Abstract
Objective: The purpose of this study was to evaluate the efficacy and safety of an automated microneedling device (Exceed, Amiea Med, MT.DERM GmbH, Berlin, Germany) when used for the rejuvenation of facial skin, as well as to generate data for an FDA 510K submission for the device. Study design: This was an open-label, single-center study. Participants: Forty-eight subjects aged 35 to 75 years with signs of facial skin aging were recruited. After consenting and satisfying inclusion criteria, each subject underwent four microneedling sessions 30 days apart. Subjects were assessed at baseline and at 30, 60, 90, and 150 days after the first treatment. Measurements: Wrinkles were assessed using the Lemperle Grading Scale. Skin laxity and texture were assessed using a modified Alexiades-Armenakas Grading Scale. Digital fringe projection technology (PRIMOS) was used to determine skin topography of the periorbital and mesolabial areas. Results: Mean improvements in global wrinkle score (mean of nine facial area grades), skin laxity, and skin texture at Day 150, compared to baseline were 1.23 (99% confidence interval [CI]: 1.11, 1.34), 1.09 (99% CI: 0.93, 1.26), and 1.54 (99% CI: 1.33, 1.75), respectively. Statistically significant mean improvements in these three measures were also observed at Day 90. Improvements in wrinkle grading and skin texture were confirmed by the PRIMOS profilometry. The treatment was well tolerated with minimal pain, discomfort, and downtime. Side effects were minor and easily managed. Conclusion: This study demonstrates that four microneedling treatments of facial skin, spaced four weeks apart, significantly improve lines, wrinkles, skin laxity and skin texture, 90 and 150 days after the first treatment. The treatment was well tolerated with minimal pain, discomfort, and downtime. Side effects were minor and easily managed compared to other invasive technologies, such as laser ablation and radiofrequency.
Keywords: Microneedling, device; aging skin; safety; effectiveness; single-center; open-label; minimally invasive; percutaneous collagen induction; wrinkles; laxity; texture; skin ageing; tissue remodeling; skin rejuvenation
As the demand for effective, less invasive aesthetic procedures increases, so does the quest for successful solutions. Patients often request therapies to rejuvenate photodamaged skin, lighten abnormal pigmentation, smooth textural problems, diminish rhytides, and improve skin laxity. This search for younger-looking skin has generated many different cosmetic techniques with varying degrees of invasiveness and efficacy, such as carbon dioxide laser resurfacing, deep chemical peels, and fractionated laser technology.1–4 Predominantly, these techniques work by damaging or causing the destruction of the epidermis, which leads to changes in the dermis that consequently initiate an inflammatory response, resulting in the production of new collagen and skin.5,6 There is, however, a cost to such invasive procedures. Typically, ablative techniques produce thicker bundles of scar collagen rather than the lattice network of collagen found in normal skin. Additionally, ablative procedures can cause the skin to become more sensitive to photodamage and more susceptible to post-procedural complications.1–5,7
Microneedling, also known as percutaneous collagen induction (PCI), is a minimally invasive technique that was first described as a principle by Orentreich and Orentreich8 whereby subcutaneous subcision surgery stimulates collagen beneath retracted scars and wrinkles. Fernandes9,10 used a similar technique of inserting a 15-gauge needle into the skin under the wrinkles. The technique by Fernandes was followed by the development of the dermal roller.9 A dermal roller is a sterile plastic cylinder with stainless steel needles protruding 1 to 3mm from the surface of the cylinder. The dermal roller is rolled vigorously over the skin to create numerous needle pricks, which lead to thousands of microscopic wounds in the dermis, initiating the natural post-traumatic inflammatory response (i.e., the release of growth factors and the formation of collagen and elastin).9,10
PCI treatment preserves the epidermis while stimulating collagen deposition, thereby reducing the risk of post-treatment complications and patient downtime. Today, PCI treatment can be performed in-office, and patients can resume normal activity the next day.
Modern automated microneedling devices have increasingly replaced the dermal roller. Typically, the needle cylinder is replaced by single-use, sterile needle cartridges with a range of different needle configurations. Rather than relying on the operator to physically roll the device over the skin, the automated devices allow the operator to define the penetration depth and frequency of the needle penetration and control the treatment area and coverage. Current automated microneedling devices contain multiple fine sterile needles, typically 0.5 to 1.5mm in length.
