Abstract
Background & Aims
Disruption to endoplasmic reticulum (ER) calcium homeostasis has been implicated in obesity, however, the ability to longitudinally monitor ER calcium fluctuations has been challenging with prior methodologies. We recently described the development of a Gaussia luciferase (GLuc)- based reporter protein responsive to ER calcium depletion (GLuc-SERCaMP) and investigated the effect of a high fat diet on ER calcium homeostasis.
Methods
A GLuc-based reporter cell line was treated with palmitate, a free fatty acid (FFA). Rats intrahepatically injected with GLuc-SERCaMP reporter were fed a cafeteria diet or high fat diet. The liver and plasma were examined for established markers of steatosis and compared to plasma levels of SERCaMP activity.
Results
Palmitate induced GLuc-SERCaMP release in vitro, indicating ER calcium depletion. Consumption of a cafeteria diet or high fat pellets correlated with alterations to hepatic ER calcium homeostasis in rats, as evidenced by increased GLuc-SERCaMP release. Access to ad lib high fat pellets also led to a corresponding decrease in microsomal calcium ATPase activity and increase in markers of hepatic steatosis. In addition to GLuc-SERCaMP, we have also identified endogenous proteins (endogenous SERCaMPs) with a similar response to ER calcium depletion. We demonstrated the release of an endogenous SERCaMP, thought to be a liver esterase, during access to a high fat diet. Attenuation of both GLuc-SERCaMP and endogenous SERCaMP was observed during dantrolene administration.
Conclusions
Here we describe the use of a reporter for in vitro and in vivo models of high fat diet. Our results support that dietary fat intake correlates with a decrease in ER calcium levels in the liver and suggest a high fat diet alters the ER proteome.
Keywords: SERCaMP, endoplasmic reticulum, endoplasmic reticulum calcium, high fat diet, cafeteria diet, dantrolene, palmitate, Gaussia luciferase, endogenous SERCaMP, SERCA2b
Introduction
Metabolic disorders have plagued developing countries in the past century. Excessive nutrient intake, sedentary lifestyles, and increased food availability have all contributed to disease progression. According to the World Health Organization (WHO), in 2014 approximately 1.9 billion adults were overweight, with 600 million estimated to be obese [1]. Aside from comorbidities such as cardiovascular disease and type 2 diabetes often associated with obesity, its effects are also appreciated on a cellular level. Hepatocytes in particular, are among those most affected by obesity [2]. Within the past decade, much research has focused on the role of hepatocyte endoplasmic reticulum (ER) in obesity pathogenesis [3–6]. The ER is an intracellular organelle responsible for many intrinsic cellular functions and hepatocyte ER have four main functions: protein maturation, lipid synthesis, detoxification, and calcium storage [2]. Perturbations to these functions during pathological states, such as obesity, can lead to chronic ER stress and cell death if not ameliorated. To deal with stress and reestablish homeostasis, the ER employs an adaptive mechanism called the unfolded protein response (UPR). The UPR is a signal transduction cascade, comprised of 3 distinct arms responsive to, but not limited to, the accumulation of unfolded proteins and calcium imbalance. ER stress has been shown to contribute to the development of glucose intolerance and insulin resistance during obesity. Support for this is evidenced by decreased ER stress markers in obese livers upon the addition of chemical chaperones [5] and restoration of euglycemia upon UPR attenuation [7]. Given the presence of ER stress in the pathophysiology of obesity, it is imperative to delineate contributing mechanisms.
The endoplasmic reticulum (ER) is the main reservoir for intracellular calcium, with concentrations estimated to be 1,000–10,000-fold greater than cytosolic levels [8]. Regulation of this gradient is important for the proper function of many ER-resident chaperones and enzymes. Perturbations to ER calcium levels are associated with a variety of pathologies and has been implicated as a contributing factor to the development and progression of obesity-associated disorders [9, 10]. One of the key proteins for maintaining this crucial gradient is the sacro/endoplasmic reticulum calcium ATPase (SERCA) which pumps calcium into the ER. Three mammalian genes (ATP1-3) code for three SERCA isoforms (SERCA1-3), which can further be divided into two sub isoforms due to post-translational processing [4]. SERCA2b is highly expressed in the liver [4, 11, 12]. Recently, the relationship between obesity and ER calcium, particularly SERCA2b expression and function, has been recently identified. Fu et al., 2011 reported genes associated with de novo lipogenesis were upregulated in the livers of obese mice, which had downstream consequences on membrane composition and SERCA function [3]. Others have shown decreased mRNA and protein expression of SERCA2b in liver tissue harvested from genetically and diet-induced obese mice [4]. Restoration of SERCA2b levels by viral transgene delivery reduced pharmacologically-induced ER stress, as well as increased glucose tolerance in obese mice [4]. Additionally, in vitro models using fluorescent calcium indicators [13, 14] and genetically encoded calcium indicators (GECI) FRET-based D1ER [6] in hepatic and kidney cell lines report alterations to ER calcium in response to free fatty acids (FFA). Elevated levels of circulating FFA is a hallmark of obesity and often observed as a result of insufficient lipid storage by adipocytes, thus leading to the accumulation of lipids in non-adipose tissue, such as the liver [15]. Collectively, these data indirectly implicate ER calcium dysregulation because of impaired SERCA function and expression. Furthermore, the relationship between cellular injury and chronic ER stress remains elusive, therefore the ability to longitudinally monitor ER calcium in disease models, such as obesity, is essential for further insight into ER calcium dysregulation in disease pathology.
Our lab has previously developed a reporter protein, GLuc-SERCaMP that is responsive to ER calcium depletion [16]. Briefly, this construct consists of the final seven amino acids (ASARTDL) of mesencephalic astrocyte-derived neurotrophic factor (MANF) appended to Gaussia luciferase (GLuc) to create a secreted, ER calcium modulated protein (SERCaMP). This C-terminal appendage is similar to the canonical ER-retention sequence, KDEL, thus allowing for ER localization under homeostatic conditions. Importantly, it also confers sensitivity to ER calcium depletion by causing the release of GLuc via the secretory pathway when ER calcium is depleted. Here we highlight the use of GLuc-SERCaMP to investigate in vitro responses to FFA as well as how dietary intake influences ER calcium homeostasis in the rat liver. We also describe the measurement of an endogenous SERCaMP esterase activity which parallels the activity of our exogenous luciferase reporter.
