Abstract
Background
Escherichia coli (E. coli) is one of the best-known zoonotic bacterial species, which pathogenic strain can cause infections in humans and animals. However, existing technologies or methods are deficient for quickly on-site identifying infection of E. coli before they breakout. Herein, we present an ultrasensitive and on-site method for counting E. coli using magnetic nanoparticle (MNP) probe under a dark-field in 30 min.
Results
The antibodies functionalized MNP, binding to E. coli to form a golden ring-like structure under a dark-field microscope, allowing for counting E. coli. This method via counting MNP-conjugated E. coli under dark-field microscope demonstrated the sensitivity of 6 CFU/μL for E. coli detection. Importantly, due to the advantages such as time-saving (only 30 min) and almost free of instrument (only require a portable microscope), our MNP-labeled dark-field counting strategy has the potential of being a universal tool for on-site quantifying a variety of pathogens with size ranges from a few hundreds of nanometers to a few micrometers.
Conclusion
In summary, the MNP-labeled dark-field counting strategy is a rapid, simple, sensitive as well as low-cost assay strategy, which has the potential of being a universal tool for on-site quantification of micrometer-size pathogens like E. coli.
Electronic supplementary material
The online version of this article (10.1186/s12866-018-1241-5) contains supplementary material, which is available to authorized users.
Keywords: Rapid enumeration, Escherichia coli, Magnetic nanoparticle, Dark-field microscope
Background
E. coli is a rod-like bacterium and the genus Escherichia contains mostly motile Gram-negative bacilli in the family of Enterobacteriaceae. Most of the E. coli strains are not harmful to humans but some strains possess pathogenic virulence, which increase the ability to adapt new niches and allow them to cause broad spectrum of diseases [1]. In recent years, the foodborne diseases emerged as major concern in public [2]. Therefore, rapid, inexpensive and sensitive methods are required to detect the E. coli infection.
During last decades, several molecular and immunological methods have been developed to detect E. coli. Such as polymerase chain reaction (PCR), real-time PCR, multiplexed PCR, loop-mediated isothermal amplification, Pulsed-Field Gel Electrophoresis, Gene-chip, next generation sequencing, enzyme linked immunosorbent assay (ELISA) and immune chromatography. Despite the fact of these approaches are powerful, they have drawbacks such as laboriousness, high-cost, complexity, and requirement of expensive instruments or reagents. Most importantly, they are not able for on-site assay [3–11]. Recently, the biosensor technology plays an important role in the detection of pathogenic bacteria because they have great potential to meet the practical need of rapid, sensitive, easy-to-use, and low-cost [12, 13]. Biosensor technology implemented with the use of nanoparticles (NPs) tags and also other nanomaterials offers benefits compared with traditional methods in terms of time, sensitivity and simplicity [14–17].
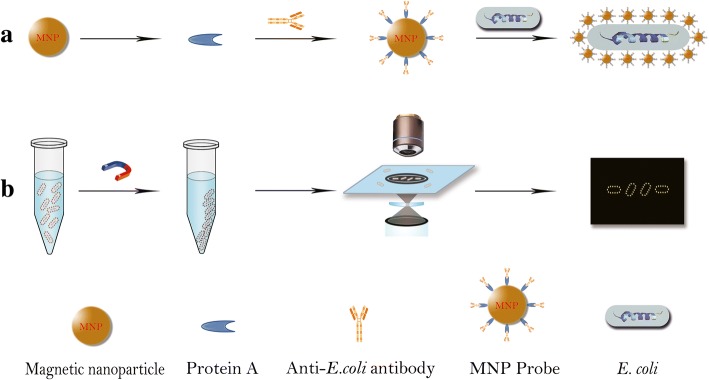
In this study, we demonstrated a quick and sensitive counting of E. coli at low concentrations without bacteria pre-enrichment by employing a MNP probe combined with dark-field microscope. The schematic diagram of our MNP-labeled dark-field counting strategy is shown in Fig. 1.
Fig. 1.
