Abstract
Purpose:
This study aims to develop liposomal formulations containing synergistic antibiotics of colistin and ciprofloxacin for the treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa.
Methods:
Colistin (Col) and ciprofloxacin (Cip) were co-encapsulated in anionic liposomes by ammonium sulfate gradient. Particle size, encapsulation efficiency, in vitro drug release and in vitro antibiotic activities were evaluated.
Results:
The optimized liposomal formulation has uniform sizes of approximately 100 nm, with encapsulation efficiency of 67.0 % (for colistin) and 85.2 % (for ciprofloxacin). Incorporation of anionic lipid (DMPG) markedly increased encapsulation efficiency of colistin (from 5.4 % to 67.0 %); however, the encapsulation efficiency of ciprofloxacin was independent of DMPG ratio. Incorporation of colistin significantly accelerated the release of ciprofloxacin from the DMPG anionic liposomes. In vitro release of ciprofloxacin and colistin in the bovine serum for 2 hours were above 70 % and 50 %. The cytotoxicity study using A549 cells showed the liposomal formulation is as non-toxic as the drug solutions. Liposomal formulations of combinations had enhanced in vitro antimicrobial activities against multidrug resistant P. aeruginosa than the monotherapies.
Conclusions:
The action of liposomal formulations of two synergistic antibiotics was promising against multidrug resistant P. aeruginosa infections.
Keywords: Ciprofloxacin, Colistin, Liposomes, Cytotoxicity, In vitro release, Antimicrobial activities, Pseudomonas aeruginosa
INTRODUCTION
Respiratory infections caused by multidrug-resistant (MDR) Pseudomonas aeruginosa are serious healthcare challenges (1), which account for approximately 20 % of ventilator-associated pneumonias (VAP) with mortality rates up to 50 % (2). Limited therapeutic options have driven the revival of colistin (Col, also known as polymyxin E, Fig. 1a) as the last-line of defense against MDR Pseudomonas infections (3). Unfortunately, with the rapid increase in the use over the last decade, colistin does not escape from the resistance development (4, 5): monotherapy of colistin may result in emergence of resistant subpopulations (6–9). Conversely, dose-dependent nephrotoxicity limits the intravenous administration of high-dose colistin monotherapy (10, 11). Colistin-based combination therapy may be a viable alternative treatment against colistin-resistant bacteria or for the purpose of minimizing colistin resistance development (12). Some colistin combinations have shown synergistic antimicrobial activities with rifampicin (13), azithromicin (14), meropenem (15), doripenem (16), and ciprofloxacin (Cip) (17). More excitingly, the combination of nebulized colistin and oral ciprofloxacin has proven to successfully eradicate P. aeruginosa from the respiratory tracts of cystic fibrosis patients with no sign of colistin resistance (18).
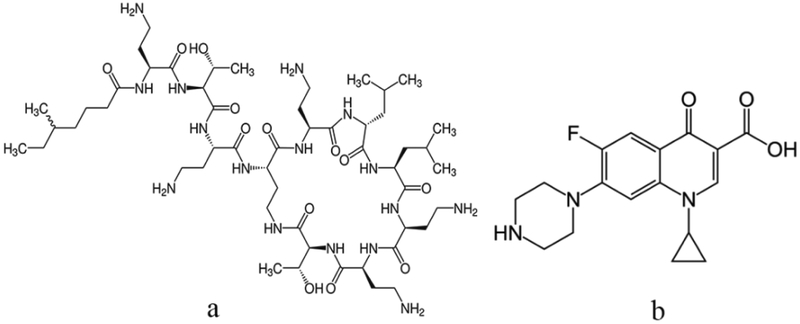
Figure 1.
Chemical structures of colistin (a) and ciprofloxacin (b).
The use of co-delivered combinations of colistin and ciprofloxacin is a promising approach for the treatment of MDR P. aeruginosa infections, particularly in pulmonary delivery for lung infections. The unique ability to encapsulate both lipophilic and hydrophilic compounds makes the liposome a promising carrier to co-deliver drugs (19). Liposomal formulations have distinct clinical advantages such as prolonged release of the drugs, resulting in a longer duration of action at the site of diseases with reduced frequency of administration, thus improving patient compliance and therapeutic effect (20). The advantage of the pulmonary route of administration is that, co-delivery liposomal formulations can reduce the irritation associated with the drug in the airways, mask the taste of bitter compounds, maximize the antimicrobial synergy by simultaneously delivery of two antibiotics to the infections sites and reduce lung clearance through nanometer particle size (21, 22).
As an amphipathic weak base (Fig.1b), ciprofloxacin can be encapsulated in liposomes through remote-loading method (23). Colistin had an amphipathic structure, carrying five positively charged amine residues and a hydrophobic acyl chain (Fig.1a). Although colistin exhibits high affinity with the phospholipid membrane (24), encapsulation efficiency (EE) of colistin in the neutral liposomes (NL) is low (25). Li et al. (26) found that through the electrostatic interaction with the anionic lipid, colistin can be effectively loaded in liposomes.
In this study, colistin and ciprofloxacin were co-loaded in anionic liposomes. The ciprofloxacin was loaded through the (NH4)2SO4 gradient and the colistin was passively loaded. Different methods for measuring the encapsulation efficiency (EE) of ciprofloxacin and colistin were evaluated. The effect of anionic lipid and ciprofloxacin concentration on the encapsulation efficiency of colistin was studied. The effect of co-existence of ciprofloxacin and colistin in liposomes on the release of each other was evaluated. The in vitro antimicrobial activities of the formulations against both colistin- and ciprofloxacin-resistant clinical isolates of P. aeruginosa H131300444 and P. aeruginosa H133880624 were assessed.
MATERIALS AND METHODS
Materials
Hydrogenated soybean phosphatidylcholine (HSPC), 1,2-Distearoyl-sn-glycero-3- phosphoglycerol, sodium salt (DSPG-Na), 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol, and sodium salt (DMPG-Na) (stored at −20 °C) were obtained from NOF America Corporation (White Plains, NY, USA). Cholesterol, Brij C20 and Tween 80 were purchased from Sigma (St. Louis, MO, USA). Ammonium sulfate was obtained from Alfa Aesar (Ward Hill, MA, USA). Colistin sulfate and ciprofloxacin hydrochloride monohydrate was purchased from Betapharma Co. Ltd. (Wujiang, Shangai, China). Dowerx® 50WX4, ion-exchange resin (200~400 mesh) was obtained from Acros Organics (Geel, Belgium). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Invitrogen (Life Technologies Corporation, Eugene, OR, USA). Penicillin streptomycin (10,000 U/mL penicillin, 10,000μg/mL streptomycin), F-12K nutrient mixture, fetal bovine serum (heat inactivated) and 0.5 % trypsin-EDTA were purchased from Gibco (Life Technologies Corporation, Eugene, OR, USA). All reagents and chemicals used for HPLC analysis were of HPLC grade.
