Abstract
The aim of the present study was to investigate whether the co-injection of hyaluronic acid (HA) and corticosteroids (CS) was superior to HA alone in the treatment of knee OA. A total of 120 participants with symptomatic knee OA were recruited and formed the intention-to-treat population for a 6-month follow-up. In the HA group, patients received a single-shot injection of 4 ml HA. In the HA&CS group, patients received a co-injection of 3 ml compound betamethasone solution and 4 ml HA. Visual analog scale (VAS), Western Ontario and McMaster University Osteoarthritis Index (WOMAC) and knee flexion motion were assessed as primary outcomes. Patients in the HA&CS group exhibited better pain relief and knee function at the time points of week 1, month 1 and month 3 (P<0.05). For the last follow-up at month 6, the values did not differ significantly between these two groups. Patients in both groups exhibited improvement in pain, knee function, and range of motion following injection. For the final follow-up at month 6, the mean VAS score, WOMAC score and knee flexion motion were still superior to that prior to treatment, but the values did not differ significantly. The co-injection of HA and CS provided a rapid improvement in pain relief, knee function, and range of motion, but did not differ significantly from that of HA alone in the long term effect.
Keywords: osteoarthritis, knee, intra-articular injection, hyaluronic acid, corticosteroids
Introduction
Osteoarthritis (OA) is one of the most prevalent chronic arthritic diseases and typically occurs in middle aged and elderly patients (1). A recent study revealed that OA is a leading cause of disability, with 10% of men and 13% of women over 60 years of age suffering from symptomatic OA of the knee (2). The incidence of OA is higher in women compared with men, and aging, obesity, genetics and biomechanical predisposing factors are risk factors for the initiation and progression of OA (3,4).
At present, the conservative therapies available for OA include oral analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and viscosupplementation, which provide short-term treatment effects (5). For patients with cardiovascular or gastrointestinal comorbidities, a number of systemic drugs, including analgesics and NSAIDs are not recommended due to their cardiovascular side effects (6). As such, injection of therapeutic agents directly into the OA joint may be more advantageous for reducing systemic complications.
A number of clinicians have confirmed that hyaluronic acid (HA) and corticosteroid (CS) supplementation is an effective means of controlling the symptoms of OA in the knee (7–9). However, the recent OA treatment guidelines from the American Academy of Orthopedic Surgeons strongly discourage the use of HA, while there is little conclusive evidence to support the use of CS injections (10). More clarity on the intra-articular supplementation of HA and CS should be determined by future studies regarding the discrepancies.
Recently, a number of studies have demonstrated that HA and CS injections are safe and effective treatments that can reduce pain and improve joint functionality in patients with OA of the knee (11–13). CS has been reported to be more effective in reducing acute pain compared with HA, due to its anti-inflammatory effect. However, the duration of pain relief is shorter in CS compared with HA (14). As such, the combined use of HA and CS may be more effective and have longer-lasting analgesic effects than either agent used alone. However, long-term use and a high frequency of injections of either agent may cause unnecessary injury and even infection in the arthritic joint. Furthermore, it has been reported that CS increases the apoptotic progression of cartilage cells when injected into the OA joint (15). Clinicians must therefore delicately balance the amount of CS used to avoid doing more damage than good.
The aim of the present study was to determine whether a single-shot co-injection of HA and CS resulted in a longer duration of pain relief and better functional improvement compared with the use of HA alone.
Materials and methods
Study design
The present study was designed as a single-center, prospective, randomized, double blind trial with parallel groups. The Ethics Committee of The Affiliated Zhongda Hospital of Southeast University (Nanjing, China) approved the present study prior to patient enrollment (approval no. 2017zdSYLL012-Y01). The study protocol was registered at ClinicalTrials.gov (registration no. NCT03047096). All enrolled subjects provided informed written consent prior to inclusion in the study. The investigation was performed at The Affiliated Zhongda Hospital of Southeast University (Nanjing, China) between February and November 2017.
