Abstract
The care of individual patients requiring anthracyclines remains challenging as uncertainty persists on predictors of cardiotoxicity. The aim of the present study was to identify potential candidate blood indicators of doxorubicin-induced heart failure. The gene expression profiles of GSE40447 and GSE9128 microarray data were downloaded from the Gene Expression Omnibus database to identify differentially expressed genes (DEGs) using the R/Limma package or GEO2R. Functional and pathway enrichment analysis on DEGs were performed using DAVID database. The cardiovascular disease (CVD)-related DEGs were screen out based on the CardioGenBase database. The protein-protein interaction (PPI) network was constructed with STRING database and visualized by using Cytoscape. Then, the CVD-related DEGs were validated by intersection analysis with DEGs in GSE9128. The overlapping DEGs with a consistent expression pattern in GSE40447 and GSE9128 were identified as candidate indicators for doxorubicin-induced heart failure. A total of 516 DEGs potentially associated with doxorubicin-induced heart failure in GSE40447 were identified, which were mainly enriched in the gene ontology terms related to B cells, leukocytes, lymphocyte activation and B cell receptor signaling pathway. Of the DEGs, 42 were screened out as CVD-related DEGs by using CardioGenBase. Seven genes with high connectivity degree were presented in the PPI network. Finally, 5/6 CVD-related DEGs revealed by the intersection analysis were validated by GSE9128 and highlighted as candidate indicators of doxorubicin-induced heart failure: CD163, CD28, SLC25A20, ANPEP and TLR5. Several genes, including the 5 previously mentioned, were proposed as potential candidate blood indicators for doxorubicin-induced heart failure. Further experimental validations are greatly warranted for future clinical application.
Keywords: cardiotoxicity, doxorubicin, differentially expressed gene, microarray, bioinformatics
Introduction
Cancer outcome is continually improved in recent years with the introduction of early detection, advances in the chemotherapy, radiotherapy, and targeted therapy strategies; and the implementation of multidisciplinary cancer care (1). In light of the prominent role of chemotherapy, anthracycline especially doxorubicin remains to be considered the footstone chemotherapy regimen of cancer treatment for a wide range of malignancies, particularly for breast cancer and lymphoma (2,3). Over decades from its discovery, the anti-tumor and cardiotoxic mechanisms of doxorubicin seem to continuously evoke considerable interest in basic science and clinical trial research. Despite the great achievement in the survival of cancer patients, there is a serious alert nowadays that today's cancer patients may be tomorrow's cardiac patients due to the increased awareness of the doxorubicin-induced cardiotoxicity (4,5).
Paradoxically, apparently prolonged survival with doxorubicin is accompanid by increased risk of cardiac damage in cancer patients, particularly in children, because the damage may not manifest for many years and sustains to be a life-long threat (6). Reportedly, the risk of cardiotoxicity varies according to the type and intensity of cancer treatment (7). A devastating cardiotoxic effect of doxorubicin is principally heart failure, with incidence rates from 0.14 to 48% (estimated risk ranges from 0.14 to 5% for doses >400 mg/m2, 7 to 26% for 550 mg/m2 and 18 to 48% for 700 mg/m2) (8). Clinically, in terms of the benefit as well as cardiotoxic effect with doxorubicin, the paradox could greatly undermine the decision making of clinicians with the lack of effective and convenient approaches to monitor and quantify the cardiotoxic effects.
Although recent emphasis on the development of biomarkers indicative of doxorubicin-induced cardiac damage has resulted in the identification of several proposed biomarkers, such as troponins, myoglobin, actate dehydrogenase, creatine phosphokinase, C-redactive protein, brain-type natriuretic peptide (BNP), and pro-BNP (6,9–11), consistent findings focusing on concerns were not always presented. For instance, administration of doxorubicin was not always associated with elevated troponin level (12). Moreover, an increase in pro-BNP level has been found early after doxorubicin use, however, elevated pro-BNP level does not predict left ventricular dysfunction (13,14).
Considering the uncertainty about the effectiveness of cardiac markers to quantify doxorubicin-induced cardiotoxicity, we therefore conducted a microarray-based study to systematically identify more potential candidate blood indicators for doxorubicin- induced heart failure via a bioinformatics analysis.
Materials and methods
Microarray data
The microarray expression profile dataset GSE40447 deposited by McCaffrey et al (15), was downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE40447) and was based on the platform of GPL16006[(HG-U133_Plus_2) Affymetrix Human Genome U133 Plus 2.0 Array]. A total of 15 blood samples from 5 women with doxorubicin chemotherapy-induced heart failure (HF) and 10 women with no HF but a history of doxorubicin chemotherapy, were used for the analysis. The microarray expression profile dataset GSE9128 including 12 blood samples from chronic heart failure patients and 12 age- and sex-matched controls was used for validation analysis (16). The GSE9128 dataset deposited by Cappuzzello et al (16) was based on the platform of GPL96 [(HG-U133A) Affymetrix Human Genome U133A Array].
