Abstract
In vivo reflectance confocal microscopy (RCM) is a noninvasive high-resolution skin imaging tool that has become an important adjunct to clinical exam, dermoscopy and histopathology assessment, in the diagnosis and management of melanoma. RCM generates a horizontal view of the skin, whereby cellular and subcellular (e.g., nuclei, melanophages, collagen) structures, to the level of the upper dermis, are projected onto a screen at near-histological resolution. Morphologic descriptors, standardized terminology, and diagnostic algorithms are well established for the RCM assessment of melanoma, melanocytic, and nonmelanocytic lesions. Clinical applications of RCM in melanoma are broad and include diagnosis, assessment of large lesions on cosmetically sensitive areas, directing areas to biopsy, delineating margins prior to surgery, detecting response to treatment and assessing recurrence. This review will provide an overview of RCM technology, findings by melanoma subtype, clinical applications, as well as explore the accuracy of RCM for melanoma diagnosis, pitfalls and emerging uses of this technology ex vivo.
Keywords: : algorithm, confocal microscopy, cutaneous oncology, diagnosis, in vivo confocal microscopy, mapping, melanoma, melanoma subtype, noninvasive skin imaging, pathology correlations
Background: reflectance technology
Optical principles
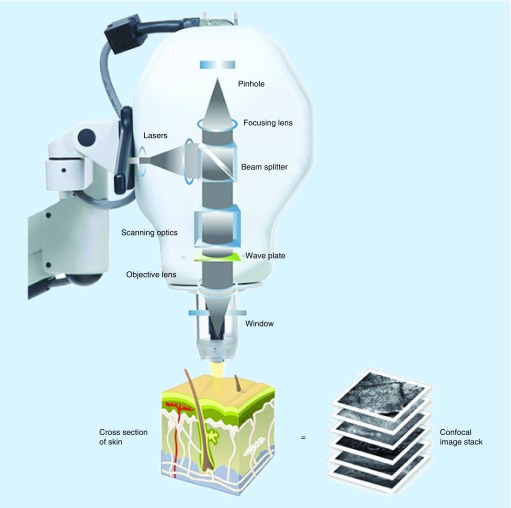
RCM (reflectance confocal microscopy) allows dynamic imaging of the skin at cellular resolution in real time, akin to an optical biopsy [1–3]. Laser light, at near infrared wavelength (830 nm), is emitted from a diode at a power up to 35 mW [4], which is nondestructive to tissue. This illuminates a point of interest on the skin to a depth approximating the upper papillary dermis (200 μm). Microscopic tissue elements reflect light with different refractive indices back through a pinhole aperture, which filters out surrounding light, allowing only light from the point of interest to be detected (Figure 1). In this way, both the point of interest and the pinhole aperture align to create a coincidence of two focal planes (hence ‘confocal’), allowing high resolution imaging (∼1 μm). As depth increases beyond 200 μm, resolution decreases, rendering images too blurry and dark for analysis [5]. Structures with a high refractive index, such as melanin, collagen, hemoglobin, and keratin, back scatter (hence the term ‘reflectance’) the light and appear brighter than other components [6,7]. Images are most often acquired in the horizontal plane, parallel to the skin surface, but raised lesions may be viewed in the oblique plane, in contrast to the vertical assessment of histopathology [8].
Figure 1. . Optical principles of reflectance confocal microscopy.
The light source is directed toward a precise point. Tissue structures backscatter the light, which is directed through a pinhole and enters a detector.
Reproduced with permission from Caliber Imaging & Diagnostics, Inc. NY, USA.
Image acquisition
Each skin layer is visualized in a stepwise manner from the stratum granulosum/spinosum, basal cell layer, and dermoepidermal junction (DEJ), followed by the papillary dermis [8]. At each level images can be captured as individual images (0.5 × 0.5 mm for Vivascope® 1500, and up to 1 × 1 mm for VivaScope 3000 [Caliber Imaging & Diagnostics, Inc., NY, USA), blocks (1 × 1 mm up to 8 × 8 mm areas of skin, made up of individual images stitched together side by side into a mosaic), or stacks (individual images captured ∼4 μm apart in depth from superficial to deep). Images are stored electronically and can be viewed at different points in time for comparison.
Technical features
Two main reflectance confocal microscopes are available for in vivo use, including VivaScope 1500 and VivaScope 3000 (Table 1) [9,10]. The major differences between the systems are the way they are operated and the way images are displayed.
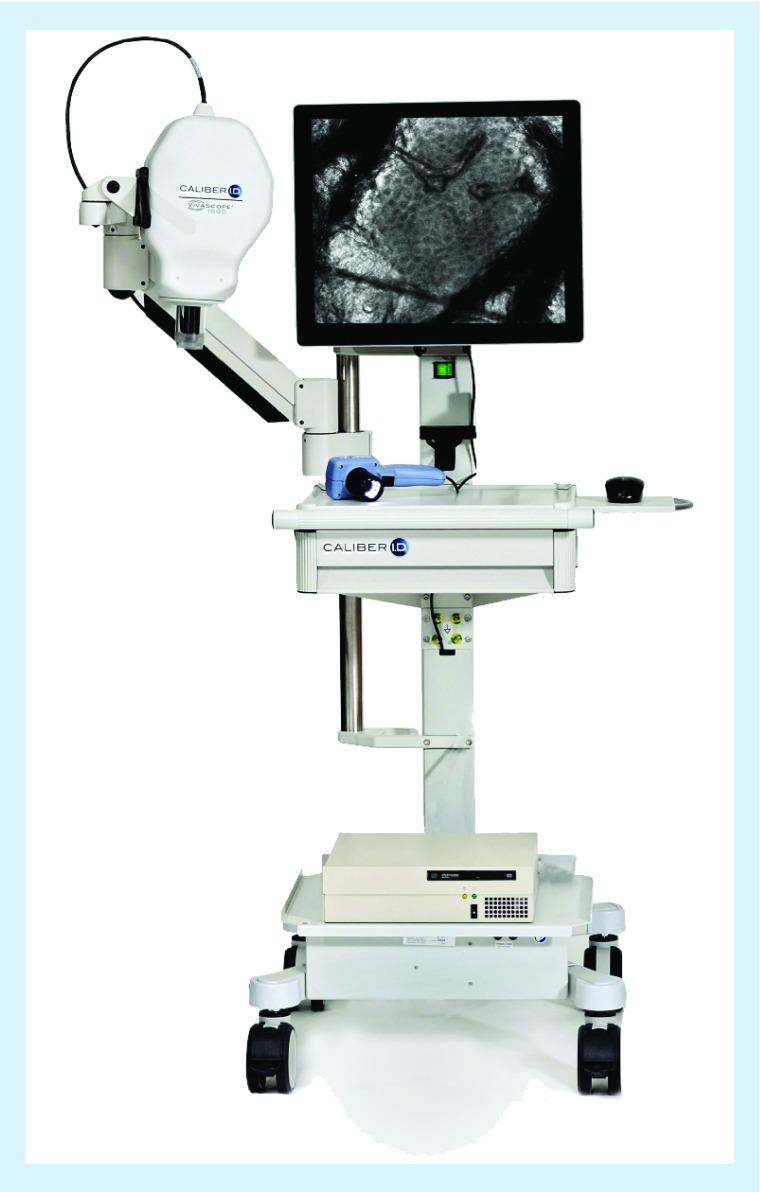
VivaScope 1500 (Figure 2) is a wide-probe microscope which uses a ringed glass window that is fixed to the patient's skin and then coupled to the RCM camera, which is attached to a movable steel arm. First, a dermoscopic image is captured and then points of interest are navigated with RCM. Individual images are captured in a 0.5 × 0.5 mm field. Mosaic imaging stitches individual images side by side to obtain a field view of up to 8 × 8 mm, allowing evaluation of the global architecture of a lesion. Additional functions include the ability to capture movies. The advantages of VivaScope 1500 are the large field of view, the fixed probe coupled with a dermoscope, allowing perfect correlation. The main disadvantages are that it cannot be used on small or curved surfaces, nor on fragile sweaty/eroded/ulcerated areas because of the need for adhesive fixation. For each confocal view, the stainless steel ring and adhesive window needs to be re-prepared and the RCM microscope does not move freely on the skin.
VivaScope 3000 (Figure 3) is a handheld device, guided by the operator, that moves freely on the skin surface. Generated images are captured in a field of view of 0.75 × 0.75 mm or 1 × 1 mm. A stack generates a series of horizontal images from the upper to the lower layer. Videos may also be captured. The main advantages of VivaScope 3000 are that it can be used on small and contoured surfaces, and that it is less time-consuming when a large area needs to be imaged. Limitations include the smaller field of view and the difficulty to determine precise orientation on the tissue once the probe is removed, with a lack of dermoscopic correlation.
Table 1. . Technical differences between VivaScope® 1500 & VivaScope 3000.
| Technical data | VivaScope 1500 | VivaScope 3000 |
|---|---|---|
| Horizontal optical resolution | <1.25 μm in center of image field | <1.25 μm in center of image field |
| Vertical optical resolution | <5.0 μm in center of image field | <5.0 μm in center of image field |
| Maximum imaging depth | Superficial dermis | Superficial dermis |
| Viewable section of individual images | 500 μm × 500 μm | 750 × 750 μm or 1000 × 1000 μm‡ |
| Maximum mapped field | 8.0 × 8.0 mm | Unlimited |
| Image resolution | 1000 × 1000 pixels | 1000 × 1000 pixels |
| Optical operating power | CDRH Class 1† | CDRH Class 1† (maximum 22 mW) |
| Imaging wavelength | 830 nm | 830 nm |
| Magnification | ca. 520× | ca. 350× |
| Objective | Caliber I.D. StableView™ 30× magnification, 0.9 NA water immersion |
Caliber I.D. StableView 30× magnification, 0.9 NA water immersion |
| Imaging types | Individual view, blocks/mosaics, movie | Individual view, stacks, movies |
| Practical uses | Assess global architecture of lesions, directly correlate dermoscopic and confocal findings | Rapid evaluation of large lesions on small and curved areas. Less time consuming than the Vivascope® 1500 |
| Limitations | Bulky steel arm. Need to re-apply adhesive window for every mosaic view |
No clear dermoscopic correlation |
†CDRH is a regulatory office within the US FDA for the safety of laser devices. Class 1 level means laser radiation is not considered to be hazardous or cause any biological damage.
