Abstract
The present study aimed to illuminate the role of circulating T follicular helper (TFH) cells in patients diagnosed with chronic immune thrombocytopenia (cITP). Fifty-four patients with cITP and 30 age-matched healthy control subjects were enrolled in the present study. TFH cell frequencies, expression of CD4+ TFH cell-associated cytokines, including interleukin (IL)-2, IL-4, IL-10 and IL-21 and associated regulatory mRNA expression levels including Bcl-6, c-Maf, Blimp-1 and PD-1 pre- and post-treatment with intravenous immunoglobulin and corticosteroids, were detected by flow cytometry, ELISA and reverse transcription-quantitative polymerase chain reaction, respectively. TFH cell frequencies of patients were significantly higher compared with healthy controls pre-treatment (P<0.05). Following treatment, significantly decreased percentages of TFH cells were present in cITP responders (P<0.05). Correlation analysis revealed that the number of TFH cells was negatively correlated with the platelet count in the peripheral blood. Furthermore, analysis of inflammatory cytokines indicated significant differences in serum interleukin (IL)-21 and IL-10 between pretreated patients and healthy controls (P<0.05). Additionally, transcription factor B-cell lymphoma (Bcl)-6, c-Maf and programmed death-ligand (PD)-1 mRNA expression levels were significantly different between cITP patients prior to treatment and the healthy controls (P<0.05). However, the expression levels of Bcl-6, C-Maf and PD-1 mRNA were significantly changed post-treatment (P<0.05). These data demonstrated that circulating TFH cells and CD4+ TFH cell-associated cytokines may serve a role in cITP. The findings suggest that the overactivation of TFH cells may contribute to the immunopathogenesis of cITP, thus blocking the pathway of TFH cells may be reasonable for therapeutic intervention.
Keywords: immune thrombocytopenia, chronic, T follicular helper cells
Introduction
Immune thrombocytopenia (ITP) is a common hematologic disorder characterized by isolated thrombocytopenia, which causes bleeding in the skin and mucosa. According to the disease duration, ITP can be classified into three types, including newly diagnosed, persistent and chronic, and chronic ITP (cITP) (1). In ~1/3 of ITP cases, the duration of thrombocytopenia will extend over 12 months, which is defined as chronic ITP (2). ITP occurs in children and adults; however, the disease is typically chronic in adults (3). The incidence primary ITP accounts for 3.3/100,000 adults per year (3). Although recent progress on the development of thrombopoietin receptor agonists have changed the management of chronic disease (4), the detailed underlying mechanisms involved in the pathophysiology of cITP remain poorly understood.
A dysfunctional proliferation of autoreactive T cells, including T helper (Th)2, Th17, Th22 and T follicular helper (TFH) cells, has been suggested to be responsible for the loss of tolerance to self-platelet antigens in ITP. TFH cells, a distinct subset of CD4+ T cells, specialize in providing critical assistance to germinal center (GC) B cells (5). TFH cells contribute to the generation and maintenance of GCs, support Ig class switching and assist in differentiation of GCs into memory B cells and long-lived plasma cells (6). Immune-mediated disease may occur due to the inability of TFH cells to maintain immune homeostasis (7). Notably, the role of TFH cells in autoimmune diseases has been studied in systemic lupus erythematosus (SLE) mouse models (7). Furthermore, the frequency of TFH cells has been indicated to be downregulated in the peripheral blood of patients with SLE (8). Additionally, the frequency of TFH is associated with the proportion of GC B cells and plasma cells. Antiplatelet antibodies of IgG, IgA and IgM produced by B cells facilitate phagocytosis by macrophages. Of note, platelet destructions are more severe with IgG compared with other isotypes (9).
Further research on the roles of TFH cells in the development of adult cITP are still required. The limited studies on pre- and post-treatment (intravenous immunoglobulin and corticosteroids) comparisons of TFH cells and the small samples sizes of patients assessed may bias the possible role of TFH cells in ITP. In the present study, the functional change of circulating TFH cells was investigated using cell counting, CD4+ TFH cell-associated cytokine profiles and expression determination of transcription factors, including Bcl-6, c-Maf, Blimp-1 and PD-1 in a cohort of patients with cITP at pre- and post-treatment time points. The present findings may provide useful insights on the role of TFH cells in cITP.
