Abstract
Background: The skin is where initial visual signs of aging manifest, including increased skin dryness and decreased firmness and elasticity. Cellulite, a skin condition characterized by changes in the skin morphology due to excessive lipid deposition in subcutaneous adipose tissue, is another characteristic of skin aging. Objective: We sought to assess the effectiveness of a topical botanical cream on cellulite, skin hydration, firmness, and elasticity after two, four, and eight weeks of use compared to an active comparator. Design: The study was a single-blind, randomized, controlled study conducted on subjects with mild-to-severe cellulite on the thighs. Subjects were treated with a topical botanical cream (UP1307) and an active comparator for eight weeks. A total of 44 women 18 to 59 years of age were enrolled. Test products were gently applied in a circular motion to the area identified by subjects as the target cellulite area twice per day. Measurements: Measurements using Corneometer® (for skin hydration) and Cutometer® (for skin elasticity and firmness) were carried out at each visit in addition to expert clinical grader evaluations for cutaneous changes and cellulite. Outcomes were also assessed by patients using subject questionnaires. Results: Patients reported significant improvement in skin hydration, firmness, and elasticity over time. Findings were corroborated with objective instrumental measurements. At Week 8, 44.4- and 42.7-percent improvement in appearance of cellulite was also observed for the UP1307 cream and the active comparator group, respectively. Conclusion: Use of UP1307 cream produced significant improvements in skin hydration, firmness, and elasticity, with associated improvement in cellulite appearance. There was overall superiority of UP1307 between groups. Progressive subject perceptions of product effects are reported.
Keywords: Skin hydration, skin firmness, skin elasticity, cellulite, UP1307, topical, cream, aging skin
The skin—the largest multifunctional organ in the body—is a dynamic structure that generally reflects the overall health of an individual.1 It is composed of two main layers, the outermost epidermis and the inner dermis, with distinct underlying structures and function. The epidermis fulfills most of the barrier functions of the skin and is predominantly made up of keratinocytes. In addition to keeping environmental threats outside of the body, the epidermal barrier also retains water. Environmental, physical, and nutritional changes can modify epidermal structure and function. The dermal skin layer provides strength and elasticity. It is made up of complex extracellular matrix proteins and is rich in collagen fibers that make up to 75 percent of the dermis.2
The skin is constantly under assault from the external environment, more so than any other organ in the body, and is where perceptible signs of aging first occur. Among the extrinsic factors causing skin damage, ultraviolet (UV)-induced reactive oxygen species (ROS)-mediated structural changes are linked to stimulation of stress-associated kinase pathways, which prompt the release of prostaglandin, proliferation of keratinocytes, loss of collagen synthesis,3,4 activation of nuclear factor (NF)-κB transcription factor (which induces the expression of inflammatory mediators such as proinflammatory cytokines and metalloproteinases), and deoxyribonucleic acid (DNA) damage.5 These aggregates of detrimental changes indicate that targeting the suppression of free radical generations and reduction of inflammation might help to spare the skin from these core triggers and prevent subsequent skin damage.
Cellulite—a skin condition characterized by changes in the skin morphology—presents as an orange-peel-like appearance around the thigh and buttock area of many post-adolescent women.6 It is a multifactorial condition thought to be associated with deficiencies in lymphatic drainage and microvascular circulation, localized abnormal fat deposition in the subcutaneous tissue, chronic inflammatory processes, and changes in connective tissues.6,7 Increasing the microcirculation flow, reducing lipogenesis and promoting lipolysis, preventing free radical formation or scavenging free radicals, and modulating inflammatory processes have been suggested as to control cellulite.6,7
Attempts to correct the appearance of cellulite have yielded limited success, and there is a lack of scientific and clinical substantiation of active ingredients of cellulite-reducing products. A plethora of over-the-counter (OTC) formulations are currently dispensed for the treatment of cellulite. Some of these preparations contain methylxanthines (caffeine, aminophylline, theophylline, and theobromine), retinol (vitamin A), alpha-tocopherol (vitamin E), and ascorbic acid (vitamin C) as the major active constituents in topical products.10,11 Extracts from Ginkgo biloba, Centella asiatica, Ruscus aculeatus, Ananas sativus, Chenopodium quinoa seed, and Yuzu seed are also available for a similar indication with diverse mechanisms of action.10,11 However, while some interventions show improvements in appearances of affected areas, none have been shown to reverse cellulite completely.10,11 These unsuccessful measures further signify the intricate nature of cellulite and have lead to the development of a combination therapy designed to target multiple mechanisms involved in the formation of cellulite.