‡The viewable section of individual images may be different depending on the generation of the Vivascope 3000 that is used.
ca: Chromatic aberration; Caliber I.D.: Caliber Imaging & Diagnostics, Inc.; CDRH: Center for Devices and Radiological Health; NA: Numerical Aperture.
Reproduced with permission from [10] © Mavig Vivascope, Munich, Germany.
Figure 2. . Vivascope® 1500: the microscope is coupled to a dermatoscope.
Prior to imaging, the large probe must be fixed to the patient's skin. Reproduced with permission from Caliber Imaging & Diagnostics, Inc., NY, USA.
Figure 3. . Vivascope® 3000: the image illustrates the hand-help probe guided by the operator, moving freely on the skin surface.
Acquired images are displayed in a similar manner than Vivascope 1500.
Reproduced with permission from Caliber Imaging & Diagnostics, Inc., NY, USA.
In vivo reflectance confocal microscopy for the diagnosis of melanoma
RCM is well suited to the imaging of melanocytic lesions, such as melanoma [6,11]. Melanin has the highest refractive index of all tissue elements (n = 1.7) and acts as a naturally occurring ‘endogenous’ contrast agent [6]. Consensus terminology for RCM evaluation of melanocytic lesions was established by a group of experts in 2007 and assembled into a glossary [8]. RCM-specific vocabulary and architectural and structural features of normal skin were defined, for example, ‘honeycomb’ and ‘cobblestone’ pattern, to describe the normal polygonal arrangement of keratinocytes in stratum spinosum/granulosum. Individual cell types including melanocytes, keratinocytes and melanophages, were described in terms of morphology (e.g., round, polygonal, etc.), as well as patterns of distribution (e.g., pagetoid spread, clustering, and nests). A summary of the basic used terms to describe melanocytic lesions is provided in Table 2.
Table 2. . Basic terminology used to describe melanocytic lesions.
| Layer | Terminology | Description |
|---|---|---|
| Superficial (suprabasal) epidermis | Regular honeycomb pattern | Normal keratinocytes appearing as polygonal cells with well-demarcated refractive cellular outlines |
| Regular cobblestone pattern | Small monomorphous round cells appearing bright because of a high amount in melanin in the keratinocytes | |
| Irregular (or atypical) honeycomb or cobblestone pattern | Irregular-shaped keratinocytes in a honeycomb or cobblestone pattern | |
| Broadened honeycomb pattern | Honeycomb pattern with enlarged intercellular spaces | |
| Epidermal disarray (or disarranged pattern) | No recognizable honeycomb or cobblestone pattern replaced by irregular bright particles | |
| Pagetoid cells (or pagetoid infiltration) | Cells twice the size of keratinocytes. May be round, dendritic or both | |
| Basal cell layer and DEJ | Edged papillae | Normal dermal papillae surrounded by a rim of refractive cells |
| Nonedged dermal papillae | Dermal papillae are not visible or not demarcated by a normal rim of bright cell but rather by large reflective structures | |
| DEJ disarray | Loss of the normal architecture of the DEJ | |
| Nests | Oval to round bright structures corresponding to aggregates of melanocytes. Nests may be further descried as being dense, sparse or cerebriform | |
DEJ: Dermoepidermal junction.
Numerous studies since have shown the diagnostic accuracy of RCM in the evaluation of pigmented lesions and melanoma diagnosis (Table 3) (12,13–17,18). RCM evaluation has the potential to improve sensitivity (improve melanoma detection) [19,20] and specificity (reduce excisions of benign lesions [12,21–23]). In one study, RCM evaluation of doubtful lesions reduced the need for unnecessary excisions by over 50% [21] and in another, it reduced the number needed to treat from 3.73 (clinical and dermoscopy evaluation) to 2.87 (addition of RCM to clinical and dermoscopy evaluation) [12]. In a meta-analysis comparing dermoscopy and RCM for the diagnosis of malignant skin tumors, RCM improved the detection rate of melanoma by 4.3% at a per-lesion level [13].
Table 3. . Selected studies evaluating melanoma diagnosis accuracy using in vivo reflectance confocal microscopy.
| Study (year) | Study design | Diagnostic accuracy |
|---|---|---|
| Gerger et al. (2008) | Prospective, observational | Sn 97.5%; Sp 99% for diagnosis of melanocytic tumors |
| Guitera et al. (2010) | Retrospective, observational | LM score ≥2 resulted in Sn 85% and Sp 76% for LM diagnosis (OR for LM: 18.6; 95% CI) |
| Stevenson et al. (2013) | Meta-analysis | Sn 93% (95% CI: 89–96); Sp 75% (95% CI: 68–83) for melanoma diagnosis |
| Alarcon et al. (2014) | Prospective, observational | Sn 97.8% (95% CI: 91.6–99.6); Sp 92.4% (95% CI: 87.2–95.7) for melanoma diagnosis |
| Lovatto et al. (2015) | Retrospective, observational | Sn 100%; Sp 69% for melanoma detection |
| Xiong et al. (2016) | Meta-analysis | Sn 92.7% (95% CI: 0.90–0.95) and Sp 78.3% (95% CI: 0.76–0.81) for melanoma detection |
| Xiong et al. (2017) | Meta-analysis | Sn 93.5% (95% CI: 0.90–0.96) and Sp 78.8% (95% CI: 0.75–0.82) |
LM: Lentigo maligna; OR: Odds ratio; Sn: Sensitivity; Sp: Specificity.
This section will outline RCM features associated with melanoma subtypes and the main algorithms for melanoma diagnosis.
Morphological features of melanoma on RCM by subtype
Some features are uniformly found in every melanoma and some are characteristic of specific subtypes.
Superficial spreading melanoma
Superficial spreading melanoma (SSM) is the most common melanoma subtype, comprising 50–80% of all melanomas [24]. RCM features are outlined in Table 4 [25].
Table 4. . Reflectance confocal microscopy features suggesting superficial spreading melanoma.
| Layer | Feature | Description |
|---|---|---|
| Superficial (suprabasal) epidermis | Atypical honeycomb pattern | Partial (poorly visible) or complete (nonvisible) loss of normal honeycomb pattern usually caused by pagetoid spread of cells |
| Atypical cobblestone pattern | Loss of normal cobblestone pattern with presence of atypical cells† | |
| Pagetoid cells (round, dendritic or spindled) | Bright, large, nucleated cells (twice the size of surrounding keratinocytes), typically round but may be pleomorphic | |
| Basal cell layer and DEJ‡ | Disarranged DEJ | In melanocytic lesions, the DEJ normally displays one of four global patterns; clod, meshwork, ringed or nonspecific patterns. In melanomas, although one of the global patterns may be recognized, the regular architecture is lost and replaced by a disorganized appearance of the DEJ |
| Nonedged dermal papillae | Dermal papillae are not visible or not demarcated by a normal rim of bright cell but rather by large reflective structures | |
| Cellular atypia | Large cells (>2x the size of surrounding keratinocytes) with large nuclei and/or irregular shape | |
| Upper dermis | Sparse nests composed of round or pleomorphic cells [26] | Aggregation of cells composed of isolated large nucleated cells |
| Cells distributed in sheet-like structures | Hyper-refractive cells distributed in the same plane and loss of dermal papillae | |
†Atypical cells are defined as irregular in size, shape or refractivity compared with their normal counterpart.
‡Patterns are best evaluated with mosaics.
DEJ: Dermoepidermal junction.
Amelanotic melanoma
The diagnosis of amelanotic melanoma is challenging. Confusions with benign skin lesions or nonmelanoma skin cancer are potential pitfalls. Dermoscopic evaluation is useful to detect subtle signs of amelanotic melanoma, such as the presence of dotted vessels, linear irregular vessels, and milky red areas [27,28]. However, these findings may be easily overlooked in areas of poikiloderma or rosacea. Amelanotic lesions are a major indication for RCM, as even small amounts of melanin will appear bright on confocal imaging [15]. When added to dermoscopy, as second step evaluative tool, Guitera and colleagues demonstrated that RCM could improve the diagnostic accuracy of 194 dermoscopically challenging lightly pigmented and amelanotic lesions in a set of 194 lesions (45 melanomas [16 in situ, 29 invasive], 68 nevi, 48 basal cell carcinomas [BCCs], 10 actinic keratosis, 10 squamous cell carcinomas, and 13 other benign lesions). With the use of RCM, sensitivity was 84.4% and specificity 56%. All melanomas misclassified by either dermoscopy or RCM were detected by the other tool.
RCM findings in amelanotic melanoma are similar to pigmented melanoma and include the loss of normal honeycomb pattern in the suprabasal epidermis, cellular atypia in the basal layer, and DEJ disarray with irregular dermal papillae [29]. Pagetoid cells may appear dendritic or round. As opposed to the bright pagetoid cells of pigmented melanomas, the pagetoid cells of amelanotic melanomas may appear hyporeflective, appearing as dark holes in the epidermis [30]. Nevertheless, hyporeflective pagetoid cells may also be found in Spitz nevi, squamous cell carcinomas, BCCs and inflamed seborrheic keratosis. In the case of nonmelanocytic tumors, the presence of other ‘benign’ confocal findings generally orient toward the right diagnosis.