Materials and Methods
Cell Culture
SH-SY5Y-SERCaMP/No-Tag generation and maintenance have been previously described [16, 17]. Cells were authenticated by expression of GLuc-reporter and RT-PCR for human ER stress genes. Cells were tested for mycoplasma after a thawing. Palmitate (Sigma-Aldrich, St. Louis, MO) was prepared as previously described [14, 18]. Briefly, palmitic acid was dissolved in ethanol to a final concentration of 195 mM, coupled to BSA (Sigma-Aldrich) and diluted to final treatment concentration (complete media exchange).
Intrahepatic Injections of AAV-GLuc-SERCaMP
Animal studies were approved by the NIDA IACUC and comply with NIH guidelines for animal research. AAV1-GLuc-SERCaMP intrahepatic injections and blood collection techniques have been previously described [16, 19]. For this study, the right medial lobe of male, Sprague-Dawley rats (6 rats per experimental group unless otherwise noted) was injected with AAV1-GLuc-SERCaMP diluted to a final concentration of 7.6 x 109 vg/mL. Injection volume did not exceed 105 μL (injected at 3 sites using approximately 33 μL per site). Animals were singly housed, on a reverse light/dark cycle (12 h off/ 12 h on) for experiment duration following surgery.
Diet
Commercially available cafeteria diet items were purchased from Peapod. Rodent high fat soft pellets and control pellets, 36% and 7.2% fat, respectively, were purchased from BioServ. NIH standard chow (Teclan) contained 5% fat. Animals had restricted access up to 1 week prior to cohort separation (standard NIH chow prior to cafeteria and BioServ control pellets for all other experiments). Restriction values were based on average ad lib intake, and reduced by 35%. Cohort assignment was based on similar percent weight gain among all animals (approximately 21–22% in each group). Food was weighed and replenished daily. For cafeteria experiments, animals had access to 1 savory item and 1 sweet item, along with NIH standard chow. Menu items changed every 2–3 days. Nutritional information found on food packaging was used for caloric calculations. For pellet experiments, animals were allowed ad lib or restricted access to either high fat pellets or control pellets. High fat pellet restriction was calorically similar to accessible control pellets.
Dantrolene Injections
Dantrolene (Cayman Chemicals, Ann Arbor, MI) was administered by i.p. injections, 15mg/kg daily for 7 days. Dantrolene powder was dissolved to a final concentration of 5mg/mL in a 10% DMSO (Sigma-Aldrich) PBS solution. Following resuspension, dantrolene was warmed in a 37°C water bath for 15 mins to ensure powder was fully dissolved. Solutions were prepared daily. Injections occurred daily, immediately following blood collection and prior to food replenishment.
Liver Function and Histopathology
For liver panel analyses, blood was collected via cardiac stick from rats deeply anesthetized with isoflurane at 3 weeks post- ad lib or restricted high fat pellet access (n=6 rats/group). Collection tubes were provided by IDEXX laboratories (Westbrook, ME). Following collection, blood samples were centrifuged at 2000 x g for 5 mins at 4°C. Serum was transferred to a separate tube and stored at −80°C until time of shipment. Comprehensive liver panel for markers of inflammation and damage was performed by IDEXX laboratories.
For histopathological examination of livers, animals fed either ad lib high fat pellets or restricted high fat pellets were anesthetized with isoflurane at 3 weeks post-high fat pellet start and perfused with saline. The right lateral lobe was removed and flash frozen in isopentane. The samples were cut into 10 μm sections on a cryostat, mounted on glass slides, and fixed in ice-cold 4% paraformaldehyde for 10 min. The sections were stained in parallel using hematoxylin (Modified Mayer’s) for 45 secs or Oil Red O lipid stain for 15 min (Abcam, Cambridge, MA). Images were acquired with a 20x objective lens using Zeiss Axioskop 2 Plus microscope (Carl Zeiss Microscopy, Oberkochen, Germany) attached to a Exi Aqua camera (QImaging, Surrey, BC, Canada) under identical acquisition settings for all sections (iVision-Mac software, BioVision Technologies, Exton, PA).
Western Blot
For western blot of microsomal preparations, resuspended microsomes (isolation methods below) were diluted in RIPA buffer and incubated on ice for 20 min. Total protein concentration was determined using DC assay according to manufacturer’s instructions (BioRad, Hercules, CA). Fifteen micrograms of protein were separated on 4–12% NuPAGE gels using MOPS running buffer (Thermo Fisher Scientific, Waltham, MA), transferred to 0.45 μm PVDF membrane (Thermo Fisher Scientific) and immunoblotted with primary antibody diluted in blocking agent (Rockland Immunochemicals, Gilbertsville, PA); rabbit anti-SERCA2b (1:1000) (Cell Signaling Technology, Danvers, MA). Secondary antibodies were DyeLight680 and DyeLight800 (1:10000) (Rockland Immunochemicals). Blots were scanned using Odyssey scanner (LI-COR). Image analysis was performed using Image J software.
GLuc Secretion Assay
Blood collection, plasma preparation, and GLuc secretion assay (in vitro and in vivo) have been previously described [16, 19]. Briefly, 10 μL of plasma or 5 μL of culture medium was transferred to a white 96-well plate. Prepared substrate, coelenterazine (CTZ; Regis Technologies, Morton Grove, IL) was diluted to a final concentration of 100μM or 10μM in 1X PBS for in vivo or in vitro assays, respectively, and injected into individual wells using an automated plate reader (BioTek Synergy II Winooski, VT). Luminescence was read on a per well basis and reading parameters included an integration time of 5 secs and a sensitivity of 150 or 0.5 secs and a sensitivity of 100 for in vivo or in vitro assays, respectively.
Endogenous SERCaMP Assay
The endogenous esterase SERCaMP assay for rat plasma is described elsewhere (Trychta, et al, under review). Blood collection and plasma preparation have been previously described [16, 19]. Twenty microliters of plasma were transferred to black-walled, clear-bottomed plates (Thermo Fisher Scientific). Fluorescein diester substrate was previously described [20] and generously synthesized by Dr. Kenner Rice, Drug Design and Synthesis Section, NIDA IRP. Substrate was diluted to a final concentration of 100 mM in DMSO (Sigma-Aldrich) and stored under dark conditions at −80°C for single use. Prepared substrate was diluted to 100 μM in esterase assay media (150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 20 mM HEPES, 1 mM CaCl2, pH 5.0). Equal volume of prepared substrate (20 μL) was added per well of plasma prior to fluorescence read. Immediately following substrate addition, fluorescence was read every minute for 60 mins at 45°C using a plate reader (BioTek Synergy H2). Fluorescence was normalized to total protein, quantified by DC assay, according to manufacturer’s instructions (BioRad). Plasma sample were diluted 1:5 in RIPA buffer prior to DC assay.