In addition to age-related skin conditions (e.g., wrinkles, laxity), there is a growing list of dermatological conditions that respond to PCI therapy, including acne scars, dyspigmentation, alopecia, and hyperhidrosis, and use of PCI in transdermal drug delivery have also been reported.11 However, most of the studies on microneedling have been case series or small randomized, controlled trials.12
Only one microneedling device, DEN160029, has been approved by the United States Food and Drug Administration (FDA) for acne scarring of the face (as of March 1, 2018). The purpose of this study was to evaluate the efficacy and safety of an automated microneedling device (Exceed, Amiea Med, MT.DERM GmbH, Berlin, Germany) when used for the rejuvenation of facial skin, as well as to generate data for an FDA 510K submission for the device.
METHODS
The study protocol was approved by IRB Co. (Buena Park, California) on January 29, 2016. This was a single-center, open-label study involving 48 healthy subjects. Screening data were reviewed to determine volunteer eligibility. After informed consent, Health Insurance Portability and Accountability Act of 1996 (HIPAA), confidentiality, and photographic release forms were completed by the study volunteers, those who met the inclusion criteria were enrolled in the study. Each subject underwent four microneedling sessions that were 30 days apart.
Subjects were assessed at baseline and before subsequent microneedling sessions at 30, 60 and 90 days. A final assessment of treatment outcomes was conducted at 150 days after the first treatment. Wrinkles, skin laxity, and skin texture were assessed by a physician. Routine VISIA photography and PRIMOS digital fringe projection technology (both from Canfield Scientific, Parsippany, New Jersey) were used to assess skin topography. Adverse events (AEs) and post-treatment erythema were assessed and recorded, and diaries were kept by the subjects.
Patient selection. Inclusion criteria. Healthy men and women aged 35 to 75 years who displayed facial rhytides (Lemperle Wrinkle Scale Grades 1–4) and skin laxity (Alexiandes Armenakas Laxity Scale Grades 1–3.5) were chosen.13,14 The grading scale by Lemperle was chosen because it is a photonumeric scale that allows reproducibility, versus a traditional descriptive scale, and it has been correlated with the measurement of wrinkle depth as determined by profilometry, which was used in this study.13
Exclusion criteria. Volunteers taking anticoagulant therapy or using aspirin or high-dose nonsteroidal anti-inflammatory drugs (NSAIDs) during the previous 14 days were excluded. Other exclusion criteria included the following:
-
•
Hepatitis
-
•
Active acne vulgaris of the face
-
•
Inflammatory skin conditions
-
•
Uncontrolled diabetes mellitus
-
•
Keloid scars
-
•
Human papillomavirus (HPV)
-
•
Birth marks
-
•
Eczema
-
•
Known malignancy
-
•
Chemotherapy, radiotherapy, or high-dose corticosteroid treatment
-
•
Known allergy to the topical anesthetic
-
•
Filler injections or neuromodulator treatment within the previous three months.
Microneedling device. The microneedling device used in this study primarily consists of a handpiece, safety needle cartridge, and control unit. The handpiece contains the motor that moves the needles of the safety needle cartridge and a needle depth gauge that allows the user to control the depth of needle incision from 0 to 1.5mm. The control unit allows the user to adjust the frequency of the needle stroke from 100 to 150hz. The safety needle cartridge contains six sterile, single-use, stainless steel microneedles (maximum 1.5mm length, 0.35mm gauge) (Figure 1).
Figure 1.