Preparation of drug loaded liposomes
HSPC, DMPG/DSPG and cholesterol (phospholipids: cholesterol = 3:1, w/w) were weighed into a 200 mL evaporating flask and dissolved in chloroform. The solvent was evaporated at reduced pressure to form a dry lipid film. The film was hydrated in 300 mM (NH4)2SO4 solution containing colistin (4 mg/mL) and 100 mg/mL sucrose at 65 °C. The resulting suspension of multilamellar liposomes (phospholipids concentration: 5 % w/v) were then sonicated with an ultrasonic probe (Sonifier 250, Branson Ultrasonics Corporation, Danbury, CT, USA) and extruded through a polycarbonate membrane with 200 nm pore size to yield colistin-loaded unilamellar liposomes (Col-LUVs). A trans-membrane NH4+gradient across the bilayer of Col-LUVs was generated after removing the extra liposomal NH4+ by passing the liposome suspension through a Dowerx® 50WX4 cation-exchange spin column (packing resin in a 5 ml plastic syringe lined with glass wool at the bottom of the barrel, pre-equilibrated with 100 mg/mL sucrose aqueous solution). The Col-LUVs suspension was mixed with the solution of ciprofloxacin (4 mg/mL), the mixture was incubated at 60 °C for 10 min for drug loading. The solvent used to create a solution of ciprofloxacin is the 100 mg/mL sucrose solution.
A modified liposome preparation procedure was also applied. The new procedure is the same as above except for the time to add colistin. The film was hydrated in 300 mM (NH4)2SO4 solution containing 100 mg/mL sucrose and then established the NH4+gradient through the cation-exchange resin. The ciprofloxacin and colistin dissolved by the 100 mg/mL sucrose solution and the concentrations of ciprofloxacin and colistin were all 4 mg/mL. The mixture drugs solution mixed with the LUVs suspension at 60 °C for 10 min for drug loading.
The preparation procedure of ciprofloxacin liposomes containing Tween 80 or Brij C20 was similar with the modified procedure. Tween 80 or Brij C20 first mixed with lipid materials and then formed a lipid film. The following procedures were same as the above.
Particle size and zeta potential measurement
The particle size and zeta potential of the liposomes were determined using a Malvern Zetasizer Nano ZS90 (Malvern Instruments Inc., Malvern, UK). Liposomes were dispersed in deionized water for particle size measurement and in phosphate buffer pH7.4 (5mM) for zeta potential measurement. Three samples of each formulation were tested, and the data were reported as mean ± SD of the triplicate measurements.
Cryogenic transmission electron microscopy (Cryo-TEM)
The morphology of the liposomes was obtained using a cryo-TEM (27). Each dilute suspension of liposomes (3 μL) was pipetted on carbon coated copper grid and then blotted with filter paper to obtain a thin aqueous film. The grid was vitrified immediately by plunging the grid (kept at 100 % humidity and 22 °C) into liquid ethane maintained at its melting point using a Vitrobot (FEI Company, Hillsboro, OR, USA). The vitreous films were transferred to a Talos TEM (FEI Company, Hillsboro, OR, USA) using a Gatan cryotransfer (Gatan, Pleasanton, CA, USA), and the samples were imaged in a low-dose mode. Images were acquired at 200 kV at a temperature around −173 °C with a complementary metal-oxide semiconductorcamera (FEI Company, Hillsboro, OR, USA).
Assay of ciprofloxacin and colistin
The HPLC system consisting of G1311C pump (1260 Quat Pump VL), G1330B thermostate (1290 Thermostate), G1329B autosampler (1260 ALS), G1316A thermostated column compartment (1260 TCC), and G1314F variable wavelength detector (1260 VWD) (Agilant, Waldbronn, Germany) was used for the analysis of ciprofloxacin and colistin. The chromatographic separation was performed using an Agilant Eclipse Plus C18 column (150 mm × 4.6 mm, 5 μm, Agilant, Waldbronn, Germany). The isocratic mobile phase was a mixture of 30mM solution of sodium sulfate (adjusted to pH 2.5 with H3PO4, 76% v/v) and acetonitrile (24% v/v). Sample was eluted at a flow rate of 1.0 mL/min, and the wavelength was set at 215 nm for detection of both drugs. The elution time for ciprofloxacin was ~2 min and for that of colistin was ~3.2 and 5.2 min corresponding to colistin A and colistin B, the two main components of colistin. Peak area for colistin A and colistin B was summed for quantification
Encapsulation efficiency
For determination of EE, liposome suspension was diluted 2-fold with 10 % sucrose aqueous solution; the free drugs and the drugs in the liposome were separated using two different methods as described below.
Method I—Centrifugal filtration (28).
An aliquot of liposome suspension (0.1 mL, drawn from total 5 mL liposome suspension) was directly diluted 10-fold with 70 % methanol (containing 0.02 mol/L HCl) and analyzed by HPLC to determine the total drug content, Mtotal.
Another aliquot of liposome suspension (0.5 mL, drawn from total 5 mL liposome suspension) was transferred to an Amicon® Ultra centrifugation filter (MWCO: 50K Da, Merck Millipore Ltd, Tullagreen, Carrigtwohill, Ireland), and centrifuged at 5000 rpm for 30 min using a MX-200 Centrifuge (Tomy Kogyo Co. LTD, Tokyo, Japan). The unencapsulated free drug was recovered in the filtrate, and the free drug (0.1 mL) was diluted 10-fold with 70 % methanol (containing 0.02 mol/L HCl) and analyzed by HPLC to determine the free drug content, Mfree.
The encapsulation efficiency, EE (%), was determined by, EE (%) = (Mtotal -Mfree)/Mtotal × 100%.
Method II—Cation-exchange resin mini-column centrifugation (29).
The mini-column was prepared by packing Dowex® 50WX4 cation-exchange resin in a 3 ml plastic syringe filled with glass wool at the bottom of the syringe. The column was placed in a test tube and spun at 3000 rpm for 3 min to remove excess buffer. The liposome suspension was diluted 2-fold with 10 % sucrose aqueous solution, and 0.1 mL of the diluted suspension was applied to the top of the column. The column was spun at 3000 rpm for 3 min, and 0.1 mL of 10 % sucrose aqueous solution was added on top of the column, the column was then centrifuged for another 3 min. The elute was collected and diluted 10-fold with 70 % methanol (containing 0.02 mol/L HCl), and the content of drug encapsulated in liposomes, Mlip, was assayed by HPLC. The total drug content, Mtotal, was separately determined with the same procedure as described in Method I. The encapsulation efficiency, EE (%), was determined by, EE (%) = Mlip/Mtotal × 100%.