Recently, we reported the development process and mechanisms of action of a standardized botanical blend topical composed of extracts from the leaf of Rosmarinus officinalis, fruit of Annona squamosa, and stem bark of Zanthoxylum clava-herculis, designated as UP1307 (Unigen, Inc., Tacoma, Washington).4,34 These botanicals separately were shown to reduce cellular lipid accumulation and improve microcirculation, in addition to demonstrating high total antioxidant capacity and significant anti-platelet aggregation activities.34 Previously, extracts and actives from these plants have showed biological functions that suggest efficacy in treating cellulite and other skin conditions.9–14 For instance, rosemary polyphenols were shown in vivo to reduce the expression of several inflammation-associated genes that are regulated by NF-kB, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and cyclooxygenase (COX)-1 and COX-2 in an inflamed mouse skin model,9 as well as photoprotective potential in UVA-irradiated human skin fibroblasts.9,10 Carnosic acid from rosemary was shown to inhibit cytokine-induced adhesion molecule expression and monocyte adhesion to endothelial cells through a mechanism that involves NFκB, which could be involved in its anti-inflammatory properties. Carnosic acid might also undergo an oxidative degradation and rearrangement cascade, giving rise to other rosemary antioxidant compounds such as carnosol, rosmanol, galdosol, and rosmariquinone.11 In addition, Rosmarinus officinalis, Annona squamosa, and Zanthoxylum Americanum have been previously reported to possess anti-inflammatory activities, reinforcing their potential use in counteracting some aspects of skin disorders such as photoaging.12–14
We believe that a formulation of these well-known botanicals at a specific ratio might yield a composition with a wide spectrum of actions that affect multiple pathways involved in several skin conditions caused by intrinsic and extrinsic detrimental factors. While some of the ingredients comprising UP1307 have been reported previously for use in mitigating skin damage (e.g., extracts from rosemary),17–20 this is the first time these three plant materials have been combined for a broader skin care application, including cellulite. To test this hypothesis, the present clinical trial was conducted in subjects with mild-to-severe cellulite on the thighs compared with a known active comparator.
METHODS
Test materials.
A detailed method for preparation of individual extracts and the standardized blend of UP1307 from Annona squamosa, Zanthoxylum clava-herculis, and Rosmarinus officinalis has been disclosed in a United States patent application published on September 15, 2016.4 Extracts were standardized to contain biomarkers squamocin and kaurenoic acid for Annona squamosa; magnoflorine and laurifoline for Zanthoxylum clava-herculis, and carnosic acid for Rosmarinus officinalis. UP1307 was formulated into a cream containing 1%, 0.05%, and 0.1% actives of Zanthoxylum clava-herculis, Annona squamosa and Rosmarinus officinalis extracts, respectively, by weight. The active comparator is a leading anticellulite contouring product containing botanical ingredients from sunflower, green coffee, and celosia (Body Lift Cellulite Control®, Clarins, Paris, France).
Study design.
This study was an eight-week, randomized, single-blind, active comparator-controlled clinical trial that was conducted at a single study center, International Research Services Inc. (IRSI, Port Chester, New York), from December 2013 to March 2014. A total of 44 subjects were enrolled in the study, and 40 subjects completed the study.
Inclusion criteria included the following (Table 1):
TABLE 1.
Expert clinical grader evaluation—monadic, comparison to baseline
| VARIABLE | UP1307 | ACTIVE COMPARATOR | ||||||
|---|---|---|---|---|---|---|---|---|
| n | MEAN± SD | MINIMUM | MAXIMUM | n | MEAN± SD | MINIMUM | MAXIMUM | |
| Age (years) | 19 | 46.52±9.35 | 29 | 57 | 21 | 45.66±8.97 | 24 | 58 |
| Height (inches)^ | 19 | 63.63±2.40 | 59 | 68 | 21 | 64.80±2.54 | 61 | 70 |
| Weight (pounds)^ | 19 | 175.10±41.92 | 120 | 250 | 21 | 184.66±45.44 | 117 | 297 |
| VARIABLE | n (%) | DEMOGRAPHIC | n (%) | DEMOGRAPHIC | ||||
| Ethnicity | 1 (5.3) | Hispanic or Latino | 2 (9.5) | Hispanic or Latino | ||||
| 18 (94.7) | Not Hispanic or Latino | 19 (90.5) | Not Hispanic or Latino | |||||
| Race | 7 (57.9) | Black or African American | 9 (42.9) | Black or African American | ||||
| 11 (36.8) | White | 11 (52.4) | White | |||||
| 1 (5.3) | No Response (see Hispanic or Latino above) | 1 (4.8) | No Response (see Hispanic or Latino above) | |||||
| Body skin type | 10 (52.6) | Normal | 16 (76.2) | Normal | ||||
| 7 (36.8) | Dry | 5 (23.8) | Dry | |||||
| 2 (10.5) | Very Dry | 0 | Very Dry | |||||
| ^No statistically significant difference found between UP1307 and the active comparator for Height and Weight. | ||||||||
-
•
Women of any race and skin type
-
•
18 to 59 years of age
-
•
Good general health
-
•
Able to read, understand, and sign an informed consent document
-
•
Able to understand and follow study instructions
-
•
Willingness to complete a brief personal and medical history
-
•
Mild-to-severe cellulite on the thighs, scoring greater than or equal to 2 on a standard ordinal scale at the baseline assessment
-
•
Willingness to abstain from extended periods of sun exposure of the skin on both thighs and all use of artificial tanning for the duration of the study.