Data preprocessing and identification of differentially expressed genes (DEGs)
For GSE40447 data, the probe-level raw data (CEL format) were preprocessed by using a robust multiarray average algorithm with the Affy package in Bioconductor (17). Missing data were imputed by the KNN-based method and median data normalization was performed using robust multichip averaging for multiple probes corresponding to a common gene symbol (18,19). The DEGs following doxorubicin chemotherapy between HF and non-HF samples were identified by using the limma package in R/Bioconductor (20). For GSE9128 data, GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/), a R-based interactive web tool commonly used to compare two independent groups of samples for identifying DEGs with lack of raw CEL format data, was used to screen DEGs between HF and control samples. Similarly, the limma package involving empirical Bayes statistics was integrated for DEG analysis. |logFC|≥0.585 (|logFC|≥0.585 indicated that fold-change >1.5) and P-value <0.05 were considered as threshold values for DEGs in Limma and GEO2R.
Functional enrichment analysis
Gene Ontology (GO) functional enrichment including biological process and molecular function and Kyoto Encyclopedia of Gene and Genomes (KEGG) pathway enrichment analyses were performed for DEGs in GSE40447 by using the Database for Annotation, Visualization and Integrated Discovery (DAVID) to study differentially expressed clusters at the functional level (https://david.ncifcrf.gov/) (21). P<0.05 was considered as the cut-off criterion for the enrichment analysis.
Screening for cardiovascular disease (CVD)-related DEGs
CVD-related DEGs were identified by mapping to a Literature Based Multi-Omics Database for Major Cardiovascular Diseases: CardioGenBase (http://cardiogenbase.com/), which collected gene-disease association data from PubMed and MEDLINE and covered major CVDs such as cerebrovascular disease, coronary artery disease (CAD), hypertensive heart disease, inflammatory heart disease, ischemic heart disease and rheumatic heart disease (22). The CardioGenBase database documented ~1,500 CVD genes from ~2,4000 research articles.
Construction of protein-protein interaction (PPI) network
The DEGs in GSE40447 data were mapped to the overall PPIs by using the database Search Tool for the Retrieval of Interacting Genes (STRING, http://string-db.org/), which integrates a variety of predicted and experimentally validated interactions of proteins (23). Then, the primary PPI network was constructed with a threshold value of combined score >0.4 output by the STRING database and visualized by using Cytoscape (http://cytoscape.org/) (24).
Microarray-based validation for CVD-related DEGs