In vitro drug release
Phosphate buffered saline (PBS pH 7.4) and the mixed solution of PBS and bovine serum (1:1 v/v) (28) were selected as the in vitro release media. An aliquot of 2 mL liposome suspension containing approximately 2 mg/mL ciprofloxacin and/or 2 mg/mL colistin was mixed with 8 mL of PBS or mixed solution at 0~4 °C and then incubated in water bath at 37 ◦C and stirred gently (50 rpm). Each sample (0.2 mL) was withdrawn for the determination of the total drug content at each time point, and another 0.5 mL of sample was transferred to Amicon® Ultra centrifugation filters (MWCO: 50K Da) for determining the free drug content according to Method I in Encapsulation efficiency. Three replicates of each sample were evaluated at each time point of 5, 30, 60, 120, and 240 min.
Cytotoxicity study
A549 human epithelial alveolar cell line was obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA). The cells were grown in F-12K medium supplemented with 10 % heat-inactivated fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin (ATCC recommended). Cells were cultured at 37 °C in a 5 % CO2 and 95 % humidity atmosphere (Fisher brand Isotemp CO2 Incubator, Thermo Fisher Scientific Inc, Hanover Park, IL, USA). The cell viability potential of free drug, blank liposome and drug-loaded liposome were performed by the MTT assay (30). Briefly, the cells were seeded into 96-well plated at a density of 1×104 cells/well and cultured for 24 hrs. Then, the free drug (ciprofloxacin/colistin, 2 mg/ 2 mg, 1 mL) which was dissolved in 10 % sucrose solution and drug-loaded liposome were diluted with culture medium to obtain a series of drug solution, i.e. 20/20, 10/10, 5/5 and 2.5/2.5 μg/mL. For the blank liposomes, the lipid concentration were kept as the same to that of corresponding drug-loaded liposome (i.e. 36, 18, 9 and 4.5 μg/mL). The culture medium and 10% sucrose were added as the positive control and the cells were incubated for 24 hrs. At the end of incubation, the culture medium containing drugs was aspirated out of the 96 well plates and serum free medium and MTT solution (5 mg/mL dissolved in PBS) were added. The incubation time was 4 hrs. The medium was removed, the formazan crystals were dissolved by the addition of DMSO and the absorbance was measured at 570 nm using a microplate absorbance reader (BioTek, Bio Tek Instruments, Inc., Winooski, VT, USA).
Minimum inhibitory concentration
Clinical isolates of P. aeruginosa H131300444 and P. aeruginosa H133880624 were employed for MIC and time-kill studies. MICs of colistin and ciprofloxacin solutions were determined the broth microdilution method according to Clinical and Laboratory Standards Institute guidelines (31). Briefly, an aliquot of an overnight culture was diluted in cation-adjusted Mueller-Hinton broth (Oxoid, Hampshire, England) giving an approximately 106 cfu/mL as the inocula. Drug concentrations of 0, 0.125, 0.25, 0.5, 1, 2, and 4 mg/L were achieved by diluting the stock drug solution (5.12 mg/mL) with CAMHB. Microplates were incubated at 35 °C in a humidified incubator for approximately 20 h. MICs were determined as the lowest concentration without visible bacterial growth.
In vitro static time-kill experiment
Static time-kill studies were conducted to examine the antimicrobial activity of colistin-ciprofloxacin liposomal formulation using cation adjusted Mueller Hinton broth (CAMHB; Mg2+ at 12.2 mg/L and Ca2+ at 23.0 mg/L [Oxoid, Hampshire, England]). All experiments were performed with an initial inoculum of ~106 CFU/mL in 20 mL of CAMHB in 50 mL pyrogen-free and sterile polypropylene tubes. Based on our preliminary data, drug concentrations of 4 mg/L and 8 mg/L were tested for both solutions and liposomal formulations. Drug concentrations were made by diluting the liposomal formulation (colistin: ciprofloxacin = 2 mg/mL:2 mg/L) with the broth. Samples (50 µL) were collected at 0, 1, 3, 6 and 24 hrs, centrifuged at 12000 × g for 10 min and cell pellets were resuspended in 0.9% saline and diluted accordingly for viable counting on nutrient agar plates. The limit of detection was 20 CFU/mL (equivalent to one colony per plate). A ProtoCOL automated colony counter (Synbiosis, Cambridge, United Kingdom) was used to quantify bacteria after 24 hrs of incubation at 37 °C.
RESULTS
Characteristics of ciprofloxacin/colistin Liposomes
EE and particle size distribution (z-average size and polydispersity index (PDI)) of the liposomes are shown in Table 1. The EE determined by these two different methods was similar for ciprofloxacin in the combination formulations. No difference was observed in EE of ciprofloxacin between neutral liposomes and those anionic liposomes. However, the EE of colistin in the neutral liposomes was much lower than that in the anionic liposomes. Meanwhile, the EE of colistin determined by the Method II (Cation-exchange resin mini-column centrifugation) was much lower than that determined by the Method I (Centrifugal filtration); and it was even found to be zero for the neutral liposomes as determined by the Method II.
Table 1.
Characterization of Liposomes containing 2 mg/mL of colistin and/or ciprofloxacin (n=3, mean ± SD).
| Average diameter (nm) | EE of Col (%) | EE of Cip (%) | ||||
|---|---|---|---|---|---|---|
| PDI | Method I | Method II | Method I | Method II | ||
| Cip/Col-NL | 107±3 | 0.149±0.040 | 5.4±1.5 | 0 | 86.4±3.2 | 89.3±5.5 |
| Cip/Col-AL | 107±2 | 0.164±0.049 | 67.0±5.2 | 18.4±7.2 | 89.6±2.7 | 83.4±4.2 |
| Cip-AL | 110±3 | 0.163±0.021 | - | - | 85.6±2.8 | - |
| Col-AL | 103±2 | 0.202±0.038 | 68.8±4.4 | - | - | - |
| Blank-AL | 107±4 | 0.047±0.032 | - | - | - | - |
Neutral liposomes (NL): DMPG: HSPC= 0:5, w/w; Anionic liposomes (AL): DMPG: HSPC= 2:3, w/w
For the anionic liposomes, incorporation of colistin in the formulation led to an statistically insignificant increase in the EE of ciprofloxacin slightly from 85.6 % to 89.6 %; while the existence of ciprofloxacin in the formulation at the concentration of 2 mg/mL did not affect the EE of colistin in the anionic liposomes (DMPG: HSPC= 2:3, w/w). Additionally, the encapsulation of ciprofloxacin and/or colistin did not change the size of the liposomes, with the average particle sizes being approximately 100 nm.