Schematic diagram of counting E. coli under a dark-field by hatting them golden ring structure with MNP probes. a The MNP probes were obtained by incubating anti-E. coli antibodies and the MNP under gently shaking for 4 h at room temperature, followed by washing three times with PBS. b E. coli samples were first mixed with MNP probes to develop probe-E. coli complexes, followed by magnetic separation for counting with dark-field microscope
Results
Validation of MNP probes
The electrophoresis result clearly showed the presence of two bands (a ~ 50 kDa band of antibody heavy chain and a ~ 20 kDa band of antibody light chain) in MNP probes sample (Fig. 2, lane 3), indicating successful bio-functionalization of MNP probes.
Fig. 2.

SDS-PAGE analysis of prepared MNP probes. Column 1: 10 μL of as-synthesized MNP; Column 2: 5 μg anti-E. coli antibody; Column 3: 10 μL of MNP probes
Detection of E. coli cultured in laboratory with our counting strategy
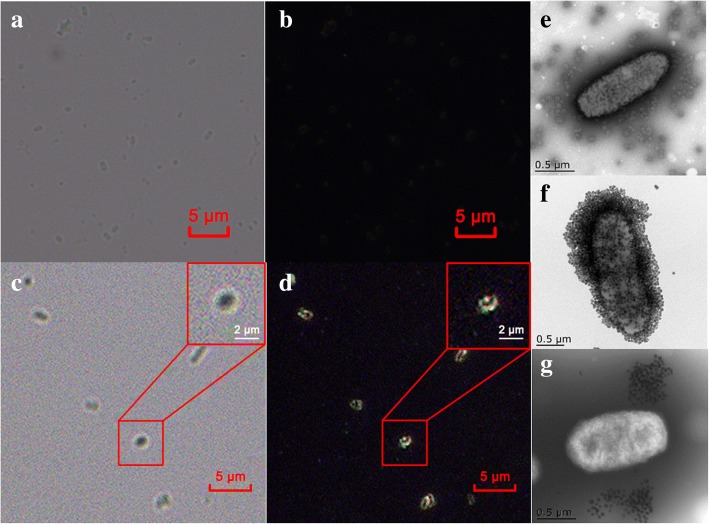
The results show that each bacteria was encircled with MNP probes to form dim bacilliform structures under light field (Fig. 3c) and bright gold ring structure under dark-field microscope (Fig. 3d), which was also confirmed by TEM (Fig. 3f). On the contrary, E. coli mixed with MNP without anti-E. coli antibodies were observed under a bright and dark-field microscope. E. coli were dim and indistinct under bright field (Fig. 3a) and were completely invisible under dark field (Fig. 3b) due to no recognition between E. coli and MNP particles, which was verified by TEM (Fig. 3e). The Fig. 3g clearly showed that Salmonella bacteria did not bind to MNP probes, which suggests that our MNP probes has a specific affinity to target species.
Fig. 3.
Microscope images of E. coli at different conditions. a E. coli without MNP probes under a bright field; b E. coli without MNP probes under a dark-field; c E. coli mixed with MNP probes under a bright field; d E. coli mixed with MNP probes under a dark-field; e Transmission Electronic Microscope (TEM) image of E. coli mixed with naked MNPs. f TEM image of . coli with MNP probes. g Salmonella mixed with MNP probes; Red box in the upper right corner of d and e is the enlargement of the selected area, respectively
Determination of limit of detection of our counting assay
Figures 4a (102 CFU/μL) and 4b (10 CFU/μL) show that many monodisperse golden ring-like structures can be counted under dark-field. Even in Fig. 4c (1 CFU/μL of E. coli sample), we could also occasionally find one or two bright golden ring structure in a certain field of view. The limit of detection is defined as three times the signal-to-noise ratio, which is calculated by counting the number of golden ring-like E. coli found in 10 random fields of view of background experiment (naked MNP mixed with E. coli). Because 10 μL of sample solution covers the area of 200 field of views (each view area equal to 100 μm2) and the background experiments showed that one dim ring-like structure could be occasionally found in 10 random field of views, LOD of our counting method could be defined as 6 CFU/μL. The maximum quantity of E. coli in single field of view can be used to clearly count is approximated to 300 CFU, the suitable concentration range by our MNP-based counting method is from 6 to 6000 CFU/μL. The results show the LOD of PCR in this study is 100 CFU/μL (Fig. 4d, line 4) because the band from 10 CFU/μL sample is invisible to the naked eye. These results indicate that the theoretical sensitivity of our counting strategy is 15 times more sensitive than that of PCR technique. Moreover, it is worth to mention that, strictly speaking, no false positive happened using our MNP-based dark-field microscopy counting method because no bright gold ring structures formation in the absence of target pathogens.