The CVD-related DEGs were validated by an intersection analysis with DEGs in the GSE9128 data. The overlap with consistent expression pattern of DEGs in GSE40447 and GSE9128 were identified as candidate indicators for doxorubicin- induced heart failure. Meanwhile, the expression files of candidate indicators in experiment and control groups in GSE9128 were obtained from the GEO database, of which the relative expression corresponded to the results of DEG analysis was graphically presented.
Results
Identification of DEGs in GSE40447 microarray data
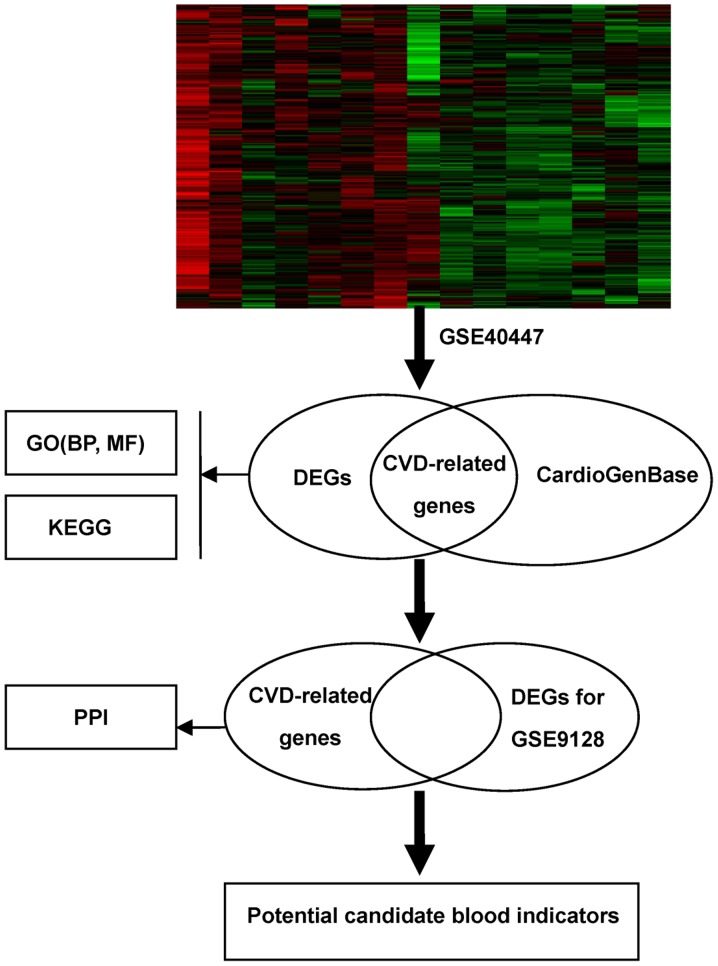
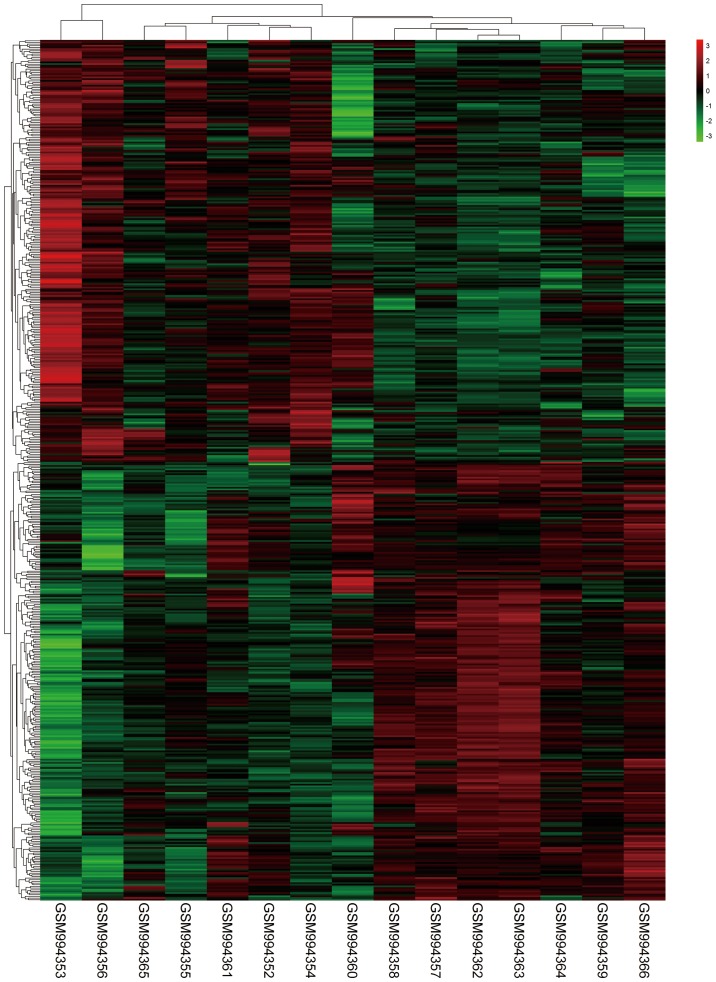
The workflow of the proposed analysis was performed as Fig. 1. As a result shown in Fig. 2, a total of 516 DEGs in GSE40447 including 263 down-regulated genes and 253 up-regulated genes were identified in HF group following doxorubicin chemotherapy compared to non-HF group with a history of doxorubicin chemotherapy according to the established cut-off criterion. The heatmap for DEGs is shown in Fig. 3.
Figure 1.
Workflow of the proposed analysis.
Figure 2.
Intersection analysis between DEGs in GSE40447 microarray data and cardiovascular genes in CardioGenBase; the 42 overlapped genes were CVD-related genes. DEGs, differentially expressed genes; PPI, protein-protein interaction; GO, Gene Ontology; BP, biological process; MF, molecular function; CVD, cardiovascular disease.
Figure 3.
Heat maps of blood DEGs in patients with doxorubicin-induced heart failure compared to patients without heart failure but with a history of doxorubicin treatment. DEGs, differentially expressed genes.
GO and KEGG pathway enrichment analyses of DEGs in GSE40447 data
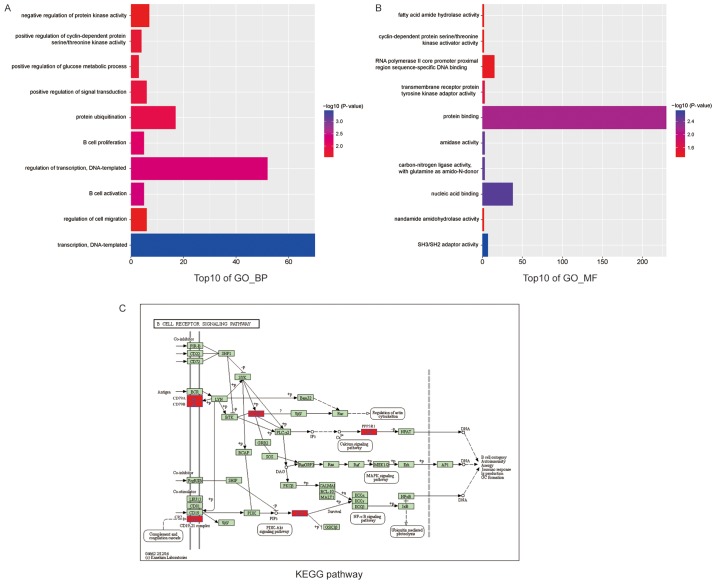
DAVID provided comprehensively functional annotation to understand the biological meaning behind the large list of genes. In the current study, GO enrichment analysis using DAVID was performed to explore DEGs functions from two aspects, including molecular function and biological process. As presented in Fig. 4, the results showed that the top10 overrepresented GO terms in biological processes were enriched in B-cell activation, leukocyte activation, cell activation, lymphocyte activation, regulation of transcription, transcription, negative regulation of B-cell proliferation, inflammatory response, regulation of lymphocyte proliferation and regulation of mononuclear cell proliferation. Additionally, the top10 enriched GO terms in molecular function were protein domain-specific binding, molecular adaptor activity, carbon-nitrogen ligase activity, with glutamine as an amido-N-donor, copper ion binding, phospholipid binding, SH3/SH2 adaptor activity, zinc ion binding, phosphoinositide binding, UDP-galactose: Glucosylceramide beta-1,4-galactosyltrans- ferase activity and transcription factor binding. On the other hand, we adopted KEGG enrichment analysis by clustering similar genes into a same network to understand the DEGs action mode. The B-cell receptor signaling pathway containing 6 DEGs (BLNK, CD79A, CD79B, CR2, PPP3R1, AKT1) indicated with red was enriched (Fig. 4).