Samples
Patients that presented themselves to the Department of Orthopaedics at The Affiliated Zhongda Hospital of Southeast University with knee pain were diagnosed radiographically and those who were suffering from knee OA with a duration of >3 months were graded as stage II–IV by a senior radiologist based on the Kellgren-Lawrence (KL) grade (16). Symptomatic knee OA was diagnosed based on the American Rheumatism Association classification criteria for knee OA (17). Patients were excluded if they had a diagnosis of rheumatoid arthritis or other inflammatory OA, trauma or pain-causing diseases, had received treatment with oral medications within 3 days, or had received physiotherapy or intra-articular injections of HA or CS within 6 months. Participants with severe diabetes were excluded due to the increased risk of developing serious side effects. Participants who were allergic to any of the medications used in the present study or were diagnosed with current systemic infection were also excluded.
Interventions
A total of 120 patients met the inclusion criteria and were randomized into two groups (n=60/group) via the following method. The age range of the enrolled patients was 45–80 years (mean, 63.05±6.40 years) and 75% of the patients were female (90/120). A computer-generated list of random numbers was used. This method created 120 study cards, which were titled as either HA&CS or HA. Each card was sealed in an opaque envelope. An assistant nurse opened each envelope and assigned patients to the corresponding group. The disposition of the patients in the present study was processed according to a Consolidated Standards of Reporting Trials flow diagram (Fig. 1). In the HA group, 4 ml high molecular weight (600,000–1,500,000 g/mol) HA (Shandong FREDA Pharmaceutical Industry Group Co., Ltd., Jinan, China) was administered to patients. In the HA&CS group, 4 ml HA was administered, followed by 3 ml compound betamethasone solution (Schering-Plough Labo NV, Heist-op-den-Berg, Belgium), which comprised 1 ml compound betamethasone (2 mg betamethasone sodium phosphate and 5 mg betamethasone dipropionate) and 2 ml 0.9% normal saline.
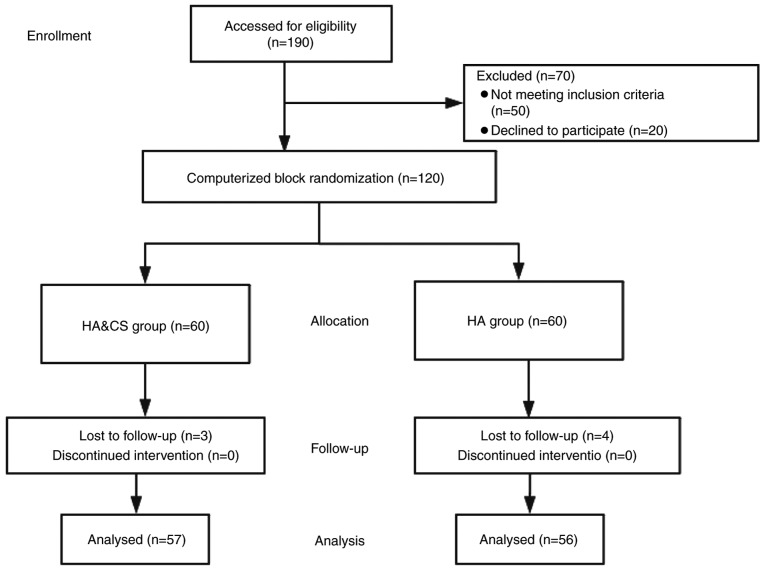
Figure 1.
Consolidated Standards of Reporting Trials flow diagram in the present study. HA, hyaluronic acid; CS, corticosteroids.
All procedures were performed in the injection room of the Orthopedic Department at The Affiliated Zhongda Hospital of Southeast University. An experienced orthopedic surgeon administered injections. Patients were placed in sitting position with the eyes covered. Knees were flexed to ~90 degrees and sterilized prior to injection. A 22-gauge needle (external diameter, 0.71 mm) was used to puncture the lateral soft spot above the joint line into the joint capsule. A small amount of air was injected to ensure that the needle was accurately positioned in the joint capsule. The 22-gauge needle was left in the injection spot for further synovial fluid aspiration and drug delivery. The effusion, if present, was removed using the inserted 22-gauge needle prior to administration.
In the present study, both the evaluators and patients were blinded. Evaluators were not informed of the patient allocations to reduce bias in the assessment. Patients were blinded using an eye mask during the injection process and were unaware which group they were assigned to. The use of any additional medications associated with OA, including NSAIDs and analgesics, was prohibited following treatment.