Fig. 4.
Sensitivity analysis of E.coli at different dilution concentrations and count of two real samples by our MNP-based strategy. a 103 dilution of E. coli stock solution (equal to 102 CFU/μL) captured by MNP probe; b 104 dilution of E. coli stock solution (equal to 10 CFU/μL) captured by MNP probe; c 105 dilution of E. coli stock solution (equal to 1 CFU/μL) captured by MNP probe. d PCR analysis of E.coli at different dilution concentrations. M: DNA Marker; Line 1: E. coli stock solution; Line 2–6: 101, 102, 103, 104 and 105 dilution of E. coli stock solution, respectively; Red box in the upper right corner of a, b and c is the enlargement of the selected area, respectively. e A representative dark-field image of E. coli in ten-fold concentration dilute soup sample captured by MNP probes; f A representative dark-field image of E. coli in ten-fold concentration rice sample captured by MNP probes
Detection of real samples with MNP-labeled dark-field counting strategy
Two food samples that were exposed to the air for 24 h in our lab were quantified with our MNP-labeled dark-field counting method. As shown in the Table 1, 163 ± 16 CFU/10 μL from soup sample and 126 ± 14 CFU/10 μL from rice sample could be obtained by counting the clones in petri dishes (The raw counting images were shown in Additional file 1: Figures. S1 and S2). Using our counting method, 142 ± 14 CFU/10 μL and 112 ± 8 CFU/10 μL could be counted, respectively (the raw dark-field images by our counting strategy were shown in Additional file 1: Table S1). The high coincidence rate (~ 87%) compared to the clone counting method and efficient detection time of 30 min suggest that our strategy will be a more advantageous method of enumeration of E. coli in real samples than the traditional method that normally takes 10 h for analysis. Figure 4e and Fig. 4f are the representative dark-field images of MNP-E. coli from the ten-fold concentration dilute soup and rice, respectively (Additional file 1: Figures. S3 to S8).
Table 1.
Detection of real samples by traditional colony counting and MNP-labeled dark-field counting strategy
| Samples | Colony counting method (CFU of 10 μL of sample) |
Our counting strategy (CFU of 10 μL of sample) |
|---|---|---|
| Dilute soup | 163 ± 16 | 142 ± 14 |
| Rice | 126 ± 14 | 112 ± 8 |
Statistical analysis: The experimental counting results are reported as means ± SE of triplicate independent experiments. Ten fields of view were counted for each sample. Data were analyzed using the SPSS (Statistical Package for the Social Sciences) software (Version 13.0; SPSS, Inc., Chicago, IL)
Discussion
Since the quantity of E. coli is used as a potential indicator for the presence of other pathogens in many samples [18], the number of E. coli is essential for evaluating the extent of enteric disease by pathogenic bacteria such as enterohemorrhagic strains of E. coli [19, 20] that contaminates food or water [20, 21]. Conventional identification methods for bacteria involves several steps: selective culture, Gram staining, as well as biochemical and/or serological tests, which are still considered as gold standard for E. coli detection [22]. However, the conventional assay is labor-intensive and often time-consuming and strenuous to obtain a confirmed result. Theoretically, the sensitivity of our MNP-labeled dark-field counting strategy can reach 1 CFU in 10 μL of sample solution because the structure of non-specific adsorption formation is far from the bright gold ring. However, considering the convenience of practical assay, the LOD of our counting method can be determined approximate to 6 CFU/μL. And the suitable concentration range by our MNP-based counting method is from 6 to 6000 CFU/μL. The wide range of detection (6 to 6000 CFU/μL) can cover a variety of E. coli samples even without concentration or dilution; therefore, our MNP-labeled dark-field counting strategy is an efficient technique for counting the E. coli from different samples.
In this study, a rapid, reliable and effective assay has been demonstrated for the detection of E. coli in samples with different concentrations. The sensitivity of the MNP-based counting method was thoroughly investigated and compared to the conventional PCR assay. The data showed that LOD of our counting method is down to 6 CFU/μL, which greatly exceeds the PCR assay (the LOD of PCR assay performed in this study is 100 CFU/μL). Owing to the advantages such as time-saving (~ 30 min) and almost free of instrument (only the requirement of a portable microscope), MNP-labeled dark-field counting strategy has the potential of being a universal platform for on-site quantifying a variety of pathogens in all sizes ranging from hundreds of nanometers to micrometers. The MNP-based counting method potentially allows for on-site use in the monitoring of water quality, food safety and environmental parameters.
