva A, Zhan T, Xiao H, Cao X, Gursky A, Siddiqui J, Chinnayan AM, Jiang H, Lee CT. Expression of PDL1 (B7-H1) before and after neoadjuvant chemotherapy in urothelial carcinoma. Eur Urol Focus. 2016;1(2):265–268. doi: 10.1016/j.euf.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Gong W, Song Q, Lu X, Gong W, Zhao J, Min P, Yi X. Paclitaxel induced B7-H1 expression in cancer cells via the MAPK pathway. J Chemother. 2011;23(5):295–299. doi: 10.1179/joc.2011.23.5.295. [DOI] [PubMed] [Google Scholar]
- 10.Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69(2):277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Tang JC, Wan TS, Wong N, Pang E, Lam KY, Law SY, Chow LM, Ma ES, Chan LC, Wong J. Establishment and characterization of a new xenograft-derived human esophageal squamous cell carcinoma cell line SLMT-1 of Chinese origin. Cancer Genet Cytogenet. 2001;124(1):36–41. doi: 10.1016/s0165-4608(00)00317-4. [DOI] [PubMed] [Google Scholar]
- 12.Ng HY, Ko JM-Y, Yu VZ, Ip JCY, Dai W, Cal S. DESC1, a novel tumor suppressor, sensitizes cells to apoptosis by downregulating the EGFR/AKT pathway in esophageal squamous cell carcinoma. Int J Cancer. 2016;138(12):2940–2951. doi: 10.1002/ijc.30034. [DOI] [PubMed] [Google Scholar]
- 13.Schettino C, Bareschino MA, Ricci V, Ciardiello F. Erlotinib: An EGF receptor tyrosine kinase inhibitor in non–small-cell lung cancer treatment. Expert Rev Respir Med. 2008;2(2):167–178. doi: 10.1586/17476348.2.2.167. [DOI] [PubMed] [Google Scholar]
- 14.Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, Hatton C, Chopra R, Oberholzer PA, Karpova MB. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106(48):20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu VZ, Wong VC-L, Dai W, Ko JM-Y, Lam AK-Y, Chan KW, Samant RS, Lung HL, Shun WH, Law S. Nuclear localization of DNAJB6 is associated with survival of patients with esophageal cancer and reduces AKT signaling and proliferation of cancer cells. Gastroenterology. 2015;149(7):1825–1836.e5. doi: 10.1053/j.gastro.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Ip JC, Ko JM, Yu VZ, Lam AK, Law S, Tong DK, Lung ML. A versatile orthotopic nude mouse model for study of esophageal squamous cell carcinoma. Biomed Res Int. 2015;2015:910715. doi: 10.1155/2015/910715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung AK, Ip JC, Chu AC, Leong MM, Ko JM, Shuen WH, Lung HL, Lung ML. PTPRG suppresses tumor growth and invasion via inhibition of Akt signaling in nasopharyngeal carcinoma. Oncotarget. 2015;6(15):13434–13447. doi: 10.18632/oncotarget.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis. 2017;8(8) doi: 10.1038/cddis.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, He X, Zhou T, Qin T. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 20.Frydenlund N, Mahalingam M. PD-L1 and immune escape: insights from melanoma and other lineage-unrelated malignancies. Hum Pathol. 2017;66:13–33. doi: 10.1016/j.humpath.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Lo AWI, Wong A, Chen W, Wang Y, Lin L, Xu J. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017;8(18):30175–30189. doi: 10.18632/oncotarget.15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito S, Okano S, Morita M, Saeki H, Tsutsumi S, Tsukihara H, Nakashima Y, Ando K, Imamura Y, Ohgaki K. Expression of PD-L1 and HLA class I in esophageal squamous cell carcinoma: prognostic factors for patient outcome. Ann Surg Oncol. 2016;23:508–515. doi: 10.1245/s10434-016-5376-z. [DOI] [PubMed] [Google Scholar]
- 23.Chen M-F, Chen P-T, Chen W-C, Lu M-S, Lin P-Y, Lee KDer. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. 2016;7(7):7913–7924. doi: 10.18632/oncotarget.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Cheng G, Zhang F, Zhang N, Li D, Jin J, Wu J, Ying L, Mao W, Su D. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget. 2016;7(21):30772–30780. doi: 10.18632/oncotarget.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leng C, Li Y, Qin J, Ma J, Liu X, Cui Y, Sun H, Wang Z, Hua X, Yu Y. Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8+ T cells. Oncol Rep. 2015;35(2):699–708. doi: 10.3892/or.2015.4435. [DOI] [PubMed] [Google Scholar]