Materials and methods
Patients and healthy volunteers
A total of 54 patients diagnosed with cITP who received treatment with intravenous immunoglobulin, corticosteroids or a combination, were enrolled in the study for blood analyses. There were 32 female patients (59%) and 22 male patients (41%), aged 28–61 years. Only patients who fit the international guidelines for ITP (10) were included in the present study. Cases that refused data collection or had autoimmune disorders were excluded from the present study. Based on the efficacy of the treatment, the patients were divided into responders and non-responders. A total of 30 healthy controls were recruited at the same time as patients, there were 16 female (53.3%) and 14 male (46.7%), aged 24–60 years. A total of 5 ml blood samples were collected from the patients at the Hematology Outpatient Department in The First Affiliated Hospital of Soochow University (Suzhou, China) between January 2016 and July 2017. Ethylenediaminetetraacetic acid (EDTA)-stabilized venous blood (5 ml) was obtained from patients and healthy adults. Whole blood (2 ml) samples were stored at 4°C for flow cytometric analysis. Serum isolated from venous blood samples was used to investigate the change of CD4+ TFH cell-associated factors by comparing responders (n=30) to non-responders (n=24). Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples using gradient centrifugation (3,000 × g/min for 5 min at room temperature) and stored at −80°C for further use. Patients who did not achieve a platelet count >30 G/l were considered as non-responders. Expression of serum platelet-associated immunoglobulin G (PAIgG) in all patients was examined. All participants provided their informed consent in accordance with the declaration of Helsinki. The study was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University (Suzhou, China).
Flow cytometry
The following antibodies were used for flow cytometry analysis: Cluster of differentiation (CD)4-phycoerythrin (PE)-CY5, CD3-PE-CY7 (Beckman Coulter, Inc., Miami, FL, USA), C-X-C chemokine receptor type 5 (CXCR5)-fluorescein and inducible costimulatory molecule (ICOS)-PE (BioLegend, San Diego, CA, USA). Whole blood (50 µl) was incubated with specific antibodies for 30 min at room temperature. Following incubation, red blood cells were lysed and washed twice by phosphate-buffered saline (PBS) solution. Cells were acquired on Beckman Coulter FC500 and analyzed with CXP analysis software 2.2 (Beckman Coulter, Inc.). For each tube, at least 10,000 events were collected in a gate created around the viable lymphocyte population. Data were presented as percentage of cells in the lymphocyte population. Flow cytometric measurement of PAIgG was performed using LEGENDplex Human Immunoglobulin Isotyping Panel-IgGs (4-plex) with V-bottom Plate (cat. no. 740715; BioLegend). The platelet-rich plasma (PRP) was obtained by centrifugation at 180 × g for 15 min. PRP was washed twice with PBS containing 10 mM EDTA and 0.5% bovine albumin. Notably, the platelet concentration was adjusted to 1×108/ml. The platelet population was gated with forward scatter light and side scatter light. Quantification of four human immunoglobulins, including IgG1, IgG2, IgG3 and IgG4 was performed according to the manufacturer's protocol.
ELISA detection of cytokines
Serum IL-2, IL-4, IL-10 and IL-21 levels were detected using ELISA with a xMark Microplate Absorbance Spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to manufacturer's protocol. All samples were analyzed in duplicate. The ELISA kits used in the experiment were Human IL-2 ELISA MAX™ Deluxe (cat. no. 431804), Human IL-4 ELISA MAX™ Deluxe (cat. no. 430304), Human IL-10 ELISA MAX™ Standard (cat. no. 430601) and Human IL-21 ELISA MAX™ Deluxe (cat. no. 433804), all purchased from BioLegend.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from PBMCs was extracted using an AllPrep RNA/DNA/miRNA Universal kit (Qiagen AB, Sollentuna, Sweden) according to the manufacturer's instructions. cDNA was synthesized using an RT-qPCR kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). qPCR was performed on an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The sequences of the primers used were as follows: Bcl-6, forward 5′-AGTTTATTAAGGCCAGTGA-3′ and reverse 5′-GATAGGCCATGATGTCTT-3′; c-Maf, forward 5′-ACTGGCAATGAGCAACTCCG-3′ and reverse 5′-GCTGATGATGCGGTCGGTCT-3′; B lymphocyte-induced maturation protein-1 (Blimp-1), forward 5′-TCCAGCACTGTGAGGTTTCA-3′ and reverse 5′-TCAAACTCAGCCTCTGTCCA-3′; and PD-1, forward 5′-GACAACGCCACCTTCACCT-3′ and reverse 5′-GCTTGTCCGTCTGGTTGCT-3′. GAPDH, forward 5′-AATCCCATCACCATCTTCCA-3′ and reverse 5′-TGGACTCCACGACGTACTCA-3′. qPCR was performed with SYBR Select Master Mix (Thermo Fisher Scientific Inc.) and 500 nM forward and reverse primers. Thermal cycle conditions used were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 1 sec and 60°C for 30 sec Gene expression values were calculated by the comparative threshold cycle method (11). Stable expressed GAPDH served as endogenous control.