Conclusion
In summary, a rapid, simple, sensitive as well as low-cost assay has been developed for on-site enumeration of micrometer-size pathogens like E. coli.
Methods
Bacterial culture
E. coli O78 used in this study was a gift from Dr. Fang Tang (College of veterinary medicine, Nanjing Agricultural University). The bacteria were cultured in Luria Bertani (LB) medium and kept shaking at 220 rpm at 37 °C over night. Then the cells were harvested and washed for several times with 10 mM PBS solution (pH = 7.4). Optical density at 600 nm (OD600) was measured and adjusted to around 0.60, corresponding to bacteria concentration of 108 CFU/mL.
Preparation of MNP probes
To demonstrate the proof-of-concept above, the MNP probes were prepared as follows: 1) Protein A functionalized MNPs (Creative Diagnostics, USA) at diameter of ~ 50 nm were incubated with anti-E. coli polyclonal antibody (5 μg/μL) (PA1–7213, Thermo Fisher, USA) for 4 h at room temperature. 2) The MNP probes were washed three times with PBS by magnetic stand to remove the unattached E. coli antibodies and then were stored at 4 °C until use. 3) To investigate the successful modification of antibody, MNP probes were assayed using the sodium dodecyl sulfate−polyacrylamide gel electrophoresis (SDS-PAGE).
Detection of E. coli with MNP-labeled dark-field counting strategy
With the achievement of prepared MNP probes, we next examined the feasibility of counting E. coli under dark-field microscope. The counting assay solution containing 50 μL of MNP probes (~ 8 × 1011 particles), 10 μL of E. coli (~ 1.0 × 106 CFU) and 140 μL of PBS (pH 7.4) was gently mixed for 25 min at room temperature, and then immediately separated by magnetic stand. The resulting solution was further washed three times with PBS and subjected to dark-field microscopy and transmission electron microscopy (TEM, Tecnai 12, Philips, Holland). A Salmonella strain (BNCC103307, purchased from ATCC) was employed as a control to investigate the specificity of MNP probes.
Investigation of sensitivity of MNP-labeled dark-field counting strategy
In addition, the sensitivity of the proposed method was subsequently investigated. A serial of E. coli samples cultured in LB medium at different concentrations were employed to investigate the limit of detection (LOD) using our MNP-based dark-field microscopy counting method. Briefly, the E. coli stock solution was diluted with PBS buffer into five samples at different concentration from 100 to 104 CFU/μL. 10 μL of E. coli sample solution was mixed with 50 μL of MNP probes and 140 μL of PBS (pH = 7.4) for 25 min, and then separated by magnetic stand, finally subjected to dark-field microscopy. Meanwhile, PCR assay was employed as a compared method to evaluate the sensitivity of our counting strategy. To this end, a pair of primers (forward primer: 5′-TAGGTATTCCTGTTGCGGAGTATAT-3′; reverse primer: 5′-TACTATACATCAAACCCTCAGCATT-3′) were designed to amplify a fragment of specific E. coli gene (6-phosphoguuconate dehydrogenase). PCR were performed according to standard procedure and 2 μL of each of E. coli samples at different concentration was used as template in each PCR reaction.
Detection of real samples with MNP-labeled dark-field counting strategy
Furthermore, two real samples, one of which is a dilute meat soup and the other is a rice exposed to air for two days, were employed to demonstrated the availability of our MNP-labeled dark-field counting strategy. Firstly, two samples were washed with PBS under gently mixing for 5 min and then the supernatants were collected by centrifugation at 10,000 g for 3 min. 10 time-fold condensed sample solutions were prepared at the same time. 50 μL of condensed samples were mixed with 100 μL of anti-E. coli antibody functionalized MNP probe under gently shaking for 25 min at room temperature and then washed three times with PBS, followed by subjecting to dark-field microscopy for enumeration. In the meantime, the conventional colony enumeration method was employed as a comparison to evaluate the feasibility of our MNP-labeled dark-field counting strategy.
