Abstract
Purpose
To retrospectively assess whether there is an association between screening mammography and the use of a variety of preventive services in women who are enrolled in Medicare.
Materials and Methods
U.S. Medicare claims from 2010 to 2014 Research Identifiable Files were reviewed to retrospectively identify a group of women who underwent screening mammography and a control group without screening mammography in 2012. The screened group was divided into positive versus negative results at screening, and the positive subgroup was divided into false-positive and true-positive findings. Multivariate logistic regression models and inverse probability of treatment weighting were used to examine the relationship between screening status and the probabilities of undergoing Papanicolaou test, bone mass measurement, or influenza vaccination in the following 2 years.
Results
The cohort consisted of 555 705 patients, of whom 185 625 (33.4%) underwent mammography. After adjusting for patient demographics, comorbidities, geographic covariates, and baseline preventive care, women who underwent index screening mammography (with either positive or negative results) were more likely than unscreened women to later undergo Papanicolaou test (odds ratio [OR], 1.49; 95% confidence interval: 1.40, 1.58), bone mass measurement (OR, 1.70; 95% confidence interval: 1.63, 1.78), and influenza vaccine (OR, 1.45; 95% confidence interval: 1.37, 1.53). In women who had not undergone these preventive measures in the 2 years before screening mammography, use of these three services after false-positive findings at screening was no different than after true-negative findings at screening.
Conclusion
In beneficiaries of U.S. Medicare, use of screening mammography was associated with higher likelihood of adherence to other preventive guidelines, without a negative association between false-positive results and cervical cancer screening.
© RSNA, 2018
See also the editorial by Whitman and Cantor in this issue.
Introduction
Preventive services are commonly recommended to patients according to clinical practice guidelines, and mammography is among the offered screening tests for women aged 40 years and older (1). Use of mammography has been reported to be high among age groups recommended to undergo screening, in the range of 60%–72% in countries with programs in place to encourage testing (2,3). As patients decide whether or not to follow preventive guidelines, they may weigh their personal perceptions of population-level risks presented to them, nonprobabilistic factors such as personal experience with a disease (eg, friend or family member diagnosed with the disease), or their own previous experience with the test.
Despite controversies surrounding false-positive results and their influence on policy decisions and guidelines regarding screening mammography (4,5), to our knowledge little is known about the effect of false-positive findings at mammography on patients’ use of other nonbreast cancer preventive services. Aside from the question of whether to continue undergoing screening mammography, false-positive results at mammography may also potentially influence patient perception of screening for other diseases. A recent study (6) suggested that women who undergo a biopsy with results negative for cancer after positive findings at mammography may delay their next screening mammogram, with the potential consequence of delayed diagnosis for early stage cancers. Thus, patients’ experiences with an invasive procedure as a result of false-positive screening mammography could plausibly affect their desire to adhere to other, nonbreast cancer screening guidelines. Of note, such guidelines are developed on the basis of population-level benefits and harms and are typically discussed by physicians as a series of recommendations at a given visit (7,8). However, it is also possible that results at mammography do not affect—or even heighten—women’s awareness and willingness regarding adherence to other screening studies, and that the so-called harms of false-positive results at mammography may be a transient phenomenon.
The potential for the use and results of screening mammography to affect women’s adherence to other preventive guidelines has broad implications for population health. Thus, the purpose of the study was to retrospectively assess whether there is an association between screening mammography and use of a variety of preventive services in women who are enrolled in Medicare.
Materials and Methods
Data
By adhering to a data use agreement from the U.S. Centers for Medicare and Medicaid Services, we used retrospective Medicare claims from 2010 to 2014 research identifiable files. Our data include all fee-for-services claims associated with a 5% nationally representative sample of Medicare beneficiaries. The claims data provide beneficiaries’ enrollment, demographic, and health services utilization information. In addition, we obtained county-level income and health resource variables from the Area Health Resource File linked to Medicare claims by ZIP code. The datasets were studied with an exemption from the institutional review board of the American College of Radiology.
Study Population
The study cohort included beneficiaries who were women (≥65 years), who had continuous Medicare part A and part B coverage without managed care enrollment between 2010 and 2014, who resided in one of the 50 U.S. states or the District of Columbia, and who were alive as of December 31, 2014. The treatment group consisted of patients who underwent two-dimensional screening mammography in 2012 (hereafter, referred to as index screen), whereas the control group consisted of patients who underwent no screening mammography in 2012. During the period of interest, the American College of Radiology and American Cancer Society guidelines recommended yearly mammography for women in good health over age 40 years without an upper age limit, and the Affordable Care Act required full coverage of mammography, including complete coverage by Medicare plans starting in 2011 (9–12). Yearly Papanicolaou test and biannual bone mass testing were also covered by Medicare during this time frame, though guidelines regarding frequency of Papanicolaou test began to change in 2012 (13,14). Patients were excluded if they had a diagnosis of breast cancer or mastectomy before the index screen during the study period (dating back to 2010), or if they underwent breast-related imaging within 9 months of the index screen; these groups may have a higher adherence to subsequent screening and represent a potential source of bias in favor of screening, and in addition these patients’ mammograms are often not considered screening studies.