Subjects were instructed to continue the use of their usual skin cleansing products and not to initiate use of any new cleansing products during the study period. Women who were pregnant, breastfeeding, or planning a pregnancy during the study period and/or women with any history of sensitivity to skin treatment products or known allergies to personal care products (self-reported) were excluded from the study.
Measurements.
The eight-week clinical study included four visits: one for baseline screening, qualification, evaluations and three others for Week 2, Week 4, and Week 8 evaluations. Following completion of baseline procedures, which included obtaining informed consent and medical history, screening for inclusion and exclusion criteria, and completing baseline measurements, subjects were randomly assigned to either a botanical cream containing UP1307 or an active comparator, according to a predetermined randomization scheme. All subjects were blinded to the test materials. Subjects were instructed to apply the test products in a gentle, circular motion to the area identified by the study personnel as the target cellulite area (back and side areas of thighs) twice-daily after thoroughly washing and drying their hands. At each visit, subjects underwent instrumental monitoring for skin hydration using Corneometer® CM 825 (Courage + Khazaka electronic GmbH, Germany) and for skin elasticity and firmness using Cutometer® dual MPA 580 (Courage + Khazaka electronic GmbH, Germany).16 The Corneometer® CM 825 is the most frequently used instrument to determine the hydration level of the skin surface, mainly the stratum corneum.16 The measurement is based on capacitance measurement of a dielectric medium that records the variation in the dielectric constant due to skin surface hydration changes. The Cutometer® MPA 580 is a standard device used to measure elasticity and firmness of the skin by creating negative pressure using suction that draws the skin into the aperture of the probe. The resistance of the skin to be sucked up by the negative pressure (firmness) and its ability to return to its original position (elasticity) are displayed as curves in real time. In each case, data were analyzed using the device software. Skin conditions of subjects’ thighs were also evaluated by expert clinical grading using a standard ordinal scale and visual analogue scales (VAS) at each visit. The Cellulite Ordinal Grading Scale includes 0=no visible cellulite or dimpling; 1=very little visible cellulite and hardly discernible, little to no dimpling; 2=mild presence of visible cellulite and evidence of shallow dimpling; 3=moderate dimpling that is quite pronounced and easily visible; and 4=extremely visible cellulite with heavy, deep dimpling.
Ethics.
This study was performed in accordance to IRSI final signed clinical study protocol number 3747UI0813 version 2.2 dated December 18, 2013. The informed consent process was completed prior to all subjects’ involvement in any study related activity. The process was documented using a written informed consent form (ICF) conforming to the United States Food and Drug Administration (FDA) 21 CFR 50.25. The study was conducted in accordance with FDA Good Clinical Practice (GCP) regulations and the International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines in as much as they apply to cosmetic research with the following noted: This was not an Investigational New Drug/New Drug Application (IND/NDA) clinical trial and not intended for submission to the FDA.
Statistical analysis.
The mean and standard deviation of instrumental data and visual scores from subjects at all visits were provided and compared to that of baseline, utilizing paired t-Test. The significance value for each parameter was set at p≤0.05. The mean percent-improvement of individual scores and the percent of subjects scores improving from baseline results were also presented for each visit. Similarly, response frequency percentages were tabulated for each question at each visit, and product group data were compared utilizing the Wilcoxon Rank Sum Test or a Chi-Square test depending on structure of self-assessment questionnaire.
RESULTS
Enrollment and demographics.
Out of the 44 subjects enrolled in the study, 40 subjects completed the study. Four subjects that were initially enrolled in the study did not return for scheduled Visits 2 and 4. The UP1307 group contained 19 subjects and the active comparator product group contained 21 subjects. A total of 40 subjects, 18 to 59 years of age, were required to complete study participation, with a minimum of 20 subjects in each of the two product groups. The study was completed by 40 subjects ranging in age from 24 to 58 years, with an average age of 46.07 years. Fifty-five percent of the subjects were Caucasian, 40.0 percent were African-American, and 5.0 percent were Hispanic/Latina (Table 1).
Skin hydration as measured by Corneometer.
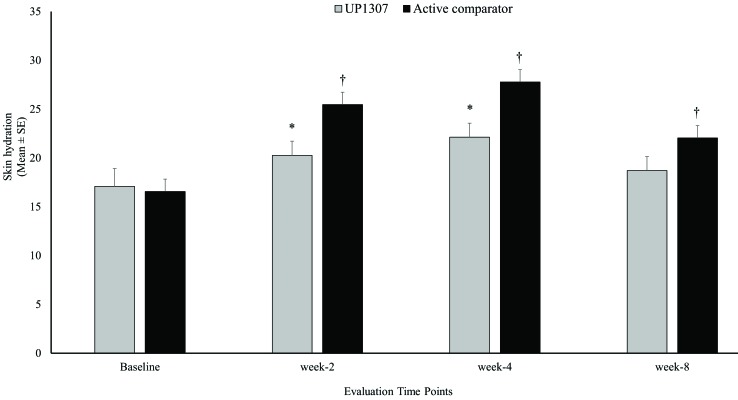
Statistically significant improvements from mean baseline score for skin hydration were observed for subjects treated with UP1307 at the Week 2 and Week 4 visits and for the active comparator at the Week 2, Week 4, and Week 8 visits. The subjects showed improvement from baseline measurement at rates of 72.2, 77.8, and 63.2 percent for the UP1307 group and 95.0, 94.1, and 75.0 percent for the active comparator, at Weeks 2, 4, and 8, respectively (Figure 1). Comparative analysis of results from both groups showed a statistically significant difference between their mean change from baseline scores for hydration at Weeks 2 and 4, where improvement in the active comparator was significantly greater than that in the UP1307. No other statistically significant differences between mean changes from baseline scores were observed for the two products.