Additional file
Counting clones of dilute soup and rice samples in petri dishes. Dark-field images of real samples using the MNP-based counting method. Counting clones of dilute soup samples in petri dishes. Counting clones of rice samples in petri dishes. 10 random views of dark-field images of MNP-E. coli from the ten-fold concentration dilute soup. Three repetitions were performed. 10 random views of dark-field images of MNP-E. coli from the ten-fold concentration rice. Three repetitions were performed. The counting numbers were marked on respective dark-field images. (DOCX 666 kb)
Acknowledgments
Availability of data and material
All data generated or analyzed during this study are included in this published article and the Additional file 1.
Funding
This work was supported by National Key R&D Program of China (2016YFD0500800), the startup funds to X. Zhou provided by the Yangzhou University (137011016) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Veterinary Medicine).
Abbreviations
- CFU
Colony forming units
- E. coli
Escherichia coli
- ELISA
Enzyme linked immunosorbent assay
- h
hour
- LB
Luria Bertani
- LOD
Limit of detection
- min
minute
- MNP
Magnetic nanoparticle
- NPs
Nanoparticles
- PBS
Phosphate buffered solution
- PCR
Polymerase chain reaction
- rpm
revolution per minute
- SDS-PAGE
Sodium dodecyl sulfate−polyacrylamide gel electrophoresis
- TEM
Transmission electron microscopy
Authors’ contributions
XZ conceived the research work, guided the experiments and wrote the manuscript. HX performed most of the experiments, analyzed the experimental data and drafted the manuscript. FT Screened the pathogenic bacteria from chicken feces and participated in partial testing experiments. JD and CW critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Chan KY, Ye WW, Zhang Y, Xiao LD, Leung PH, Li Y, Yang M. Ultrasensitive detection of E. Coli O157:H7 with biofunctional magnetic bead concentration via nanoporous membrane based electrochemical immunosensor. Biosens Bioelectron. 2013;41:532–537. doi: 10.1016/j.bios.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Roy D, Biswas B, Islam HM, Ahmed MS, Rasheduzzaman M, Sarower MG. Rapid identification of enterovirulent Escherichia coli strains using polymerase chain reaction from shrimp farms. Pak J Biol Sci. 2013;16(21):1260–1269. doi: 10.3923/pjbs.2013.1260.1269. [DOI] [PubMed] [Google Scholar]
- 4.Miszczycha SD, Ganet S, Duniere L, Rozand C, Loukiadis E, Thevenot-Sergentet D. Novel real-time PCR method to detect Escherichia coli O157:H7 in raw milk cheese and raw ground meat. J Food Prot. 2012;75(8):1373–1381. doi: 10.4315/0362-028X.JFP-11-498. [DOI] [PubMed] [Google Scholar]
- 5.Son I, Binet R, Maounounen-Laasri A, Lin A, Hammack TS, Kase JA. Detection of five Shiga toxin-producing Escherichia coli genes with multiplex PCR. Food Microbiol. 2014;40:31–40. doi: 10.1016/j.fm.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Jiang L, Ge B. Loop-mediated isothermal amplification assays for detecting Shiga toxin-producing Escherichia coli in ground beef and human stools. J Clin Microbiol. 2012;50(1):91–97. doi: 10.1128/JCM.05612-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uysal A, Durak Y. Pulsed-field gel electrophoresis typing, antibiotic resistance, and plasmid profiles of Escherichia coli strains isolated from foods. Can J Microbiol. 2012;58(11):1278–1287. doi: 10.1139/w2012-108. [DOI] [PubMed] [Google Scholar]
- 8.Dowd SE, Ishizaki H. Microarray based comparison of two Escherichia coli O157:H7 lineages. BMC Microbiol. 2006;6:30. doi: 10.1186/1471-2180-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson SA, Kotewicz ML, Patel IR, Lacher DW, Gangiredla J, Elkins CA. Rapid genomic-scale analysis of Escherichia coli O104:H4 by using high-resolution alternative methods to next-generation sequencing. Appl Environ Microbiol. 2012;78(5):1601–1605. doi: 10.1128/AEM.07464-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan S, Liu D, Guo Q, Wu S, Chen R, Luo K, Hu L, Xiong Y, Lai W. Sensitive detection of Escherichia coli O157:H7 based on cascade signal amplification in ELISA. J Dairy Sci. 2016;99(9):7025–7032. doi: 10.3168/jds.2016-11320. [DOI] [PubMed] [Google Scholar]