Ca2+ ATPase activity assay
Ca2+ ATPase activity in the microsomal fraction was measured using a standard NADH enzyme-linked assay. Briefly, after a short perfusion with heparinized saline the left lateral lobe from rat liver was removed and homogenized in a buffer consisting of 10 mM HEPES (pH 7.8), 250 mM sucrose, 25 mM KCl, 1 mM EGTA and protease inhibitors (Sigma-Aldrich). After centrifugation at 1,000 x g for 10 min and 12,000 x g for 15 min at 4°C, the supernatant was further transferred to ultracentrifugation tubes and centrifuged at 100,000g for 1 h (protocol adapted from Sigma-Aldrich Endoplasmic Reticulum Isolation Kit). The pellet was resuspended in the homogenization buffer, and samples stored at −80°C until further analysis.
Microsomal protein concentration was determined with a DC assay (BioRad, Hercules, CA), and 50 μg protein was loaded onto UV-transparent 96-well plates (Corning, Corning, NY) in technical triplicates. Samples were incubated in a modified reaction buffer [21] (20 mM HEPES, 100 mM KCl, 5 mM MgCl2, 0.2 mM EGTA, 1mM CaCl2, 4 μM ionophore A23187, 0.6 mM phospho(enol)pyruvate, 0.27 mM NADH, 2.4 U/ml pyruvate kinase, 10 U/mL lactate dehydrogenase) at 37°C for 10 min, after which the baseline absorption at 340 nm was measured using Biotek Synergy II plate reader (Winooski, VT). Following addition of 1 mM ATP to the samples, absorbance was again read every 5 min. Serial dilutions of ADP served as a standard curve.
Results
GLuc-SERCaMP can be used to monitor ER calcium over time
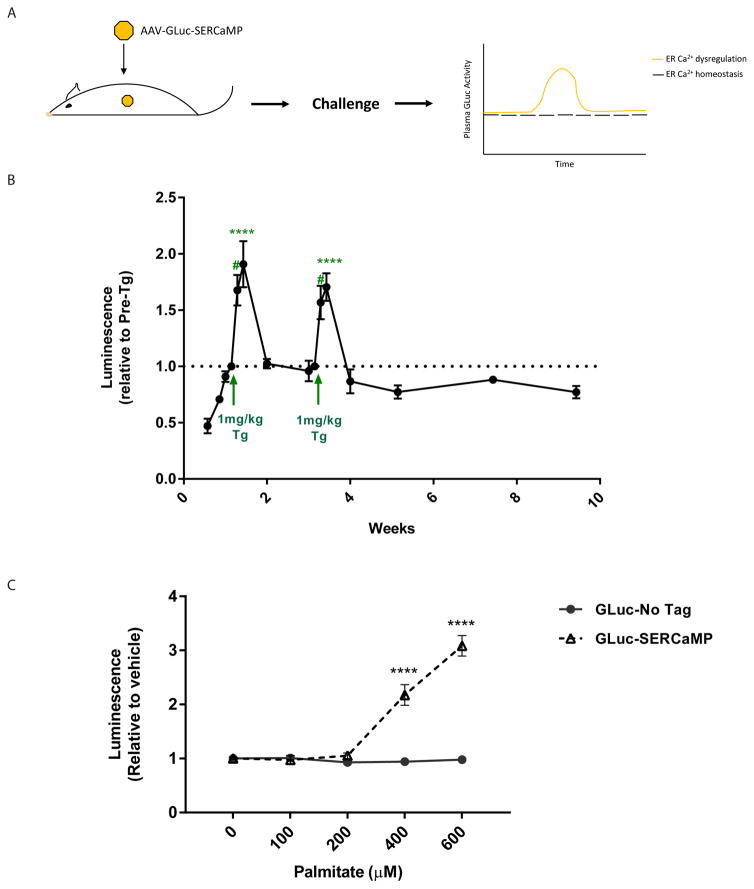
To determine the temporal extent to which our sensor could monitor ER calcium in vivo, rats expressing GLuc-SERCaMP (Figure 1A) were monitored over an extended period compared to previous in vivo experiments [16]. Luminescence in the plasma was consistently detected in the plasma for at least 9.5 weeks post- intrahepatic injections with transient increases due to thapsigargin-induced ER calcium depletion (Figure 1B). This data supports the use of GLuc-SERCaMP for monitoring ER calcium depletion in models of chronic diseases.
Fig. 1. GLuc-SERCaMP is responsive to ER calcium perturbations.
(A) Schematic of viral-mediated delivery of GLuc-SERCaMP to the liver and expected outcomes from challenges that alter ER calcium homeostasis in the liver. (B) Temporal profile of GLuc-SERCaMP activity in plasma over time with two thapsigargin injections (1mg/kg i.p.) (mean ± SEM, n=4 rats, *p<0.05, **p<0.01, 1-way ANOVA, Dunnett’s multiple comparison test). (C) SH-SY5Y-GLuc-SERCaMP or SH-SY5Y-GLuc-No Tag stable cell lines were treated with palmitate (0–600μM), and luminescence in the cell media was read 24hrs post-treatment (mean ± SEM, n=6 wells/treatment/cell line, ****p<0.0001, 2-way ANOVA, Tukey’s multiple comparison test, 0μM vs other concentrations).
Free fatty acid induces GLuc-SERCaMP response
Previous studies found that FFA cause a decrease in ER calcium [6, 13]. Using our GLuc-SERCaMP reporter cell line (SH-SY5Y-GLuc-SERCaMP) or a control cell line that constitutively secretes GLuc (SH-SY5Y-GLuc-No Tag) we tested the effect increasing concentrations of palmitate has on GLuc activity in cell culture media. Palmitate is the most prevalent circulating FFA in obese individuals and has been used in previous in vitro reports [22]. For the SH-SY5Y-GLuc-SERCaMP cells, we observed a dose-dependent increase in GLuc activity in the media after 24 hrs of treatment (Figure 1C). There was no change in GLuc activity in the media of the SH-SY5Y-GLuc-No Tag control cells suggesting the increase is not due to changes in overall secretion.