FIGURE 1.
Skin hydration improvements as a result of topical application of UP1307—subjects were monitored with Corneometer at baseline and Weeks 2, 4, and 8. *p≤0.05 improved from bassline, †p≤0.001 improved from baseline.
Skin firmness as measured by Cutometer.
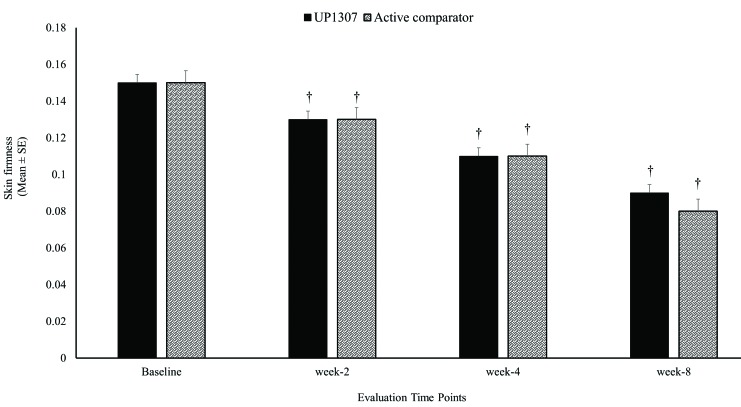
Subjects were monitored for changes in skin firmness using Cutometer following daily application of UP1307 and the active comparator at Weeks 2, 4, and 8. As seen in Figure 2, statistically significant improvements from mean baseline score for skin firmness were observed in both product groups at the Week 2, 4, and 8. Subjects showed improvement from baseline measurement at rates of 82.2, 100, and 100 percent for the UP1307 and 85.0, 100, and 100 percent for the active comparator at Weeks 2, 4, and 8, respectively. In the comparative analysis, the improvements observed for the active comparator in skin firmness were not statistically significantly different from that of UP1307 at any timepoint.
FIGURE 2.
Skin firmness improvements as a result of topical application of UP1307 —subjects were monitored with Cutometer at baseline and Weeks 2, 4, and 8. †p≤0.001 improved from baseline.
Skin elasticity as measured by Cutometer.
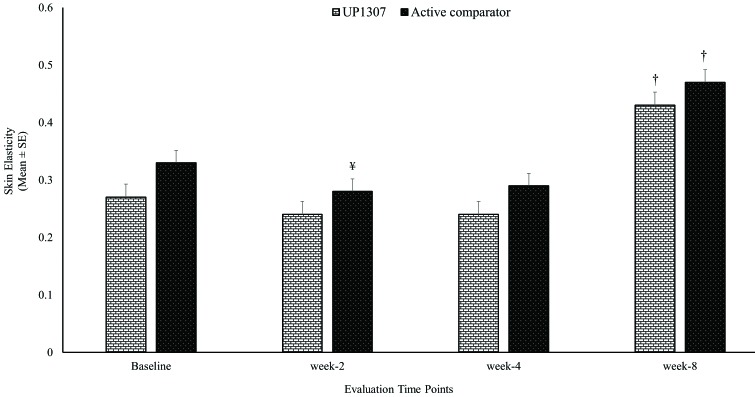
While time-dependent improvement in skin elasticity was observed for subjects treated with UP1307 from Week 2 to Week 8, the statistical significance was only achieved at Week 8 (Figure 3). In contrast, there was statistically significant worsening of skin elasticity for the active comparator group at Week 2 and significant improvement was also observed at Week 8. Subjects showed improvement from baseline measurements at rates of 47.1, 44.4, and 94.4 percent for UP1307 and 28.6, 22.2 and 95.2 percent for the active comparator at Weeks 2, 4, and 8, respectively. Comparative analysis of the results from both test groups showed no statistically significant differences between their mean changes from baseline scores for elasticity.
FIGURE 3.
Skin elasticity improvements as a result of topical application of UP1307—subjects were monitored with Cutometer at baseline and Weeks 2, 4, and 8. †p≤0.001 improved from baseline; ¥p≤ 0.05 worsened from baseline.
Subjective questionnaire.