Measures
The primary outcomes of treatment were evaluated at a 6-month follow-up. Pain in the knee with OA was measured using a 100-mm visual analog scale (VAS), whereas knee function was measured using 3 dimensions of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (18). The WOMAC score was validated in Chinese language. VAS and WOMAC questionnaires were completed at the baseline (prior to treatment), and at week 1 and months 1, 3 and 6 following treatment. An independent, blinded therapist assessed the active flexion motion of the treated knees using a goniometer at the baseline, week 1 and months 1, 3 and 6.
Statistical analysis
A calculation was performed as previously described to determine the sample size needed to provide 80% power to demonstrate a difference of >1.2 points in the VAS score at the 5% significance level in a 2-sided hypothesis test (19). A total of 50 patients were required from each group to ensure adequate power to detect a similar between-group difference. As such, the study was designed to enroll 120 participants at the baseline (n=60/group), anticipating that 20% may drop out. The analysis was performed on the intention-to-treat populations. Data are presented as mean ± standard deviation in tables and as mean ± standard error of the mean in figures. Demographic variables were tested using a χ2 test. VAS score, WOMAC score and the range of motion observed at each time point was evaluated using independent Student's t-tests. Comparisons in each group prior to the injection and 6 months following the injection were performed using paired Student's t-tests. Data were analyzed using SPSS 19 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics and follow-up
A total of 120 patients were enrolled in the present study and allocated to the HA or HA&CS treatment groups (n=60/group). No significant differences were observed in basic characteristics, including sex distribution, mean age, mean VAS score, mean body mass index, mean WOMAC score and mean knee range of motion, between the groups (Table I). At the end of the trial, the majority of enrolled patients underwent clinical assessments (57 patients in the HA&CS group and 56 patients in the HA group). In the HA&CS group, 3 patients were lost to follow-up at month 6. In the HA group, 2 patients were lost to follow-up at month 3 and 2 patients were lost to follow-up at month 6.
Table I.
Baseline demographic data and clinical parameters of patients.
| Parameters | HA&CS group (n=60) | HA group (n=60) | P-value |
|---|---|---|---|
| Sexa | 0.833 | ||
| Female | 46 (76.67) | 44 (73.33) | |
| Male | 14 (23.33) | 16 (26.67) | |
| Ageb | 63.60±6.24 | 62.50±6.55 | 0.348 |
| Body mass index (kg/m2)b | 25.30±3.22 | 26.00±4.15 | 0.304 |
| Smokinga | 1.000 | ||
| Yes | 5 (8.33) | 6 (10.00) | |
| No | 55 (91.67) | 54 (90.00) | |
| Living situationa | 1.000 | ||
| Live with partner/spouse | 54 (90.00) | 55 (8.33) | |
| Live alone | 6 (10.00) | 5 (91.67) | |
| Kellgren-Lawrence gradea | 0.872 | ||
| II | 12 (20.00) | 10 (16.67) | |
| III | 37 (61.67) | 39 (65.00) | |
| IV | 11 (18.33) | 11 (18.33) | |
| VAS pain (mean points)b | 7.13±1.00 | 7.15±0.99 | 0.927 |
| WOMAC score (mean points)b | 40.95±8.016 | 41.08±6.828 | 0.922 |
| Active knee range of motion (mean flexion°)b | 126.40±6.35 | 125.93±6.31 | 0.455 |
Data are presented as n (%), evaluated by χ2 test
Data are presented as the mean ± standard deviation, evaluated by Student's t-test. HA, hyaluronic acid; CS, corticosteroids; VAS, visual analog scale; WOMAC, Western Ontario and McMaster University Osteoarthritis Index.
Pain relief and VAS score
Prior to treatment, no significant difference in VAS score was observed between the HA&CS and HA groups (7.45±2.05 vs. 7.30±1.96, respectively; Table II). Following treatment, the VAS score in the HA&CS group decreased significantly compared with the HA group (Fig. 2). At month 6, the mean VAS score in both groups increased and were not significantly different. Patients in both groups exhibited decreased VAS scores following injection; however, there was no significant difference in the VAS score at month 6 compared with the baseline in either group (Table III).