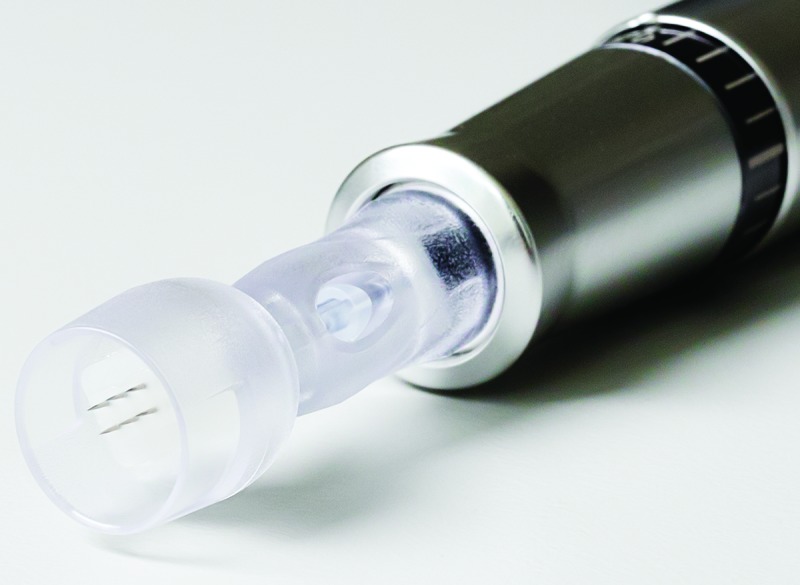
Safety needle cartridge of microneedling device comprising 6 sterile, single-use, stainless steel microneedles (maximum 1.5mm length, 0.35mm gauge)
Treatment protocol. Each subject underwent four microneedling sessions that were 30 days apart. After cleansing the face, a topical anesthetic (LMX 4, Eloquest Healthcare, Ferndale, Michigan) was applied to the entire face and jawline. After 30 minutes, the topical anesthetic was removed by wiping the skin with a cotton pad soaked in 70% isopropyl alcohol, and the skin was allowed to dry. A liberal quantity of Sterile Clear Image singles gel (NEXT Medical Products Company, Branchburg, New Jersey) was applied to the subject’s face to assist the movement of the needle cartridge over the skin, and the needle cartridge was then moved in circular motion across the face. The following needle depths were used to achieve pinpoint bleeding: forehead 0.7 to 1.0mm; cheek 0.9 to 1.5mm; eye area 0.5 to 0.8mm; chin 0.9 to 1.3mm; glabella and jawline 0.7 to 1.3mm; upper lip 0.7 to 1.0mm. After treatment, the skin was cleansed with warm water and sterile gauze. Subjects were provided instructions for post-skin care, including the use of moisturizer and sun block.
Recorded data. Efficacy. Wrinkles for nine separate facial areas were assessed by the physician using the grading scale developed by Lemperle.13 Skin laxity and texture were also assessed using a modified Alexiades-Armenakas14 Grading Scale.
The PRIMOS digital fringe projection technology was used to determine skin topography of the periorbital and mesolabial areas (i.e., wrinkle depth and texture). The following common topographic parameters were chosen based on previous publications:15–19
-
•
Ra (average roughness of skin)
-
•
Rmax (maximum roughness of skin)
-
•
Rz (average of single roughness depths)
-
•
Sa (arithmetic mean of surface roughness of skin)
-
•
Smax (height difference between highest peak and lowest valley on the entire measuring field)
-
•
Sz (mean of 5 highest peaks and deepest valleys).
Subjects completed diaries of self-assessment of treatment response. Subjects were asked to assess the effect of the treatment with respect to pigmentation, fine lines and wrinkles, texture, and pores. The four parameters were graded by each subject at baseline and at each follow-up using a five-point numerical grading scale, where 0=the most favorable outcome (e.g., no visible wrinkles, skin feels smooth) and 4=the worst outcome (e.g., very visible lines and wrinkles, very rough and grainy).
Safety. The number, type, and severity of AEs were recorded during the duration of the study period. Immediately after each microneedling procedure, the principle investigator graded erythema in the treatment area. Grading was carried out using a five-point scale where 0=no erythema or redness of the skin and 4=severe erythema (i.e., bright or dark red color to the skin).
Subjects graded their erythema, pain, and discomfort immediately after treatment and up to seven days post-treatment using a subject diary. A descriptive photonumeric grading scale was used to evaluate erythema. Pain and discomfort were recorded using an 11-point visual-analogue scale (0–10) where 0=no pain/discomfort and 10=most intense pain/discomfort ever.