Consumer perception of product efficacy was collected using subject questionnaires at each visit. The number of positive responses grew over time and product usage for both UP1307 and the active comparator (Table 2). At Week 8, most subjects in the UP1307 group indicated that the test product improved skin’s hydration/moisture (78.9%), improved skin texture/smoothness (52.6%), and improved firmness and elasticity (57.9%). Similarly, after eight weeks of daily topical use, subjects treated with UP1307 agreed that the test product improved the appearance of contours and tone on the skin of the upper thigh (52.9%). In the active comparator group, the majority of the subjects (>50%) reported improvements in skin hydration, texture, contour, firmness, and elasticity at Week 8. At the end of the study period, 47.4 and 52.4 percent of subjects reported an overall appearance of a slimmer upper thigh for the UP1307 and the active comparator groups, respectively. In addition, comparative analysis of results from both groups showed statistically significant differences between subjects mean response scores for questions regarding improvements only in overall appearance of a slimmer upper thigh at all visits, favoring the active comparator over the UP1307 group. No statistically significant differences in efficacy regarding skin elasticity, firmness, and/or hydration were observed between the UP1307 and the active comparator groups at the end of the study period.
TABLE 2.
Consumer Perception – Subjective Questionnaire at Week 8
| QUESTION | UP1307 (N=19) | ACTIVE COMPARATOR (N=21) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YES | NO | UNCERTAIN | PERCENT IMPROVEMENT* | YES | NO | UNCERTAIN | PERCENT IMPROVEMENT* | ||||||||||
| The test product improved skin's hydration/moisture? | 15 (78.9%) | 0 (0.0%) | 4 (21.1%) | 45.3 | 16 (76.2%) | 1 (4.8%) | 4 (19.0%) | 50.0 | |||||||||
| The test product improved texture/smoothness? | 10 (52.6%) | 2 (10.5%) | 7 (36.8%) | 49.0 | 14 (66.7%) | 3 (14.3%) | 4 (19.0%) | 50.7 | |||||||||
| The test product improved the appearance of upper thigh skin contours and tone? | 10 (52.6%) | 3 (15.8%) | 6 (31.6%) | 40.0 | 14 (66.7%) | 2 (9.5%) | 5 (23.8%) | 40.7 | |||||||||
| The test product improved the firmness/elasticity? | 11 (57.9%) | 0 (0.0%) | 8 (47.5%) | 39.1 | 15 (71.4%) | 1 (4.8%) | 5 (23.8%) | 43.3 | |||||||||
| The test product improved the overall appearance of a slimmer upper thigh area? | 9 (47.4%) | 2 (10.5%) | 8 (42.1%) | 44.4 | 11 (52.4%) | 2 (9.5%) | 8 (38.1%) | 45.5 | |||||||||
| *Only subjects responding YES to the question answered percent-improvement portion of the question. | Bold/Shaded: the majority of subjects responded favorably (>50%) | ||||||||||||||||
Anticellulite activity.
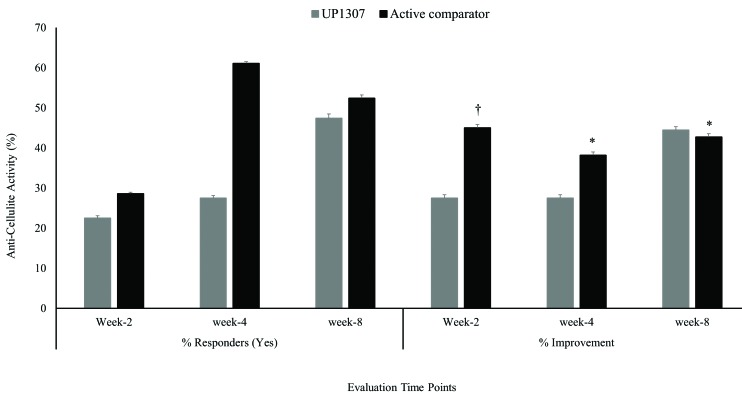
At each visit, subjects were asked to grade their perception of improvement in the appearance of cellulite. The number of subjects with positive responses increased as a function of time. While 22.2 versus 28.6 percent, 27.8 versus 61.1 percent, and 47.4 versus 52.4 percent of subjects gave a “Yes” response, percentage improvements were determined as 27.5 versus 45.0 percent, 30.0 versus 38.2 percent, and 44.4 versus 42.7 percent for these subjects compared to baseline at Week 2, Week 4 and Week 8 for UP1307 and the active comparator, respectively (Figure 4). It is worth noting that the changes observed in the UP1307 product group were consistently improved from visit to visit without fluctuation, unlike the active comparator product group. At Week 8, almost half of the subjects reported improved appearance of cellulite, suggesting longer study duration for significant clinical outcome. Statistically nonsignificant similar trends of time-dependent improvements in cellulite appearance were also observed in the expert clinical grader evaluations for both the UP1307 treated and the active comparator (data not shown).
FIGURE 4.
Anticellulite activity of a topical cream, UP1307. Significance was compared with in the subjects that responded “Yes” for perception at each visits. *p≤0.05 improved from bassline, †p≤0.001 improved from baseline.