Key Variables
Screening positive versus negative results.—The study design was aligned with clinical definitions of screening results according to current auditing and quality standards of the American College of Radiology (15), and as applied in previous investigations (5,16,17). In clinical practice, a screening test is classified as positive for recall if the result indicates physical examination, additional imaging, or biopsy instead of routine screening (Breast Imaging Reporting and Data System, or BI-RADS, 0, 3, 4, or 5; in practice, BI-RADS categories 3, 4, and 5 are used rarely in the context of screening). A negative screening result is defined as that which shows no findings or benign findings (BI-RADS 1 or 2) (15,18).
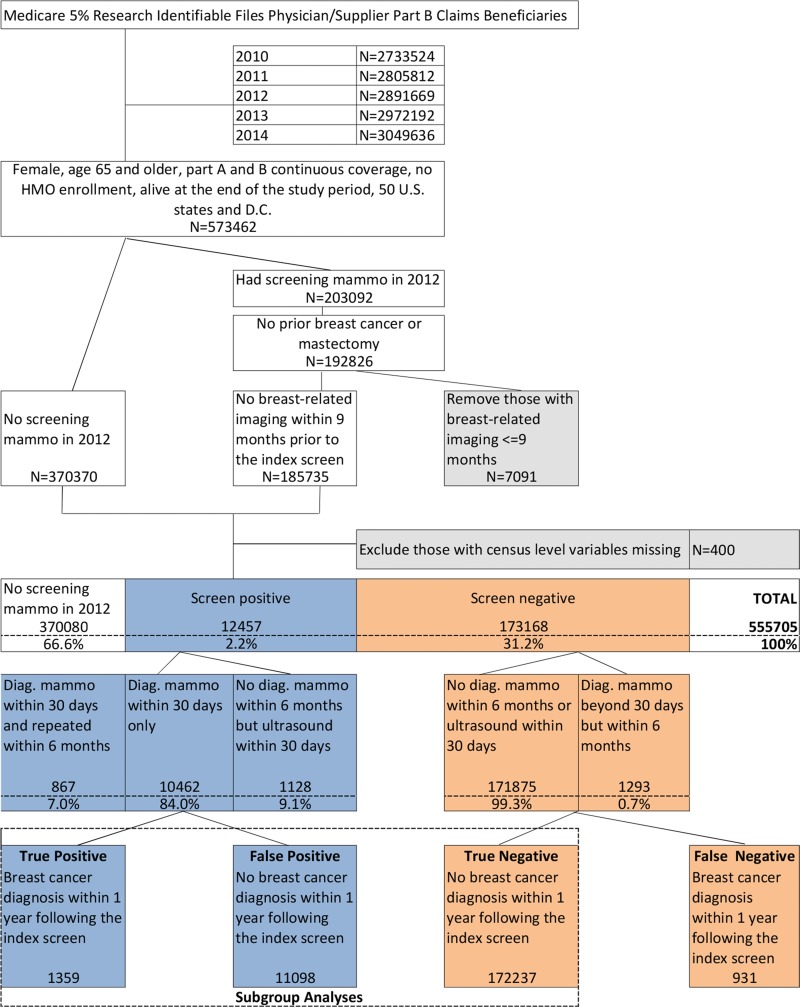
With Medicare claims, the clinical outcomes of the initial screening tests are unavailable and results are defined as positive or negative for cancer based on subsequent breast imaging services and breast cancer diagnoses in billing claims. We defined screening-positive results as patients who underwent diagnostic mammography or breast US within 30 days after the index screen, and screening-negative results otherwise (Figure), on the basis of published multicenter data at the completion of additional imaging within 30 days of screening (19,20).
Figure:
Breast cancer screening cohort selection flowchart: 2012 Medicare beneficiaries. Diag. = diagnostic, HMO = health maintenance organization, mammo = mammography.
True-positive results were defined by using Medicare claims for women in the positive screening category who also had a diagnosis of breast cancer within 1 year after the index screen. False-positive results were defined as results from patients who were in the positive screening category but without any breast cancer diagnosis during the same period. True-negative results were defined as negative screening results and no breast cancer diagnosis within 1 year after the index screen.