Nodular melanoma
Nodular melanoma (NM) has a fast growth rate and is associated with a higher rate of death from metastatic melanoma [31]. Amelanotic or hypomelanotic presentation of NM is especially challenging [32] and can be mistaken for benign tumors. A retrospective study by Cicchiello et al. (120 patients, including 60 NM and 60 SSM) found that patients with NM were more likely to be falsely reassured that the lesion was benign (47% NM vs 27% SSM, p = 0.02) with only 43% of NM biopsied at the first visit (vs 70% of SSM, p = 0.01) [31].
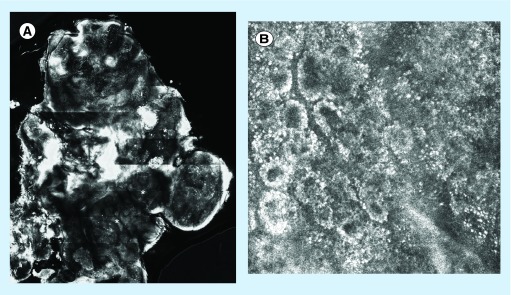
NM findings more frequently seen include cerebriform nests (Figure 4), which are highly specific of deep/NM but rarely found [33,34] and enlarged dermal vessels [3,35]. Pure NMs will lack typical pagetoid cells and show a preserved epidermal architecture [35]. A summary of the RCM features of NMs is provided in (Table 5).
Figure 4. . Melanoma with cerebriform nests compared with the normal dermoepidermal junction.
(A) Cerebriform nests compared with (B) normal dermoepidermal junction. (A) Data taken from A/Professor Caterina Longo (Dermatology and Skin Cancer Unit, Arcispedale Santa Maria Nuova IRCCS, Reggio Emilia, Italy).
Table 5. . Reflectance confocal microscopy features in favor of nodular melanoma.
| Layer | Feature | Description |
|---|---|---|
| Suprabasal epidermis | Few pagetoid cells | Pure nodular melanomas have fewer pagetoid cells than SSMs |
| Typical epidermal architecture | Preserved honeycomb pattern (differing from SSM that exhibit distortion of the normal epidermal pattern) | |
| Basal cell layer and DEJ | No visible dermal papillae | Substituted by sheets of atypical cells |
| Upper dermis | Cerebriform nests | Aggregation of small compact cells with global cerebriform appearance |
| Plump bright cells | Bright refractive particles with no visible nucleus, correlating to melanophages | |
| Enlarged vessels | – | |
DEJ: Dermoepidermal junction; SSM: Superficial spreading melanoma.
Lentigo maligna & lentigo maligna melanoma
Lentigo maligna (LM) presents as a slowly progressive pigmented macule on sun-exposed areas, most commonly on the face and neck and lentigo maligna melanoma (LMM) is its invasive counterpart. In clinical practice, one must differentiate LM from benign pigmented macules. Dermoscopy has improved diagnosis of these lesions but they remain a challenge owing to overlapping features [36]. Histopathology is also difficult due to atypical melanocytic proliferation on sun-damaged skin [37]. RCM has proved to be helpful to enhance diagnosis of these lesions.
Features of LM/LMM using RCM are well described [15,38–42]. In 2010, Guitera et al. developed the LM score to assist in differentiating LM from equivocal pigmented macules of the face [15]. This was developed on a set of 81 LM and 203 benign pigmented macules. The sensitivity and specificity of 64 confocal findings were evaluated. Six features that independently correlate with the diagnosis of LM were identified. The score consists of two major and four minor criteria (Table 6). With a score more than or equal to two points, a sensitivity and specificity of 85 and 76%, respectively (odds ratio: 18.6; 95% CI: 9.3–37.1) were achieved for LM diagnosis. The method was developed using the traditional wide-probe RCM. Further studies have applied these criteria using the handheld RCM and obtained similar results [41,43].
Table 6. . Lentigo maligna score to distinguish benign pigmented macules from lentigo maligna and lentigo maligna melanoma.
| Criteria type | RCM feature | Point(s) |
|---|---|---|
| Major criteria | Nonedged dermal papillae | +2 |
| Large round pagetoid cells | +2 | |
| Minor criteria | Nucleated cells in the dermal papillae | +1 |
| Atypical cells at the DEJ | +1 | |
| Adnexal spread of atypical cells | +1 | |
| Broadened honeycomb pattern | -1 | |
DEJ: Dermoepidermal junction; RCM: Reflectance confocal microscopy.
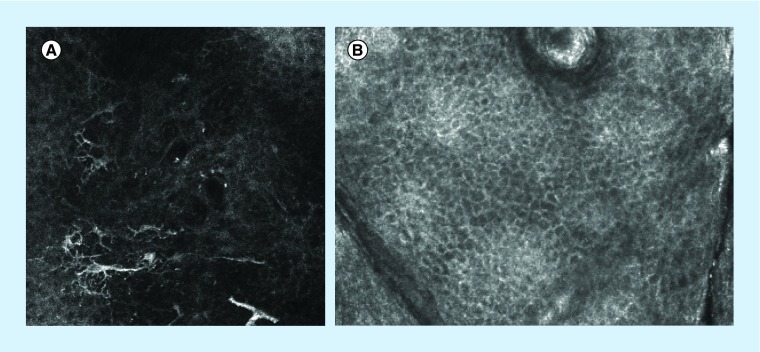
The earliest histopathological finding in LM is the proliferation of atypical melanocytes at the dermal–epidermal junction [44]. On RCM, this correlates with large atypical cells at the DEJ. Evaluation of this layer is thus usually prioritized when there is clinical suspicion of LM. Round large pagetoid cells (Figure 5), epidermal disarray, and follicular localization of atypical cells (Figure 6) are other recognized findings. Advanced cases will show large nucleated cells and nests in the upper dermis. A summary of features of LM and LMM is in Table 7.
Figure 5. . (A) Large dendritic pagetoid cells in the superficial epidermis of lentigo maligna compared with (B) normal superficial epidermis: the normal honeycomb pattern expected in the supra-basal epidermis with well-demarcated cells that resemble a honeycomb.
Figure 6. . Atypical cells around a hair follicle.
This feature is typically found in lentigo maligna and corresponds to atypical melanocytes descending along the hair follicle.
Table 7. . Reflectance confocal microscopy features of lentigo maligna and lentigo maligna melanoma.
| Layer | Feature | Description |
|---|---|---|
| Suprabasal epidermis | Large round pagetoid cells | Round bright nucleated cells twice the size of keratinocytes in the suprabasal epidermis |
| Epidermal disarray | No recognizable honeycomb or cobblestone pattern | |
| Atypical cobblestone pattern | Loss of normal cobblestone pattern with atypical cell size and/or refractivity | |
| Basal cell layer and DEJ | Nonedged papillae | Loss of normal rim of bright cells around the dermal papillae |
| Atypical cells at the junction | Including cells distributed in sheet-like structures | |
| Follicular localization of atypical cells | Atypical cells around the hair follicles | |
| Medusa head-like structures | Elongated bundles, composed of atypical cells, extending from the hair follicles | |
| Upper dermis | Large nucleated cells in the dermal papillae | – |
| Dermal nests | Aggregation of cells | |
| Plump bright cells | Bright refractive particles with no visible nucleus; represent melanophages on histopathology | |
DEJ: Dermoepidermal junction.
RCM is also useful for presurgical delineation of LM and LMM and post-treatment surveillance. This is discussed in the section clinical applications.
Desmoplastic melanoma
Desmoplastic melanoma is a rare melanoma subtype that most commonly occurs on chronically sun-exposed areas of elderly patients. Misdiagnosis is common as it is often amelanotic and may mimic a scar or benign cutaneous tumor, such as dermatofibroma [45].
General melanoma features, such as pagetoid cells and cytologic atypia, are generally found in this melanoma subtype. Features more commonly seen in desmoplastic melanoma include spindle cells in the superficial dermis, nucleated cells in the dermis, and dermal inflammation (Table 8) [46]. These findings may be especially useful to distinguish desmoplastic melanoma from melanoma in situ. Nevertheless, the presence of spindle and nucleated cells in the dermis is not specific for desmoplastic melanoma and may also be found in any type of invasive melanoma [46].
Table 8. . Reflectance confocal microscopy features that may suggest the diagnosis of desmoplastic melanoma in the upper dermis.
| Feature | Description |
|---|---|
| Spindle cells | Elongated nucleated cells |
| Large nucleated cells | – |
| Inflammation | Small bright rounded structures |
Differentiating nevi from melanoma
Benign nevi have an overall homogeneous preserved architecture (e.g., typical honeycomb pattern), no pagetoid cells, and no cytologic atypia [47]. At the DEJ, benign patterns are recognized by features, such as a ringed pattern (created by bright cells around the papillae) or a meshwork pattern (formed by junctional thickening and widening of the interpapillary spaces) in junctional nevi, a clod pattern (formed by dense nests in the dermis) in dermal nevi, and a mixture of meshwork and/or ringed and clod pattern in compound nevi.
Dysplastic nevi can be very challenging to assess on RCM, as is dermoscopy and pathology. Pellacani et al. developed an algorithm, based on the histological Duke criteria of grading melanocytic dysplasia, to assist in creating an equivalent RCM-based method to distinguish dysplatic nevi from nondysplastic nevi and melanoma (Table 9) [48]. 60 dermoscopically equivocal melanocytic lesions (19 nondysplastic nevi, 27 dysplastic nevi, and 14 SSM) were evaluated with RCM and histopathology. RCM correlates of Duke criteria were established and a two-step algorithm was developed based on the features that were independently associated with dysplastic nevi, nondysplastic nevi and melanoma. The first step involves distinguishing dysplastic from nondysplastic nevi. The presence of at least one feature of architectural and cytological atypia is suggestive of a dysplastic nevus. The second step allows differentiation of dysplastic nevi from melanoma by qualifying the extent of atypia. Using this approach, all melanomas, 12 of 27 dysplastic nevi and 15 of 19 nondysplastic nevi were correctly classified. This was a pilot study and sensitivity and specificity are yet to be validated in a test set.