Table II.
Differences in mean outcome scores between groups.
| Treatment | HA&CS group (n=60) | HA group (n=60) | P-value |
|---|---|---|---|
| VAS pain (points) | |||
| Baseline | 7.13±1.00 | 7.15±0.99 | 0.927 |
| 1 week | 3.77±1.23 | 6.82±1.10 | <0.001 |
| 1 month | 4.60±1.20 | 6.40±0.96 | <0.001 |
| 3 months | 5.30±1.11 | 6.07±1.06 | <0.001 |
| 6 months | 7.07±0.73 | 7.13±0.81 | 0.638 |
| WOMAC score (points) | |||
| Baseline | 40.95±8.02 | 41.08±6.83 | 0.922 |
| 1 week | 19.42±4.49 | 30.67±6.37 | <0.001 |
| 1 month | 21.27±5.00 | 29.52±6.31 | <0.001 |
| 3 months | 22.65±7.01 | 27.43±8.12 | 0.008 |
| 6 months | 39.02±6.88 | 40.95±7.70 | 0.150 |
| Flexion motion of knee (°) | |||
| Baseline | 126.37±6.35 | 125.50±6.31 | 0.455 |
| 1 week | 129.83±4.73 | 129.16±4.53 | 0.432 |
| 1 month | 130.17±4.78 | 129.63±4.40 | 0.526 |
| 3 months | 132.20±4.71 | 131.93±4.58 | 0.754 |
| 6 months | 126.90±4.52 | 126.73±4.29 | 0.559 |
Data are presented as the mean ± standard deviation. HA, hyaluronic acid; CS, corticosteroids; VAS, visual analog scale; WOMAC, Western Ontario and McMaster University Osteoarthritis Index.
Figure 2.
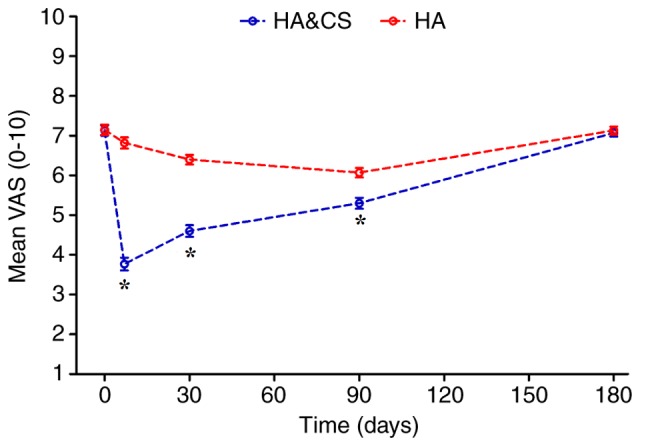
The mean VAS score over time for the HA&CS group and the HA group in the intention-to-treat population. The error bars indicate standard error of the mean. *P<0.05 vs. HA. VAS, visual analog scale; HA, hyaluronic acid; CS, corticosteroids.
Table III.
Differences in mean outcome scores over time.
| Treatment | HA&CS group (n=60) | HA group (n=60) |
|---|---|---|
| VAS pain (points) | ||
| Baseline | 7.13±1.00 | 7.15±0.99 |
| 6 months | 7.07±0.73 | 7.13±0.81 |
| P-value | 0.677 | 0.927 |
| WOMAC score (points) | ||
| Baseline | 40.95±8.02 | 41.08±6.83 |
| 6 months | 39.02±6.88 | 40.95±7.70 |
| P-value | 0.129 | 0.915 |
| Flexion motion of knee (°) | ||
| Baseline | 126.37±6.35 | 125.50±6.31 |
| 6 months | 126.90±4.52 | 126.73±4.29 |
| P-value | 0.494 | 0.201 |
Data are presented as the mean ± standard deviation. HA, hyaluronic acid; CS, corticosteroids; VAS, visual analog scale; WOMAC, Western Ontario and McMaster University Osteoarthritis Index.