Outcome measures.—Our study outcomes were three binary indicators of whether patients underwent a Papanicolaou test, bone mass measurement, or influenza vaccine within 2 years after the index screen (for screened patients) or during 2012 and 2013 (for not screened patients). These outcomes represent other preventive services relevant to women other than mammography that may be affected by the result of mammography screening. We further excluded the preventive services of interest performed within 60 days after the index screen to exclude services that likely were ordered at the same time and performed just after screening mammography while preserving capture of the months after completed diagnostic evaluation when potential psychologic effects are most pronounced (21,22).
Covariates.—To minimize possible confounding, we adjusted for patient demographic variables such as patient age groups, race, state of residence, urban or rural residence (23), and Medicaid receipt. We also included a Charlson comorbidity index, which is a summary score of 17 comorbid conditions with assigned weights of either 1, 2, 3, or 6 based on the risk of dying from the associated conditions (24). The Charlson comorbidity index was calculated by using the previous 12 months’ inpatient claims for each patient following an established algorithm (25). Furthermore, we included indicators for two sets of baseline preventive service use: whether patients attended an annual wellness visit or welcome-to-Medicare visit, and whether patients underwent any Papanicolau test bone mass measurement or influenza vaccine within 2 years before the index screen (for patients who underwent mammography) or during 2010 and 2011 (for unscreened patients). Finally, we included three county-level variables: median household income, the number of hospitals within each county, and the rate of primary care providers per 100 000 persons. A list of International Classification of Disease, Ninth Revision codes and Health care Common Procedure Coding System codes for key medical terms is provided (Table E1 [online]).
Statistical Analysis
Patient characteristics were first compared among those who were not screened at mammography during 2012, those who were screened with positive results, and those who were screened with negative results by using a 30-day window for diagnostic evaluation to define positive screening. A bivariate logistic regression model was then used to examine the relationship between the probabilities of receiving a Papanicolaou test, bone mass measurement, or influenza vaccine 2 years after the index screen and the group indicator. Next, a multivariate logistic regression model was constructed by adjusting for all the covariates described with forward model selection. We also applied inverse probability of treatment weighting by using the propensity score for estimation of causal effects of screening mammography and results on use of the selected preventive measures (13). To assess for potential negative associations between false-positive mammography results and later use of preventive care, we compared subgroups of women who were screened and obtained false-positive results with those who had negative screening results for use of the three preventive services we examined during the following 2-year period. A comparison was also performed between false-positive and true-positive results for use of these services.
Finally, we conducted a sensitivity analysis to examine the stability of the findings. First, a window of 60 days for diagnostic mammography or breast US after the index screen was used to determine screening-positive results for assessment of the effect of women who may have been delayed in obtaining additional examinations. Second, baseline use of preventive services may involve unobserved factors that are not adjusted by our covariates (eg, health intervention–seeking behavior) and the sensitivity analysis examined whether the results differed among women who had not recently undergone the preventive services. Accordingly, we restricted the sample to those individuals who had not undergone the particular service of interest within 2 years before the index screen (with adjustment for the other two preventive services). For example, when we compared the difference in the utilization rates of Papanicolaou testing among the three original groups of patients (positive results, negative results, no screening), we restricted the sample to include only those individuals who did not undergo a Papanicolaou test within 2 years before the index screen. This comparison was designed to provide a direct estimate of the net effect of undergoing screening mammography on later preventive service use because all the groups had not undergone the preventive service of interest at baseline.
Analyses were conducted by using statistical software (SAS version 9.4; SAS Institute, Cary, NC), and a two-sided P value less than .05 indicated statistical significance.
Results
Characteristics of the Cohort
Our final cohort was composed of 555 705 patients. Of the cohort, 66.6% (370 080 patients) did not undergo screening mammography in 2012, 31.2% (173 168 patients) underwent screening mammography in 2012 with negative results, and 2.2% (12 457 patients) underwent screening mammography in 2012 with positive results. Thus, among women who underwent screening, 6.7% (12 457 of 185 625) obtained a positive screening result. The cohort selection process is in the Figure. Descriptive statistics of the three patient subgroups of nonscreened patients, patients with positive screening results, and patients with negative screening results are in Table 1. The overall sensitivity and specificity of screening mammography for detection of breast cancer were 63.7% and 93.5%, respectively. The patients who did not undergo screening versus each screened group differed in terms of higher proportions of older age (≥80 years; Charlson comorbidity index of at least 1) and lower rate of baseline preventive care among patients who did not undergo mammography (P values <.001).