Table 9. . Two-step algorithm to distinguish dysplastic nevi from melanoma and nondysplastic nevi.
| Step | Description | Features |
|---|---|---|
| Step 1. Dysplastic versus nondysplastic nevus | Architectural atypia (≥1 feature present) | 1. Irregular junctional nests 2. Short interconnections between junctional nests 3. Nonhomogeneous junctional nests |
| Cytological atypia (≥1 feature present) | 1. Round pagetoid cells 2. Atypical cells at the DEJ |
|
| Step 2. Melanoma versus dysplastic nevus | Degree of atypia (≥1 feature present suggestive of melanoma) | 1. Atypical cells involving at least 50% of DEJ 2. Round pagetoid cells encompassing at least 50% of spinous layer 3. Nonedged papillae involving at least 10% of the lesion |
DEJ: Dermoepidermal junction.
Diagnosis of nevoid melanoma may be challenging due to features mimicking dermal nevi. In a retrospective study, four nevoid melanomas were misdiagnosed [34]. When this diagnosis is suspected clinically, subtle cytologic atypia in dermal nests may be the only finding and should raise suspicion for melanoma [49].
Melanoma in special sites
Mucosal melanoma (oral & genital)
RCM is a promising modality to distinguish mucosal melanoma from mucosal melanosis [50–57]. Due to the lack of a keratinized stratum corneum, mucosa is well suited for RCM evaluation. A summary of RCM features of lip and genital melanoma is presented in Table 10.
Table 10. . Reflectance confocal microscopy features of mucosal (i.e., lip and genital) melanoma.
| Layer | Feature | Description |
|---|---|---|
| Suprabasal epidermis | Round, dendritic or fusiform pagetoid cells with plump body | Bright nucleated cells twice the size of keratinocytes in the suprabasal epidermis |
| Epidermal disarray | No recognizable honeycomb or cobblestone pattern | |
| Basal cell layer and DEJ | High density of dendritic cells | Bright dendritic cells at the basal cell layer |
| Loss of the normal architecture of the papillae | Dishomogeneous distribution of the papillae | |
| Atypical cells in sheet-like structures in the papillae | Bright atypical cells distributed in the same plan in the chordion papillae | |
DEJ: Dermoepidermal junction.
As opposed to cutaneous melanoma, nonedged papillae and atypical dendritic cells at the DEJ are not useful to distinguish between benign melanocytic macules and melanoma of the lip [15,58]. A recent retrospective observational study analyzed RCM images from 16 benign melanocytic macules of the lip (five biopsy proven) and six melanomas of the lip. They identified features that independently correlate with lip melanoma to create the ‘Lip score’ (Table 11) [50]. A score greater than or equal to four correctly identified lip melanoma with 100% sensitivity and 88% specificity. Validation of the score on a larger cohort is needed.
Table 11. . The ‘Lip score’ to differentiate lip melanoma from benign melanocytic macules of the lip.
| RCM feature | Points |
|---|---|
| Regular honeycomb pattern | -1 |
| Epidermal disarray | +1 |
| Presence of dendritic or round pagetoid cells | +2 |
| Homogenous distribution of the papillae | -1 |
| Dishomogeneous distribution of the papillae | +1 |
| Marked cellular atypia at the DEJ | +1 |
| Atypical cells distributed in the interpapillary space | +1 |
DEJ: Dermoepidermal junction; RCM: Reflectance confocal microscopy.
Cinotti et al. reported a series of ten pigmented lesions of the vulva, including eight benign melanocytic macules and two melanomas (both multifocal with invasive and in situ components). They found that benign melanocytic macules are characterized by draped (elongated) or ringed (round) papillae surrounded by a rim of bright cells and occasional bright dendritic cells at the basal layer. Vulvar melanomas featured atypical cells in the suprabasal epidermis and loss of normal architecture of the papillae [56].
RCM diagnostic algorithms for melanoma diagnosis
Five main scoring systems and algorithms have been developed for the diagnosis of melanoma using RCM [15,33–34,59–63]. Four of them are described below and the fifth is the LM score, previously discussed in the section on LM and LMM.
The Modena algorithm: Pellacani et al. identified six RCM criteria that independently correlate with the diagnosis of melanoma and may be used to differentiate melanomas from nevi (Table 12) [58,64]. These were originally developed in a cohort of 37 melanomas (including four melanoma in situ) and 65 nevi. The sensitivity and specificity were validated in 351 equivocal melanocytic lesions (136 melanomas and 215 nevi) and used to develop a scoring algorithm for diagnosis of melanoma [58]. The 136 melanomas included, 42 in situ (excluding LM), 86 SSM and 8 NM, with a median Breslow's thickness of 0.49 mm (interquartile range: 0–0.89 mm). The scoring algorithm is composed of two major criteria (two points each) and four minor criteria (one point each). A score of greater than or equal to three is strongly associated with the diagnosis of melanoma (97.3% sensitivity, 72.3% specificity). Five of 136 (3 in situ, 2 invasive: 0.3 and 0.4 mm Breslow) melanomas were falsely negative on RCM and were characterized by mildly atypical melanocytes, occasional pagetoid cells and nested proliferation, indicating the need for follow-up of lesions with subtle RCM features.
The Barcelona algorithm: Segura et al. developed a two-step method for differentiating melanocytic from nonmelanocytic lesions and melanoma from nevi using RCM (Table 13) ) [59]. Their cohort consisted of 154 cutaneous tumors (100 melanocytic, 54 nonmelanocytic). Melanocytic lesions included 36 melanomas (eight in situ, five LM, four NM and 19 SSM) and 64 nevi. The first step is to determine if the lesion is melanocytic or nonmelanocytic. A melanocytic lesion is suspected based on the presence of at least one of four confocal features. The second step is to determine if the lesion is a nevus or a melanoma using a scoring system. Benign features, such as edged papillae, are given a score of -1, while malignant features, such as pagetoid roundish cells, a score of +1. Lesions with a score of greater than or equal to zero, have a sensitivity and specificity, respectively, of 86.1 and 95.3%, for melanoma. Those with a score of greater than or equal to two have an increased sensitivity of 100% but specificity reduced to 57%. The authors calculated that, despite a lower specificity, 50% of biopsies could be avoided, without missing a melanoma. There were five false-negative results in their study, including four in situ and one SSM, indicating the possible limitation of the algorithm in noninvasive melanoma.
Guitera et al. described a two-step method to diagnose melanoma and BCC (Table 14) [34]. It was developed in a cohort of 216 melanomas, 119 BCC, 266 nevi, 67 benign macules of the face and 42 nonmelanocytic lesions. Independently significant RCM features were identified to establish the following criteria in a two-step algorithm. The first step is to determine if the lesion is a BCC by the analysis of positive and negative features. The second step is to determine if the lesion is a melanoma using the Modena algorithm. Using this method, they obtained a sensitivity and specificity respectively, of 87.6 and 70.8%, for the diagnosis of melanoma.
Borsari et al. recently proposed a diagnostic score for melanoma in situ (MIS) combining dermoscopic and RCM features (Table 15) [63]. It was developed on a test set of 333 melanocytic lesions (including 120 in situ melanomas, 167 common melanocytic nevi and 46 Spitz or spitzoid nevi) and validated on a set of 100 lesions (including 50 MIS) matched for age, gender and body site. Facial and acral lesions were excluded. Significant positive and negative predictors of MIS were determined with multivariate analysis and used to develop their two-step score. Dermoscopic finding of atypical pigment network and regression gives one point each. On RCM, the observation of pagetoid cells gives one point and cytologic atypia one point if it is focal and two points if it is widespread. The presence of dense nests and melanophages was found to be protective of MIS and given -1 point each. A score of two or more had a sensitivity of 92.5% and a specificity of 61% for melanoma.
Table 12. . Modena algorithm: a reflectance confocal microscopy score for the diagnosis of malignant melanoma.
| Criteria (score) | RCM feature |
|---|---|
| Major criteria (+2 points per feature) | Nonedged dermal papillae |
| Atypical cells at the DEJ | |
| Minor criteria (+1 point per feature) | Roundish pagetoid cells |
| Pagetoid cells widespread throughout the lesion | |
| Cerebriform clusters in the papillary dermis | |
| Isolated nucleated cells within dermal papilla | |
DEJ: Dermoepidermal junction; RCM: Reflectance confocal microscopy.
Table 13. . Barcelona algorithm: a two-step method for the diagnosis of melanoma by reflectance confocal microscopy.
| Step | Description | Features |
|---|---|---|
| Step 1. | Determine if the lesion is melanocytic or nonmelanocytic | Features of a melanocytic lesion: 1. Cobblestone pattern 2. Pagetoid spread 3. Mesh appearance at DEJ 4. Presence of dermal clusters of cells or dermal nests |
| Step 2. | Determine if the lesion is a nevus or a melanoma | Protective features (i.e., associated with nevi; -1 point per feature) 1. Typical basal cells 2. Edged papillae Risk features (i.e., associated with melanoma; +1 point per feature) 2. Roundish pagetoid cells 3. Atypical nucleated cells in the dermis |
DEJ: Dermoepidermal junction.