Functional improvement and WOMAC score
The mean WOMAC score were markedly decreased in the HA&CS and HA groups for 6 months following treatment, compared with baseline (Fig. 3). In terms of pain, stiffness and physical function, better knee function was observed in the HA&CS group compared with the HA group during the first 3 months post-injection (P<0.05). However, no significant differences in WOMAC score were observed between groups at month 6. WOMAC scores were improved in both groups post-injection; however, the mean WOMAC score at month 6 was not significantly different to the baseline score in either group (Table III).
Figure 3.
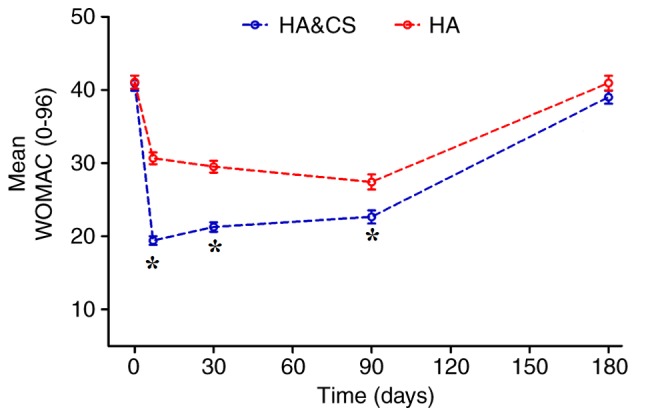
The mean WOMAC score over time for the HA&CS group and the HA group in the intention-to-treat population. The error bars indicate standard error of the mean. *P<0.05 vs. HA. WOMAC, Western Ontario and McMaster University Osteoarthritis Index; HA, hyaluronic acid; CS, corticosteroids.
Active flexion motion of the knee
Patients in both groups reported improved flexion compared with the baseline for the first 3 months post-injection. No significant difference in mean flexion angle of the knee was observed between groups at any time point (Fig. 4). There was no significant difference in the mean flexion angle at month 6 compared with the baseline in either group (Table III).
Figure 4.
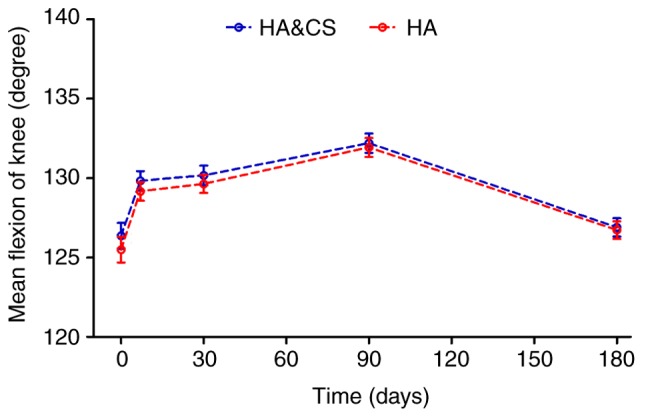
The mean flexion angle of the knee over time for the HA&CS group and the HA group in the intention-to-treat population. The error bars indicate the standard error of the mean. HA, hyaluronic acid; CS, corticosteroids.
Adverse events
No severe systemic or local adverse events were observed in either group.
Discussion
In the present study, prompt pain relief was clearly observed in the HA&CS group, whereas the VAS score was decreased gradually in the HA group. At month 6, the mean change in VAS score was not significantly different between groups. The fast action of intra-articular CS injection is well recognized, however the long-term outcome of repetitive injections remains unknown (11). CS is able to block the synthesis and activation of matrix metalloproteinases, slowing down decomposition of the cartilage matrix (15,20). However, a repetitive high dose of CS can hinder the regeneration of articular cartilage by downregulating proteoglycan and HA synthesis. Intra-articular CS injection typically reduced pain associated with OA for 3–4 weeks (21), whereas HA has been reported to have a greater efficacy beyond week 8 (22). A clinical trial revealed that HA controls pain better at 12 and 26 weeks compared to CS (23). A similar trend in pain experience and VAS scores was observed in the present study. However, the duration of pain relief was less than 6 months regardless of the use of combined treatment with HA&CS or HA alone. In the present study, the indication for intra-articular CS and HA injection is symptomatic knee OA, which is mainly associated with pain, stiffness, joint warmth and swelling. However, as knee joint puncture is an invasive operation, patients with severe diabetes or systemic infection should be excluded due to the risk of skin and joint capsule infection.