Table 1:
Descriptive Statistics of the Study Sample
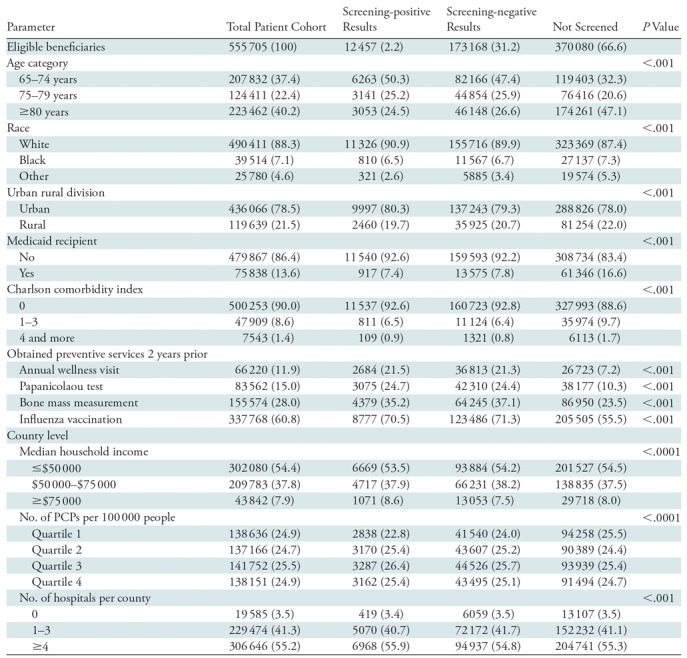
Note.—Unless otherwise indicated, data are number of patients; data in parentheses are percentages. P values are for comparison of means across screening positive, screening negative, and not screened groups. PCP = primary care providers.
Comparative Use of Preventive Services according to Screening Status
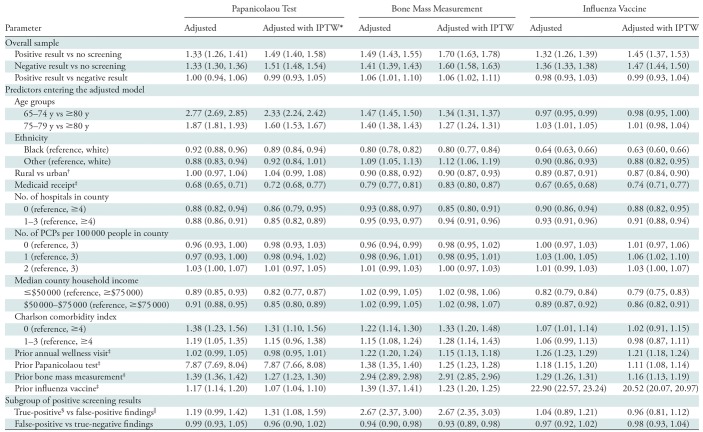
The odds of use of each of the three preventive services compared between patients who had positive screening results versus no screening, negative screening results versus no screening, and positive versus negative screening results are provided by using the estimated odds ratios (ORs) (Table 2). After inverse probability of treatment weighting adjustment for patient demographics, comorbidity status, geographic covariates, and baseline preventive care, women who underwent index screen mammography (with either positive or negative results) were more likely than women without screening to undergo the Papanicolaou test, bone mass measurement tests, and to be administered the influenza vaccine in the following 2-year period. For example, the breast screening group with positive findings had a higher likelihood than the group without screening of undergoing Papanicolaou test (OR, 1.49; 95% confidence interval: 1.40, 1.58), bone mass measurement (OR, 1.70; 95% confidence interval: 1.63, 1.78), and administration of the influenza vaccine (OR 1.45; 95% confidence interval: 1.37, 1.53). Patients with positive results at screening mammography were also slightly more likely than those with negative results at screening to undergo bone mass measurement (OR, 1.06; 95% confidence interval: 1.02, 1.11), whereas there was no difference in the likelihood of undergoing the Papanicolaou test (OR, 0.99; 95% confidence interval: 0.93, 1.05) or administration of the influenza vaccine (OR, 0.99; 95% confidence interval: 0.93, 1.04).
Table 2:
Adjusted Odds Ratio Estimates with Model Selection
Note.—Data are odds ratios; data in parentheses are 95% confidence intervals. IPTW = inverse probability of treatment weighting using propensity score, PCP = primary care providers.
*Model was adjusted by using multivariable regression and inverse probability of treatment weighting.
†Because of sample size limitation, geographic division variable instead of state was used in the subgroup analysis.
‡Indicated variables are binary (ie, yes vs no).