Table 14. . Guitera's two-step method for the diagnosis of basal cell carcinomas and melanoma.
| Step | Description | Features |
|---|---|---|
| Step 1. | Determine if the lesion is a basal cell carcinoma | Positive features 1. Polarized elongation 2. Telangiectasia and convoluted vessels 3. Horizontal clefting 4. Basaloid nodules Negative features 1. Cerebriform nests 2. Nonvisible papillae 3. Epidermal disarray |
| Step 2. | Determine if the lesion is a melanoma | 1. Cerebriform nests 2. Atypical cobblestone pattern with small nucleated cells 3. Epidermal disarray 4. Pagetoid cells 5. No broadened interpapillary space 6. No dense nests |
Table 15. . Multistep algorithm to identify MIS using dermoscopy and confocal features.
| Imaging modality | Criteria | Point(s) |
|---|---|---|
| Dermoscopy | Atypical network | +1 |
| Regression | +1 | |
| RCM | Pagetoid infiltration | +1 |
| Cytologic atypia | Focal: +1 Widespread: +2 |
|
| Dense nests | -1 | |
| Melanophages | -1 | |
RCM: Reflectance confocal microscopy.
Clinical applications of RCM in melanoma
Clinicians may choose to use RCM to increase diagnostic accuracy of clinically equivocal pigmented or amelanotic lesions (e.g., no straightforward clinical or dermoscopic sign of melanoma or of a benign lesion but report of recent change) in the following situations:
Lesions located in sensitive areas (e.g., face, genitalia).
Location on patient or site at risk of hypertrophic and keloid scarring, if surgical excision anticipated, for example, décolletage.
Patient and/or clinician reluctant for biopsy/excision (e.g., pregnant woman and children).
In an Italian cohort of 1279 lesions, the major indications for RCM in melanoma diagnosis were lesions located on the head and neck, in chronically sun-damaged skin and lesions with regression on dermoscopy [65].
Histopathologic examination remains the gold standard and should be performed if RCM evaluation reveals uncertain features suggesting melanoma. If the lesion presents equivocal or benign features on RCM, digital dermoscopic monitoring can be considered [19,21,66].
RCM & digital dermoscopic surveillance
Digital dermoscopy monitoring of equivocal atypical pigmented lesions improves the sensitivity and specificity for melanoma diagnosis while minimizing unnecessary excisions [67–69]. However, 8% of monitored lesions excised due to change are benign [70]. RCM can be used as an adjunct in atypical melanocytic lesions exhibiting changes on digital dermoscopy follow-up to further improve diagnostic accuracy of melanoma [17,19,71]. Lovatto et al. retrospectively evaluated the impact of RCM evaluation in melanocytic lesions exhibiting changes on digital dermoscopy surveillance [17]. In a series of 64 lesions (13 MIS, 5 SSM, 51 benign nevi), confocal microscopy analysis showed a sensitivity and specificity of 100 and 69%, respectively, for the diagnosis of melanoma and could have avoided excision of 35 of 51 benign nevi.
RCM versus dermoscopy & their use as complementary tools
Dermoscopy and RCM each have features that the other modality lacks, enhancing the use of these tools in tandem for the noninvasive assessment of melanocytic and nonmelanocytic lesions (Table 16).
Table 16. . Contrasting and complementary strengths of dermoscopy and reflectance confocal microscopy in assessing melanocytic and nonmelanocytic lesions.
| Variable | Dermoscopy | RCM |
|---|---|---|
| Melanoma subtype | Able to view deeply located chromophores indicative of melanoma, e.g., blue–white veil | Able to view the superficial layers of the skin in multiple detailed horizontal layers ideal for in situ melanomas |
| Lesion color | Best for pigmented lesions with increased difficulty of amelanotic lesions | Ease in assessment of amelanotic lesions |
| Location | Acral | Acral assessment not possible. Ideal for face and mucosa |
RCM: Reflectance confocal microscopy.
Dermoscopy is suited to assess deep melanoma features, such as a blue–white veil and a gray color, while RCM lacks resolution to view these deeply reflected pigments or evaluation of dermal tumors [65]. Instead, RCM can view in detail the superficial layers of the skin, containing subtle clues for in situ melanomas [63], which may not yet be obvious on dermoscopy. Interpretation of colors and patterns is key to dermoscopy and the absence of pigmentation increases the degree of difficulty of lesion assessment. In contrast, RCM views the skin in black and white, and relies on refractive properties of subsurface structures, the color of which may not be detected on the surface for ease of dermoscopic assessment [72]. The dermoscopic patterns of melanocytic lesions on acral sites are well established [73]. RCM cannot be used for acral lesions, due to thick stratum corneum. In contrast, RCM is well-suited detailed examination of mucosal lesions [50,52,57] and complex pigmented areas of the face [15].
Clinical application of RCM to LM & LMM
Among the clinical applications of all melanoma subtypes, RCM is most applicable to the management of LM and LMM.
Guide biopsies: LM/LMM often has a large surface area, not amenable to a single biopsy and is located on sensitive to biopsy areas of the face. RCM may be used to assist in targeting the area for incisional biopsy to the worst area, increasing diagnostic yield. In practice, RCM is also used to guide biopsy sites to the most suspicious area, assessing for invasion prior to the decision to treat with nonsurgical modalities (although there are no large studies demonstrating this) [74].
Preoperative mapping
LM has the highest risk of residual disease and highest recurrence rate of all melanoma subtypes after surgical excision [75]. RCM allows detection and delineation of subclinical tumor extension [42,76,77,42]. Presurgical mapping of the involved area helps guide the clinician and the patient toward the most appropriate management options, assists surgeons planning reconstruction, and helps the patient understand the size of anticipated postoperative defect if surgery is undertaken. RCM also reduces the risk of positive margins after surgical excision [42,77]. RCM has been used successfully intraoperatively to achieve negative surgical margins in a case of standard wide excision [78], and also in combination with the staged excision spaghetti technique [79]. Margin delineation of LM and LMM with the handheld RCM (HRCM) is limited by the lack of precise orientation during imaging and the small field of view in absence of mosaics. Video mosaicking of HRCM images is a novel technique developed to overcome this limitation [80]. Yélamos et al. used this technique to estimate the surgical defect area in a series of 23 lesions (19 LM and four LMM) of the head and neck prior to staged excision [77]. Navigation was guided by the use of adhesive rings placed along clinical margins. They found that the mean surgical defect area estimated with HRCM correlated but were smaller than the actual defect (6.34 [4.02] vs 7.74 [5.28], p = 0.01).
Monitoring of nonsurgical therapies
RCM can be used to assess response to treatment and allow detection of treatment failure, especially after nonsurgical treatments (e.g., radiation therapy or topical imiquimod) [41,81–82]. Using the LM score, RCM has a sensitivity and specificity respectively of 100 and 94% in detection of treatment failure [41]. In comparison, dermoscopy has a sensitivity and specificity of 80 and 56%, respectively [82]. An advantage of RCM is that it enables the clinician to follow such difficult cases with serial noninvasive imaging of the skin. This may be a more suitable approach since it is associated with less repeated biopsies.
Limitations & pitfalls
In skin areas with a thick stratum corneum (e.g., palms and soles) and hyperkeratotic lesions, backscattering of the light by keratin restricts proper evaluation of deeper layers and represents a limitation for RCM. Similarly, ulcerated lesions are not evaluable because of backscattering of the light by keratin debris and blood [3,83]. Dendritic cells represent a major pitfall for RCM because they may represent melanocytes or Langerhans cells [84]. Limitations and pitfalls are summarized in Box 1 [3,34,49,83,85].
Box 1. . Limitations & pitfalls.
Acral sites
Hyperkeratotic lesions
Ulcerated lesions
Deep dermal lesions (e.g., blue nevi, melanoma satellite lesions and intransit, dermal nevi)
Spitz nevi or spitzoid tumors
Nevoid melanoma
Pagetoid spread: may be found in other melanocytic lesions, such as atypical nevi, traumatized or inflamed nevi, Spitz and Reed nevi, congenital nevi and recurrent nevi
Dendritic cells: a feature of benign and malignant lesions. In benign lesions they usually represent activated langerhans cells and in malignant lesions, pagetoid spread of melanocytes. Both langerhans cells and melanocytes have a similar shape and refractive index
Assessing the exact depth and at times discerning dermoepidermal junction from upper papillary dermis
Confocal unable to determine Breslow thickness
Practical aspects
Cost–effectiveness
As discussed above, RCM improves specificity and reduces unnecessary excisions. Formal cost-benefit studies are limited, and the main limitation to a more global implementation of RCM in the dermatology practice is the cost of the equipment. A retrospective study conducted in the Province of Modena (Italy) by Pellacani et al. evaluated the cost-benefit value of adding RCM to assist in melanoma diagnosis [23]. A decrease in the number of benign lesions needed to excise a melanoma from 19.41 (dermatologists using dermoscopy alone in nontertiary centres) to 6.25 (in university-based dermatologists using combination of dermoscopy and RCM) was found, corresponding to a decrease in 70% of unnecessary excisions. Further cost analysis found potential annual savings of 280,000 euros to the European healthcare system.
Standard of care
In Australia, The Cancer Council Melanoma Guidelines Working Party state that sufficient evidence is available to promote the use of RCM for margin assessment, in addition to dermoscopy, for melanoma in situ (LM subtype) [85]. Conversely, in the UK, National Institute for Health and Care Excellent (NICE) guidelines state there is insufficient evidence to recommend the routine use of RCM in deciding whether to biopsy cutaneous lesions and to map margins of cutaneous tumors [86].
Since 2016, RCM imaging has been reimbursed on a per-lesion basis by Centers for Medicare and Medicaid services (CMS) in the USA [87]. Images must be recorded using Vivascope® 1500 and include multiple mosaic views at different depths (one at the suprabasal epidermis, one at the DEJ and one at the dermis) and a report must be generated.
Although the technology shows real promise, further work is needed to determine the best way to integrate the technology as a standard of care [88].
Training
The mastery of RCM requires extensive training to recognize and become familiar with the normal and abnormal imaging patterns. For dermatologists and pathologists, a basic knowledge of cutaneous diseases, dermoscopy and normal histopathology facilitates learning. The clinician may choose to develop their knowledge by training in a specific field that suits their practice. Another barrier for the adoption of RCM is that training opportunities are limited. Training can be gained in atlases [89,90], online and at international conferences and courses.