Figure 4.
Functional enrichment analysis and KEGG pathway analysis of DEGs in GSE40447. (A) GO analysis of biological process. (B) GO analysis of molecular function. (C) KEGG pathway enrichment analysis. DEGs, differentially expressed genes.
Screening for CVD-related DEGs
Many DEGs generated may result from various responses to doxorubicin chemotherapy with multifarious etiologies but not just cardiac damage. In order to screen the CVD-related DEGs, the CardioGenBase database containing 1556 CVD-related genes was applied. Finally, the intersection analysis showed 42 DEGs associated with CVD (Fig. 2 and Table I). Of note, all 42 genes were literarily confirmed with a tight relevance to CVD in MEDLINE.
Table I.
CVD-related DEGs mapped in the CardioGenBase database.
| Gene Symbol | HGNC ID | Gene Location | No. of articles |
|---|---|---|---|
| ABCB1 | 40 | 7q21.12 | 3 |
| AKT1 | 391 | 14q32.33 | 22 |
| ANPEP | 500 | 15q25-q26 | 1 |
| ARHGAP5 | 675 | 14q12 | 2 |
| BGLAP | 1043 | 1q22 | 11 |
| CD163 | 1631 | 12p13 | 2 |
| CD28 | 1653 | 2q33 | 1 |
| CD40 | 11919 | 20q12-q13.2 | 30 |
| CD79A | 1698 | 19q13.2 | 2 |
| CD83 | 1703 | 6p23 | 1 |
| CDKN2D | 1790 | 19p13 | 1 |
| CELSR2 | 3231 | 1p13.3 | 4 |
| CHML | 1941 | 1q43 | 3 |
| CTNS | 2518 | 17p13 | 1 |
| EBF1 | 3126 | 5q34 | 1 |
| F5 | 3542 | 1q23 | 54 |
| GAA | 4065 | 17q25.2-q25.3 | 3 |
| GABPA | 4071 | 21q21-q22.1 | 1 |
| GATA2 | 4171 | 3q21 | 3 |
| GEN1 | 26881 | 2p24.2 | 1 |
| HDC | 4855 | 15q21.2 | 1 |
| IDUA | 5391 | 4p16.3 | 1 |
| IGFBP4 | 5473 | 17q21.2 | 3 |
| IL3RA | 6012 | Xp22.3 and Yp13.3 | 1 |
| KLRB1 | 6373 | 12p13 | 1 |
| MACROD2 | 16126 | 20p12.1 | 1 |
| MPO | 7218 | 17q21.3-q23 | 82 |
| NEFL | 7739 | 8p21.2 | 1 |
| OLR1 | 8133 | 12p13.1-p12.3 | 27 |
| PAWR | 8614 | 12q21.2 | 6 |
| PEX6 | 8859 | 6p22-p11 | 1 |
| PPP3R1 | 9317 | 2p14 | 1 |
| PTGDS | 9592 | 9q34.2-q34.3 | 2 |
| SASS6 | 25403 | 1p21.3 | 4 |
| SCN3A | 10590 | 2q24 | 1 |
| SLC22A16 | 20302 | 6q21 | 1 |
| SLC25A20 | 1421 | 3p21.31 | 3 |
| SPRY1 | 11269 | 4q | 1 |
| TBXAS1 | 11609 | 7q34-q35 | 52 |
| TLR5 | 11851 | 1q32.3-q42 | 1 |
| UNC13D | 23147 | 17q25.3 | 1 |
| XRCC1 | 12828 | 19q13.2 | 5 |
CVD, cardiovascular disease; DEGs, differentially expressed genes; HGNC, HUGO Gene Nomenclature Committee.