Response criteria
Respondents were classified as responders who were responding to intravenous immunoglobulin and corticosteroids therapy, and non-responders. Responders were defined as a normal platelet count of >30×109/l, a doubling of baseline platelet count and no bleeding symptoms. Non-responders were defined as a platelet count of <30×109/l or below twice as much as the pre-treatment platelet count.
Statistical analysis
All statistical analyses were performed using SPSS for Microsoft Windows (version 13.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Quantitative data are presented as mean ± standard deviation. The unpaired Student's t-test or one-way analysis of variance with the Tukey multiple-comparison post hoc test, χ2 test and the Pearson correlation coefficient were employed. P<0.05 was considered to indicate a statistically significant difference.
Results
Circulating TFH cells in the peripheral blood of patients with cITP and healthy controls
The baseline characteristics of patients with cITP and healthy controls are indicated in Table I. There was no significant difference in the distribution of age, sex and platelet count between the cITP group and healthy controls. At least 10,000 events were collected in a gate around the lymphocytes. In order to identify TFH cells, CD4+ lymphocytes were gated on. Subsequently, the percentages of CD4+ CXCR5+ICOS+ cells were gated and determined by flow cytometry.
Table I.
Baseline characteristics of patients with chronic immune thrombocytopenia and healthy controls.
| Variables | Healthy controls, n=30 (%) | Responders, n=30 (%) | Non-responders, n=24 (%) | P-value |
|---|---|---|---|---|
| Sex (n, %) | ||||
| Female | 16 (53.3) | 18 (60.0) | 14 (58.3) | NS |
| Male | 14 (46.7) | 12 (40.0) | 10 (41.7) | NS |
| Age (years, range) | 41 (24–60) | 45 (25–61) | 43 (28–60) | NS |
| Disease duration (months, range) | – | 28 (13–124) | 27 (15–120) | NS |
| Platelet count (109/l, mean ± SD) | 112.5±10.9 | 34.9±14.1 | 35.3±15.7 | 0.05 |
SD, standard deviation; NS, not significant.
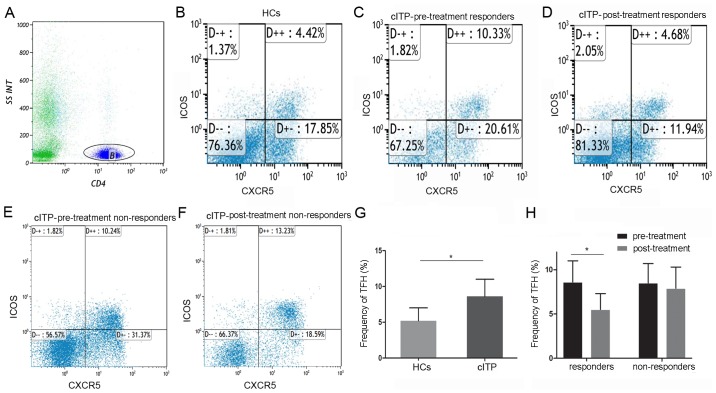
As indicated in Fig. 1, the proportion of circulating TFH cells in all pretreated patients with cITP was significantly higher compared with healthy controls (8.6±2.4 and 5.2±1.8%, P<0.05). Compared with pre-treatment, the frequencies of circulating TFH cells in the responders were significantly reduced post-treatment (8.8±2.5 and 5.5±1.8%, P<0.05), whereas no significant difference was identified in the non-responders (8.5±2.2 and 7.9±2.4%, P>0.05).
Figure 1.
Flow cytometry analysis of proportions of circulating TFH cells in patients with cITP and HCs. (A) CD4+ lymphocytes were gated and the CD4+ CXCR5+ICOS+ T subsets were gated using flow cytometry. Plots in the inter box of D++ represent circulating TFH cells. (B) Circulating TFH in healthy controls, (C) circulating TFH in cITP patient responders at pre-treatment, (D) circulating TFH in cITP patient responders at post-treatment, (E) circulating TFH in cITP patient non-responders at pre-treatment and (F) circulating TFH cITP patient non-responders at post-treatment were indicated. (G) Bar graph quantification of circulating TFH cells in HCs and patients with cITP. (H) Bar graph quantification of circulating TFH cells in patient responders and non-responders. Normalized values were presented as the mean ± standard deviation. *P<0.05 as indicated. TFH, T follicular helper; HCs, healthy controls; cITP, chronic immune thrombocytopenia.