Primary and secondary objectives. The primary objectives of the study were a reduction of facial wrinkles by one grade relative to baseline, 90, and 150 days after the first treatment and a reduction in common line roughness characteristics (R-parameters of PRIMOS) relative to baseline, 90, and 150 days after the first treatment. The secondary objectives of the study were improvement in skin laxity and texture (Alexiades Armenakas) by one grade relative to baseline, 90, and 150 days after the first treatment and reduction in common surface roughness characteristics (S-parameters of PRIMOS) relative to baseline, 90, and 150 days after the first treatment.
Statistical considerations. Sample size calculation. A pre-trial estimate of mean grade improvement (1.0) was based on information obtained from post-market vigilance and related literature. No directly relevant published clinical data were available on the variability of grade improvement, but three published trials of some relevance were found. Two of these, Majid20 and Fabbrocini,21 related to microneedling for acne scarring, and the third, Alexiades-Armenakas,22 described trials of fractional microneedling for skin laxity. The coefficient of variation of grade improvement for each of these studies was evaluated, and the largest of these (0.5) was used to compute the sample size required to give a 95-percent confidence interval (CI) of ±15 percent for mean wrinkle grade improvement, resulting in a sample size of 43.20
Data analysis. All eligible patients who were enrolled into the study and received at least one microneedling treatment were included in the safety analysis. All eligible patients who were enrolled into the study and received at least three microneedling treatments were included in the analysis of efficacy.
For each subject, the mean of the wrinkle grades over all nine facial areas was evaluated as a global score. Global scores and physician-assessed grades for wrinkles, skin texture, and skin laxity and changes from baseline were summarized by mean values and ranges.
For changes in global wrinkle score, skin laxity, and skin texture at Days 90 and 150, 99-percent CIs were computed for the mean changes from baseline. The Bonferroni correction applied to six 95-percent CIs and gave a corrected level of 99.2 percent. The correction was based on statistical independence of the outcome measures, but since there was substantial dependence among the measures, with correlation coefficients varying from 0.36 to 0.9, it was considered safe to round down the confidence level to 99 percent. The single-sample, one-sided t-test was used to assess the statistical significance of mean values of improvement in relation to the target improvement of one grade at Days 90 and 150. For each test, the Bonferroni-corrected significance level was 0.0083, giving a family-wise Type 1 error rate of five percent.
Baseline and follow-up skin topography measures and percentage changes from baseline were summarized by mean values. Mean values were also used to summarize subject-reported outcomes at baseline and follow-up and changes from baseline.
RESULTS
Forty-eight subjects were enrolled in the study, all of whom completed treatment and assessment visits. No subjects withdrew from the study due to issues related to study protocol or AEs. Data from all subjects were included for analysis.
The mean age of the subjects was 55.1 years (range 39–67 years), and the subjects were predominantly female. Fitzpatrick phototypes ranged from I to V with a mean of 2.2. Baseline scores and range for wrinkles, skin laxity, and skin texture were 3.2 (2.2–4), 2.8 (2–3.5), and 2.7 (1.5–3.5), respectively.
Table 1 shows the mean wrinkle grade for each of the nine assessment areas at baseline and Days 90 and 150, together with summarized changes at follow-up.
TABLE 1.
Summary statistics for wrinkle severity and for changes at Days 90 and 150
| FACIAL AREA | BASELINE | DAY 90 | DAY 150 | CHANGE AT DAY 90 | CHANGE AT DAY 150 | ||
|---|---|---|---|---|---|---|---|
| MEAN | RANGE | MEAN | RANGE | ||||
| Global score | 3.17 | 2.24 | 1.95 | 0.93 | n/a | 1.23 | n/a |
| Horizontal forehead lines | 2.6 | 1.7 | 1.5 | 0.9 | 0–2 | 1.1 | 0–3 |
| Glabella frown lines | 3.2 | 1.9 | 1.6 | 1.3 | 0–2 | 1.6 | 0–3 |
| Periorbital lines | 3.3 | 1.8 | 1.4 | 1.5 | 0–3 | 1.9 | 0–3 |
| Preauricular lines | 3.0 | 2.7 | 2.6 | 0.3 | 0–1 | 0.4 | 0–1 |
| Cheek folds | 3.0 | 1.5 | 1.2 | 1.5 | 0–2 | 1.8 | 0–3 |
| Nasolabial folds | 3.5 | 3.2 | 3.0 | 0.4 | 0–1 | 0.6 | 0–2 |
| Upper lip lines | 3.2 | 1.8 | 1.4 | 1.5 | 0–3 | 1.9 | 0–3 |
| Marionette lines | 3.2 | 2.9 | 2.5 | 0.3 | 0–2 | 0.7 | 0–2 |
| Labiomental crease | 3.5 | 2.8 | 2.4 | 0.7 | 0–2 | 1.1 | 0–2 |
n/a: not applicable
At Day 90, four facial areas had improved by at least one grade—glabella frown lines, periorbital lines, cheek folds, and upper lip lines. The mean improvement in global score was 0.93 (99% CI: 0.81, 1.06).