PPI network construction for CVD-related DEGs
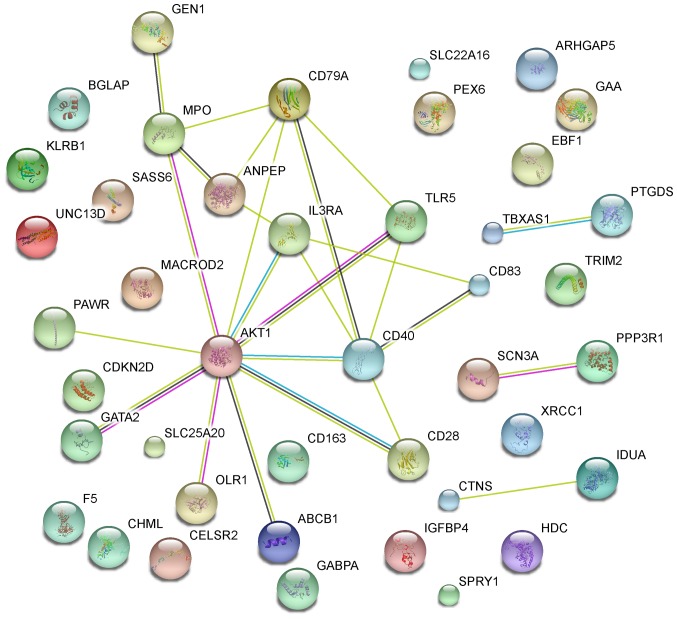
The primary PPI network including all CVD-related DEGs was constructed using STRING database (Fig. 5) and was further constructed with a threshold value of a combined score >0.4 and visualized by Cytoscape. Overall, 20 nodes and 25 edges were mapped in the PPI network of identified DEGs, including 11 up-regulated genes and 9 down-regulated genes (Fig. 6). The 7 nodes with the higher degrees were screened as hub genes (more than 3 interactions for each hub gene), including AKT1, MPO, ANPEP, CD79A, CD40, IL3RA and TLR5.
Figure 5.
PPI of DEGs in GSE4047 constructed by STRING database. PPI, protein-protein interaction; DEGs, differentially expressed genes.
Figure 6.
PPI network of CVD-related DEGs constructed with STRING database and displayed with Cytoscape. Genes in orange were upregulated and those in blue were downregulated. PPI, protein-protein interaction; CVD, cardiovascular disease; DEGs, differentially expressed genes.
Validation for CVD-related DEGs by GSE9128
The study involving GSE9128 was designed to identify diagnostic/prognostic blood markers and gene expression profiles of chronic heart failure. Based on the established cut-off criterion for DEGs, 651 DEGs (330 down-regulated and 321 up-regulated) were identified in HF group compared to healthy control. To validate the CVD-related DEGs induced by doxorubicin treatment, an intersection analysis with DEGs in GSE9128 was performed, and 6 genes (CD163, CD28, SLC25A20, ANPEP, TLR5, CD40) was shown in the overlap (Fig. 7). However, 5 genes (CD163, CD28, SLC25A20, ANPEP, TLR5) were highlighted with a consistent expression pattern in GSE40447 and GSE9128, and were finally identified as potential candidate blood indicators for doxorubicin-induced heart failure (Table II). Corresponded to the detailed results of DEG analysis, the expression files of the candidate indicators obtained from GSE9128 in experiment and control groups were graphically presented. As shown in Fig. 8, 2.14, 0.52, 1.53, 1.74 and 1.68 fold-change transcriptional level for CD163, CD28, SLC25A20, ANPEP and TLR5 were observed compared to the non-HF healthy control, respectively.
Figure 7.
Intersection analysis between DEGs in GSE9128 data and CVD-related DEGs, proposing some potential indicators for heart failure. CVD, cardiovascular disease; DEGs, differentially expressed genes.
Table II.
| LogFC | P-value | Expression pattern | ||||
|---|---|---|---|---|---|---|
| Gene symbol | GSE40447 | GSE9128 | GSE40447 | GSE9128 | GSE40447 | GSE9128 |
| CD163 | 0.7023137 | 1.0998679 | 0.0254562 | 0.0410781 | Up | Up |
| CD28 | −0.700993 | −0.9513838 | 0.049 | 0.0097194 | Down | Down |
| SLC25A20 | 0.6808281 | 0.6178648 | 0.0299684 | 0.0449655 | Up | Up |
| ANPEP | 0.9295412 | 0.7951052 | 0.0275249 | 0.0012539 | Up | Up |
| TLR5 | 0.6858654 | 0.7478113 | 0.0332472 | 0.0127178 | Up | Up |
| CD40 | −1.36061145 | 0.8154462 | 0.002348725 | 0.04237951 | Down | Up |
An absolute LogFC>0.585 indicates 1.5-fold change. CVD, cardiovascular disease; FC, fold change.
Figure 8.
Expression validation of CVD-related DEGs by GSE9128 compared to that in GSE40447. CVD, cardiovascular disease; DEGs, differentially expressed genes.
Discussion
Currently, bioinformatics analysis with high-throughput gene expression profiling has provided systematic insights into the mechanism of doxorubicin-induced cardiotoxic effects, related to the underlying gene activity changes and more importantly, has enabled the identification of targets for a diagnosis and prevention policy. In the present study, a total of 516 blood DEGs potentially related to doxorubicin-induced HF were initially identified with the GSE40447 microarray data. Functional enrichment analysis showed that these DEGs were mainly related to B -cell receptor signaling pathway. Of the DEGs, 42 were literarily evidenced as CVD-related genes. Importantly, the further validation analysis revealed that 5 CVD-related DEGs (CD163, CD28, SLC25A20, ANPEP, TLR5) may served as potential candidate blood indicators for doxorubicin-induced heart failure.