High fat diet induces GLuc-SERCaMP and endogenous SERCaMP response
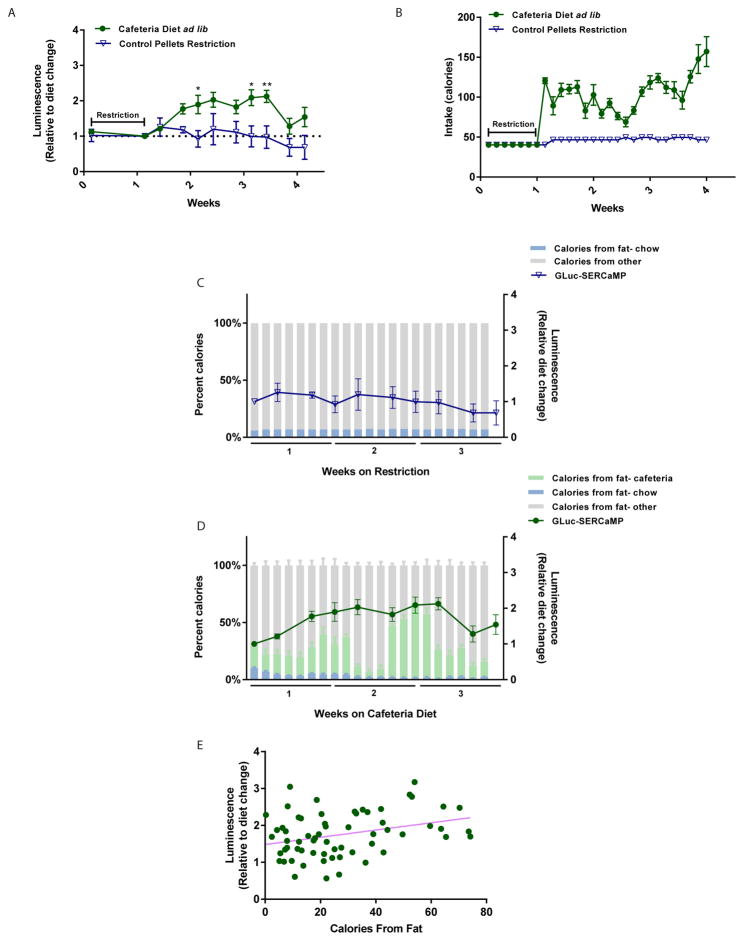
ER stress in the liver has been linked to excessive nutrient intake [22, 23], but little is known about the state of ER calcium during various feeding conditions. To determine if dietary intake influences ER calcium homeostasis in liver, rats were injected intrahepatically with an AAV vector expressing the GLuc-SERCaMP reporter (Figure 1A) as described previously [19] and allowed ad lib or restricted access to a cafeteria diet plus standard chow or standard chow only. The cafeteria diet is an established experimental paradigm for feeding animals a variety of energy-dense, palatable human food items [24]. Following AAV injections, a food restriction paradigm that entailed a 35% reduction in available food for 7 days was applied to ensure equivalent weights between test groups. Rats were sorted into feeding cohorts based on similar percent weight gain after 1 week of restriction, and blood samples were collected throughout the study to monitor plasma levels of GLuc-SERCaMP. Rats allowed ad lib access to cafeteria diet had significantly elevated plasma levels of GLuc-SERCaMP activity when compared to restricted chow (Figure 2A), indicating dietary intake can alter ER calcium homeostasis. As expected, those on the cafeteria diet consumed more calories than restricted chow (Figure 2B) and gained more weight (Supplemental Figure 1A). The cafeteria diet was composed of different menu groups with varying macronutrient composition, and, interestingly, the number of calories from fat paralleled GLuc-SERCaMP plasma levels in animals on cafeteria diet (Figure 2D). Similarly, GLuc-SERCaMP remained constant for animals on restricted diet where fat levels were kept constant (Figure 2C). Figure 2E shows a significant correlation (r= 0.3217, p= 0.0101, n= 6) between average fat calories from each menu group on the cafeteria diet and the level of GLuc-SERCaMP in plasma relative to start of diet. No significant correlation was observed for restricted chow rats (r= 0.1598, p= 0.3517, n= 4 rats) where the calories from fat remained relatively constant (92–94 calories per day). Collectively, these data suggest that high fat diet with increased caloric intake alter ER calcium homeostasis in liver.
Fig. 2. Cafeteria diet induces GLuc-SERCaMP response.
(A) Effect of dietary intake on plasma levels of GLuc-SERCaMP. Rats intrahepatically expressing GLuc-SERCaMP were allowed ad lib access to a cafeteria diet plus chow (green circles) or restricted access to standard chow (blue triangles). Rats allowed ab lib access to cafeteria diet plus chow showed increased release of GLuc-SERCaMP (mean ± SEM, n=7 rats/cafeteria group, n= 4 rats/restricted chow group, *p<0.05, **p<0.01, 2-way ANOVA, Sidak’s multiple comparison test). (B) Caloric intake of ad lib cafeteria diet plus chow versus restricted chow. (C, D) Fat intake tracks with changes in plasma GLuc-SERCaMP (percent of calories from fat calculated as percentage of total caloric intake based off nutritional labels). (E) Increased fat intake (calculated based on average per day on menu item) correlates with increase in GLuc-SERCaMP release in cafeteria diet-fed rats (Pearson’s correlation, r= 0.3217, p= 0.0101, n= 6 rats).