Image processing time
The time required to process images depends on the extent of the lesion and will usually vary from two (immediate diagnosis of a small BCC of the face) to approximately 45 min (very large area of LM, needed to be mapped) [15,42,91]. In a busy clinical setting, this may limit the use of RCM but most melanoma units in the world have now integrated it in their work flow and see clear time benefit in terms of triage, accuracy of targeting biopsies, mapping and follow-up. Some practices are organized with image technicians and/or image readers, who work in collaboration with the practitioner.
Conclusion & future perspective
RCM technology is constantly developing; becoming smaller, lighter, and easier to use with expanding software capacities. New software allows the potential use of confocal in a telemedicine capacity [92,93].
RCM is being integrated with other technologies. Scientists are working on combining in vivo RCM and optical coherence tomography (OCT) [94]. OCT is an emerging noninvasive method for cutaneous imaging that allows imaging at a greater depth (1–3 mm) than RCM (∼200 μm) [95]. It has been mostly used for BCCs but its use for the evaluation of pigmented lesions, including melanoma, is in early phase [95]. Preliminary work suggests that OCT could become a useful adjunct to assess the presence of dermal involvement, guiding the clinician to choose a complete excision over shave biopsy in suspicious melanocytic tumors [96]. The combination of OCT and RCM would take advantage of depth and resolution of both technologies, respectively. This combination has been on freshly excised tissues (ex vivo) to delineate deep and lateral margins of BCCs [94].
To help navigation during imaging with the HRCM, promising modalities are being developed including automated video mosaicking [97] and combination of HRCM with a wide-field camera [98]. Also, preliminary studies have evaluated lesions in mucosal cavities by adding a telescopic probe to the confocal microscope [99,100].
Identification of the DEJ during stacks is important for accurate interpretation of confocal images but may be challenging. To overcome this problem, scientists are working on algorithms for automated detection of the DEJ [101].
Ex vivo confocal microscopy is being introduced for assessment of surgical margins on freshly excised tissues, mostly of BCCs [102–104] but also for breast cancer core biopsies [105] and intraoperative assessment of conjunctival [106], breast [107] and brain tumors [108]. For this purpose, a fluorescence mode confocal microscope is used. Prior to imaging, a fluorescence agent (e.g., acridine orange) is applied to the tissue and captured by the cell nuclei (not by collagen). The contrast created with the surrounding dermis allows analysis of nuclear morphology. The procedure is nondestructive and does not impair further histopathology evaluation on frozen or paraffin sections. In a prospective study by Bennassar et al., the use of FCM for margin assessment of 80 BCCs allowed detection of residual tumor with a sensitivity and specificity of 88 and 99%, respectively. Interestingly, the technology could save two-thirds of processing time compared with conventional analysis on frozen sections in the setting of Mohs surgery. The use of ex vivo confocal microscopy for melanocytic lesions is harder to investigate and still at a preliminary stage. For this purpose, combination with standard immunohistochemistry staining is currently being investigated [109].
In the next 5–10 years we envisage that the miniaturization of the imaging tool will continue with integration of algorithms and artificial intelligence that will greatly guide the clinician. The ex vivo diagnosis should be routine use in the surgical theater to guide the surgeon through complete clearance of margins.
In conclusion, RCM is a complimentary technology in the diagnosis and management of melanoma and has continually expanding roles in cutaneous disease. As a secondary diagnostic evaluative tool, correlation with clinical, dermoscopic and pathology findings is essential in appropriately integrating it into melanoma diagnosis and management and practice workflow. In vivo and ex vivo applications of RCM in the surgical setting, both to guide conventional wide local excision margins, staged excision and Mohs suggest an exciting future for RCM in the multidisciplinary management of melanoma at all stages.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Rajadhyaksha M, González S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J. Invest. Dermatol. 1999;113(3):293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 2.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions – improvement in melanoma diagnostic specificity. J. Am. Acad. Dermatol. 2005;53(6):979–985. doi: 10.1016/j.jaad.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Longo C, Farnetani F, Ciardo S, et al. Is confocal microscopy a valuable tool in diagnosing nodular lesions? A study of 140 cases. Br. J. Dermatol. 2013;169(1):58–67. doi: 10.1111/bjd.12259. [DOI] [PubMed] [Google Scholar]
- 4.Calzavara-Pinton P, Longo C, Venturini M, Sala R, Pellacani G. Reflectance confocal microscopy for in vivo skin imaging. Photochem. Photobiol. 2008;84(6):1421–1430. doi: 10.1111/j.1751-1097.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- 5.Rajadhyaksha M. Confocal microscopy of skin cancers: translational advances toward clinical utility. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009;2009:3231–3233. doi: 10.1109/IEMBS.2009.5333600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J. Invest. Dermatol. 1995;104(6):946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 7.Busam KJ, Charles C, Lee G, Halpern AC. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod. Pathol. 2001;14(9):862–868. doi: 10.1038/modpathol.3880402. [DOI] [PubMed] [Google Scholar]
- 8.Scope A, Benvenuto-Andrade C, Agero A-LC, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J. Am. Acad. Dermatol. 2007;57(4):644–658. doi: 10.1016/j.jaad.2007.05.044. [DOI] [PubMed] [Google Scholar]; •• Important glossary of the terminology used to describe normal cutaneous features and melanocytic lesions.
- 9.Que SKT, Grant-Kels JM, Rabinovitz HS, Oliviero M, Scope A. Application of handheld confocal microscopy for skin cancer diagnosis: advantages and limitations compared with the wide-probe confocal. Dermatol. Clin. 2016;34(4):469–475. doi: 10.1016/j.det.2016.05.009. [DOI] [PubMed] [Google Scholar]; •• Suggested article to help choose the appropriate reflectance confocal microscopy (RCM) device for your practice.
- 10.MAVIG GmbH. VivaScope® 1500/3000 confocal laser scanning microscope for in vivo use (830 nm) 2017. www.vivascope.de/fileadmin/user_upload/Downloads/DATASHEET_VS_1500–3000_ENG_03_2017.pdf
- 11.Yamashita T, Kuwahara T, González S, Takahashi M. Non-invasive visualization of melanin and melanocytes by reflectance-mode confocal microscopy. J. Invest. Dermatol. 2005;124(1):235–240. doi: 10.1111/j.0022-202X.2004.23562.x. [DOI] [PubMed] [Google Scholar]
- 12.Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br. J. Dermatol. 2014;170(4):802–808. doi: 10.1111/bjd.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong YQ, Ma SJ, Mo Y, Huo ST, Wen YQ, Chen Q. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J. Cancer Res. Clin. Oncol. 2017 doi: 10.1007/s00432-017-2391-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Well-conducted meta-analysis compares the diagnostic accuracy of RCM and dermoscopy.
- 14.Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br. J. Dermatol. 2008;158(2):329–333. doi: 10.1111/j.1365-2133.2007.08389.x. [DOI] [PubMed] [Google Scholar]
- 15.Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J. Invest. Dermatol. 2010;130(8):2080–2091. doi: 10.1038/jid.2010.84. [DOI] [PubMed] [Google Scholar]; •• A key study describing the lentigo maligna score and confirming the value of RCM in evaluating equivocal pigmented and nonpigmented facial macules.
- 16.Stevenson AD, Mickan S, Mallett S, Ayya M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol. Pract. Concept. 2013;3(4):19–27. doi: 10.5826/dpc.0304a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovatto L, Carrera C, Salerni G, Alós L, Malvehy J, Puig S. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J. Eur. Acad. Dermatol. Venereol. 2015;29(10):1918–1925. doi: 10.1111/jdv.13067. [DOI] [PubMed] [Google Scholar]
- 18.Xiong YD, Ma S, Li X, Zhong X, Duan C, Chen Q. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J. Eur. Acad. Dermatol. Venereol. 2016;30(8):1295–1302. doi: 10.1111/jdv.13712. [DOI] [PubMed] [Google Scholar]; •• Well-conducted meta-analysis provides level 2 evidence on the diagnostic accuracy of RCM.
- 19.Stanganelli I, Longo C, Mazzoni L, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br. J. Dermatol. 2015;172(2):365–371. doi: 10.1111/bjd.13373. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J. Eur. Acad. Dermatol. Venereol. 2015;29(6):1135–1140. doi: 10.1111/jdv.12769. [DOI] [PubMed] [Google Scholar]
- 21.Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br. J. Dermatol. 2014;171(5):1044–1051. doi: 10.1111/bjd.13148. [DOI] [PubMed] [Google Scholar]
- 22.Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J. Invest. Dermatol. 2009;129(1):131–138. doi: 10.1038/jid.2008.193. [DOI] [PubMed] [Google Scholar]; • Provides evidence for increased diagnostic accuracy of melanocytic lesions with RCM analysis. The Modena algorithm is tested and shows a high sensitivity and specificity.