The pathogenesis of doxorubicin-induced HF is a complex process driven by specific genetic alterations and susceptible drug toxicity. Hemoglobin scavenger receptor CD163 is a macrophage-specific protein and its up-regulation is one of the major changes in the macrophage switch to alternative activated phenotypes in inflammation (25). This upregulation may echo the activation of inflammation triggered by doxorubicin-related cardiac damage in that enhanced inflammation has been confirmed as a hallmark of cardiac injury (26). In support of this, several previous studies have revealed increased serum level of CD163 along with elevated inflammatory cytokines in patients with atrial fibrillation (27) and coronary heart disease (28). In addition, elevated serum CD163 level was also found in patients with chronic heart failure with reduced ejection fraction (29). Hence, the results of the current study are in line with previous findings, suggesting the potential role of increased CD163 expression in doxorubicin-induced cardiotoxicity. Moreover, the CD28 molecule is a type I transmembrane protein expressed on the surface of 80% of human CD4+ T cells and 50% of human CD8+ T cells (30), which is well known as one of the most important co-stimulatory receptors for T-lymphocyte activation and T-cell receptor signal transduction, thereby regulating the production of inflammatory cytokine/chemokines (31). Several separate studies have reported that CD28 might act as an accomplice but not always a defender by demonstrating the deleterious effect of CD4+CD28 null T-lymphocytes in patients with atrial fibrillation, chronic heart failure, coronary artery disease, atherosclerosis and other CVDs (32–34). Investigators have uncovered partial mechanisms responsible for the deleterious effect of activation of cytotoxic lymphocytes secreting pro-inflammatory cytokines to promote inflammation and the development of cardiovascular inflammatory diseases (35). Therefore, it is not difficult to speculate the detrimental role of CD28 down-regulation in doxorubicin-induced cardiotoxicity.
The present study also suggested a role for Toll-like receptor 5 (TLR5) up-regulation in doxorubicin-induced cardiotoxicity. In the heart, previous research found that TLR5 activation may condition vascular dendritic cells (DCs) to support perivasculitic infiltrates possibly by increasing the suppressive activity of Treg cells and inducing the synthesis of immunosuppressive interleukins IL-10, IL-35, and transforming growth factor β (36,37). Supportively, Zhu et al (38) found that TLR5 polymorphism might be associated with rheumatic heart disease risk in a Chinese Han population. Platelet TLR5 was found associated with ratio of total cholesterol to high-density lipoprotein, thus contributing to cardiovascular risk (39). Therefore, it could not rule out the link between TLR5-involved vasculitic and doxorubicin-induced cardiotoxicity although the underlying mechanism remains uninvestigated.
For the solute carrier family 25 member 20 (SLC25A20 or CACT), a key carnitine-acylcarnitine translocase exchanging for free carnitine across the mitochondrial membrane in mitochondrial beta-oxidation, little information has been acknowledged on its pathophysiologic mechanisms in CVD except for several case reports about the CACT-deficiency in cardiomyopathy, arrythmias and cardiogenic shock (40–42). However, the current study found for the first time a markedly up-regulation of SLC25A20 transcriptional level in doxorubicin-induced HF, which was further validated with a 1.74-fold change in a HF cohort compared to the healthy control. Nevertheless, the underlying mechanism remains unknown and that whether this is a compensatory upregulation to prevent injury needs to be studied.
The present study also suggested a potential role of upregulated alanyl aminopeptidase (ANPEP, APN or CD13) in doxorubicin-induced heart failure. ANPEP is a conserved type II integral membrane zinc-dependent metalloprotease in the M1 family of ectoenzymes (43), widely expressed on all myeloid cells, activated endothelial cells and epithelium of the kidney and intestine (44). Evolving evidence supported a role for ANPEP in controlling arterial blood pressure and the pathogenesis of hypertension by inhibiting renal tubule Na flux and modulating salt-adaptation (43,45). Interestingly, circulating ANPEP was found to be up-regulated in patients with essential hypertension and in the angiogenic vessels in the infarct area and border zone after myocardial infarction (44,46). Further exploration revealed that ANPEP is essential for promoting optimal post-infarction healing for the ischaemic heart via compensatory mechanisms including the preparation and sustainment of the reparative response to relieve potential angiogenic defects (44). Thus, it is biologically reasonable to speculate that the increase of ANPEP expression may be compensatory, which might be predictive for doxorubicin-induced cardiotoxicity.
Several limitations for the study should be acknowledged. The underlying molecular mechanism of doxorubicin-induced HF is not completely consistent with non-pharmaceutical HF. Some potential more important indicators specified for doxorubicin-induced HF may be concealed by the validation with the study GSE9128 designed for the identification of non-pharmaceutical HF biomarkers. For instance, the present study showed that DEGs in GSE40447 were mainly related to B cell receptor signaling pathway in KEGG enrichment analysis, whereas the focused indicators were not implicated, which may suggest the specificity of some DEGs that needed to be experimentally confirmed. Moreover, the potential blood indicators were initially proposed by the intersection between GSE40447 and CardioGenBase, which may exclude other important predictors of doxorubicin-induced heart injury that has not been documented before in CardioGenBase, and therefore may result in limited ability for diagnosis of doxorubicin-induced HF. Nevertheless, the highlighted indicators are biologically reasonable and reliable because of the identical molecular basis of heart failure and a systematic and scientific study approach in the study. Moreover, in light of applying predicament of the current proposed biomarkers in the clinic, these indicators may contribute to the diagnosis and prevention of doxorubicin-induced HF and the decision-making for clinicians when combined with several routine markers for cardiac injury such as troponins and pro-BNP. Definitely, the aforementioned results were based on microarray data with limited sample size and a lack of experimental verification, which was also a limitation of the study and required to be addressed by future studies.