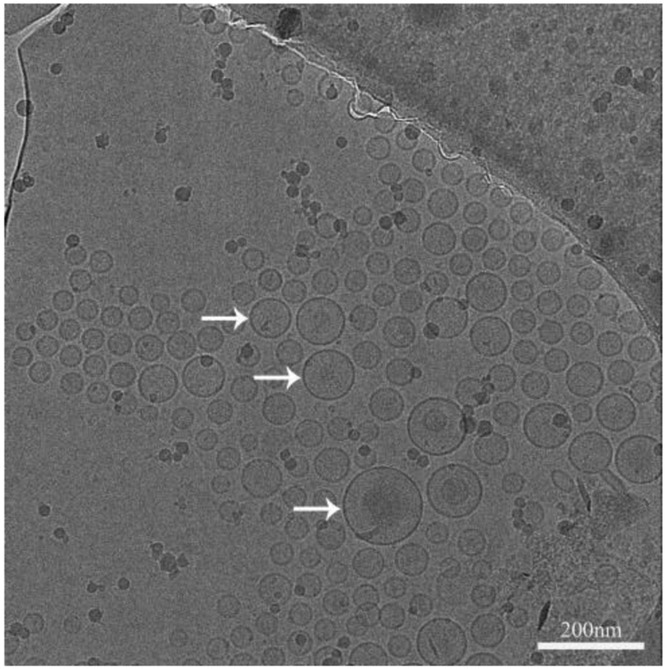
The representative morphology of Cip/Col anionic liposomes was shown in Fig. 2. The anionic liposomes had a spherical shape with a distinct lipid membrane (indicated by the arrows) showing average particle sizes around 100 nm, which was in agreement with the Zetasizer measurements.
Figure 2.
The cryo-TEM image of Cip/Col anionic liposomes. Scale bar represents 200 nm. Arrows indicate typical liposomes.
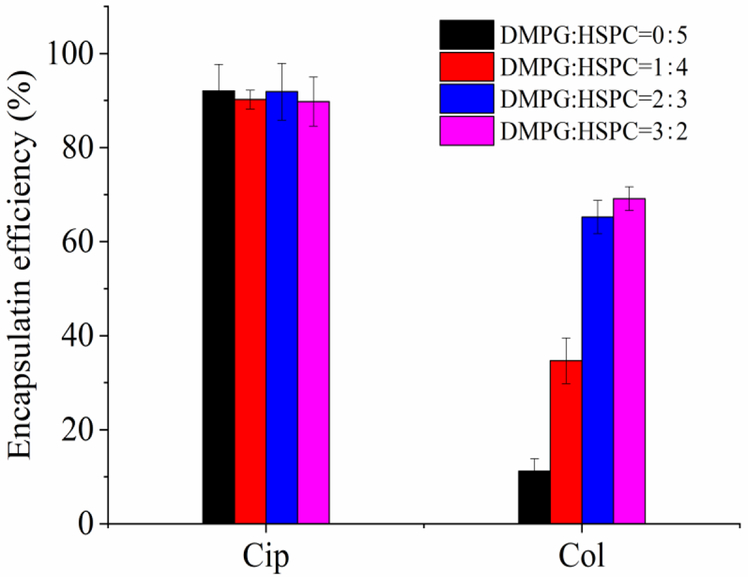
Influence of the DMPG/HSPC ratio on the EE of ciprofloxacin and colistin
As shown in Fig. 3, the EE of ciprofloxacin was independent to the DMPG/HSPC ratio; on the contrary, the EE of colistin was greatly impacted by the content of DMPG. The EE of colistin increased significantly from about 5.4 % to 67.0 % with the increase of DMPG/HSPG ratio from 0:5 to 2:3 (p < 0.05). But when the DMPG/HSPG ratio was 3:2, the encapsulation efficiency (69.1 ± 2.6 %) did not further increase, as compared to that of DMPG/HSPG ratio of 2:3 (p > 0.1). Therefore, the DMPG/HSPG ratio of 2:3 was chosen for the following studies.
Figure 3.
Encapsulation efficiency of ciprofloxacin and colistin in liposomes with various DMPG/HSPC ratios. The EE values were determined by Method I (Centrifugal filtration).
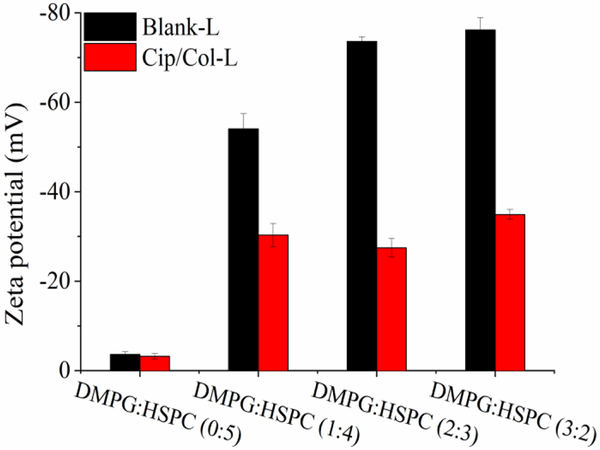
Interaction of colistin with liposomes
From Fig. 3, the EE of colistin is dependent on the concentration of DMPG in the liposomes, indicating significant interactions between colistin and DMPG. In this study, zeta potential was used to characterize the interactions of colistin with DMPG. Fig. 4 illustrated the zeta potential of blank liposomes without DMPG was −4 mV, and the incorporation of ciprofloxacin and colistin did not significantly change the zeta potential. Incorporation of negatively charged lipid, DMPG, to the blank liposomes decreased the zeta potential profoundly to around −56 mV (DMPG/HSPC=1:4), −74 mV (DMPG/HSPC=2:3), and −76 mV (DMPG/HSPC=3:2). Interestingly, after addition of ciprofloxacin and colistin, the potential of all anionic liposomes decreased to approximately −30 mV, regardless the ratio of DMPG to HSPC.
Figure 4.
Zeta potential of liposomes with various DMPG/HSPC ratios (n=3, mean ± SD).