Correlation analysis between circulating TFH cell proportions, PAIgG levels and platelet counts in patients with cITP
Following the observational changes indicated with regards to the circulating TFH cell ratio in patients with cITP and respondents, the possible correlation between the proportion of circulating TFH cells and platelet counts was assessed. Correlation analysis revealed no correlation between the circulating TFH cell ratio and PAIgG (r=0.21, P>0.05; data not shown). Furthermore, the TFH cell percentage was negatively correlated with the platelet count in peripheral blood (r=−0.84, P<0.05; Fig. 2). These results suggested that the TFH cell ratio may be involved in decreasing the platelet count in patients with cITP.
Figure 2.
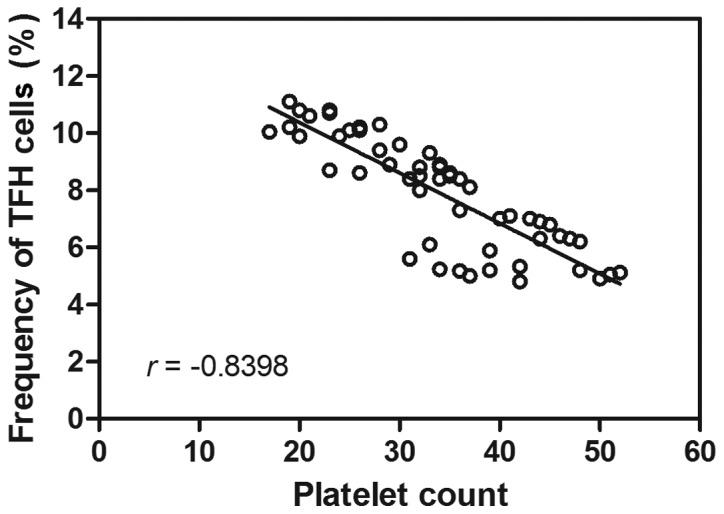
Correlation analysis between the percentage of circulating TFH cells and platelet count (109/l). TFH, T follicular helper.
Serum IL-2, IL-4, IL-10 and IL-21 levels of cITP-associated cytokines
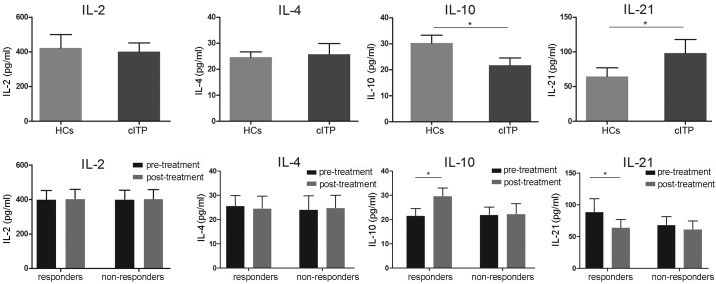
Following the results regarding the circulating TFH cell ratio, the level of associated serum cytokines IL-2, IL-4, IL-10 and IL-21 were evaluated using ELISA. As indicated in Fig. 3, serum levels of IL-21 in patients with cITP that were pretreated were significantly higher compared with healthy controls, whereas the levels were significantly lower in the pretreated responders compared with responders post-treatment (P<0.05). Serum levels of IL-10 in pretreated patients with cITP were significantly reduced compared with the healthy controls, while the levels of IL-10 were significantly elevated following treatment in the responders (P<0.05). No significance differences in IL-10 and IL-21 expression levels were observed in non-responders (P>0.05). Furthermore, no significant differences in IL-2 and IL-4 levels were identified between pretreated patients and healthy controls or responders and non-responders (P>0.05; Fig. 3).
Figure 3.
Serum concentrations of IL-2, IL-4, IL-10 and IL-21 were determined by ELISA. Normalized values were presented as the mean ± standard deviation.*P<0.05 as indicated. IL, interleukin; HCs, healthy controls; cITP, chronic immune thrombocytopenia.