DISCUSSION
This study highlights findings from a randomized, active comparator-controlled clinical trial of a botanical composition (UP1307) on subjects with mild-to-severe cellulite on the thighs. In addition to the cellulite outcome, data on skin hydration, elasticity, and firmness have been reported. The topical application of UP1307 cream led to significant improvements in skin hydration, firmness, and elasticity. Subject perception of product effects became increasingly positive over timeand product usage. For some attributes, such as skin hydration, the active comparator performed better than UP1307. However, no overall superiority of either product effect was observed in comparative data analysis.
Several external factors contribute to skin aging in women, including decreased skin hydration, firmness, and elasticity. As aging progresses, loose skin will present itself as wrinkles or dimples. In the present study, subjects reported improvement over time in their skin appearance with increased skin hydration, firmness, and elasticity. The clinical improvements of the various skin properties observed by the subjects were in accordance with the instrumental measurement data objectively verified by Corneometer and Cutometer.
The major components of UP1307 have been observed to have significant skin-related protective properties, which supports their usage in various cutaneous conditions. For example, in a study by López-Jiménez et al,17 rosemary extracts rich in polyphenols and diterpenes given orally to subjects in combination with citrus flavonoids produced significant reduction in ultraviolet (UV)-induced erythema and lipoperoxides, as well as improvements in wrinkles and elasticity after Week 8 of treatment. The combination of these extracts in vitro also decreased UVB-induced intracellular radical oxygen species and prevented DNA damage while decreasing chromosomal aberrations in X-irradiated human lymphocytes.18 In addition, Park et al19 reported that carnosic acid (a phenolic diterpene from rosemary) significantly inhibited UVA- and UVB-induced expression of matrix metalloproteinase (MMP-1, MMP-3, and MMP-9), suppressed UVB-induced extracellular signal-regulated kinase activation and the formation of transcription factor Activator protein (AP)-1, and attenuated UVB-induced ROS generation in human skin fibroblasts and keratinocytes in a concentration-dependent manner. These characteristics are especially relevant in that even at an extremely low level of UVB irradiation, there will be a breakdown of the major structural components of the skin, such as collagen and the elastic network, as a result of up-regulation of MMP activity. Therefore, the observed increase in skin elasticity, hydration, and firmness in the present study could be an indication of maintenance of the extracellular matrix that is composed of collagen and elastic fibers, which is responsible for skin elasticity and firmness. Presence of an intact abundance of collagen in the matrix will also help trap more water into the skin, improving hydration.
Similarly, extracts from fruits of Annona Squamosa have been shown to exhibit strong free radical scavenging activity using various techniques, including total reducing power estimation, total phenolic count, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging effect, evaluation of 2,2’-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) cation decolonization capacity, ferric reducing antioxidant power (FRAP) assay, hydroxyl radical scavenging assay, super oxide assay, and nitric oxide radical scavenging assay, suggesting its valuable contribution to the composition in skin protection.20 It has also been reported that extracts from the fruits of Annona squamosa increase synthesis of collagen and glycosaminoglycan, two integral parts of skin extracellular matrix, during wound healing in rats, which suggests the possibility of improved skin hydration, elasticity, and firmness.21
In this study, subjects in UP1307 group also experienced improved cellulite appearance as a function of time during the course of the treatment period. The current treatment for cellulite ranges from topical cream applications to invasive procedures (e.g., laser-assisted lipolysis and liposuction). Despite the significant advancements in the treatment of cellulite, at this time, there are no efficacious methods that target the underline causes of cellulite. The primary etiological factors that influence cellulite include deficiencies in lymphatic drainage and microvascular circulation, localized abnormal fat deposition in the subcutaneous tissue, chronic inflammatory process, and changes in connective tissues.6,7 Many commercially available products have been created to target these disorders. For example, Centella asiatica extracts for microcirculation and an anti-inflammation,22 Ruscus aculeatus and common ivy (Hedera helix) for improving lymphatic drainage;23,24 methylxanthines [(e.g., caffeine and aminophylline)] preparation to promote lipolysis;24–26 Ginkgo biloba as an anti-inflammatory and antioxidant,27 red grapes (Vitis vinifera) as an antioxidant,24 and Carica papaya as anti-inflammatory28 are some of the topical botanical extracts with limited success in managing cellulite. For instance, when the effectiveness of 2% aminophylline formulated with 10% glycolic acid in a cream was evaluated in a 12-week, randomized, controlled clinical trial, no improvement in the appearance of cellulite was observed. Only three of the 35 patients using aminophylline treatment showed improvement by self evaluation.29 Similarly, a two-month, parallel, placebo-controlled clinical study comparing the effects of Cellesene (a formulation that containing Ginko biloba, sweet clover, seaweed, grapeseed oil, lecithins, and evening primrose oil) to those of a control cream failed to show significant changes in the appearance of cellulite.30 On the other hand, Wu et al31 applied a topical retinol twice-daily on one thigh of study subjects for a period of six months and observed improvement in the feel and appearance of cellulite in 13 of the 19 patients.The investigators ratings were in concordance with 12 of the 13 who reported a beneficial effect. Their findings clearly indicate the need for longer duration of topical application to achieve desired outcome in cellulite treatment.