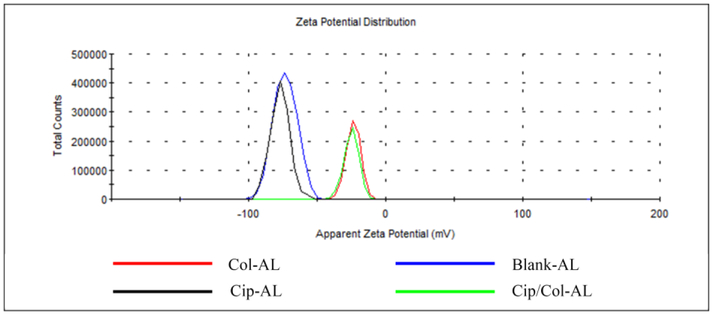
To verify the interaction of negatively charged liposomes with colistin, the zeta potential of anionic liposomes (DMPG/HSPC=2:3) encapsulated with colistin and/or ciprofloxacin was measured separately. In Fig. 5, ciprofloxacin liposomes had the similar zeta potential to the blank liposomes; while the zeta potential of the colistin liposomes and Cip/Col liposomes shifted from −74 mV (for the blank liposomes) to −26 mV for both formulations. This result was consistent with the encapsulation results (Fig. 3) indicating that electrostatic interaction of colistin with liposomes containing DMPG played a pivotal role in the encapsulation of colistin.
Figure 5.
Zeta potential distribution of ciprofloxacin and/or colistin liposomes (DMPG: HSPC = 2:3).
Influence of ciprofloxacin concentration on the encapsulation of ciprofloxacin and colistin
One of the most important advantages of remote-loading was that drugs can be encapsulated into liposomes with very little loss even at a high drug/lipid ratio (22). In this study, with the increase of ciprofloxacin concentrations from 2 mg/mL to 12 mg/mL (the ciprofloxacin/lipid ratios increased from 0.17 to 1.02), no change of the EE for ciprofloxacin was observed. The EE of colistin retained above 60 % with the concentration of ciprofloxacin from 2 mg/mL to 8 mg/mL; while it sharply decreased to 45.9 % when the ciprofloxacin concentration in liposomes increased to 12 mg/mL.
In vitro drug release
Drug release from liposomes containing ciprofloxacin and colistin each at the concentration of 2 mg/mL, was measured at 37 °C in PBS (pH7.4) to evaluate whether the presence of both drugs in liposomes resulted in dependent release.
In vitro release of ciprofloxacin in PBS
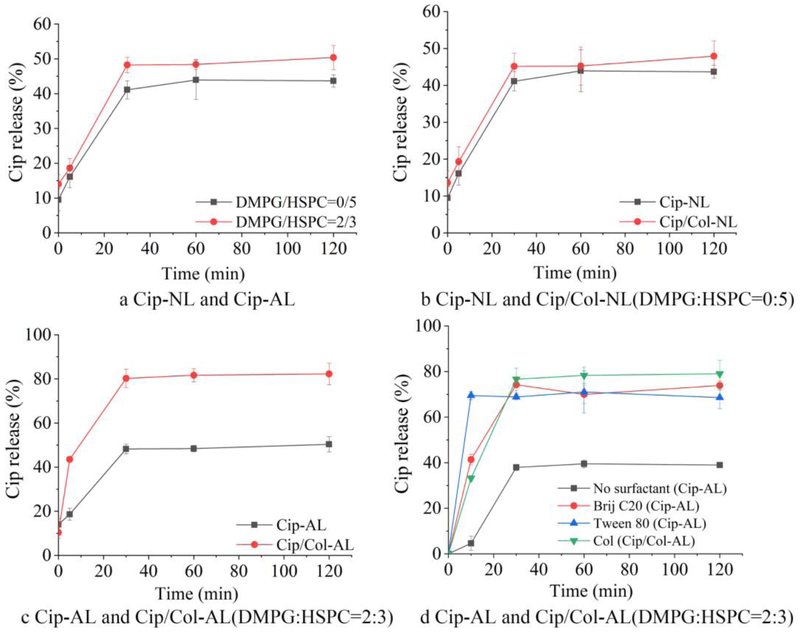
The effect of DMPG, colistin and surfactants on the release of ciprofloxacin in liposomes are shown in Fig. 6. In Fig. 6a, it can be seen that the release of ciprofloxacin from the anionic liposomes was similar to that from the neutral liposomes. Rapid release was observed within the first 30 min, and then plateaued at approximately 50% release.
Figure 6.
In vitro ciprofloxacin release profiles of ciprofloxacin and ciprofloxacin/colistin loaded liposomes (n=3, mean ± SD).
Wallace et al. (32) reported that colistin could accelerate the release of co-formulated azithromycin from liposomes. For the neutral liposomes, only 5 % colistin was encapsulated in liposomes (Fig. 3), which had no obvious influence on the release of ciprofloxacin (Fig. 6b). Colistin significantly increased the release of ciprofloxacin from anionic liposomes as shown in Fig. 6c, which may be due to the colistin encapsulation efficiency of ~65%. Compared to the Cip-AL that drug release plateaued at approximately 50%, more than 40 % of ciprofloxacin was released from Cip/Col-AL at the 5 min time point, and the drug release plateaued at 80 % from around 30 min.
Fig. 6c clearly shows that incorporation of colistin accelerated the release of ciprofloxacin from the anionic liposomes. Cipolla et al. reported that surfactants can modulate the release of ciprofloxacin from liposomes (33). As colistin had an amphipathic surfactant-like structure, we hypothesized that colistin acts as surfactants in liposomes, and compared the effects of colistin and the surfactants on the release behavior of the liposomes. In this study, Tween 80 and Brij C20, with the concentration of 0.2 % (g/mL), were investigated (Fig. 6d). For the liposomes with Tween 80, an enormously burst release was observed with 75 % of ciprofloxacin being released from the liposomes within 5 min. Conversely, the release profile of the liposomes with Brij C20 was similar to the Cip/Col-AL.
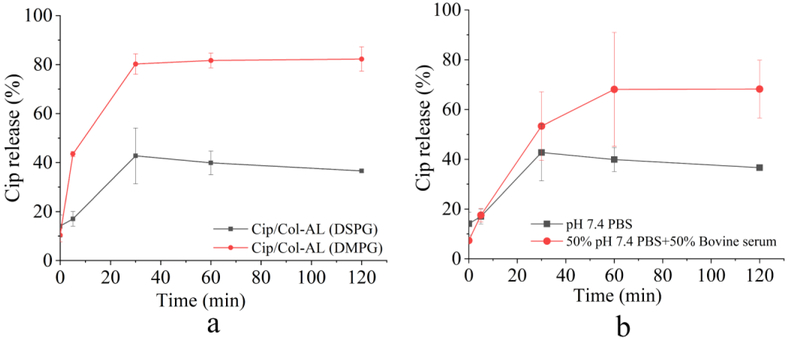
Alinaghi et al. reported that the use of phospholipid with a higher transition temperature created a more rigid membrane bilayer and decreased the leakage rate of liposomes (34). Therefore, DMPG was replaced with DSPG that has a higher transition temperature, in order to decrease the release rate of ciprofloxacin. The transition temperatures of DMPG and DSPG are 23 °C and 55 °C, respectively. As shown in Fig. 7a, the release of ciprofloxacin from the Cip/Col-AL containing DSPG was less than 20 % at the 5 min time point and eventually plateaus in a 30–120 min time frame at a value approximately 40 %, which was much slower compared to those made of DMPG.