- 23.Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J. Eur. Acad. Dermatol. Venereol. 2016;30(3):413–419. doi: 10.1111/jdv.13408. [DOI] [PubMed] [Google Scholar]
- 24.Tuong W, Cheng LS, Armstrong AW. Melanoma: epidemiology, diagnosis, treatment, and outcomes. Dermatol. Clin. 2012;30(1):113–124. doi: 10.1016/j.det.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Longo C, Pellacani G. Melanomas. Dermatol. Clin. 2016;34(4):411–419. doi: 10.1016/j.det.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Benati E, Argenziano G, Kyrgidis A, et al. Melanoma and naevi with a globular pattern: confocal microscopy as an aid for diagnostic differentiation. Br. J. Dermatol. 2015;173(5):1232–1238. doi: 10.1111/bjd.14049. [DOI] [PubMed] [Google Scholar]
- 27.Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. Br. J. Dermatol. 2004;150(6):1117–1124. doi: 10.1111/j.1365-2133.2004.05928.x. [DOI] [PubMed] [Google Scholar]
- 28.Menzies SW, Kreusch J, Byth K, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch. Dermatol. 2008;144(9):1120–1127. doi: 10.1001/archderm.144.9.1120. [DOI] [PubMed] [Google Scholar]
- 29.Gill M, González S. Enlightening the pink: use of confocal microscopy in pink lesions. Dermatol. Clin. 2016;34(4):443–458. doi: 10.1016/j.det.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br. J. Dermatol. 2014;171(1):48–54. doi: 10.1111/bjd.12781. [DOI] [PubMed] [Google Scholar]
- 31.Cicchiello M, Lin MJ, Pan Y, McLean C, Kelly JW. An assessment of clinical pathways and missed opportunities for the diagnosis of nodular melanoma versus superficial spreading melanoma. Australas. J. Dermatol. 2016;57(2):97–101. doi: 10.1111/ajd.12416. [DOI] [PubMed] [Google Scholar]
- 32.Moloney FJ, Menzies SW. Key points in the dermoscopic diagnosis of hypomelanotic melanoma and nodular melanoma. J. Dermatol. 2011;38(1):10–15. doi: 10.1111/j.1346-8138.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 33.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions – improvement in melanoma diagnostic specificity. J. Am. Acad. Dermatol. 2005;53(6):979–985. doi: 10.1016/j.jaad.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J. Invest. Dermatol. 2012;132(10):2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- 35.Segura S, Pellacani G, Puig S, et al. In vivo microscopic features of nodular melanomas: dermoscopy, confocal microscopy, and histopathologic correlates. Arch. Dermatol. 2008;144(10):1311–1320. doi: 10.1001/archderm.144.10.1311. [DOI] [PubMed] [Google Scholar]
- 36.Lallas A, Argenziano G, Moscarella E, Longo C, Simonetti V, Zalaudek I. Diagnosis and management of facial pigmented macules. Clin. Dermatol. 2014;32(1):94–100. doi: 10.1016/j.clindermatol.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Star P, Guitera P. Lentigo maligna macules of the face, and lesions on sun-damaged skin. Dermatol. Clin. 2016;34(4):421–429. doi: 10.1016/j.det.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Langley RGB, Burton E, Walsh N, Propperova I, Murray SJ. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J. Am. Acad. Dermatol. 2006;55(1):88–97. doi: 10.1016/j.jaad.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Ahlgrimm-Siess V, Massone C, Scope A, et al. Reflectance confocal microscopy of facial lentigo maligna and lentigo maligna melanoma: a preliminary study. Br. J. Dermatol. 2009;161(6):1307–1316. doi: 10.1111/j.1365-2133.2009.09289.x. [DOI] [PubMed] [Google Scholar]
- 40.Tannous ZS, Mihm MC, Flotte TJ, González S. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J. Am. Acad. Dermatol. 2002;46(2):260–263. doi: 10.1067/mjd.2002.118345. [DOI] [PubMed] [Google Scholar]
- 41.Guitera P, Haydu LE, Menzies SW, et al. Surveillance for treatment failure of lentigo maligna with dermoscopy and in vivo confocal microscopy: new descriptors. Br. J. Dermatol. 2014;170(6):1305–1312. doi: 10.1111/bjd.12839. [DOI] [PubMed] [Google Scholar]
- 42.Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149(6):692–698. doi: 10.1001/jamadermatol.2013.2301. [DOI] [PubMed] [Google Scholar]
- 43.Gómez-Martín I, Moreno S, Andrades-López E, et al. Histopathologic and immunohistochemical correlates of confocal descriptors in pigmented facial macules on photodamaged skin. JAMA Dermatol. 2017;153(8):771–780. doi: 10.1001/jamadermatol.2017.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch. Pathol. Lab. Med. 2011;135(7):838–841. doi: 10.5858/2011-0051-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 45.Chen LL, Jaimes N, Barker CA, Busam KJ, Marghoob AA. Desmoplastic melanoma: a review. J. Am. Acad. Dermatol. 2013;68(5):825–833. doi: 10.1016/j.jaad.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maher NG, Solinas A, Scolyer RA, Puig S, Pellacani G, Guitera P. Detection of desmoplastic melanoma with dermoscopy and reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2017;31(12):2016–2024. doi: 10.1111/jdv.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol. Clin. 2016;34(4):395–409. doi: 10.1016/j.det.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J. Am. Acad. Dermatol. 2012;66(3):e109–e121. doi: 10.1016/j.jaad.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Jain M, Marghoob AA. Integrating clinical, dermoscopy, and reflectance confocal microscopy findings into correctly identifying a nevoid melanoma. JAAD Case Rep. 2017;3(6):505–508. doi: 10.1016/j.jdcr.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uribe P, Collgros H, Scolyer RA, Menzies SW, Guitera P. In vivo reflectance confocal microscopy for the diagnosis of melanoma and melanotic macules of the lip. JAMA Dermatol. 2017;153(9):882–891. doi: 10.1001/jamadermatol.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maher NG, Solinas A, Scolyer RA, Guitera P. In vivo reflectance confocal microscopy for evaluating melanoma of the lip and its differential diagnoses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017;123(1):84–94. doi: 10.1016/j.oooo.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Cinotti E, Labeille B, Cambazard F, Thuret G, Gain P, Perrot JL. Reflectance confocal microscopy for mucosal diseases. G. Ital. Dermatol. Venereol. 2015;150(5):585–593. [PubMed] [Google Scholar]
- 53.Lucchese A, Gentile E, Romano A, Maio C, Laino L, Serpico R. The potential role of in vivo reflectance confocal microscopy for evaluating oral cavity lesions: a systematic review. J. Oral Pathol. Med. 2016;45(10):723–729. doi: 10.1111/jop.12454. [DOI] [PubMed] [Google Scholar]
- 54.Cinotti E, Couzan C, Perrot JL, et al. In vivo confocal microscopic substrate of grey colour in melanosis. J. Eur. Acad. Dermatol. Venereol. 2015;29(12):2458–2462. doi: 10.1111/jdv.13394. [DOI] [PubMed] [Google Scholar]
- 55.Cinotti E, Couzan C, Perrot JL, et al. Reflectance confocal microscopy for the diagnosis of vulvar naevi: six cases. J. Eur. Acad. Dermatol. Venereol. 2016;30(1):30–35. doi: 10.1111/jdv.12924. [DOI] [PubMed] [Google Scholar]
- 56.Cinotti E, Perrot JL, Labeille B, Adegbidi H, Cambazard F. Reflectance confocal microscopy for the diagnosis of vulvar melanoma and melanosis: preliminary results. Dermatol. Surg. 2012;38(12):1962–1967. doi: 10.1111/dsu.12009. [DOI] [PubMed] [Google Scholar]
- 57.Debarbieux S, Perrot JL, Erfan N, et al. Reflectance confocal microscopy of mucosal pigmented macules: a review of 56 cases including 10 macular melanomas. Br. J. Dermatol. 2014;170(6):1276–1284. doi: 10.1111/bjd.12803. [DOI] [PubMed] [Google Scholar]
- 58.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J. Invest. Dermatol. 2007;127(12):2759–2765. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 59.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J. Am. Acad. Dermatol. 2009;61(2):216–229. doi: 10.1016/j.jaad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Langley RGB, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared with dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215(4):365–372. doi: 10.1159/000109087. [DOI] [PubMed] [Google Scholar]
- 61.Gerger A, Wiltgen M, Langsenlehner U, et al. Diagnostic image analysis of malignant melanoma in in vivo confocal laser-scanning microscopy: a preliminary study. Skin Res. Technol. 2008;14(3):359–363. doi: 10.1111/j.1600-0846.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- 62.Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107(1):193–200. doi: 10.1002/cncr.21910. [DOI] [PubMed] [Google Scholar]
- 63.Borsari S, Pampena R, Benati E, et al. In vivo dermoscopic and confocal microscopy multi-step algorithm to detect in situ melanomas. Br. J. Dermatol. 2018 doi: 10.1111/bjd.16364. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J. Invest. Dermatol. 2005;125(3):532–537. doi: 10.1111/j.0022-202X.2005.23813.x. [DOI] [PubMed] [Google Scholar]
- 65.Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152(10):1093–1098. doi: 10.1001/jamadermatol.2016.1188. [DOI] [PubMed] [Google Scholar]; •• Studies the best clinical indications for confocal microscopy.