In conclusion, the present study aimed to identify potential candidate blood biomarkers to predict doxorubicin-induced heart failure with comprehensive bioinformatics analysis. A set of significantly altered genes were identified and five (CD163, CD28, SLC25A20, ANPEP, TLR5) were highlighted by bioinformatics validation. Our results suggest that data mining and integration could be a useful tool to predict doxorubicin-induced heart failure. Despite the absence of experimental validation, this study still has significance for further study, providing potential new key genes for further experimental validation, and shedding light on the molecular mechanisms of doxorubicin-induced cardiotoxicity.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
GXW conceived and designed the study, and wrote the manuscript. LHJ, WBX and LC performed the statistical analysis, and data collation and interpretation. YGZ designed the study, and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–325. doi: 10.3322/caac.21341. [DOI] [PubMed] [Google Scholar]
- 2.Shabalala S, Muller CJF, Louw J, Johnson R. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017;180:160–170. doi: 10.1016/j.lfs.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Argun M, Üzüm K, Sönmez MF, Özyurt A, Derya K, Çilenk KT, Unalmış S, Pamukcu Ö, Baykan A, Narin F, et al. Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anatol J Cardiol. 2016;16:234–241. doi: 10.5152/akd.2015.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahadir A, Kurucu N, Kadıoğlu M, Yenilme E. The role of nitric oxide in Doxorubicin-induced cardiotoxicity: Experimental study. Turk J Haematol. 2014;31:68–74. doi: 10.4274/Tjh.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant J, Picot J, Baxter L, Levitt G, Sullivan I, Clegg A. Use of cardiac markers to assess the toxic effects of anthracyclines given to children with cancer: A systematic review. Eur J Cancer. 2007;43:1959–1966. doi: 10.1016/j.ejca.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Conway A, McCarthy AL, Lawrence P, Clark RA. The prevention, detection and management of cancer treatment-induced cardiotoxicity: A meta-review. BMC Cancer. 2015;15:366. doi: 10.1186/s12885-015-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2011;37:300–311. doi: 10.1016/j.ctrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Bai AD, Agarwal A, Steinberg M, Showler A, Burry L, Tomlinson GA, Bell CM, Morris AM. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteraemia: A systematic review and meta-analysis. Clin Microbiol Infect. 2017;23:900–906. doi: 10.1016/j.cmi.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D'Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Jain D, Russell RR, Schwartz RG, Panjrath GS, Aronow W. Cardiac complications of cancer therapy: Pathophysiology, identification, prevention, treatment, and future directions. Curr Cardiol Rep. 2017;19:36. doi: 10.1007/s11886-017-0846-x. [DOI] [PubMed] [Google Scholar]
- 12.Cardinale D, Sandri MT. Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis. 2010;53:121–129. doi: 10.1016/j.pcad.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: Systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008;130:688–695. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- 14.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 15.McCaffrey TA, Tziros C, Lewis J, Katz R, Siegel R, Weglicki W, Kramer J, Mak IT, Toma I, Chen L, et al. Genomic profiling reveals the potential role of TCL1A and MDR1 deficiency in chemotherapy-induced cardiotoxicity. Int J Biol Sci. 2013;9:350–360. doi: 10.7150/ijbs.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cappuzzello C, Napolitano M, Arcelli D, Melillo G, Melchionna R, Di Vito L, Carlini D, Silvestri L, Brugaletta S, Liuzzo G, et al. Gene expression profiles in peripheral blood mononuclear cells of chronic heart failure patients. Physiol Genomics. 2009;38:233–240. doi: 10.1152/physiolgenomics.90364.2008. [DOI] [PubMed] [Google Scholar]
- 17.Gautier L, Cope L, Bolstad BM, Irizarry RA. Affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 18.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17:520–525. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 19.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Nayar PG, Murugesan R, Mary BPD, Ahmed SS. CardioGenBase: A literature based Multi-Omics database for major cardiovascular diseases. PLoS One. 2015;10:e143188. doi: 10.1371/journal.pone.0143188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database Issue):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohl M, Wiese S, Warscheid B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303. doi: 10.1007/978-1-60761-987-1_18. [DOI] [PubMed] [Google Scholar]
- 25.Etzerodt A, Moestrup SK. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18:2352–2363. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landis RC, Philippidis P, Domin J, Boyle JJ, Haskard DO. Haptoglobin genotype-dependent anti-inflammatory signaling in CD163(+) macrophages. Int J Inflam. 2013;2013:980327. doi: 10.1155/2013/980327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong SM, Qin YH, Li ZC, Wei YS. Clinical value of detecting serum soluble CD163 level in patients with atrial fibrillation. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:1406–1409. (In Chinese) [PubMed] [Google Scholar]