Transcription factor expression levels in patients with cITP
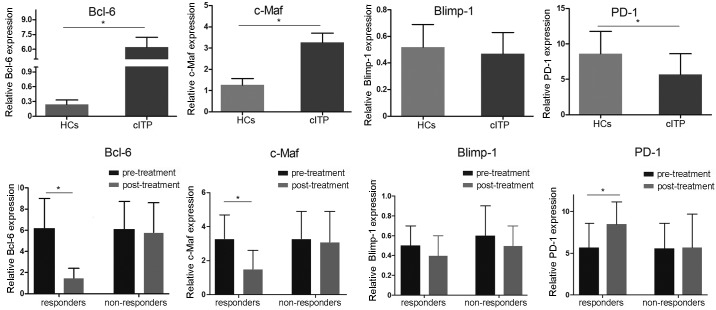
To investigate the mRNA expression levels of the possible involvement of Bcl-6, c-Maf, Blimp-1 and PD-1 transcription factors, RT-qPCR was applied. Compared with healthy controls, the mRNA expression levels of Bcl-6 and c-Maf in pretreated patients with cITP were significantly elevated, whereas the expression levels of PD-1 were significantly decreased (P<0.05; Fig. 4). There was no significant difference in Blimp-1 mRNA expression between pretreated patients with cITP and healthy controls (P>0.05). Furthermore, the mRNA expression levels of Bcl-6 and c-Maf in cITP responders post-treatment were significantly downregulated while PD-1 in cITP responders post-treatment was significantly increased compared with pretreated patients with cITP (P<0.05; Fig. 4), whereas no significant difference was observed in expression level of Blimp-1 (P>0.05).
Figure 4.
Expression levels of Bcl-6, c-Maf, Blimp-1 and PD-1 mRNA in patients with cITP and healthy controls were determined by reverse transcription-quantitative polymerase chain reaction using GAPDH as an endogenous control. Normalized values were presented as the mean ± standard deviation. *P<0.05 as indicated. Bcl-6, B-cell lymphoma-6; PD-1, programmed death-ligand-1; Blimp-1, B lymphocyte-induced maturation protein-1; HCs, healthy controls; cITP, chronic immune thrombocytopenia.
Discussion
Increasing evidence has revealed the association of TFH cells and autoimmune diseases. In the present study, the proportions of circulating TFH cells in patients with cITP were significantly increased in the peripheral blood; however, this was decreased to control levels following treatment. Additionally, a negative correlation was indicated between the percentage of circulating TFH cells and platelet count, demonstrating circulating TFH cells may serve a role in the pathogenesis of adult cITP.
IL-2 is an essential factor in preventing autoimmune disease development and inhibiting TFH differentiation (12). Previous data has demonstrated TFH cells can express cytokines, such as TH2-associated cytokine IL-4 (13). However, changes in IL-4 levels were not observed in cITP and ITP responders in the present study. In light of these findings, it was suggested that these two cytokines may be involved in the early stage of antibody generation, however, their role in patients with cITP may be minor.
Notably, IL-10 is abundantly produced in healthy persons (14). Xin et al (15) identified that IL-10-producing TFH cells have an increased capacity to form stable TFH-B cell conjugates compared with their IL-10-TFH counterparts, suggesting that IL-10+TFH cells may specialize in providing distress signals to B cells during chronic infection. Importantly, depletion of IL-10+/IL-21+-coproducing CD4+ T cells or deletion of IL-10, specifically from TFH cells, resulted in impaired GC B cell responses, lymphocytic choriomeningitis virus-specific antibody production and viral control (16). Subsequently, a heterogeneous population of TFH cells was determined and a critical role for TFH-derived IL-10 in promoting humoral immunity during persistent viral infection was elucidated (17). In agreement with these findings, the present study revealed a significant change of IL-10 in cITP and ITP responders. However, further flow cytometry analysis of IL-10+ TFH cells may be required to confirm its role.
IL-21 is a type I cytokine that signals via a specific receptor protein, IL-21 receptor (18,19), and the common cytokine receptor γ-chain, γc, which is shared by the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 (20). In addition, it has been recognized that IL-21 may be a master cytokine that promotes the expression of Bcl-6 in CD4+ T cells (21–23). TFH cells produce high levels of IL-21, a cytokine that is critical for GC formation and also for the generation of TFH cells (24). In the present study, IL-21 was significantly increased in cITP and significantly decreased in ITP responders post-treatment. These results further confirmed the possible regulatory role of IL-21 in ITP.