At Day 150, six of the nine facial areas had improved by at least one grade—horizontal forehead lines, glabella frown lines, periorbital lines, cheek folds, upper lip lines, and labiomental crease. The mean improvement in global score was 1.23 (99% CI: 1.11, 1.34) with p=2.5×106 (i.e., highly significant).
Mean values for line roughness and surface roughness for the periorbital regions and mesolabial regions are shown in Tables 2 and 3. At both Day 90 and Day 150 assessment points, mean improvements were observed for all roughness parameters (R# and S#) in both the periorbital and mesolabial regions. Table 4 shows mean skin laxity and skin texture grades at baseline and Days 90 and 150 and mean changes at follow-up. Mean improvement in skin laxity was 0.82 (99% CI: 0.69, 0.95) at Day 90 and 1.09 (99% CI: 0.93, 1.26) at Day 150. A significance test of the mean improvement at Day 150 against 1.0 gave p=0.07, exceeding the study significance level of 0.0083. Mean improvement in skin texture was 1.33 (99% CI: 1.16, 1.51) at Day 90 and 1.54 (99% CI: 1.33, 1.75) at Day 150. Both of these mean values were significantly greater than 1, with p=7.0×106 and p=1.3×108, respectively.
TABLE 2.
Mean values for surface and line roughness characteristics and changes (periorbital)
| PARAMETER | BASELINE | DAY 90 | DAY 150 | PERCENT CHANGE AT DAY 90 | PERCENT CHANGE AT DAY 150 |
|---|---|---|---|---|---|
| Sa | 45.2 | 33.8 | 33.3 | 25.0 | 26.0 |
| Smax | 806.5 | 554.4 | 522.0 | 31.0 | 35.0 |
| Sz | 740.6 | 502.3 | 476.7 | 32.0 | 36.0 |
| Ra | 31.9 | 22.9 | 22.3 | 28.0 | 30.0 |
| Rmax | 236.8 | 165.4 | 156.8 | 30.0 | 34.0 |
| Rz | 172.2 | 121.0 | 116.2 | 30.0 | 33.0 |
Ra: average roughness of skin; Rmax: maximum roughness of skin; Rz: average of single roughness depths; Sa: arithmetic mean of surface roughness of skin; Smax: height difference between highest peak and lowest valley on the entire measuring field; Sz: mean of 5 highest peaks and deepest valleys
TABLE 3.
Mean values for surface and line roughness characteristics and changes (mesolabial)
| PARAMETER | BASELINE | DAY 90 | DAY 150 | PERCENT CHANGE AT DAY 90 | PERCENT CHANGE AT DAY 150 |
|---|---|---|---|---|---|
| Sa | 65.0 | 39.1 | 36.5 | 40.0 | 44.0 |
| Smax | 1207.5 | 603.6 | 531.8 | 50.0 | 56.0 |
| Sz | 1123.9 | 546.9 | 486.8 | 51.0 | 57.0 |
| Ra | 40.1 | 26.0 | 24.5 | 35.0 | 39.0 |
| Rmax | 319.2 | 182.5 | 171.0 | 43.0 | 46.0 |
| Rz | 216.8 | 133.4 | 124.9 | 38.0 | 42.0 |
Ra: average roughness of skin; Rmax: maximum roughness of skin; Rz: average of single roughness depths; Sa: arithmetic mean of surface roughness of skin; Smax: height difference between highest peak and lowest valley on the entire measuring field; Sz: mean of 5 highest peaks and deepest valleys
TABLE 4.