- 28.Zou LY, Peng CQ, Li CZ, Zhao CL, Zhu JM, Liu JL, Zhang CX. Association between hemoglobin scavenger receptor CD163 expression and coronary atherosclerotic severity in patients with coronary heart disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37:605–609. (In Chinese) [PubMed] [Google Scholar]
- 29.Ptaszynska-Kopczynska K, Marcinkiewicz-Siemion M, Lisowska A, Waszkiewicz E, Witkowski M, Jasiewicz M, Miklasz P, Jakim P, Galar B, Musial WJ, Kaminski KA. Alterations of soluble TWEAK and CD163 concentrations in patients with chronic heart failure. Cytokine. 2016;80:7–12. doi: 10.1016/j.cyto.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci USA. 1987;84:8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porciello N, Tuosto L. CD28 costimulatory signals in T lymphocyte activation: Emerging functions beyond a qualitative and quantitative support to TCR signalling. Cytokine Growth Factor Rev. 2016;28:11–19. doi: 10.1016/j.cytogfr.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Sulzgruber P, Koller L, Winter MP, Richter B, Blum S, Korpak M, Hulsmann M, Goliasch G, Wojta J, Niessner A. The impact of CD4+CD28null T-lymphocytes on atrial fibrillation and mortality in patients with chronic heart failure. Thromb Haemost. 2017;117:349–356. doi: 10.1160/TH16-07-0531. [DOI] [PubMed] [Google Scholar]
- 33.Dumitriu IE, Araguás ET, Baboonian C, Kaski JC. CD4+ CD28 null T cells in coronary artery disease: When helpers become killers. Cardiovasc Res. 2009;81:11–19. doi: 10.1093/cvr/cvn248. [DOI] [PubMed] [Google Scholar]
- 34.Kyaw T, Tipping P, Toh BH, Bobik A. Killer cells in atherosclerosis. Eur J Pharmacol. 2017;816:67–75. doi: 10.1016/j.ejphar.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Kyaw T, Peter K, Li Y, Tipping P, Toh BH, Bobik A. Cytotoxic lymphocytes and atherosclerosis: Significance, mechanisms and therapeutic challenges. Br J Pharmacol. 2017;174:3956–3972. doi: 10.1111/bph.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piggott K, Biousse V, Newman NJ, Goronzy JJ, Weyand CM. Vascular damage in giant cell arteritis. Autoimmunity. 2009;42:596–604. doi: 10.1080/08916930903002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garib FY, Rizopulu AP. T-regulatory cells as part of strategy of immune evasion by pathogens. Biochemistry (Mosc) 2015;80:957–971. doi: 10.1134/S0006297915080015. [DOI] [PubMed] [Google Scholar]
- 38.Zhu L, Zou LJ, Hua R, Li B. Association of single-nucleotide polymorphisms in toll-like receptor 5 gene with rheumatic heart disease in Chinese Han population. Int J Cardiol. 2010;145:129–130. doi: 10.1016/j.ijcard.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Koupenova M, Mick E, Mikhalev E, Benjamin EJ, Tanriverdi K, Freedman JE. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2015;35:1030–1037. doi: 10.1161/ATVBAHA.114.304954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahapatra S, Ananth A, Baugh N, Damian M, Enns GM. Triheptanoin: A rescue therapy for cardiogenic shock in carnitine-acylcarnitine translocase deficiency. JIMD Rep. 2018;39:19–23. doi: 10.1007/8904_2017_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choong K, Clarke JT, Cutz E, Pollit RJ, Olpin SE. Lethal cardiac tachyarrhythmia in a patient with neonatal carnitine-acylcarnitine translocase deficiency. Pediatr Dev Pathol. 2001;4:573–579. doi: 10.1007/s10024001-0101-7. [DOI] [PubMed] [Google Scholar]
- 42.Rubio-Gozalbo ME, Bakker JA, Waterham HR, Wanders RJ. Carnitine-acylcarnitine translocase deficiency, clinical, biochemical and genetic aspects. Mol Aspects Med. 2004;25:521–532. doi: 10.1016/j.mam.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Danziger RS. Aminopeptidase N in arterial hypertension. Heart Fail Rev. 2008;13:293–298. doi: 10.1007/s10741-007-9061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira FE, Cronin C, Ghosh M, Zhou SY, Agosto M, Subramani J, Wang R, Shen JB, Schacke W, Liang B, et al. CD13 is essential for inflammatory trafficking and infarct healing following permanent coronary artery occlusion in mice. Cardiovasc Res. 2013;100:74–83. doi: 10.1093/cvr/cvt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sánchez-Agesta Ortega R, Arias de Saavedra-Alías JM, Liébana-Cañada A, Sánchez-Muñoz B, Martínez-Martos JM, Ramírez-Expósito MJ. Circulating aminopeptidase activities in men and women with essential hypertension. Curr Med Chem. 2013;20:4935–4945. doi: 10.2174/15672050113109990205. [DOI] [PubMed] [Google Scholar]
- 46.Buehler A, van Zandvoort MA, Stelt BJ, Hackeng TM, Schrans-Stassen BH, Bennaghmouch A, Hofstra L, Cleutjens JP, Duijvestijn A, Smeets MB, et al. cNGR: A novel homing sequence for CD13/APN targeted molecular imaging of murine cardiac angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2681–2687. doi: 10.1161/01.ATV.0000245807.65714.0b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.