Figure 7.
The ciprofloxacin release profiles of Cip/Col-AL containing different phospholipids in (a) PBS and (b) the ciprofloxacin release profiles of Cip/Col-AL containing DSPG in different release media (n=3, mean ± SD).
In vitro release of ciprofloxacin in bovine serum
In order to make a closer dissolution environment to the in vivo conditions (28), the release profiles of ciprofloxacin in the bovine serum were also determined. As shown in Fig. 7b, ciprofloxacin was released gradually in the bovine serum and reached the plateaus in 60 min at a value above 70 %, which is much higher than those in PBS. These results demonstrated our liposomes containing DSPG could sustain the release of ciprofloxacin. Therefore, the DSPG was chosen for further investigations.
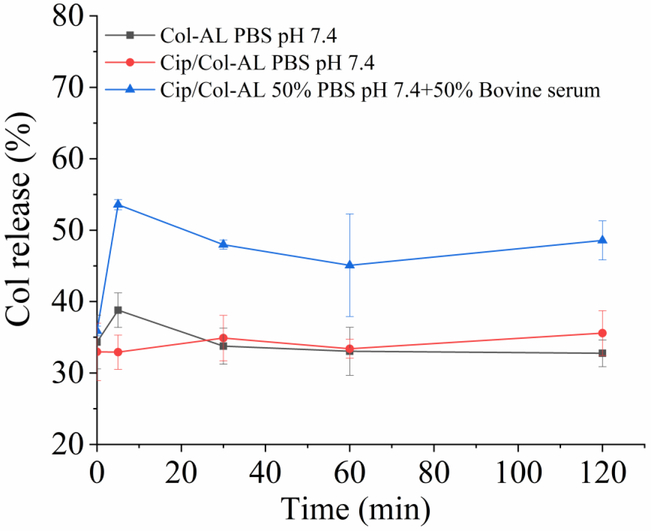
In vitro release of colistin
Fig. 8 shows the in vitro release of colistin from anionic liposomes (DSPG/HSPC=2/3) with and without ciprofloxacin in the different release media. There was ~35% unencapsulated colistin measured throughout the sample period of 120 min when the release medium was PBS (pH 7.4). However, the release of colistin increased two folds when 50 % w/w bovine serum was added into the PBS.
Figure 8.
In vitro release profiles of colistin in the Col-AL or Cip/Col-AL in different release media (DSPG/HSPC=2:3) (n=3, mean ± SD).
Cytotoxicity study
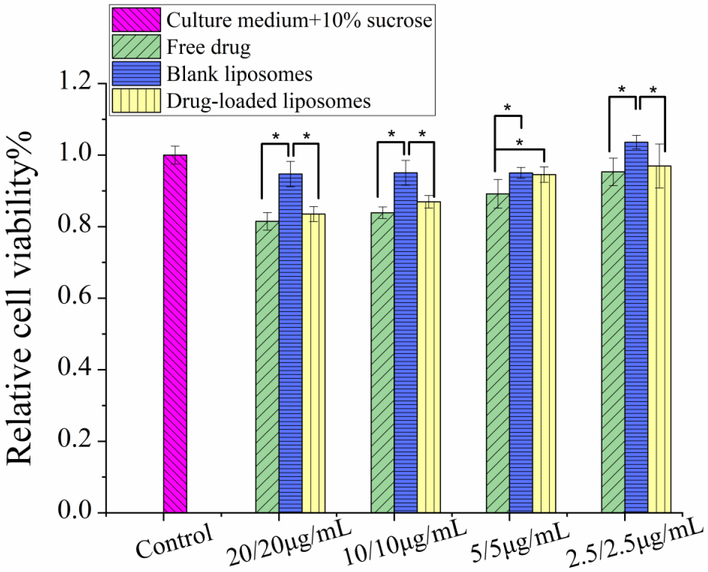
The cell viability of liposome formulation is shown in Fig. 9. For the blank liposomes, there is almost no inhibitory effect on the cell proliferation showing no cytotoxicity. Therefore, the lipid materials used in the liposome formulation is safe for use in pulmonary drug delivery. The drug-loaded liposomes have comparable relative cell viability with the free drugs and blank liposomes at 5 μg/mL or lower, suggesting non-apparent cytotoxicity of the liposomal formulations to the human airway epithelial cells.
Figure 9.
Cytotoxicity of free drugs, blank liposomes and Cip/Col-AL liposomes (DSPG/HSPC=2: 3) in A549 human airway cells (n = 3, mean ± SD).
Notes: One-way ANOVA, followed by Dunnett’s multiple comparison test, was performed to calculate statistical significance. *: significant difference, P < 0.05.
In vitro antimicrobial activities
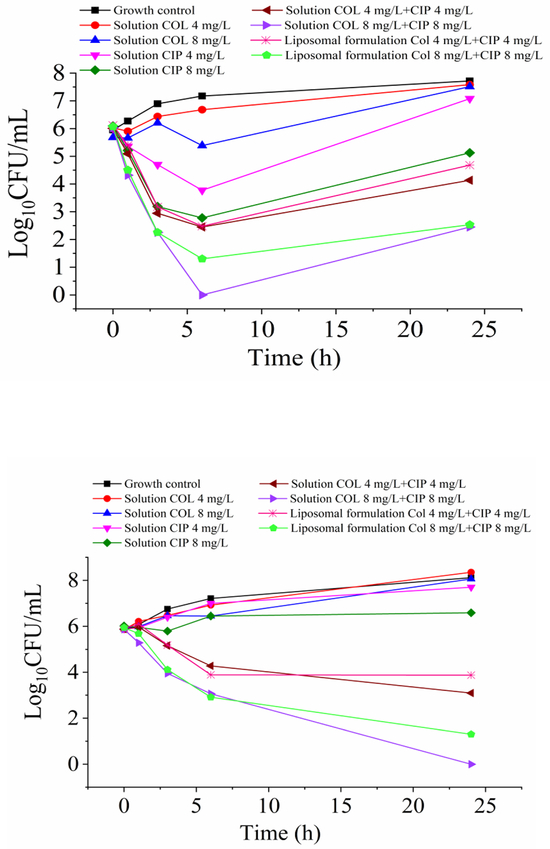
MICs of colistin and ciprofloxacin are summarized in Table 3. The clinical isolates studied were resistant to both colistin and ciprofloxacin. The static time-kill kinetics of colistin and ciprofloxacin solution and liposomal formulation are shown in Fig. 10. Colistin (8 mg/L) and ciprofloxacin (4 and 8 mg/L) monotherapy achieved moderate bactericidal activity against P. aeruginosa H131300444, but with rapid regrowth. For P. aeruginosa H133880624, no killing was observed following colistin solution (4 and 8 mg/L) and ciprofloxacin solution (4 and 8 mg/L) monotherapies. However, combination solutions of colistin and ciprofloxacin resulted in extensive enhanced bacterial killing. More prominent killing was initially observed for P. aeruginosa H131300444 than P. aeruginosa H133880624 following treatments with the colistin-ciprofloxacin combinational solutions. For P. aeruginosa H133880624, a total eradication of bacteria was achieved with the combination solution of colistin 8 mg/L + ciprofloxacin 8 mg/L. Interestingly, no major difference was observed in the time-kill kinetics between the colistin-ciprofloxacin combinational solutions and liposomal combinations, showing the antimicrobial activities are preserved for the liposomal formulations.