Bcl-6 is a selectively expressed transcription factor in murine and human TFH cells (25,26). Notably, Bcl-6 was previously demonstrated to be inhibitory in Th2 responses by blocking STAT6 from binding to DNA (27). Furthermore, a previous study revealed that Bcl-6-deficient mice developed multi-organ inflammatory diseases, exhibited enhanced IgE production and defective GC reaction (28). Previous results have suggested that Bcl-6 deficiency in T cells resulted in impaired TFH cell development in vitro and in vivo and that Bcl6 expression in B and T cells is required for GC reactions (29–31). Notably, transcriptional repressor Bcl-6 was considered as the critical gene involved with TFH cell differentiation (29). It has been suggested that overexpression of Bcl-6 promotes the mRNA expression of several TFH cell-associated genes in the absence of exogenous cytokines. Furthermore, Bcl-6 has been indicated to suppress the expression of various microRNAs that are considered to control TFH cell generation, including miR-17-92 (29). These findings suggest that Bcl-6 regulates TFH cell development through repression of microRNAs and Th1, Th2 and Th17 lineage-specific transcription factors (32). The present study revealed significantly decreased levels of Bcl-6 and IL-21 and increased levels of IL-10 in responders, indicating the Th1 and Th2 modulating effect of the Bcl-6 gene.
c-Maf is a transcription factor in the AP-1 family with a basic region/leucine zipper that is highly expressed by mature TFH cells and is thought to primarily function as a regulator of cytokines that can promote B cell proliferation and differentiation (33–35). The present study revealed c-Maf was significantly elevated in cITP and decreased in ITP responders post-treatment, suggesting a possible association of TFH cells in cITP and ITP responders. Furthermore, Sahoo et al (35) recently reported that c-Maf promotes IL-4 secretion in TFH cells through direct binding to the CNS2 region in the IL-4 locus and via induction of interferon regulatory factor 4, thus revealing a distinct role of c-Maf in IL-4 secretion between Th2 and TFH cell subsets. However, significant changes in IL-4 levels in cITP and ITP responders were not indicated in the present study, suggesting an alternative influence of Th2 and TFH cell subsets on IL-4 secretion.
PD-1, an immunoreceptor that belongs to the CD28/CTLA-4 family, has been demonstrated as an important molecule expressed on TFH cells (36). Typically, PD-1 is a negative regulatory signaling molecule that results in the inhibition of effector T cells via their specific ligands (PD-L1 and PD-L2), which are expressed on target cells (37). Furthermore, PD-1 signaling contributes to induce memory B cell differentiation and promotes the generation of high-affinity, long-lived plasma cells (38). Previous studies have indicated an increased frequency of PD-1+ TFH cells in several autoimmune renal diseases, including Henoch-Schönlein purpura nephritis (39), IgA nephropathy (40) and diabetic nephropathy (41). A significant downregulation of PD-1 expression levels was observed in patients with cITP in the present study compared with healthy controls; however, PD-1 levels were significantly increased in responders post-treatment compared with pre-treatment. Taken together, these results indicate possible involvement of TFH cells in ITP.
Bcl-6 and Blimp-1 present vital but opposing influences in the development of TFH cells, mutual antagonism between Bcl-6 and Blimp-1 is a primary mechanism for commitment to the T effector and TFH cell fates (42). Although Blimp-1 was previously indicated to be involved in Th cell differentiation (43), the present results indicate a remarkable change in the Bcl-6 gene levels and did not demonstrate a significant change of Blimp-1 levels at transcription level in cITP patients.
In conclusion, the present study demonstrated that the expansion of circulating TFH cells may serve a role in the immunopathogenesis of cITP. These data aided to elucidate that transcription factors such as Bcl-6, c-Maf and PD-1 and specific cytokine signaling of IL-10 and IL-21 may correlate with the abnormal activation of TFH cells in cITP. The present data revealed a novel mechanism in patients with cITP and provided an understanding of the role of TFH cells in cITP in offering diagnostic values and potential novel therapeutic strategies. Further research is required to fully clarify the clinical outcome of respondent and non-respondent cITP patients with expanded TFH cells.
Acknowledgements
Not applicable.
Funding
The present work is supported by the National Natural Science Foundation of China (grant no. 81700132) and the Natural Science Foundation of Jiangsu Province (grant no. BK20170324).
Availability of data and materials
The data and materials used to support the findings of this study are included within the published article.