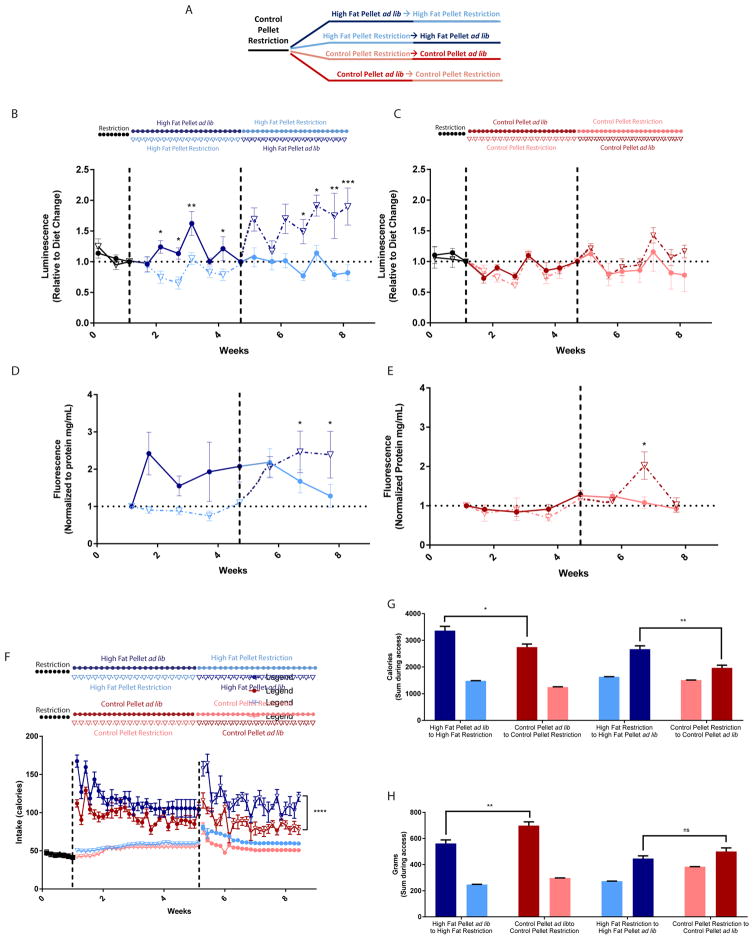
Although the cafeteria diet is a tangible model of human obesity [24], the nutritional variability among diet items makes it challenging to control fat intake. We switched to high fat pellets to address the effects of dietary fat intake on ER calcium homeostasis. Rats expressing GLuc-SERCaMP in liver were allowed ad lib or restricted access to high fat pellets or control pellets (nutritionally matched aside from fat content). Access to ad lib high fat pellets increased plasma levels of GLuc-SERCaMP and the endogenous esterase SERCaMP, an effect which could be reversed by switching rats to restricted access (Figure 3B, D). However, it should be noted that plasma levels of GLuc-No Tag also increased slightly in response to an ad lib high fat pellet diet, albeit not the extent of GLuc-SERCaMP (Supplemental Figure 3D). Access to control pellets appeared to have no effect on GLuc-SERCaMP, as shown by similar fluctuations among ad lib and restricted groups (Figure 3C). Endogenous SERCaMP levels in control pellet groups had the same trend as GLuc-SERCaMP levels, however, a transient but significant increase in endogenous SERCaMP activity was observed following the switch to ad lib access (Figure 3E). Caloric intake was significantly higher in ad lib high fat pellets when compared to ad lib control pellets (Figure 3F, G), despite the rats consuming less food mass (Figure 3H). Together, these data suggest that food content plays a larger role in ER calcium homeostasis than the amount of food.
Fig. 3. High fat pellets induce GLuc-SERCaMP and endogenous SERCaMP response.
(A) Schematic of experimental paradigm color coded to match subsequent panels. (B) Ad lib access to high fat pellets increases GLuc-SERCaMP release (mean ± SEM, n=6 rats/group, *p<0.05, **p<0.01, ***p<0.001, 2-way ANOVA, Sidak’s multiple comparison test). Color change indicates access change (dark blue= ad lib access, light blue= restricted access). Symbols and line pattern (closed circles and solid line versus open triangles and dotted line) represent same group of animals throughout experiment. (C) Control pellet intake has no effect on GLuc-SERCaMP release regardless of food access (mean ± SEM, n=6 rats/group, n.s. p=0.2760, 2-way ANOVA, Sidak’s multiple comparison test). Color change indicates access change (dark red= ad lib access, pink= restricted access). (D) Switch from restricted high fat pellet to ad lib access increases endogenous SERCaMP release (mean ± SEM, n=6 rats/group, *p<0.05, 1-way ANOVA, Dunnett’s multiple comparison test). (E) Switch from restricted control pellet to ad lib access increases endogenous SERCaMP release (mean ± SEM, n=6 rats/group, *p<0.05, 1-way ANOVA, Dunnett’s multiple comparison test). (F) Caloric intake over the course of the study in rats allowed ad lib and restricted access to high fat pellets and control pellets. Color, symbol, and line key same as previous panels. (G) Sum of caloric intake among feeding cohorts. Rats with ad lib access to high fat pellets consumed more calories than rats with ad lib access to control pellets (mean ± SEM, n=6 rats/group, *p=0.0127, **p=0.0016, unpaired t-test with Welch’s correction). (H) Rats with ad lib access to high fat pellets consumed less food than rats with ad lib access to control pellets (mean ± SEM, n=6 rats/group, **p=0.0066, n.s. p=0.1588, Unpaired t-test with Welch’s correction).
Liver function and SERCA activity is altered in high fat diet
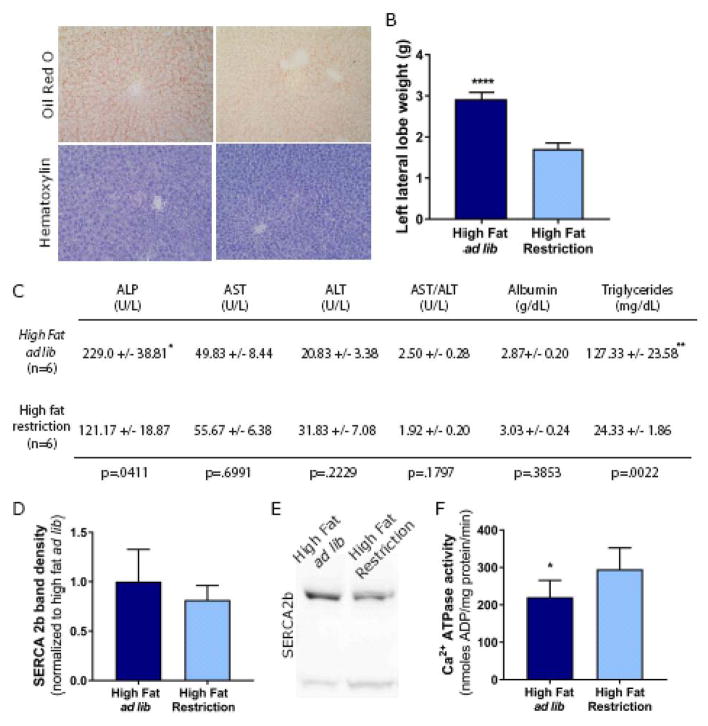
To investigate a potential mechanism contributing to ER calcium dysregulation, rats were allowed ad lib access or restricted access to high fat pellets for 3 weeks. Ad lib intake of high fat pellets increased the volume and mass of livers, caused liver discoloration and altered surface texture of the liver compared to restricted intake of high fat pellets (Figure 4A, B). Histopathological evidence from hematoxylin and Oil Red O staining suggests mild hepatic steatosis caused by the ad lib high fat pellets compared to restricted high fat pellets (Figure 4A). Additionally, liver panel analyses indicated elevated levels of triglycerides, ALP and the AST/ALT ratio in plasma of rats on ad lib high fat pellets versus restricted high fat pellets (Figure 4C). These indicators of hepatic steatosis corresponded to the increased SERCaMP levels in plasma of rats fed ad lib high fat pellets compared to their restricted counterparts (Figure 3B, D). In light of previous studies that have linked obesity to alterations in SERCA2b expression [4] and function [3], we hypothesized decreased SERCA2b expression contributes to SERCaMP release. On the contrary, expression remained relatively unchanged (Figure 4D, E). However, calcium ATPase activity, which is reflective of SERCA function was significantly decreased in liver microsomes isolated from rats with an ad lib high fat pellet diet compared to those on a restricted high fat pellet diet (Figure 4F).