§True-positive findings were defined as positive findings at screening and a breast cancer diagnosis within 1 year after the index screen; true-negative findings at screening did not result in breast cancer diagnosis within the next year.
||False-positive findings were defined as positive findings at screening and no breast cancer diagnosis within 1 year after the index screen.
The strongest predictors of use of the Papanicolaou test and bone mass measurement test were the patient age group and whether or not the patient underwent the respective test in the 2 years before the index screen. Younger age groups showed significantly greater odds ratios for undergoing a Papanicolaou test and bone mass measurement than did those older than 80 years. In addition, having undergone a previous Papanicolaou test or bone mass measurement within 2 years before the index screen increased the respective odds of undergoing a Papanicolaou test (OR, 7.87; 95% confidence interval: 7.66, 8.08) or bone mass measurement (OR, 2.91; 95% confidence interval: 2.85, 2.96) within 2 years after the index screen. The patient age group was not a significant predictor for administration of the influenza vaccine. Instead, the history of a previous influenza vaccine was the strongest predictor of obtaining this service after the index screen (OR, 20.52; 95% confidence interval: 20.07, 20.97), and patients who received Medicaid were less likely to be administered the vaccine (OR, 0.74; 95% confidence interval: 0.71, 0.77).
We also examined subgroups of patients who were screened with positive results (true-positive versus false-positive results) (Table 2). Women with false-positive results showed no difference in odds ratios for use of Papanicolaou test or influenza vaccines compared with women with negative screening results in the subsequent 2-year period. False-positive screening results compared with negative screening was associated with a slight difference in the likelihood of undergoing bone mass measurement (OR, 0.93; 95% confidence interval: 0.89, 0.98). Women with true-positive results compared with women with false-positive results had increased odds of having bone mass measurement (OR, 2.67; 95% confidence interval: 2.35, 3.03). True-positive results were also associated with a slight increase in the odds of undergoing Papanicolaou test compared with false-positive results (OR, 1.31; 95% confidence interval: 1.08, 1.59). There was no difference in administration of the influenza vaccine between patients with true-positive and false-positive screening results (OR, 0.96; 95% confidence interval: 0.81, 1.12).
Sensitivity Analysis Results
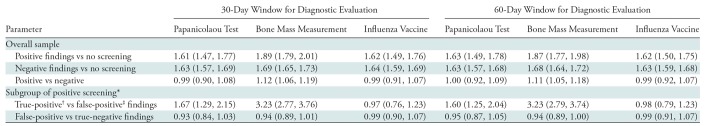
In our sensitivity analysis, we varied the length of time in which diagnostic mammography or breast US after the index screen would determine a positive screening result. Use of a 60-day window for subsequent diagnostic imaging instead of a 30-day window yielded a small increase in the number of patients with positive results at screening (Fig E1 [online]): 872 more patients had positive screening results with a 60-day window than with use of a 30-day window, leading to a positive index screening rate of 7.2% (13 329 patients) instead of 6.7%. The propensity score–adjusted ORs for the overall sample with 60-day window to define positive screening (Table E2 [online]) were consistent with the 30-day window results: OR for all three preventive services of interest indicated higher likelihood of utilization in women who underwent screening mammography compared with those who did not (Table E2 [online]). Likewise, results for the subgroup analysis for use of preventive services between false-positive and true-positive findings, and also false-positive and true-negative screening results, were similar by using the 60-day period. Use of Papanicolaou test, bone mass test, and influenza vaccine did not differ among women with false-positive versus negative findings at screening.
When we restricted our sample to women who did not undergo the preventive service of interest in the 2 years before the index screen, we found that women who underwent screening mammography (with either positive or negative results at screening) again showed higher odds than the nonscreened group of undergoing a Papanicolaou test, bone mass measurement test, and administration of the influenza vaccine in the 2 years after undergoing the index screen, and the effects were larger than in the analyses of the overall sample (Table 3). Compared with negative screening results, positive screening results were again associated with greater odds of having a bone mass measurement. Finally, we found no difference between women who obtained false-positive versus true-negative results at screening regarding their utilization of cervical cancer screening (OR, 0.93; 95% confidence interval: 0.84, 1.03), bone mass measurement (OR, 0.94; 95% confidence interval: 0.89, 1.01), or influenza vaccines (OR, 0.99; 95% confidence interval: 0.90, 1.07). The results remained consistent with expansion of the diagnostic evaluation window to 60 days for determining a positive screening result.
Table 3:
Propensity Score–adjusted Odds Ratio Estimates for Sensitivity Analyses
Note.—Data are odds ratio; data in parentheses are 95% confidence intervals.