Mean values for skin laxity and skin texture grades and changes at follow-up
| PARAMETER | BASELINE | DAY 90 | DAY 150 | CHANGE AT DAY 90 | CHANGE AT DAY 150 |
|---|---|---|---|---|---|
| Mean skin laxity grade | 2.84 | 2.02 | 1.75 | 0.82 | 1.09 |
| Percent of subjects with improvement | n/a | n/a | n/a | 97.9 | 97.9 |
| Mean skin texture grade | 2.71 | 1.38 | 1.17 | 1.33 | 1.54 |
| Percent of subjects with improvement | n/a | n/a | n/a | 100 | 100 |
n/a: not applicable
Table 5 lists the improvements seen in all four patient evaluated parameters at Days 90 and 150, with the later assessment point demonstrating greater improvement. At Day 150, there was at least a one-grade improvement in lines, wrinkles, texture, and pores; 73 percent of the subjects reported visible improvement in lines, wrinkles, and texture and 83 percent reported visible improvement in pores.
TABLE 5.
Mean values for patient subjective evaluation of treatment response
| PARAMETER | BASELINE GRADE | DAY 90 | DAY 150 | CHANGE AT DAY 90 | CHANGE AT DAY 150 |
|---|---|---|---|---|---|
| Pigmentation | 1.9 | 1.2 | 1.1 | 0.7 | 0.8 |
| Line and wrinkles | 3.2 | 2.4 | 2.1 | 0.7 | 1.0 |
| Texture | 1.3 | 0.4 | 0.3 | 1.0 | 1.1 |
| Pores | 2.8 | 2.0 | 1.6 | 0.8 | 1.2 |
No AEs were observed. There were nine recorded device-related AEs during the study, summarized in Table 6, all of which all were considered mild. Of the nine AEs, eight were herpes simplex labialis (HSL) outbreaks. Four outbreaks were recorded 5 to 9 days post-treatment. Three of the four subjects received treatment in addition to prophylactic antiviral therapy for the remainder of the study. One subject did not receive treatment for the outbreak but received prophylactic antiviral therapy for the remainder of the study. Four additional subjects reported HSL outbreaks 1 to 2 days post-treatment but were already receiving prophylactic antiviral therapy; all cases resolved in 4 to 5 days. There was no recurrence of HSL outbreaks in any of the subjects. One subject reported an isolated incident of excessively dry skin after treatment and was given instructions for post-treatment skin care. Additionally, there were four reported AEs that were not related to the device or the procedure. There were no incidents related to the gel that was applied to each subject’s face to assist the movement of the needle cartridge over the skin.
TABLE 6.
Adverse events
| EVENT | RELATED (R, n=9) | NON-RELATED (NR, n=4) | TOTAL (R+NR, n=13) |
|---|---|---|---|
| Herpes labialis | 8 | 0 | 8 |
| Dry skin | 1 | 0 | 1 |
| Cold/Flu | 0 | 2 | 2 |
| Surgery | 0 | 2 | 2 |
Physician-assessed erythema was graded predominantly as mild after each treatment. Seventy percent of subjects were graded as having mild erythema, and the remaining 30 percent were graded as having moderate erythema. No subjects were graded as severe. Subject evaluation of pain and discomfort during treatment indicated a mean pain score of 5.2 over the four treatments (range 3.8–6.1) and a mean discomfort score of 1.3 (range 0.4–2.1). Subjects reported gradual cessation of pain after treatment. By the evening of the fourth day following treatment, an average of 41 subjects (85%) recorded no pain, and by the sixth day, an average of 39 subjects (81%) recorded no discomfort.
DISCUSSION
Microneedling, or PCI, is a relatively simple in-office procedure that appears to be effective in the treatment of facial wrinkles, skin laxity, and skin texture. Past studies using the technique have lacked sufficient numbers of test subjects. Our study, presented here, evaluated a microneedling system for the treatment of facial aging among a suitably sized sample of patients.