- 11.Yonekita T, Fujimura T, Morishita N, Matsumoto T, Morimatsu F. Simple, rapid, and reliable detection of Escherichia coli O26 using immunochromatography. J Food Prot. 2013;76(5):748–754. doi: 10.4315/0362-028X.JFP-12-423. [DOI] [PubMed] [Google Scholar]
- 12.Afonso AS, Perez-Lopez B, Faria RC, Mattoso LH, Hernandez-Herrero M, Roig-Sagues AX, Maltez-da Costa M, Merkoci A. Electrochemical detection of Salmonella using gold nanoparticles. Biosens Bioelectron. 2013;40(1):121–126. doi: 10.1016/j.bios.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Liebana S, Lermo A, Campoy S, Barbe J, Alegret S, Pividori MI. Magneto immunoseparation of pathogenic bacteria and electrochemical magneto genosensing of the double-tagged amplicon. Anal Chem. 2009;81(14):5812–5820. doi: 10.1021/ac9007539. [DOI] [PubMed] [Google Scholar]
- 14.Baeumner AJ, Cohen RN, Miksic V, Min J. RNA biosensor for the rapid detection of viable Escherichia coli in drinking water. Biosens Bioelectron. 2003;18(4):405–413. doi: 10.1016/S0956-5663(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 15.Sanvicens N, Pascual N, Fernandez-Arguelles MT, Adrian J, Costa-Fernandez JM, Sanchez-Baeza F, Sanz-Medel A, Marco MP. Quantum dot-based array for sensitive detection of Escherichia coli. Anal Bioanal Chem. 2011;399(8):2755–2762. doi: 10.1007/s00216-010-4624-5. [DOI] [PubMed] [Google Scholar]
- 16.Hassan AR, de la Escosura-Muniz A, Merkoci A. Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags. Biosens Bioelectron. 2015;67:511–515. doi: 10.1016/j.bios.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Huang CJ, Dostalek J, Sessitsch A, Knoll W. Long-range surface plasmon-enhanced fluorescence spectroscopy biosensor for ultrasensitive detection of E. Coli O157:H7. Anal Chem. 2011;83(3):674–677. doi: 10.1021/ac102773r. [DOI] [PubMed] [Google Scholar]
- 18.Kuhnert P, Boerlin P, Frey J. Target genes for virulence assessment of Escherichia coli isolates from water, food and the environment. FEMS Microbiol Rev. 2000;24(1):107–117. doi: 10.1111/j.1574-6976.2000.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan RL. Identifying and controlling emerging foodborne pathogens: research needs. Emerg Infect Dis. 1997;3(4):517–521. doi: 10.3201/eid0304.970416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokarskyy O, Marshall DL. Immunosensors for rapid detection of Escherichia coli O157:H7 - perspectives for use in the meat processing industry. Food Microbiol. 2008;25(1):1–12. doi: 10.1016/j.fm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Lin YH, Chen SH, Chuang YC, Lu YC, Shen TY, Chang CA, Lin CS. Disposable amperometric immunosensing strips fabricated by au nanoparticles-modified screen-printed carbon electrodes for the detection of foodborne pathogen Escherichia coli O157:H7. Biosens Bioelectron. 2008;23(12):1832–1837. doi: 10.1016/j.bios.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Afrasiabi R, Fathi F, Wang N, Xiang C, Love R, She Z, Kraatz HB. Impedance based detection of pathogenic E. Coli O157:H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens Bioelectron. 2014;58:193–199. doi: 10.1016/j.bios.2014.02.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Counting clones of dilute soup and rice samples in petri dishes. Dark-field images of real samples using the MNP-based counting method. Counting clones of dilute soup samples in petri dishes. Counting clones of rice samples in petri dishes. 10 random views of dark-field images of MNP-E. coli from the ten-fold concentration dilute soup. Three repetitions were performed. 10 random views of dark-field images of MNP-E. coli from the ten-fold concentration rice. Three repetitions were performed. The counting numbers were marked on respective dark-field images. (DOCX 666 kb)