*Because of sample size limitation, geographic division variable instead of state was used in the subgroup analysis.
†True-positive findings were defined as positive findings at screening and a breast cancer diagnosis within 1 year after the index screen.
‡False-positive findings defined as positive findings at screening and no breast cancer diagnosis within 1 year after the index screen.
Discussion
The potential effect of the use of breast cancer screening and results on patient adherence to other preventive service recommendations is relevant not only to routine clinical practice, but also to policy-level discussions of how preventive services are bundled and recommended to patients. Because of the current attention to consequences of false-positive screening results in terms of invasive procedures, patient distress, and willingness to adhere to breast cancer screening recommendations, we conducted a large-scale retrospective examination of whether the use of screening mammography may influence the use of other preventative cancer services and preventive services unrelated to cancer.
To our knowledge, this is the first reported study on this topic. In the overall U.S. Medicare population, women not undergoing screening mammography were older and had more severe comorbidities. However, after adjusting for factors such as patient age and comorbidity status, undergoing screening mammography was associated with increased use of screening for cervical cancer and osteoporosis, even if the patients did not have these other screening tests in the 2 years before the index screen. An increase in the odds of undergoing Papanicolaou test and bone mass testing in patients with true-positive versus false-positive results is likely attributable to baseline testing for gynecologic cancers and assessment of fracture risk before commencement of therapy with tamoxifen and aromatase inhibitors (because of risks of bone mass loss), respectively (26–28). Furthermore, women with false-positive results at mammography were no less likely than women with negative results at mammography to undergo another form of cancer screening (ie, the Papanicolaou test) in the following 2 years. False-positive findings at screening mammography showed a borderline association with decreased use of bone mass measurement, and then no difference when a preceding 2-year period without preventive services was applied; the initial finding may possibly be because of a scheduling delay after completing breast evaluation, but the reasons for this questionable difference are unclear. Overall, the implications of our findings are relevant to policy discussions about the inclusion of breast cancer screening among key preventive service recommendations (29) because women in the U.S. Medicare population may be more likely to adhere to preventive service guidelines unrelated to breast cancer in the period after screening mammography.
The reasons for increased use of cervical cancer and osteoporosis screening among U.S. Medicare beneficiaries who underwent breast cancer screening are not clear. There may be a heightened awareness of preventive measures after screening mammography or a decisiveness to act proactively to curtail disease. Referring physicians who counsel patients about preventive services may consider that patients’ willingness to undergo screening mammography may be tied to an understanding or acceptance of the favorable benefit-risk profile of recommended preventive services, and hesitancy or confusion about screening mammography can prompt supportive discussions about the reasons preventive measures are recommended even if unnecessary tests or interventions are possible. Furthermore, the lack of an association between false-positive results at mammography and the use of other screening tests suggests that, overall, potential distress or desire to delay or discontinue screening mammography after a false-positive finding at mammography does not clearly extend to other screening tests (6). This lack of a negative association may be further supported by some findings from previous studies (22,30) that false-positive results at screening mammography led to neither decreased willingness to adhere to breast cancer screening recommendations nor long-term increased anxiety. Although we hypothesized that patients with false-positive results might adhere less often to other screening recommendations such as Papanicolaou test to avoid the possibility of additional testing or invasive procedures, no trend was found to support such a potential effect.
Dabbous et al (6) and McCann et al (31) previously examined the effect of false-positive findings at mammography on subsequent use of breast cancer screening and cancer diagnoses, and they showed a negative association between false-positive results and adherence to screening mammography recommendations, and risk for breast cancers detected at more advanced stage. Our findings question the generalizability of these findings on overall adherence to screening recommendations and suggest that further research is needed to assess potential benefits in regarding the use of preventive services.
Our study had several limitations inherent to the use of retrospective administrative Medicare claims. First, we restricted our sample to be fee-for-service beneficiaries age 65 and older with continuous part A and B coverage from 2010 to 2014. The findings therefore cannot necessarily be generalized to other U.S. Medicare groups (ie, those enrolled in managed care settings) or to non-Medicare populations in the U.S. (eg, younger women with private insurance, Medicaid, or no insurance) or in other countries where preventive services may differ in terms of timing, insurance coverage, and access. The proportion of women with Medicare who routinely undergo screening mammography may vary according to the specific plan, and in addition the 1-year period in this study may underestimate the proportion of women who undergo mammography (32). We note that the demographic characteristics of the studied population may affect the results, and access to or use of preventive services may differ where the demographics of our population do not apply. In addition, our data are observational in nature, and though we performed a propensity score–based analysis, we do not establish causality in the relationship between mammography and later use of preventive services. We excluded preventive services performed within 60 days of screening mammography because we assumed the mammography and preventive measures were ordered concurrently, and this assumption may result in underestimation of an association between screening mammography and other preventive services in a shorter term than was examined. Patients who underwent screening mammography also may be different from the control group in ways we did not fully capture (ie, their health literacy or level of motivation to preserve their health). Patients who did not undergo breast cancer screening may also have been counseled less effectively or they may be less amenable to screening tests for other reasons that were not studied. Counseling for preventive services has the potential to be time consuming and impeded by barriers such as patient literacy, probabilistic reasoning, and comprehension of population level benefits and harms. With potential for short-term distress and possible hesitancy to continue screening mammography after false-positive results, health care providers should remain dedicated to a dialogue for accurate patient understanding of risk estimates, screening benefits and harms, and shared decision making regarding preventive recommendations.