- 66.Pellacani G, Scope A, Farnetani F, et al. Towards an in vivo morphologic classification of melanocytic nevi. J. Eur. Acad. Dermatol. Venereol. 2014;28(7):864–872. doi: 10.1111/jdv.12181. [DOI] [PubMed] [Google Scholar]
- 67.Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch. Dermatol. 2006;142(9):1113–1119. doi: 10.1001/archderm.142.9.1113. [DOI] [PubMed] [Google Scholar]
- 68.Moloney FJ, Guitera P, Coates E, et al. Detection of primary melanoma in individuals at extreme high risk. JAMA Dermatol. 2014;150(8):819. doi: 10.1001/jamadermatol.2014.514. [DOI] [PubMed] [Google Scholar]
- 69.Salerni G, Carrera C, Lovatto L, et al. Characterization of 1152 lesions excised over 10 years using total-body photography and digital dermatoscopy in the surveillance of patients at high risk for melanoma. J. Am. Acad. Dermatol. 2012;67(5):836–845. doi: 10.1016/j.jaad.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 70.Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J. Eur. Acad. Dermatol. Venereol. 2013;27(7):805–814. doi: 10.1111/jdv.12032. [DOI] [PubMed] [Google Scholar]
- 71.Carrera C. High-risk melanoma patients: can unnecessary naevi biopsies be avoided? Br. J. Dermatol. 2015;172(2):313–315. doi: 10.1111/bjd.13527. [DOI] [PubMed] [Google Scholar]
- 72.Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions. Br. J. Dermatol. 2016;175(6):1311–1319. doi: 10.1111/bjd.14749. [DOI] [PubMed] [Google Scholar]
- 73.Altamura D, Altobelli E, Micantonio T, Piccolo D, Fargnoli MC, Peris K. Dermoscopic patterns of acral melanocytic nevi and melanomas in a white population in central Italy. Arch. Dermatol. 2006;142(9):1123–1128. doi: 10.1001/archderm.142.9.1123. [DOI] [PubMed] [Google Scholar]
- 74.Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99(5):346–352. [PMC free article] [PubMed] [Google Scholar]
- 75.Bolshinsky V, Lin MJ, Serpell J, et al. Frequency of residual melanoma in wide local excision (WLE) specimens after complete excisional biopsy. J. Am. Acad. Dermatol. 2016;74(1):102–107. doi: 10.1016/j.jaad.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 76.Chen C-SJ, Elias M, Busam K, Rajadhyaksha M, Marghoob AA. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br. J. Dermatol. 2005;153(5):1031–1036. doi: 10.1111/j.1365-2133.2005.06831.x. [DOI] [PubMed] [Google Scholar]
- 77.Yélamos O, Cordova M, Blank N, et al. Correlation of handheld reflectance confocal microscopy with radial video mosaicing for margin mapping of lentigo maligna and lentigo maligna melanoma. JAMA Dermatol. 2017;153(12):1278–1284. doi: 10.1001/jamadermatol.2017.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hibler BP, Cordova M, Wong RJ, Rossi AM. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol. Surg. 2015;41(8):980–983. doi: 10.1097/DSS.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Champin J, Perrot J-L, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol. Surg. 2014;40(3):247–256. doi: 10.1111/dsu.12432. [DOI] [PubMed] [Google Scholar]
- 80.Kose K, Cordova M, Duffy M, Flores ES, Brooks DH, Rajadhyaksha M. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo . Br. J. Dermatol. 2014;171(5):1239–1241. doi: 10.1111/bjd.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nadiminti H, Scope A, Marghoob AA, Busam K, Nehal KS. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol. Surg. 2010;36(2):177–184. doi: 10.1111/j.1524-4725.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 82.Alarcon I, Carrera C, Alos L, Palou J, Malvehy J, Puig S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J. Am. Acad. Dermatol. 2014;71(1):49–55. doi: 10.1016/j.jaad.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 83.Scope A, Longo C. Recognizing the benefits and pitfalls of reflectance confocal microscopy in melanoma diagnosis. Dermatol. Pract. Concept. 2014;4(3):67–71. doi: 10.5826/dpc.0403a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hashemi P, Pulitzer MP, Scope A, Kovalyshyn I, Halpern AC, Marghoob AA. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J. Am. Acad. Dermatol. 2012;66(3):452–462. doi: 10.1016/j.jaad.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guitera P, Menzies SW, Chamberlain A, Soyer HP Cancer Council Australia Melanoma Guidelines Working Party. What is the role of confocal microscopy in melanoma diagnosis? Clin. Pract. Guidelines Diagn. Manage. Melanoma. 2016 http://wiki.cancer.org.au/australia/Guidelines:Melanoma/Confocal_microscopy%0D [Google Scholar]
- 86.VivaScope 1500 and 3000 imaging systems for detecting skin cancer lesions. Natl Inst. Heal. Care Excell. 2015 www.nice.org.uk/guidance/dg19/resources/vivascope-1500-and-3000-imaging-systems-for-detecting-skin-cancer-lesions-pdf-1053638187973 [Google Scholar]
- 87.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg. Med. 2017;49(1):7–19. doi: 10.1002/lsm.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Que SKT, Grant-Kels JM, Longo C, Pellacani G. Basics of confocal microscopy and the complexity of diagnosing skin tumors: new imaging tools in clinical practice, diagnostic workflows, cost-estimate, and new trends. Dermatol. Clin. 2016;34(4):367–375. doi: 10.1016/j.det.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 89.González S, editor. Reflectance Confocal Microscopy of Cutaneous Tumors (2nd Edition) CRC Press; FL, USA: 2017. [Google Scholar]; •• The atlas contains multiple illustrations and tables and is well suited for those wishing to learn RCM.
- 90.Hofmann-Wellenhof R, Pellacani G, Malvehy J, Soyer HP, editors. Reflectance Confocal Microscopy for Skin Diseases (1st Edition) Springer-Verlag Berlin Heidelberg; 2012. [Google Scholar]
- 91.Kose K, Gou M, Yelamos O, et al. Choi B, Zeng H, Kollias N, editors. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesion. 2017. p. 100370D.http://proceedings.spiedigitallibrary.org/proceeding.aspx?doi=10.1117/12.2253085
- 92.Witkowski A, Łudzik J, Soyer HP. Telediagnosis with confocal microscopy. Dermatol. Clin. 2016;34(4):505–512. doi: 10.1016/j.det.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 93.Vivascan & Vivanet by Caliber I.D. www.caliberid.com/vivascan_Overview.html
- 94.Iftimia N, Peterson G, Chang EW, Maguluri G, Fox W, Rajadhyaksha M. Combined reflectance confocal microscopy-optical coherence tomography for delineation of basal cell carcinoma margins: an ex vivo study. J. Biomed. Opt. 2016;21(1):16006. doi: 10.1117/1.JBO.21.1.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J. Biomed. Opt. 2013;18(6):61224. doi: 10.1117/1.JBO.18.6.061224. [DOI] [PubMed] [Google Scholar]
- 96.Moraes Pinto Blumetti TC, Cohen MP, Gomes EE, et al. Optical coherence tomography (OCT) features of nevi and melanomas and their association with intraepidermal or dermal involvement: a pilot study. J. Am. Acad. Dermatol. 2015;73(2):315–317. doi: 10.1016/j.jaad.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 97.Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci. Rep. 2017;7(1):10759. doi: 10.1038/s41598-017-11072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dickensheets DL, Kreitinger S, Peterson G, Heger M, Rajadhyaksha M. Wide-field imaging combined with confocal microscopy using a miniature f/5 camera integrated within a high NA objective lens. Opt. Lett. 2017;42(7):1241–1244. doi: 10.1364/OL.42.001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peterson G, Zanoni DK, Migliacci J, Cordova M, Rajadhyaksha M, Patel S . International Society for Optics and Photonics. Progress in reflectance confocal microscopy for imaging oral tissues in vivo . In: Choi B, Kollias N, Zeng H, et al., editors. Photonic Therapeutics and Diagnostics XII. Vol. 9689. 2016. p. 96891X.http://proceedings.spiedigitallibrary.org/proceeding.aspx?doi=10.1117/12.2213692 [Google Scholar]
- 100.Yélamos O, Cordova M, Peterson G, et al. In vivo intraoral reflectance confocal microscopy of an amalgam tattoo. Dermatol. Pract. Concept. 2017;7(4):13–16. doi: 10.5826/dpc.0704a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurugol S, Rajadhyaksha M, Dy JG, Brooks DH. Validation study of automated dermal/epidermal junction localization algorithm in reflectance confocal microscopy images of skin. Proc. SPIE. 2012 doi: 10.1117/12.909227. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bennàssar A, Carrera C, Puig S, Vilalta A, Malvehy J. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149(7):839–847. doi: 10.1001/jamadermatol.2013.459. [DOI] [PubMed] [Google Scholar]
- 103.Bennàssar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br. J. Dermatol. 2014;170(2):360–365. doi: 10.1111/bjd.12671. [DOI] [PubMed] [Google Scholar]
- 104.Longo C, Rajadhyaksha M, Ragazzi M, et al. Evaluating ex vivo fluorescence confocal microscopy images of basal cell carcinomas in Mohs excised tissue. Br. J. Dermatol. 2014;171(3):561–570. doi: 10.1111/bjd.13070. [DOI] [PubMed] [Google Scholar]
- 105.Dobbs J, Krishnamurthy S, Kyrish M, Benveniste AP, Yang W, Richards-Kortum R. Confocal fluorescence microscopy for rapid evaluation of invasive tumor cellularity of inflammatory breast carcinoma core needle biopsies. Breast Cancer Res. Treat. 2015;149(1):303–310. doi: 10.1007/s10549-014-3182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iovieno A, Longo C, De Luca M, Piana S, Fontana L, Ragazzi M. Fluorescence confocal microscopy for ex vivo diagnosis of conjunctival tumors: a pilot study. Am. J. Ophthalmol. 2016;168:207–216. doi: 10.1016/j.ajo.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Larson BA, Abeytunge S, Murray M, Rajadhyaksha M . International Society for Optics and Photonics. Large area mapping of excised breast tissue by fluorescence confocal strip scanning: a preliminary feasibility study. In: Alfano RR, Demos SG, editors. Optical Biopsy XI. Vol. 8577. 2013. p. 857706.http://proceedings.spiedigitallibrary.org/proceeding.aspx?doi=10.1117/12.2005464 [Google Scholar]
- 108.Forest F, Cinotti E, Yvorel V, et al. Ex vivo confocal microscopy imaging to identify tumor tissue on freshly removed brain sample. J. Neurooncol. 2015;124(2):157–164. doi: 10.1007/s11060-015-1832-z. [DOI] [PubMed] [Google Scholar]
- 109.Hartmann D, Krammer S, Vural S, et al. Immunofluorescence and confocal microscopy for ex vivo diagnosis of melanocytic and non-melanocytic skin tumors: a pilot study. J. Biophotonics. 2017;11(3) doi: 10.1002/jbio.201700211. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 110.Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch. Dermatol. 2004;140(9):1127–1132. doi: 10.1001/archderm.140.9.1127. [DOI] [PubMed] [Google Scholar]