The anti-inflammatory effects of CS may serve an important role in alleviating the acute symptoms of OA. Patients with knee OA typically present with joint inflammation, including morning stiffness, warmth, pain and joint effusions, which occur in part due to synovial thickening or synovial fluid effusion (3,24,25). CS has a powerful anti-inflammatory effect and is able to reduce edema, capillary expansion and leukocyte infiltration in the early stages of the inflammatory reaction (26,27). A patient who dropped out of the study due to unsatisfactory pain control (lost to follow-up at month 6 following HA injection) later went on to have a total knee arthroplasty (TKA) in The Affiliated Zhongda Hospital of Southeast University. Pathological sections and radiographic images of the joint synovium were obtained, revealing chronic synovial inflammation and degenerative changes to the knee joint. For patients with OA and acute synovitis, HA injection alone is typically insufficient to control the inflammation and alleviate pain. As such, co-administration of a CS injection may be necessary to achieve the desired outcome (28).
The results of the present study revealed that combined treatment with HA and CS resulted in a greater improvement knee function compared with HA alone for the first 3 months post-injection. However, at month 6 there was no significant difference in WOMAC scores between groups. These results suggest that, in the long-term, combined treatment with HA and CS is not superior to HA alone. For patients with acute pain, however, the use of HA and CS together may provide more effective immediate pain relief. Usually, the use of cross-linked HA results in a significant reduction in pain and improved knee function from 6->12 months (29,30), as cross-linking is a proven means for prolonging the intra-articular residence time of HA (31). In the present study, a linear HA was used, which has a shorter degradation half-life compared with cross-linked HA. The shorter effect duration may be due to the intrinsic characteristic of the viscosupplementation injected. In future studies, researchers should evaluate the efficacy of co-application of cross-linked HA and CS, which may have better clinical outcomes.
With regards to the knee range of motion, patients in both groups reported improved flexion for the first 3 months post-injection. At month 6, the mean change was greater in the HA&CS group compared with the HA group, however there was no statistical significance. For patients with limited knee extension, a compound betamethasone solution (1 ml compound betamethasone in 4 ml 1% lidocaine hydrochloride) was used for local injection into the posterior joint capsule and gastrocnemius tendons. The majority of patients reported a prompt improvement in extension capabilities, even from the second day following the injection. However, this procedure may cause unnecessary injury to the posterior tibial nerve and blood vessels, and patients who underwent this treatment were not included in the present study.
Prolonging the interval between injections and decreasing the dosage of CS used may reduce the risk of injury and drug-related adverse events. In the present study, HA and CS were used in combination and patients were followed up for 6 months. The CS used in the present study was a compound betamethasone, which is primarily composed of betamethasone sodium phosphate and betamethasone dipropionate. Following administration, the soluble betamethasone sodium phosphate is rapidly absorbed and often reaches a peak plasma concentration within 1 h, relieving acute pain within 6 h. Fat-soluble betamethasone dipropionate was absorbed slowly in the knee joint capsule, prolonging the duration of pain management. Betamethasone was injected directly into the OA knee and the majority of patients experienced rapid pain relief and improved knee function within 3 days. However, the duration of pain relief varied significantly among patients, especially in those diagnosed with advanced knee OA (KL grading III–IV). In a recent systematic review and meta-analysis, Jüni et al (32) concluded that CS injection had a negligible effect after 6 months. Due to the potential cardiovascular side effects and cartilage erosion associated with CS, single CS injections are not recommended in the Department of Orthopaedics at The Affiliated Zhongda Hospital of Southeast University. The results of the present study suggest that, even when combined with HA, CS is unable to maintain pain relief for >6 months. As such, co-injection of HA and CS once every 6 months may be an effective treatment for patients with knee OA. The combined use of HA and CS can provide short-term and comparative long-term effects in terms of pain relief and knee function improvement. Meanwhile, HA may serve to protect the cartilage from CS erosion by adhering to the joint cartilage, thus improving the safety of CS application.