Authors' contributions
LD and CR conceived and designed the study. LD, LH, LC, ZW and MZ performed the experiments. LH reviewed and edited the manuscript. XB and YH analyzed the data. CR gave final approval of the version to be published. All authors read and approved the manuscript.
Ethics approval and consent to participate
Approval for the present study was obtained by the Ethics Committee of The First Affiliated Hospital of Soochow University.
Patient consent for publication
All patients admitted to the study provided informed consent for their participation of the present study and publication of the data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nomura S. Advances in diagnosis and treatments for immune thrombocytopenia. Clin Med Insights Blood Disord. 2016;9:15–22. doi: 10.4137/CMBD.S39643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16:620–632. doi: 10.1016/j.autrev.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129:2829–2835. doi: 10.1182/blood-2017-03-754119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper N. State of the art-how i manage immune thrombocytopenia. Br J Haematol. 2017;177:39–54. doi: 10.1111/bjh.14515. [DOI] [PubMed] [Google Scholar]
- 5.Audia S, Rossato M, Trad M, Samson M, Santegoets K, Gautheron A, Bekker C, Facy O, Cheynel N, Ortega-Deballon P, et al. B cell depleting therapy regulates splenic and circulating T follicular helper cells in immune thrombocytopenia. J Autoimmun. 2017;77:89–95. doi: 10.1016/j.jaut.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Eivazi S, Bagheri S, Hashemzadeh MS, Ghalavand M, Qamsari ES, Dorostkar R, Yasemi M. Development of T follicular helper cells and their role in disease and immune system. Biomed Pharmacother. 2016;84:1668–1678. doi: 10.1016/j.biopha.2016.10.083. [DOI] [PubMed] [Google Scholar]
- 7.Shekhar S, Yang X. The darker side of follicular helper T cells: From autoimmunity to immunodeficiency. Cell Mol Immunol. 2012;9:380–385. doi: 10.1038/cmi.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259:103–114. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan H, Moore JC, Finch CN, Warkentin TE, Kelton JG. The IgG subclasses of platelet-associated autoantibodies directed against platelet glycoproteins IIb/IIIa in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2003;122:818–824. doi: 10.1046/j.1365-2141.2003.04509.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanz MÁ, García Vicente V, Fernández A, López MF, Grande C, Jarque I, Martínez R, Mingot ME, Monteagudo E, Ribera JM, et al. Guidelines for diagnosis, treatment and monitoring of primary immune thrombocytopenia. Med Clin (Barc) 2012;138:261.e1–261.e17. doi: 10.1016/j.medcli.2011.11.011. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Ballesteros-Tato A, León B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, Randolph GJ, Taylor JJ, Pearce EJ. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J Immunol. 2015;194:2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Hartog G, van Osch TLJ, Vos M, Meijer B, Savelkoul HFJ, van Neerven RJJ, Brugman S. BAFF augments IgA2 and IL-10 production by TLR7/8 stimulated total peripheral blood B cells. Eur J Immunol. 2018;48:283–292. doi: 10.1002/eji.201646861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin G, Schauder DM, Zander R, Cui W. Two is better than one: Advances in pathogen-boosted immunotherapy and adoptive T-cell therapy. Immunotherapy. 2017;9:837–849. doi: 10.2217/imt-2017-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, Kaech SM. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol. 2015;16:871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, Reyes-Teran G, Bosinger SE, Silvestri G. Decreased T follicular regulatory cell/T follicular helper cell (TFH) in Simian Immunodeficiency virus-infected rhesus macaques may contribute to accumulation of TFH in chronic infection. J Immunol. 2015;195:3237–3247. doi: 10.4049/jimmunol.1402701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong J, Kim WH, Yoo J, Lee C, Kim S, Cho JH, Jang HK, Kim DW, Lillehoj HS, Min W. Identification and comparative expression analysis of interleukin 2/15 receptor β chain in chickens infected with E. tenella. PLoS One. 2012;7:e37704. doi: 10.1371/journal.pone.0037704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis KE, Selby MJ, Masters G, Valle J, Dito G, Curtis WR, Garcia R, Mink KA, Waggie KS, Holdren MS, et al. Interleukin-21 combined with PD-1 or CTLA-4 blockade enhances antitumor immunity in mouse tumor models. Oncoimmunology. 