Fig. 4. Ad lib access to high fat pellets alters liver morphology, function and calcium ATPase activity.
(A) Representative images of left lateral lobe harvested from rats allowed ad lib or restricted access to high fat pellets. (B) Weights of left lateral lobe harvested from rats allowed ad lib or restricted access to high fat pellets (mean ± SEM, n=6 rats/group, t-test, P<0.0001, t=13.52, df=10). (C) Table of peripheral markers of liver inflammation and damage (mean ± SEM, n=6 rats/group, t-test, *p<0.05, **p<0.01). (D, E) Western blot analysis of SERCA2b from liver microsomes (15 μg). No significant changes in the expression levels of SERCA 2b (mean ± SEM, n=6 rats/group, t-test, p=0.2375). (F) Ca2+ ATPase activity reflecting SERCA activity in rats allowed ad lib access to high fat pellets (mean ± SEM, n=6 rats/group, t-test, *p=0.0308).
Dantrolene attenuates GLuc-SERCaMP response in high fat diet
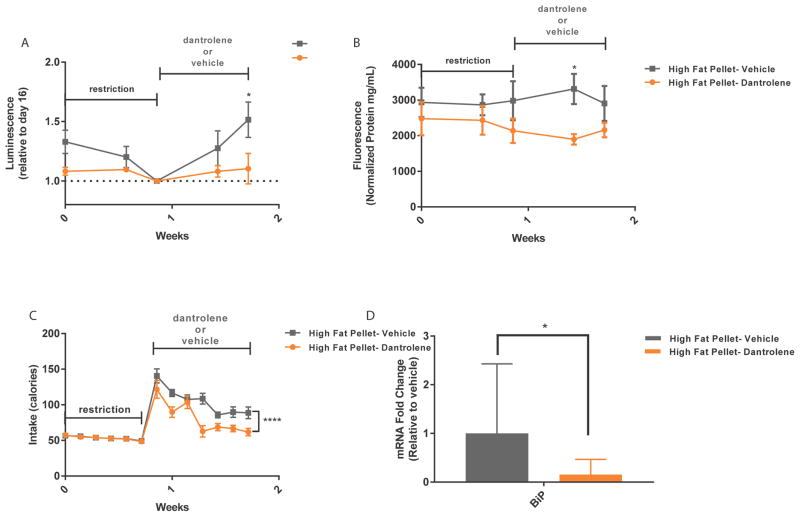
Dantrolene is a FDA-approved drug that stabilizes intracellular calcium by selectively antagonizing ryanodine receptors (RyRs). It has been vastly successful for the treatment of malignant hyperthermia, as evident by decreased mortality rates [25, 26]. Ryanodine receptors are located within the membrane of the ER and serve as one of the main release mechanisms for intracellular calcium [27]. We hypothesized that dantrolene administration during a high fat diet could stabilize ER calcium levels and reduce SERCaMP release. Following a food restriction period, rats were allowed ad lib access to high fat pellets and administered either vehicle or 15mg/kg (i.p.) dantrolene daily for 7 days. Dantrolene administration decreased plasma GLuc-SERCaMP activity and endogenous SERCaMP activity when compared to vehicle during ad lib high fat diet (Figure 5A, B). Interestingly, rats administered dantrolene gained less weight compared to vehicle treated rats (Supplemental Figure 1B), and had decreased caloric intake (Figure 5C). Excessive nutrient intake and ER stress have been extensively linked [3, 5, 28–32], with a high fat diet leading to increased expression levels of ER stress response genes, such as BiP [29]. Indeed, BiP mRNA was significantly decreased with the addition of dantrolene (Figure 5D), supporting the results from our SERCaMP assays indicating stabilization of ER calcium.
Fig. 5. Dantrolene attenuates GLuc-SERCaMP and endogenous SERCaMP.
(A, B) Dantrolene decreases GLuc-SERCaMP and endogenous SERCaMP release during ad lib access to high fat pellets. Rats were administered dantrolene (15mg/kg) or vehicle for 7 days during diet (mean ± SEM, n=6 rats/group, *p<0.05, 2-way ANOVA, Sidak’s multiple comparison test). (C) Decreased food consumption among dantrolene injected rats (mean ± SEM, n=6 rats/group, ****p<0.0001, 2-way ANOVA). (D) Dantrolene decreases BiP mRNA levels in liver tissue (data expressed as fold change (2^-ΔΔCt) relative to vehicle-treated rats (mean ± upper and lower limits (2ΔΔCt±SD), n=6 rats/group, multiple t-test, Holm-Sidak method (dantrolene versus vehicle) transformed from ΔCt±SD, *p=0.01).
Discussion
Our results demonstrate that dietary intake, particularly of high fat content can alter ER calcium homeostasis in rat liver. This was observed in two models of diet-induced obesity; cafeteria diet and high fat pellets. Animals with ad lib access to these diets rapidly gained weight and consumed more fat calories when compared to their control counterparts. Likewise, the significantly elevated plasma levels of GLuc-SERCaMP activity and endogenous SERCaMP activity and the compromised calcium ATPase activity suggest ER calcium homeostasis during these periods of high fat intake. Dantrolene attenuated GLuc-SERCaMP and endogenous SERCaMP in response to high fat diet, however, we cannot fully attribute this to its activity on the RyR, as evident by decreased food intake. While this is not the initial use of SERCaMP technology in vivo [16, 19], it is the first to demonstrate its use in an animal model of disease.