We believe that the improved cellulite appearance observed by subjects in UP1307 group in this study could be the result of the composition having a much wider spectrum of mechanisms of action that could affect multiple pathways involved in cellulite onset, continuation, or exacerbations. Reduced cellular lipid accumulation by the extract from fruits of Annona squamosa; microcirculation improvement from the extract of the stem barks of Zanthoxylum clava-herculis, and anti-platelet aggregation, nitric oxide inhibition, and antioxidant activity from leaf extract of Rosmarinus officinalis have been reported,34 all of which are significant mechanisms for anticellulite activities of UP1307.8 Recently it has also been shown that the major bioactive component of zanthoxylum extract, called magnoflorine, had dual target effects in NF-κB inhibition and β2-adrenoceptor agonist activities, both of which are primary contributiors to the pathophysiology of cellulite.32 The β2- adrenoceptor agonist alkaloid extract from magnoflorine might also promote lipolysis positioning with additional benefit in cellulite intervention. Extracts from all the three components of the composition UP1307, Rosmarinus officinalis,12 Annona squamosa,13 and Zanthoxylum americanum,14 have been previously reported to possess anti-inflammatory properties that potentially strengthen their usage in cellulite intervention. Hence, UP1307 composition containing Annona squamosa, Zanthoxylum clava-herculis, and Rosmarinus officinalis extracts appears to have protective cellular properties against the destructive free radical species, improves microcirculation, and promotes lipolysis, suggesting use for cellulite and other skin conditions.
CONCLUSION
UP1307 has shown significant improvements of skin hydration and firmness after as early as two weeks of application. Based on the subjective questionnaires, after eight weeks of UP1307 topical usage, the skin was significantly more hydrated and firm with improved elasticity. These subjective improvements from UP1307 were corroborated objectively by instrument data from the cutometer (firmness and elasticity) and corneometer (hydration). In addition, within the given period, almost half of the subjects using UP1307 reported reductions in cellulite appearance. No significant adverse effects at the site of UP1307 cream application were reported by the subjects. This study is the first to show that topical application of a combination of three medicinal extracts from Rosmarinus officinalis, Annona squamosa, and Zanthoxylum clava-herculis can improve skin hydration, firmness, and elasticity while reducing cellulite. If the indication is solely intended for cellulite reduction, longer duration of application is warranted.
REFERENCES
- 1.Alanen E, Nuutinen J, Nicklén K, et al. Measurement of hydration in the stratum corneum with the MoistureMeter and comparison with the Corneometer. Skin Res Technol. 2004;10(1):32–37. doi: 10.1111/j.1600-0846.2004.00050.x. [DOI] [PubMed] [Google Scholar]
- 2.Bertin C, Zunino H, Pittet JC, et al. A double-blind evaluation of the activity of an anticellulite product containing retinol, caffeine, and ruscogenine by a combination of several non-invasive methods. J Cosmet Sci. 2001;52:199–210. [PubMed] [Google Scholar]
- 3.Blume-Peytavi U, Kottner J, Sterry W, et al. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist. 2016;56(Suppl 2):S230–S242. doi: 10.1093/geront/gnw003. [DOI] [PubMed] [Google Scholar]
- 4.Brownell LA, Chu M, Corneliusen B, et al. Skin care compositions and methods of use thereof. USPT application # 20160263013. 15 Sep 2016. [Google Scholar]
- 5.Carle R. Anti-inflammatory and spasmolytic botanical drugs. Breast J Phytother. 1990;1:32–39. [Google Scholar]
- 6.Cellulite meltdown? Harv Womens Health Watch. 1998:12–7. Aug;5. [PubMed] [Google Scholar]
- 7.Collis N, Elliot LA, Sharpe C, Sharpe DT. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104(4):1110–1114. discussion 1115—1117. [PubMed] [Google Scholar]
- 8.Facino RM, Carini M, Stefani R, et al. Anti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus. factors contributing to their efficacy in the treatment of venous insufficiency. Arch Pharm (Weinheim). 1995;328:720–724. doi: 10.1002/ardp.19953281006. [DOI] [PubMed] [Google Scholar]
- 9.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 10.Ghasemzadeh Rahbardar M, Amin B, et al. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed Pharmacother. 2016;86:441–449. doi: 10.1016/j.biopha.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Hexsel D, Orlandi C, Zechmeister do Prado D, et al. Botanical extracts used in the treatment of cellulite. Dermatol Surg. 2005;31:866–873. doi: 10.1111/j.1524-4725.2005.31733. [DOI] [PubMed] [Google Scholar]