In the present study, no local anesthetic was used in the injection spot, as the injection of local anesthetic itself often causes unnecessary pain that lasts longer than the joint capsule penetration process. According to clinical observations, patients who received prompt puncture of the needle into the joint capsule experienced minor or acceptable pain experience. The use of a thinner needle also alleviated the pain of penetration. Therefore, a 22-gauge needle (external diameter, 0.71 mm), instead of a commonly used 21-gauge needle (external diameter, 0.81 mm) (19), was used in the present study. The adverse events and pain experience in this study proved minimal. However, a patient in the HA&CS group experienced CS-induced facial flushing and dizziness. This patient recovered from the symptoms within 24 h without any pharmaceutical interventions.
No placebo group was used in the present study, as it has previously been demonstrated that the use of HA and/or CS is superior compared with a placebo injection (33–35). One limitation of the present study was that the effect of a combined use of HA and CS treatment on the cartilage metabolism was not investigated. Further basic studies are required to determine whether co-treatment with CS and HA can cause the long-term cartilage deterioration. The CS injections used in the present study were able to induce prompt analgesia for acute pain, thus prolonging the necessity for possible surgical interventions. However, prior intra-articular injection of CS was reported to be associated with an increased infection risk for subsequent TKA (36,37).
In conclusion, patients who received co-treatment with HA and CS experienced pain relief and improved knee function faster than those who received HA alone. However, the combined use of HA and CS was not overall superior to HA in terms of pain control, knee function and range of motion at month 6 post-injection. By adhering to the joint cartilage, HA may protect the cartilage from CS erosion, improving the safety of CS application. However, further in vivo studies are required to investigate the biological mechanisms underlying this protective effect.
Acknowledgements
The authors would like to thank Dr Chang Su from the Department of Medical Database, Dr Li-Wei Huang and Dr Jing-Yuan Xu from the Department of Intensive Care Unit, and Dr. Jun Lu from the Department of Orthopaedics of Zhongda Hospital, Medical School of Southeast University (Nanjing, China) for help with the present statistical analysis.
Funding
The present study was supported by National Natural Science Foundation of China (grant no. 81572188) and Science and Technology Project of Jiangsu Province (grant no. BK20161439).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SZW collected data and drafted the manuscript. DYW participated in the experimental design and experimentation, and collected data. QC and YDG participated in designing the study and interpreting the results. CW took part in the study design, provided clinical perspectives for the findings of the study and revised the manuscript. WMF conceived the study and interpreted the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Affiliated Zhongda Hospital of Southeast University (Nanjing, China) Ethics Committee approved the present study prior to patient enrollment (approval no. 2017zdSYLL012-Y01). All enrolled subjects provided written informed consent prior to inclusion in the present study.
Patient consent for publication
Informed consent was obtained from all individual participants to publish the material in the present study. Data of all individual participants were kept private and confidential.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12:92–101. doi: 10.1038/nrrheum.2015.135. [DOI] [PubMed] [Google Scholar]
- 5.McArthur BA, Dy CJ, Fabricant PD, Valle AG. Long term safety, efficacy, and patient acceptability of hyaluronic acid injection in patients with painful osteoarthritis of the knee. Patient Prefer Adherence. 2012;6:905–910. doi: 10.2147/PPA.S27783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16:821–847. doi: 10.18433/J3VW2F. [DOI] [PubMed] [Google Scholar]
- 7.Adams ME, Lussier AJ, Peyron JG. A risk-benefit assessment of injections of hyaluronan and its derivatives in the treatment of osteoarthritis of the knee. Drug Saf. 2000;23:115–130. doi: 10.2165/00002018-200023020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: A 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20:410–423. doi: 10.1016/S0149-2918(98)80052-0. [DOI] [PubMed] [Google Scholar]
- 9.Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: A 1 year placebo-controlled trial. Osteoarthritis Cartilage. 1993;1:97–103. doi: 10.1016/S1063-4584(05)80024-X. [DOI] [PubMed] [Google Scholar]