The seven C-terminal amino acids (ASARTDL) of mesencephalic astrocyte-derived neurotrophic factor (MANF) confer secretion in response to ER calcium depletion when appended to reporter proteins [16, 17]. MANF is an ER-localized protein that is secreted upon ER stress [33] and implicated as a marker and therapeutic of neurodegeneration [34–36]. Recently MANF’s potential as a disease biomarker has been investigated in both type I diabetes and kidney disease [37, 38]. Both found increased levels of MANF in serum and urine in disease-associated samples, respectively. We have recently identified other C-terminal tails of endogenous proteins (~80 proteins) within the human proteome with similar C-terminal sequences to MANF that also act as SERCaMPs in that they are localized to the ER and secreted in response to ER calcium depletion (Trychta et al, under review). One of the proteins identified with a SERCaMP tail is carboxylesterase 1 (CES1) which has a specific enzymatic activity for a previously described fluorescent substrate [20]. Using this substrate, we find an ER calcium dependent change in extracellular activity (Trychta et al, under review). Here, we show that the endogenous esterase SERCaMP activity in plasma tracks with the activity of our exogenously expressed GLuc-SERCaMP. Both are significantly increased in rats fed ad lib high fat pellets when compared to rats on a restricted control pellet diet (Figure 2D, Supplemental Figure 2A). Of the multiple endogenous SERCaMPs identified, many have enzymatic activities important for lipid metabolism and protein processing within the ER (Trychta et al, under review). Increased plasma levels of endogenous SERCaMP imply that the ER proteome is changing and losing key proteins necessary for ER function. In the liver where fat metabolism, drug metabolism and carbohydrate metabolism require proper ER function, the disruption of ER calcium homeostasis may contribute to dysfunction of the liver because of the subsequent secretion of necessary ER resident proteins. We observed a slight increase in the plasma of rats intrahepatically injected with a constitutively secreted, non-SERCaMP GLuc (GLuc -No Tag) reporter in response to ad lib high fat pellets (Supplemental Figure 3D). Although general secretion may be increased, the increase in GLuc-SERCaMP was greater. Taken with our previously published work showing GLuc-SERCaMP secretion is regulated by ER calcium levels, our data described herein suggest that a high fat diet can alter the ER proteome as a consequence of ER calcium depletion.
Although the results pertaining to SERCA2b protein expression appear inconsistent with previous work [4, 39], previous feeding paradigms where SERCA2b expression was altered in response to high fat diet ranged from 6–16 weeks [4, 39]. Our study, however, examined changes at 3 weeks on high fat diet. Despite unaltered protein expression (Figure 4D, E), calcium ATPase activity reflecting SERCA activity was significantly decreased in microsomes isolated from rats allowed ad lib access to a high fat diet compared to those on restricted high fat diet (Figure 4F). This data suggests altered ER calcium influx, because of dietary intake, may contribute to increased GLuc-SERCaMP release.
Hepatic ER function is altered in obese livers [3]. Genes associated with de novo lipogenesis and phospholipid synthesis are upregulated in livers of obese mice, while genes involved with ER-associated protein synthesis are downregulated [3]. Membrane lipid composition affects ER calcium transporters within the membrane [3, 32]. Notably, the ratio of phosphatidylcholine (PC) to phosphotidylethanolamine (PE) is increased in obese livers when compared to control [3]. Elevated levels of PC have been reported to hinder SERCA activity [3, 40, 41]. There is also growing evidence for changes in cholesterol composition of the ER membrane with UPR activation. Cholesterol is estimated to comprise 1% of the ER membrane; a minute amount when compared to the 60–80% found within the plasma membrane [42]. In cholesterol-enriched macrophages SERCA2b activity was inhibited, suggesting a role for cholesterol content in ER calcium homeostasis [40]. Moreover, palmitate treatment has been shown to alter lipid raft composition and distribution in a pancreatic cell line [43]. This disorganization within the membrane also altered PC:PE ratio [43]. Our in vitro data suggest that the addition of the free fatty acid, palmitate, can cause GLuc-SERCaMP release which is consistent with other reports showing palmitate can alter ER calcium homeostasis [6, 13, 14]. In vivo, we also observed changes in liver morphology and gross appearance, histopathology, triglyceride levels, ALP levels and AST/ALT ratio that were all consistent with mild hepatic steatosis suggesting the increased fat in the diet over a relatively short time course can cause dysregulation of ER calcium in the liver. It would be of great value, to further investigate whether the changes we observed with GLuc-SERCaMP and endogenous SERCaMP during a high fat diet correspond to changes in ER membrane biochemistry that can alter ER calcium. Towards this goal, we did observe a decrease in microsomal SERCA activity after 3 weeks of diet between ad lib and restricted access to high fat pellets, corresponding to the difference in SERCaMP (both GLuc and endogenous SERCaMP; Figure 3B, D). However, further studies are needed to understand the interplay of high fat content and ER calcium dysfunction.
Dantrolene’s specificity for RyRs and its current use for the treatment of malignant hyperthermia made it an obvious choice for the pharmacological manipulation of diet-induced ER calcium dysregulation. While we did observe a significant decrease in GLuc-SERCaMP, endogenous SERCaMP and BiP expression in animals on high fat diet that received systemic dantrolene administration, we cannot attribute these findings solely to its effect on RyR in liver ER. We observed a decrease in overall caloric consumption (Figure 5C) and less weight gain for dantrolene treated animals on ad lib high fat diet compared to the vehicle-treated controls (Supplemental Figure 1B). Minimal literature is available corroborating this finding, however dantrolene has been reported to prevent the contraction of masseter muscles, one of the major muscles involved in chewing, in rats [44]. What is clear however, is that consumption of fewer fat-related calories results in decreased SERCaMP release and BiP levels in liver. Another regulator of ER calcium homeostasis in the liver is the inositol-1,4,5-triphosphate (IP3) receptors (IP3Rs). Pharmacological manipulation of IP3Rs is an alternative approach to modulating ER calcium but has caveats for the current study. While IP3Rs are indeed expressed in hepatocytes, previous work has shown the IP3R selective antagonist, xestospongin C, to be ineffective for inhibiting IP3 calcium release [45] and we previously observed minimal attenuation of GLuc-SERCaMP release using xestospongin C [16]. An alternative to xestospongin such as heparin could be potentially life-threatening to the animal [45]. Future studies examining other ER calcium modulating drugs may further advance our understanding of ER calcium homeostasis and liver dysfunction associated with high fat diets or other liver-related diseases.
Collectively, our results demonstrate high fat consumption alters ER calcium homeostasis in the liver. Furthermore, our study supports existing literature regarding the effects of excessive nutrient intake on the ER using new methodologies.
Supplementary Material
Lay Summary.
ER calcium dysregulation was observed in rats fed a cafeteria diet or high fat pellets, with fluctuations in sensor release correlating with fat intake. Attenuation of sensor release, as well as food intake was observed during administration of dantrolene, a drug that stabilizes ER calcium. The study describes a novel technique for liver research and provides insight into cellular processes that may contribute to the pathogenesis of obesity and fatty liver disease.
Highlights.
A novel technique for monitoring ER calcium dysfunction in liver
High fat diet decreases lumenal endoplasmic reticulum calcium in the liver
Levels of GLuc-SERCaMP and SERCaMP esterase track with markers of hepatic steatosis
Calcium ATPase activity in the liver is decreased by high fat diet
Acknowledgments
This work was supported by the Intramural Research Program, National Institute on Drug Abuse.