2017;7:e1377873. doi: 10.1080/2162402X.2017.1377873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharibi T, Majidi J, Kazemi T, Dehghanzadeh R, Motallebnezhad M, Babaloo Z. Biological effects of IL-21 on different immune cells and its role in autoimmune diseases. Immunobiology. 2016;221:357–367. doi: 10.1016/j.imbio.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: Predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 22.Vyas AK, Trehanpati N. Commentary: IL-21 receptor antagonist inhibits differentiation of B cells toward plasmablasts upon alloantigen stimulation. Front Immunol. 2017;8:934. doi: 10.3389/fimmu.2017.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Audia S, Rossato M, Santegoets K, Spijkers S, Wichers C, Bekker C, Bloem A, Boon L, Flinsenberg T, Compeer E, et al. Splenic TFH expansion participates in B-cell differentiation and antiplatelet-antibody production during immune thrombocytopenia. Blood. 2014;124:2858–2866. doi: 10.1182/blood-2014-03-563445. [DOI] [PubMed] [Google Scholar]
- 24.Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol. 2010;22:7–12. doi: 10.1093/intimm/dxp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 27.Harris MB, Chang CC, Berton MT, Danial NN, Zhang J, Kuehner D, Ye BH, Kvatyuk M, Pandolfi PP, Cattoretti G, et al. Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL-6: Specific regulation of iepsilon transcription and immunoglobulin E switching. Mol Cell Biol. 1999;19:7264–7275. doi: 10.1128/MCB.19.10.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RS, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 29.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Y, Hou M. T cells in the pathogenesis of immune thrombocytopenia. Semin Hematol. 2016;53(Suppl 1):S13–S15. doi: 10.1053/j.seminhematol.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Xin N, Fu L, Shao Z, Guo M, Zhang X, Zhang Y, Dou C, Zheng S, Shen X, Yao Y, et al. RNA interference targeting Bcl-6 ameliorates experimental autoimmune myasthenia gravis in mice. Mol Cell Neurosci. 2014;58:85–94. doi: 10.1016/j.mcn.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S, Ikeda K, Hirose K, Watanabe N, Grusby MJ, Iwamoto I, Nakajima H. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 35.Sahoo A, Alekseev A, Tanaka K, Obertas L, Lerman B, Haymaker C, Clise-Dwyer K, McMurray JS, Nurieva R. Batf is important for il-4 expression in T follicular helper cells. Nat Commun. 2015;6:7997. doi: 10.1038/ncomms8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger KN, Pu JJ. PD-1 pathway and its clinical application: A 20 year journey after discovery of the complete human PD-1 gene. Gene. 2018;638:20–25. doi: 10.1016/j.gene.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 37.Guo Y, Walsh AM, Canavan M, Wechalekar MD, Cole S, Yin X, Scott B, Loza M, Orr C, McGarry T, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One. 2018;13:e0192704. doi: 10.1371/journal.pone.0192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greisen SR, Rasmussen TK, Stengaard-Pedersen K, Hetland ML, Hørslev-Petersen K, Hvid M, Deleuran B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol. 2014;43:101–108. doi: 10.3109/03009742.2013.823517. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Zhao S, Zhang L, Crew R, Zhang N, Sun X, Jiang Y. A higher frequency of CD4(+)CXCR5(+) T follicular helper cells in patients with newly diagnosed Henoch-Schönlein purpura nephritis. Int Immunopharmacol. 2016;32:8–15. doi: 10.1016/j.intimp.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Wang Y, Shi X, Zou H, Jiang Y. A higher frequency of CD4+CXCR5+ T follicular helper cells in patients with newly diagnosed IgA nephropathy. Immunol Lett. 2014;158:101–108. doi: 10.1016/j.imlet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Zhang N, Tai J, Qu Z, Zhang Z, Zhao S, He J, Zhang S, Jiang Y. Increased CD4+CXCR5+ T follicular helper cells in diabetic nephropathy. Autoimmunity. 2016;49:405–413. doi: 10.1080/08916934.2016.1196677. [DOI] [PubMed] [Google Scholar]
- 42.Krishnamoorthy V, Kannanganat S, Maienschein-Cline M, Cook SL, Chen J, Bahroos N, Sievert E, Corse E, Chong A, Sciammas R. The IRF4 gene regulatory module functions as a read-write integrator to dynamically coordinate T helper cell fate. Immunity. 2017;47(481–497):e7. doi: 10.1016/j.immuni.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu SH, Yeh LT, Chu CC, Yen BL, Sytwu HK. New insights into Blimp-1 in T lymphocytes: A divergent regulator of cell destiny and effector function. J Biomed Sci. 2017;24:49. doi: 10.1186/s12929-017-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials used to support the findings of this study are included within the published article.