- 10.Jevsevar DS. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:571–576. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen C, Lefèvre-Colau MM, Poiraudeau S, Rannou F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann Phys Rehabil Med. 2016;59:184–189. doi: 10.1016/j.rehab.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45:S28–S33. doi: 10.1016/j.semarthrit.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Koenig KM, Ong KL, Lau EC, Vail TP, Berry DJ, Rubash HE, Kurtz S, Bozic KJ. The use of hyaluronic acid and corticosteroid injections among medicare patients with knee osteoarthritis. J Arthroplasty. 2016;31:351–355. doi: 10.1016/j.arth.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Askari A, Gholami T, NaghiZadeh MM, Farjam M, Kouhpayeh SA, Shahabfard Z. Hyaluronic acid compared with corticosteroid injections for the treatment of osteoarthritis of the knee: A randomized control trail. Springerplus. 2016;5:442. doi: 10.1186/s40064-016-2020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandeweerd JM, Zhao Y, Nisolle JF, Zhang W, Zhihong L, Clegg P, Gustin P. Effect of corticosteroids on articular cartilage: Have animal studies said everything. Fundam Clin Pharmacol. 2015;29:427–438. doi: 10.1111/fcp.12137. [DOI] [PubMed] [Google Scholar]
- 16.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 18.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthritis Rheum. 2001;45:453–461. doi: 10.1002/1529-0131(200110)45:5<453::AID-ART365>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 19.Levin PE. Utilizing Health-Care Resources Wisely: Understanding the Efficacy of Our Interventions: Commentary on an article by Nattapol Tammachote, MD, MSc, et al: Intra-articular, single-shot hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis. A double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:e47. doi: 10.2106/JBJS.16.00020. [DOI] [PubMed] [Google Scholar]
- 20.Tehranzadeh J, Booya F, Root J. Cartilage metabolism in osteoarthritis and the influence of viscosupplementation and steroid: A review. Acta Radiol. 2005;46:288–296. doi: 10.1080/02841850510016027. [DOI] [PubMed] [Google Scholar]
- 21.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Arthritis Rheum. 2009;61:1704–1711. doi: 10.1002/art.24925. [DOI] [PubMed] [Google Scholar]
- 23.Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31:333–343. [PubMed] [Google Scholar]
- 24.Maricar N, Callaghan MJ, Parkes MJ, Felson DT, O'Neill TW. Clinical assessment of effusion in knee osteoarthritis-A systematic review. Semin Arthritis Rheum. 2016;45:556–563. doi: 10.1016/j.semarthrit.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards MM, Maxwell JS, Weng L, Angelos MG, Golzarian J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Phys Sportsmed. 2016;44:101–108. doi: 10.1080/00913847.2016.1168272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laev SS, Salakhutdinov NF. Anti-arthritic agents: Progress and potential. Bioorg Med Chem. 2015;23:3059–3080. doi: 10.1016/j.bmc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5:351–361. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iannitti T, Rottigni V, Palmieri B. A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. Int J Immunopathol Pharmacol. 2012;25:1093–1098. doi: 10.1177/039463201202500426. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Park D, Chmell SJ. Viscosupplementation with hylan G-F 20 (Synvisc): Pain and mobility observations from 74 consecutive patients. J Knee Surg. 2004;17:73–77. doi: 10.1055/s-0030-1248202. [DOI] [PubMed] [Google Scholar]
- 31.Henrotin Y, Raman R, Richette P, Bard H, Jerosch J, Conrozier T, Chevalier X, Migliore A. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45:140–149. doi: 10.1016/j.semarthrit.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Jüni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, da Costa BR. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev: CD005328. 2015 doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev: CD005321. 2006 doi: 10.1002/14651858.CD005328.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev: CD005328. 2006 doi: 10.1002/14651858.CD005328.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Godwin M, Dawes M. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Can Fam Physician. 2004;50:241–248. [PMC free article] [PubMed] [Google Scholar]
- 36.Marsland D, Mumith A, Barlow IW. Systematic review: The safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21:6–11. doi: 10.1016/j.knee.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Papavasiliou AV, Isaac DL, Marimuthu R, Skyrme A, Armitage A. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2006;88:321–323. doi: 10.1302/0301-620X.88B3.17136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.