- 12.Hexsel D, Zechemeister do Prado D, Goldman MP. Topical management of cellulite [Google Scholar]
- 13.Goldman M, Hexsel D. London: England, Informa Health Care; 2010. Cellulite: Pathophysiology and Treatment; pp. 62–68. [Google Scholar]
- 14.Khan MH, Victor F, Rao B, Sadick NS. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62(3):361–370. doi: 10.1016/j.jaad.2009.10.042. quiz 371—372. [DOI] [PubMed] [Google Scholar]
- 15.Kligman AM, Pagnoni A. Stoudemeyer. Topical retinol improves cellulite. J Dermatol Treat. 1999;10:119–125. [Google Scholar]
- 16.Lesser T, Ritvo E, Moy LS, et al. Modification of subcutaneous adipose tissue by a methylxanthine formulation. a double-blind controlled study. Dermatol Surg. 1999;25:445–462. doi: 10.1046/j.1524-4725.1999.08243.x. [DOI] [PubMed] [Google Scholar]
- 17.Lis-Balchin M. Parallel-placebo-controlled clinical study of a mixture of herbs sold as a remedy for cellulite. Phytother Res. 1999;13:627–629. doi: 10.1002/(sici)1099-1573(199911)13:7<627::aid-ptr561>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.López-Jiménez A, García-Caballero M, Medina MÁ, Quesada AR. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur J Nutr. 2013;52(1):85–95. doi: 10.1007/s00394-011-0289-x. [DOI] [PubMed] [Google Scholar]
- 19.Mengoni ES, Vichera G, Rigano LA, et al. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia. 2011;82(3):414–421. doi: 10.1016/j.fitote.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Nobile V, Michelotti A, Cestone E, et al. Skin photoprotective and antiaging effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr Res. 2016;60:31871. doi: 10.3402/fnr.v60.31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Offord EA, Gautier JC, Avanti O, et al. Photoprotective potential of lycopene, beta-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic Biol Med. 2002;32(12):1293–1303. doi: 10.1016/s0891-5849(02)00831-6. [DOI] [PubMed] [Google Scholar]
- 22.Oriowo Ma. Anti-inflammatory activity of piperonyl-4-acrylic isobutyl amide, an extractive from Zanthoxylum zanthoxyloides. Planta Med. 1982;44(1):54–56. doi: 10.1055/s-2007-971402. [DOI] [PubMed] [Google Scholar]
- 23.Park M, Han J, Lee CS, et al. Carnosic acid, a phenolic diterpene from rosemary, prevents UV-induced expression of matrix metalloproteinases in human skin fibroblasts and keratinocytes. Exp Dermatol. 2013;22(5):336–341. doi: 10.1111/exd.12138. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-SÁnchez A, Barrajón-CatalÁn E, et al. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J Photochem Photobiol B. 2014;136:12–18. doi: 10.1016/j.jphotobiol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Pierard GE. Commentary on cellulite: skin mechanobiology and the waist-to-hip ratio. J Cosmet Dermatol. 2005;4:151–152. doi: 10.1111/j.1473-2165.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 26.Ponrasu T, Suguna L. Efficacy of Annona squamosa L. in the synthesis of glycosaminoglycans and collagen during wound repair in streptozotocin induced diabetic rats. Biomed Res Int. 2014:124352. doi: 10.1155/2014/124352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 28.Rubanyi G, Marcelon G, Vanhoutte PM. Effect of temperature on the responsiveness of cutaneous veins to the extract of Ruscus aculeatus. Gen Pharmacol. 1984;15:431–434. doi: 10.1016/0306-3623(84)90045-4. [DOI] [PubMed] [Google Scholar]
- 29.Sun D, Han Y, Wang W, et al. Screening and identification of Caulis Sinomenii bioactive ingredients with dual-target NF-κB inhibition and β2- AR agonizing activities. Biomed Chromatogr. 2016;30(11):1843–1853. doi: 10.1002/bmc.3761. [DOI] [PubMed] [Google Scholar]
- 30.Velasco MV, Tano CT, Machado-Santelli GM, et al. Effects of caffeine and siloxanetriol alginate caffeine, as anticellulite agents, on fatty tissue: histological evaluation. J Cosmet Dermatol. 2009;7:23–29. doi: 10.1111/j.1473-2165.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- 31.Vikas B, Akhil B S, P R, Sujathan K. Free radical scavenging properties of Annona squamosa. Asian Pac J Cancer Prev. 2017;18(10):2725–2731. doi: 10.22034/APJCP.2017.18.10.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu P, Wu M, Xu L, et al. Anti-inflammatory cyclopeptides from exocarps of sugar-apples. Food Chem. 2014;152:23–28. doi: 10.1016/j.foodchem.2013.11.100. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J Biol Chem. 2006;281(37):27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157(5):874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 35.Yimam M, Lee YC, Jiao P, et al. A standardized composition comprised of extracts from Rosmarinus officinalis, Annona squamosa and Zanthoxylum clava-herculis for cellulite. Phcog Res. 2017;9:319–24. doi: 10.4103/pr.pr_70_17. [DOI] [PMC free article] [PubMed] [Google Scholar]