Table 3.
MICs of colistin and ciprofloxacin for MDR P. aeruginosa isolates examined in this study.
| Strain | Colistin Susceptibility |
Colistin MIC (mg/L) |
Ciprofloxacin Susceptibility |
Ciprofloxacin MIC (mg/L) |
|---|---|---|---|---|
|
P. aeruginosa H133880624 |
Resistant | 128 | Resistant | 16 |
|
P. aeruginosa H131300444 |
Resistant | 128 | Resistant | 8 |
EUCAST breakpoints for colistin: Susceptibility and resistance to colistin were defined as MICs of ≤ 2 mg/L and > 2 mg/L, respectively.
EUCAST breakpoints for ciprofloxacin: Susceptibility and resistance to ciprofloxacin were defined as MICs of < 0.5 mg/L for susceptibility and >1 mg/L for resistance.
Figure 10.
Static time-kill kinetics for colistin in combination with ciprofloxacin against (A) P. aeruginosa H131300444 and (B) P. aeruginosa H133880624. The limit of detection is 1.30-Log10CFU/mL (equivalent to one colony per plate).
DISCUSSION
Determination of the encapsulation efficiency
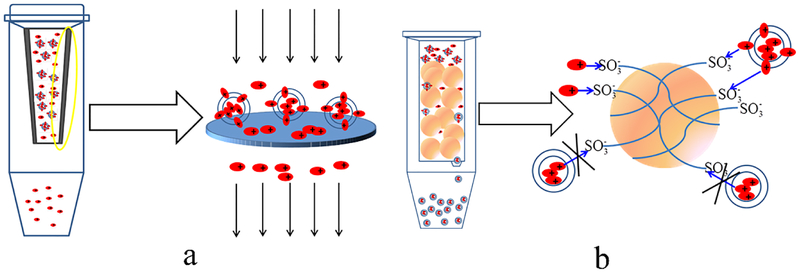
Various methods have been developed to determine the encapsulation efficiency of liposomes. In this study, encapsulation efficiency measured by the centrifugal filtration (Method I) and ion-exchange resin column centrifugation (Method II) were compared for our formulations. It is interesting to note that the EE values of ciprofloxacin measured by these two methods were comparable, but varied substantially for colistin. We hypothesize that such varied EE of colistin is the consequence of different mechanisms of two methods for separation as illustrated in Fig. 11. For the centrifugal filtration method (Fig. 11a), the free drug dissolved in the outer aqueous phase flowed across the pores of the membrane filter and was found in the filtrate after centrifugation; while the encapsulated drug, whether entrapped in the inner aqueous phase or associated with the lipid bilayer, was retained by the membrane filter.
Figure 11.
Schematic diagram of (a) centrifugal filtration method, and (b) cation-exchange resin column centrifugation method for determining the EE of liposomes.
The ion-exchange resin column centrifugation method uses Dowerx® 50WX4, a strong acid (sulfonic acid) type of cation-exchange resin, in which cationic molecules can be adsorbed to the resin via exchange of hydrogen ions. When passing the formulation through the column packed with the cation-exchange resin, both the free cationic drug and the drug associated to the liposomes with the cationic residues located on the outside surface of the liposomes can be adsorbed on the resin. Only the drug encapsulated in the inner aqueous phase can be eluted through the column (Fig. 11b). The cation-exchange resin column centrifugation method is usually applied for the separation of cationic drugs from liposomes (29). Therefore, the centrifugal filtration (Method I) was chosen for determining the encapsulation efficiencies of ciprofloxacin and colistin in our study.
Co-encapsulation of ciprofloxacin and colistin into liposomes
The co-encapsulation of these two drugs in a liposomal formulation is challenging because of the different chemical natures of colistin (amphiphilic) and ciprofloxacin (weak basic). A remote loading method, e.g. (NH4)2SO4 gradient method, was effective in loading ciprofloxacin into liposomes with high EE in this study. Some passive encapsulation methods, such as dry lipid film, freeze drying, and other methods were investigated to load colistin into liposomes. The encapsulation efficiencies of colistin varied greatly ranging from 5 % to 50 % with the passive encapsulation method as reported in the literature (25, 35). At the beginning of this study, we tried to encapsulate colistin passively into liposomes using the dry lipid film method, and at the same time establish a transmembrane (NH4)2SO4 gradient for remote loading of ciprofloxacin. In such procedure, the lipid film was hydrated with (NH4)2SO4 solution containing colistin, and the EE value of colistin directly determined by the Method I was about 35 %. Surprisingly, after passing the colistin loaded “blank” liposomes through a Dowerx® 50WX4 cation-exchange spin column to build the NH4+ gradient, colistin was entirely adsorbed onto the cationic-exchange resin, and the amount of colistin loaded into liposomes could not be detected.
In the subsequent investigations, a modified procedure was introduced to co-encapsulate ciprofloxacin and colistin into liposomes. The lipid film was firstly hydrated with (NH4)2SO4 solution free of colistin. After passing the blank liposomes through cation-exchange spin column to build the NH4+ gradient, the gradient liposomes were incubated with dissolved colistin and ciprofloxacin solution to load both drugs simultaneously.