- 26.Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim JH, Jeon YK, Kwon JS, Hah JH. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget. 2016;7(13):15901–15914. doi: 10.18632/oncotarget.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Der Kraak L, Goel G, Ramanan K, Kaltenmeier C, Zhang L, Normolle DP, Freeman GJ, Tang D, Nason KS, Davison JM. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. J Immunother Cancer. 2016;4(1):65. doi: 10.1186/s40425-016-0163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An HJ, Ko GH, Lee JH, Lee JS, Kim DC, Yang JW, Kim MH, Kim JP, Jung EJ, Song SH. Programmed death-ligand 1 expression and its correlation with lymph node metastasis in papillary thyroid carcinoma. J Pathol Transl Med. 2018;52(1):9–13. doi: 10.4132/jptm.2017.07.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin G, Fan X, Zhu W, Huang C, Zhuang W, Xu H, Lin X, Hu D, Xuang Y, Jiang K. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget. 2017;8(48):83986–83994. doi: 10.18632/oncotarget.20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y-L, Yang C-Y, Huang Y-L, Wu C-T, Yang P-C. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget. 2017;8(11):18021–18030. doi: 10.18632/oncotarget.14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Quan L, Zhang C, Liu A, Tong D, Wang J. Oncology letters. Oncol Lett. 2018:3321–3328. doi: 10.3892/ol.2017.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black M, Barsoum IB, Truesdell P, Cotechini T, Macdonald-Goodfellow SK, Petroff M, Siemens DR, Koti M, Craig AW, Graham CH. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget. 2016;7(9):10557–10567. doi: 10.18632/oncotarget.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, Liu X, An G, Zhang W, Zhang J. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016;107(11):1563–1571. doi: 10.1111/cas.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu N, Lv J, Qi W, Sun L, Guo J, Zhao S, Qiu W, Liang J. Programmed death 1 induces cell chemoresistance to 5-fluorouracil in gastric cancer cell lines. Transl Cancer Res. 2016;5(6):781–788. [Google Scholar]
- 35.Wu X, Li Y, Liu X, Cao S, Harrington S, Chen C, Mansfield A, Dronca R, Park S, Yan Y. bioRxiv; 2018. B7-H1(PD-L1) confers chemoresistance through ERK and p38 MAPK pathway in tumor cells; p. 308601. [Google Scholar]
- 36.Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17(3):279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 37.Inamura K, Yokouchi Y, Sakakibara R, Kobayashi MM, Subat S, Ninomiya H, Nagano H, Nomura K, Okumura S, Ishikawa Y. Relationship of tumor PD-L1 expression with EGFR wild-type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol. 2016;46(10):935–941. doi: 10.1093/jjco/hyw087. [DOI] [PubMed] [Google Scholar]
- 38.Im JS, Herrmann AC, Bernatchez C, Haymaker C, Molldrem JJ, Hong WK, Perez-Sole R. Immune-modulation by epidermal growth factor receptor inhibitors: implication on anti-tumor immunity in lung cancer. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ock C-Y, Kim S, Keam B, Kim S, Ahn Y-O, Chung EJ, Kim JH, Kim TM, Kwon SK, Jeon YK. Changes in programmed death-ligand 1 expression during cisplatin treatment in patients with head and neck squamous cell carcinoma. Oncotarget. 2017;8(58):97920–97927. doi: 10.18632/oncotarget.18542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han JJ, Kim DW, Koh J, Keam B, Kim TM, Jeon YK, Lee SH, Chung DH, Heo DS. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non–small-cell lung cancer. Clin Lung Cancer. 2016;17(4):263–270.e2. doi: 10.1016/j.cllc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non–small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–4021. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Chen L, Xiong Y, Zheng X, Xie Q, Zhou Q, Shi L, Wu C, Jiang J, Wang H. Knockdown of PD-L1 in human gastric cancer cells inhibits tumor progression and improves the cytotoxic sensitivity to CIK therapy. Cell Physiol Biochem. 2017;41(3):907–920. doi: 10.1159/000460504. [DOI] [PubMed] [Google Scholar]
- 43.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214(4):895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328) doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material