We thank Dr. Mark Henderson (NCATS) for his technical support and critical reading of this manuscript. Dr. Donna Calu (University of Maryland, Baltimore) for advice on the cafeteria diet paradigm and Mr. Doug Howard (NIDA IRP) for virus production. This work was supported by the Intramural Research Program at the National Institute on Drug Abuse.
Footnotes
Authors’ contributions:
- Concept and design: ESW, BKH
- Experiments and Procedures: ESW, KAT, SB, AS, KCR
- Writing: ESW, SB, KAT, BKH
Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Authors in bold designate shared co-authorship.
- 1.WHO. Obesity and Overweight Fact Sheet. 2016 cited; Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Baiceanu A, Mesdom P, Lagouge M, Foufelle F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat Rev Endocrinol. 2016 doi: 10.1038/nrendo.2016.124. [DOI] [PubMed] [Google Scholar]
- 3.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SW, Zhou Y, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107:19320–19325. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu S, Nam SM, Kim JH, Das R, Choi SK, Nguyen TT, et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 2015;6:e1976. doi: 10.1038/cddis.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, Kleinridders A, et al. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem. 2010;285:6198–6207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arruda AP, Hotamisligil GS. Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes. Cell Metab. 2015;22:381–397. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashby MC, Tepikin AV. ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol. 2001;12:11–17. doi: 10.1006/scdb.2000.0212. [DOI] [PubMed] [Google Scholar]
- 12.Vangheluwe P, Louch WE, Ver Heyen M, Sipido K, Raeymaekers L, Wuytack F. Ca2+ transport ATPase isoforms SERCA2a and SERCA2b are targeted to the same sites in the murine heart. Cell Calcium. 2003;34:457–464. doi: 10.1016/s0143-4160(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y, Wang D, Gentile CL, Pagliassotti MJ. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem. 2009;331:31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metab. 2014;3:544–553. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson MJ, Wires ES, Trychta KA, Richie CT, Harvey BK. SERCaMP: a carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Mol Biol Cell. 2014;25:2828–2839. doi: 10.1091/mbc.E14-06-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson MJ, Richie CT, Airavaara M, Wang Y, Harvey BK. Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J Biol Chem. 2013;288:4209–4225. doi: 10.1074/jbc.M112.400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egnatchik RA, Leamy AK, Noguchi Y, Shiota M, Young JD. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism. 2014;63:283–295. doi: 10.1016/j.metabol.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson MJ, Wires ES, Trychta KA, Yan X, Harvey BK. Monitoring Endoplasmic Reticulum Calcium Homeostasis Using a Gaussia Luciferase SERCaMP. J Vis Exp. 2015 doi: 10.3791/53199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian L, Yang Y, Wysocki LM, Arnold AC, Hu A, Ravichandran B, et al. Selective esterase-ester pair for targeting small molecules with cellular specificity. Proc Natl Acad Sci U S A. 2012;109:4756–4761. doi: 10.1073/pnas.1111943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baez-Ruiz A, Cazares-Gomez K, Vazquez-Martinez O, Aguilar-Roblero R, Diaz-Munoz M. Diurnal and nutritional adjustments of intracellular Ca2+ release channels and Ca2+ ATPases associated with restricted feeding schedules in the rat liver. J Circadian Rhythms. 2013;11:8. doi: 10.1186/1740-3391-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism. 2016;65:1238–1246. doi: 10.1016/j.metabol.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larach MG, Brandom BW, Allen GC, Gronert GA, Lehman EB. Cardiac arrests and deaths associated with malignant hyperthermia in north america from 1987 to 2006: a report from the north american malignant hyperthermia registry of the malignant hyperthermia association of the United States. Anesthesiology. 2008;108:603–611. doi: 10.1097/ALN.0b013e318167aee2. [DOI] [PubMed] [Google Scholar]
- 26.Britt BA, Kalow W. Malignant hyperthermia: a statistical review. Can Anaesth Soc J. 1970;17:293–315. doi: 10.1007/BF03004694. [DOI] [PubMed] [Google Scholar]
- 27.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Apostolou A, Shen Y, Liang Y, Luo J, Fang S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res. 2008;314:2454–2467. doi: 10.1016/j.yexcr.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Airavaara M, Chiocco MJ, Howard DB, Zuchowski KL, Peranen J, Liu C, et al. Widespread cortical expression of MANF by AAV serotype 7: localization and protection against ischemic brain injury. Exp Neurol. 2010;225:104–113. doi: 10.1016/j.expneurol.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Airavaara M, Shen H, Kuo CC, Peranen J, Saarma M, Hoffer B, et al. Mesencephalic astrocytederived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J Comp Neurol. 2009;515:116–124. doi: 10.1002/cne.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voutilainen MH, Back S, Porsti E, Toppinen L, Lindgren L, Lindholm P, et al. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson's disease. J Neurosci. 2009;29:9651–9659. doi: 10.1523/JNEUROSCI.0833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y, Lee H, Manson SR, Lindahl M, Evans B, Miner JH, et al. Mesencephalic Astrocyte-Derived Neurotrophic Factor as a Urine Biomarker for Endoplasmic Reticulum Stress-Related Kidney Diseases. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2014100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galli E, Harkonen T, Sainio MT, Ustav M, Toots U, Urtti A, et al. Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci Rep. 2016;6:29058. doi: 10.1038/srep29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HW, Park H, Semple IA, Jang I, Ro SH, Kim M, et al. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun. 2014;5:4834. doi: 10.1038/ncomms5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M, et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 41.Cheng KH, Lepock JR, Hui SW, Yeagle PL. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. J Biol Chem. 1986;261:5081–5087. [PubMed] [Google Scholar]
- 42.Lange Y, Ye J, Rigney M, Steck TL. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J Lipid Res. 1999;40:2264–2270. [PubMed] [Google Scholar]
- 43.Boslem E, Weir JM, MacIntosh G, Sue N, Cantley J, Meikle PJ, et al. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J Biol Chem. 2013;288:26569–26582. doi: 10.1074/jbc.M113.489310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Willigen JD, Juch PJ, Ballintijn CM, Broekhuijsen ML. A hierarchy of neural control of mastication in the rat. Neuroscience. 1986;19:447–455. doi: 10.1016/0306-4522(86)90273-3. [DOI] [PubMed] [Google Scholar]
- 45.Saleem H, Tovey SC, Molinski TF, Taylor CW. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br J Pharmacol. 2014;171:3298–3312. doi: 10.1111/bph.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.