During the procedure, microscopic wounds created in the papillary dermis by the microneedles being driven into the skin result in the normal chemical cascade that follows trauma. This consists of an inflammatory stage, with its subsequent release of cellular growth factors, followed by the proliferative stage, and ultimately the remodeling stage, with the stimulation of neocollagenesis and formation of a tighter collagen matrix.
In out study, the subjects demonstrated improvement in facial wrinkles and skin texture at the end of the treatment sequence on Day 90, but with a maximal (statistically significant) effect seen in all three parameters at Day 150. This improvement in facial wrinkles demonstrates the cumulative effect of percutaneous collagen induction and ongoing dermal collagen remodeling, and it confirms previous observations described in the literature.9,10 Improvements in skin laxity are predominantly the result of Collagen III being converted to Collagen I, with the subsequent tightening of the skin. Since this process takes as long as 12 months to complete, it is not surprising that statistically significant results were not seen until Day 150 of our study. Future studies would benefit from an interim follow-up at Day 120 and an extended follow-up period of 6 to 12 months to fully appreciate the potential of this technology. Conversely, statistically significant improvements in skin texture were demonstrated by the end of the treatment course at Day 90. With conversion of Collagen III to Collagen I and subsequent skin tightening, one would expect epidermal remodeling to take place and be more measurable in the earlier stages of PCI treatment, as opposed to the later stages.
Results of wrinkle grading and skin texture improvements were confirmed by 3D profilometry. As expected, the improvement seen in the common roughness indices appeared greater than observer grading measurements. This reflects previously published data by Friedman16 and Fujiwara.17 However, the results validate the value and effectiveness of 3D profilometry in providing higher resolution of skin topography than clinical assessment alone, supporting the use of 3D profilometry in measuring improvements that cannot necessarily be identified by a trained rater.
The epidermis remained structurally intact during PCI treatment with the study device, with no damage occurring to the basal membrane.23 Side effects associated with the technique were manageable. The treatment appeared to be well-tolerated by patients, and AEs were predominantly limited to subjects with a history of HSL. No serious adverse incidents were recorded. There appeared to be no AEs related to the topical anesthetic or to the sterile gel used as a lubricant. There were no atypical skin observations made immediately after the treatment session by the physician or later by the subjects. There were no recorded incidents of hyperpigmentation, transient or otherwise, which further supports the work of Aust,23 who demonstrated that the number of melanocyctes was stable in subjects undergoing microneedling, and Bonati,24 whose review identified few cases of post-inflammatory hyperpigmentation after microneedling.
Future clinical evaluation of the technique would benefit from a study group with a more diverse ethnicity, particularly since previous authors have suggested that microneedling could be a credible alternative to more ablative therapies, particularly in Skin Phototypes IV to V, since the technique does not cause thermal damage or excessive injury to the epidermis.25
CONCLUSION
Microneedling is an uncomplicated procedure that can be performed in-office. The device is cost-effective, with the only real recurring cost to the operator being the single-use microneedling cartridges. The treatment seems well-tolerated with minimal pain, discomfort, or patient downtime. Side effects appear to be minor and easily managed compared to other invasive technologies, such as laser ablation and radiofrequency.
In this study, we demonstrated that four microneedling treatments of facial skin, spaced four weeks apart, produce noticeable improvements in lines and wrinkles, skin laxity, and skin texture at Days 90 and 150 following the first treatment.