We report a mammographic recall rate of 6.7%, which may be perceived as lower than previously reported rates (the recommended overall benchmark is less than 10%) (18). This finding may be related to the fact that we are investigating an older cohort; older women are more likely to undergo incident versus prevalent screen, and therefore they are more likely to have undergone prior mammography for comparison with abnormal interpretation rates known to decrease in this setting. However, it is also possible that use of billing claims limited our ability to determine abnormal screening results for which patients did not follow up or for which patients’ diagnostic evaluations were not billed to Medicare. Finally, our operational definitions of screening positive and negative, and true-positive and false-positive results, are on the basis of assumptions about additional examinations and diagnoses within a time frame and not on reports for the screening mammography. These definitions for screening mammography results may also lack the gravity of positive results at examination as defined by the Mammography Quality Standards Act as a test resulting in a biopsy. The majority of the women in our cohort who had false-positive findings underwent diagnostic mammography without invasive procedure. However, by employing this broader definition of false-positive results, we were able to capture patients who ultimately had benign results at diagnostic imaging and those with nonmalignant tissue sampling results. In addition, previous studies (33) described increased patient anxiety over extended periods after abnormal findings at mammography that did not result in a cancer diagnosis, regardless of whether a breast biopsy was performed.
In conclusion, women who were beneficiaries of Medicare who underwent screening mammography showed increased use of cervical cancer and osteoporosis screening tests and the influenza vaccine, all of which are recommended according to current preventive service guidelines. The positive association of use of screening mammography with the use of other screening tests may be of consideration for further studies regarding the effect of patient counseling and experiences with mammography on their comprehension, attitudes, and values regarding screening tests unrelated to mammography. In addition, the association may also be relevant to policy-level decisions regarding coverage for breast cancer screening as a key part of a bundle of recommended preventive tests.
Summary
Female U.S. Medicare beneficiaries who underwent screening mammography showed increased utilization of cervical cancer and osteoporosis screening tests, as well as the influenza vaccine, which are recommended in current preventive service guidelines.
Implications for Patient Care
■ Screening mammography potentially has a positive association with later use of other recommended preventive services.
■ In the 2 years after false-positive results at screening mammography, there was no negative association with the use of other preventive service.
APPENDIX
SUPPLEMENTAL FIGURES
S.K.K. supported by National Cancer Institute http://dx.doi.org/10.13039/100000054 (K07CA197134).
Current address: IQVIA, Fairfax, Va.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Disclosures of Conflicts of Interest: S.K.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed royalties from Wolters Kluwer. Other relationships: disclosed no relevant relationships. M.J. disclosed no relevant relationships. R.D. Activities related to the present article: disclosed money to author’s institution for a grant from the Harvey L. Neiman Health Policy Institute. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. S.L.H. disclosed no relevant relationships. D.R.H. disclosed no relevant relationships. L.M. disclosed no relevant relationships.
Abbreviation:
- OR
- odds ratio
References
- 1.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015;314(15):1599–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC/National Center for Health Statistics . Health, United States, 2015 - Women's Health. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/hus/women.htm. Accessed February 28, 2017.
- 3.Leung J, Macleod C, McLaughlin D, et al. Screening mammography uptake within Australia and Scotland in rural and urban populations. Prev Med Rep 2015;2:559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Preventive Services Task Force . Final Recommendation Statement, Breast Cancer: Screening. U.S. Preventive Services Task Force Web site. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed February 28, 2017.
- 5.Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med 1998;338(16):1089–1096. [DOI] [PubMed] [Google Scholar]
- 6.Dabbous FM, Dolecek TA, Berbaum ML, et al. Impact of a false-positive screening mammogram on subsequent screening behavior and stage at breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev 2017;26(3):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Family Physicians . Preventive Visit Algorithm: Patients Ages 18–64. American Academy of Family Physicians Web site. http://www.aafp.org/fpm/2012/0700/fpm20120700p12-rt1.pdf. Accessed February 28, 2017.