REFERENCES
- 1.Dover JS, Hruza GJ. Laser skin resurfacing. Semin Cutan Med Surg. 1996;15:177–188. doi: 10.1016/s1085-5629(96)80009-5. [DOI] [PubMed] [Google Scholar]
- 2.Fulton JE, Porumb S. Chemical peels: their place within the range of resurfacing techniques. Am J Clin Dermatol. 2004;5:179–187. doi: 10.2165/00128071-200405030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Atkins D, Frodel J. Skin rejuvenation in facial surgery. Facial Plast Surg. 2006;22(02):129–139. doi: 10.1055/s-2006-947719. [DOI] [PubMed] [Google Scholar]
- 4.Gold MH. Update on fractional laser technology. J Clin Aesthet Dermatol. 2010;3(1):42–50. [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein LJ. The short- and long-term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519–525. doi: 10.1111/j.1524-4725.1997.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 6.Kauvar AN. Histology of laser resurfacing. Dermatol Clin. 1997;15:459–467. doi: 10.1016/s0733-8635(05)70454-x. [DOI] [PubMed] [Google Scholar]
- 7.Laws RA. Alabaster skin after carbon dioxide laser resurfacing with histologic correlation. Dermatol Surg. 1998;24:633–636. doi: 10.1111/j.1524-4725.1998.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 8.Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21(6):543–549. doi: 10.1111/j.1524-4725.1995.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26(2):192–199. doi: 10.1016/j.clindermatol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17(1):51–63. doi: 10.1016/j.coms.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Vandervoort J, Ludwig A. Microneedles for transdermal drug delivery: a minireview. Front Biosci. 2008;13:1711–1715. doi: 10.2741/2794. [DOI] [PubMed] [Google Scholar]
- 12.Hou A, Cohen B, Haimovic A, Elbuluk N. Microneedling: a comprehensive review. Dermatol Surg. 2017;43(3):321–339. doi: 10.1097/DSS.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 13.Lemperle G, Holmes RE, Cohen SR, Lemperle SM. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108(6):1735–1750. doi: 10.1097/00006534-200111000-00048. [DOI] [PubMed] [Google Scholar]
- 14.Alexiades-Armenakas M. A quantitative and comprehensive grading scale for rhytides, laxity, and photoaging. J Drugs Dermatol. 2006;5(8):808–809. [PubMed] [Google Scholar]
- 15.Darvin M, Patzelt A, Gehse S, et al. Cutaneous concentration of lycopene correlates significantly with the roughness of the skin. Eur J Pharm Biopharm. 2008;69(3):943–947. doi: 10.1016/j.ejpb.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Friedman PM, Skover GR, Payonk G, et al. 3D in vivo optical skin imaging for topographical quantitative assessment of nonablative laser technology. Dermatol Surg. 2002;28(3):199–204. doi: 10.1046/j.1524-4725.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujimura T, Haketa K, Hotta M, Kitahara T. Global and systematic demonstration for the practical usage of a direct in vivo measurement system to evaluate wrinkles. Int J Cosmet Sci. 2007;29(6):423–436. doi: 10.1111/j.1468-2494.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobi U, Chen M, Frankowski G, et al. In vivo determination of skin surface topography using an optical 3D device. Skin Res Technol. 2004;10(4):207–214. doi: 10.1111/j.1600-0846.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu TH, Chen JZ, Li YH, et al. Split-face study of topical 23.8% L-ascorbic acid serum in treating photo-aged skin. J Drugs Dermatol. 2012;11(1):51–56. [PubMed] [Google Scholar]
- 20.Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2(1):26–30. doi: 10.4103/0974-2077.53096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatolog Treat. 2014;25(2):147–152. doi: 10.3109/09546634.2012.742949. [DOI] [PubMed] [Google Scholar]
- 22.Alexiades-Armenakas M, Rosenberg D, Renton B, et al. Blinded, randomized, quantitative grading comparison of minimally invasive, fractional radiofrequency and surgical face-lift to treat skin laxity. Arch Dermatol. 2010;146(4):396–405. doi: 10.1001/archdermatol.2010.24. [DOI] [PubMed] [Google Scholar]
- 23.Aust MC, Reimers K, Repenning C, et al. Percutaneous collagen induction: minimally invasive skin rejuvenation without risk of hyperpigmentation—fact or fiction? Plast Reconstr Surg. 2008;122(5):1553–1563. doi: 10.1097/PRS.0b013e318188245e. [DOI] [PubMed] [Google Scholar]
- 24.Bonati LM, Epstein GK, Strugar TL. Microneedling in All Skin Types: A Review. J Drugs Dermatol. 2017;16(4):308–313. [PubMed] [Google Scholar]
- 25.Sharad J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin.”. J Cosmet Dermatol. 2011;10(4):317–323. doi: 10.1111/j.1473-2165.2011.00583.x. [DOI] [PubMed] [Google Scholar]