- 8.Stange KC, Flocke SA, Goodwin MA. Opportunistic preventive services delivery. Are time limitations and patient satisfaction barriers? J Fam Pract 1998;46(5):419–424. [PubMed] [Google Scholar]
- 9.U.S. Department of Health & Human Services . Health Care: About the Affordable Care Act. https://www.hhs.gov/healthcare/about-the-aca/ index.html#CoveredPreventiveServicesforWomenIncludingPregnantWomen. Accessed January 9, 2018.
- 10.Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol 2010;7(1):18–27. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society . History of ACS Recommendations for the Early Detection of Cancer in People Without Symptoms. https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/ chronological-history-of-acs-recommendations.html. Last revised July 7, 2017. Accessed January 9, 2018.
- 12.Cooper GS, Kou TD, Dor A, Koroukian SM, Schluchter MD. Cancer preventive services, socioeconomic status, and the Affordable Care Act. Cancer 2017;123(9):1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services . National Coverage Determination for Bone Density Studies. https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=256&ncdver=2&SearchType=Advanced&CoverageSelection=National& NCSelection=NCA%7cCAL%7cNCD%7cMEDCAC%7cTA%7cMCD& BenftCat=7&DateFrom=01012012&DateTo=09302012&kq=true&bc=IAAAACAAAAAA&. Accessed February 19, 2018. [PubMed]
- 14.Committee on Practice Bulletins—Gynecology . ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol 2012;120(5):1222–1238. [DOI] [PubMed] [Google Scholar]
- 15.American College of Radiology . ACR BI-RADS Atlas. Follow up and Outcome Monitoring. American College of Radiology Web site. https://www.acr.org/Quality-Safety/Resources/BIRADS/Monitoring. Accessed April 18, 2017.
- 16.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Likelihood ratios for modern screening mammography. Risk of breast cancer based on age and mammographic interpretation. JAMA 1996;276(1):39–43. [DOI] [PubMed] [Google Scholar]
- 17.Robertson CL. A private breast imaging practice: medical audit of 25,788 screening and 1,077 diagnostic examinations. Radiology 1993;187(1):75–79. [DOI] [PubMed] [Google Scholar]
- 18.Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: BI-RADS Atlas ACR, Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 19.Rosenberg RD, Haneuse SJ, Geller BM, et al. Timeliness of follow-up after abnormal screening mammogram: variability of facilities. Radiology 2011;261(2):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mammography Accreditation Program Requirements . ACR Mammography Accreditation Program. American College of Radiology Web site. http://www.acraccreditation.org/∼/media/ ACRAccreditation/Documents/Mammography/Requirements.pdf?la=en. Accessed April 24, 2017.
- 21.Cockburn J, Staples M, Hurley SF, De Luise T. Psychological consequences of screening mammography. J Med Screen 1994;1(1):7–12. [DOI] [PubMed] [Google Scholar]
- 22.Tosteson AN, Fryback DG, Hammond CS, et al. Consequences of false-positive screening mammograms. JAMA Intern Med 2014;174(6):954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram DD, Franco SJ. NCHS urban-rural classification scheme for counties. Vital Health Stat 2 2012;(154):1–65. [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 26.Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 2011;22(12):2546–2555. [DOI] [PubMed] [Google Scholar]
- 27.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003;21(21):4042–4057 (Published correction appears in J Clin Oncol 2004;22(7):1351. Dosage error in article text.). [DOI] [PubMed] [Google Scholar]
- 28.Ito K, Blinder VS, Elkin EB. Cost effectiveness of fracture prevention in postmenopausal women who receive aromatase inhibitors for early breast cancer. J Clin Oncol 2012;30(13):1468–1475. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin JS, Sheffield K, Li S, Tan A. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med 2016;31(11):1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinckney RG, Geller BM, Burman M, Littenberg B. Effect of false-positive mammograms on return for subsequent screening mammography. Am J Med 2003;114(2):120–125. [DOI] [PubMed] [Google Scholar]
- 31.McCann J, Stockton D, Godward S. Impact of false-positive mammography on subsequent screening attendance and risk of cancer. Breast Cancer Res 2002;4(5):R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenkrantz AB, Fleming M, Duszak R, Jr. Variation in screening mammography rates among Medicare advantage plans. J Am Coll Radiol 2017;14(8):1013–1019. [DOI] [PubMed] [Google Scholar]
- 33.Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med 1991;114(8):657–661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.