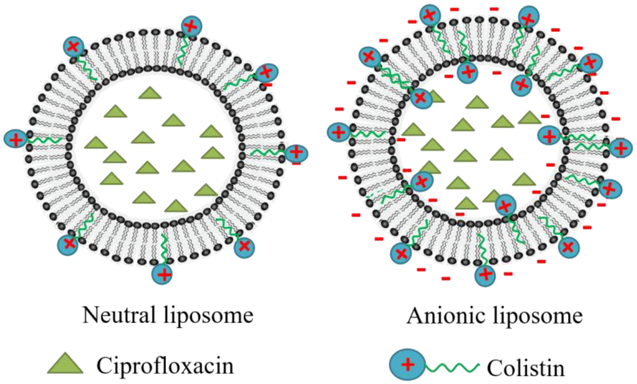
As shown in Table 1, neutral liposomes or anionic liposomes had approximately the same EE of ciprofloxacin, and the EE values determined by the two different methods were very similar. From these results, we concluded that ciprofloxacin was mainly loaded through the (NH4)2SO4 gradient into the inner aqueous phase of liposomes, less or no ciprofloxacin was associated at the outer surface of the liposomes via ionic interaction between ciprofloxacin and DMPG (Fig. 12). Thereby, it is not surprising that incorporation of ciprofloxacin had no effect on the zeta potential of anionic liposomes (Fig. 5).
Figure 12.
Illustration of the distribution of ciprofloxacin and colistin in neutral or anionic liposomes. Left: in the neutral liposomes, few colistin was associated with the outer lipid membrane with hydrophobic acyl chain inserted in the lipid bilayer and the hydrophilic residue exposed on the outer-surface of liposomes; Right: In the anionic liposomes, through the electrostatic attraction, more colistin was associated with the outer lipid membrane, and some penetrated in the inner membrane. Ciprofloxacin was located in the inner aqueous phase of liposomes through remote loading method regardless of neutral liposomes or anionic liposomes.
On the contrary, the EE of colistin in the neutral liposomes was markedly lower than that of the anionic liposomes; and interestingly, the centrifugal filtration method measured significantly higher EE of colistin than the cation-exchange resin centrifugation method. The EE of colistin in the neutral liposomes indicated that only 5.4% of colistin was associated with the neutral liposomes, and no colistin entered the inner aqueous phase of neutral liposomes (colistin was not detected after passing the liposomes through the ion-exchange resin column, see Table 1). The result revealed that colistin can’t be effectively loaded into liposomes through (NH4)2SO4 gradients and only adsorbed to the liposome surface via weak hydrophobic interaction between the hydrophobic acyl chain of colistin and the lipid membrane (Fig. 12), which was in accordance with the observations by Mestresa et al. (36). Noteworthy, such hydrophobic interactions between the colistin and neutral lipid membrane can be extremely weak or even negligible (37).
Incorporation of anionic lipid into the liposomes (DMPG: HSPC=2:3) markedly increased the EE of Colistin (67.0 % by the Method I and 18.4% by the Method II). The result also suggests that at least 73% of the loaded colistin was associated with the lipid bilayer, which can be adsorbed by the cationic-exchange resin. It is likely that the hydrophobic acyl chain of colistin inserted in the lipid bilayer; and the positively charged hydrophilic residue exposed on the outer-surface of liposomes, which was adsorbed by the cationic-exchange resin when the formulation was passed through the resin (Fig. 12). While 27% of the loaded colistin remained in the liposomes after passing the cationic-exchange resin column is mostly likely in the lipid membrane but oriented towards the inner aqueous phase (Fig. 12). It is surprising that the ionized molecules of colistin penetrate across the lipid bilayer and enter the inner aqueous phase of liposomes after the formation of liposomes. This phenomenon could be attributable to instability of the liposome outer membrane caused by electrostatic interactions, followed by hydrophobic insertion of colistin that destabilizes the outer liposome membrane, and eventually colistin entering the inner aqueous phase, which has been observed by Deris et al. on the penetration of polymyxin lipopeptides into Gram-negative bacteria (38).
In vitro drug release
Fig. 6c and 6d showed that the incorporation of colistin accelerated the release of ciprofloxacin from the anionic liposomes and the colistin, which acted as surfactants. Unlike the observations in the presence of Tween 80, the ciprofloxacin release profile of the liposomes with Brij C20 was very similar to Cip/Col-AL. The different structures of two surfactants could be the underlying factor for the varied release profiles. The hydrophobic tail of Tween 80 is much longer than that of Brij C20 (C16 vs. C10, respectively); while the polar head group for Brij C20 is much smaller than that of Tween 80. Thus, Tween 80 is likely more disruptive upon insertion into the liposome bilayer than Brij C20 (39). Colistin had an amphipathic structure carrying five positively charged amine residues and a hydrophobic acyl chain (C8), whose structure is more similar to Brij C20, leading to similar release profiles.
In vitro antimicrobial activity
Colistin, despite being an old drug, is considered as the last-line antibiotic therapy for the infections caused by MDR Gram-negative pathogens such as P. aeruginosa. The resistance to colistin has been increasingly reported among Gram-negative bacteria including P. aeruginosa (40). Similarly, ciprofloxacin resistant P. aeruginosa have been reported frequently (41, 42). Studies suggested that combination of colistin with other antimicrobials like ciprofloxacin is effective in tackling many of the colistin and ciprofloxacin resistant bacterial pathogens (40, 43). Colistin interacts with lipopolysaccharide and phospholipids present at the surface of the outer membrane disturbing the membrane permeability, thereby increasing permeability of other hydrophobic and large molecules. This could lead to the synergistic actions of colistin with other antimicrobials including ciprofloxacin. Our study shows enhanced in vitro antimicrobial activities for the combinations of colistin and ciprofloxacin against MDR P. aeruginosa strains which are resistant to either colistin or ciprofloxacin alone.
A previous study showed that the strongly negatively charged liposomes may result in very high EE of polymyxins; however, antimicrobial activities could be compromised due to the negative charge (44). The bacterial killing mechanisms of polymyxins are that positively charged polymyxin molecules bind to negatively charged lipopolysaccharide of bacterial outer membrane and rapture the bacterial cells (45). We demonstrate here that co-delivery of two synergistic antibiotics in our liposomal formulations preserves the enhanced antimicrobial activity. Although total net charges of the liposomes developed here are negative, colistin molecules are associated on the outer membrane of the liposomes and could interact with the bacterial outer membranes and lead to bacterial killing. There is likely a competitive binding to colistin between negatively charged lipopolysaccharide of bacterial outer membrane and anionic lipid of liposomes when the formulation is exposed to bacteria. With the decrease of anionic lipid ratio, the interaction of colistin with liposomes is weakened, which may increase the chance for lipopolysaccharide to deprive colistin from anionic liposomes for a stronger binding. The true mechanism warrants further investigation.
CONCLUSIONS
We have developed the liposomal formulations of colistin and ciprofloxacin for co-delivery of these two synergistic antibiotics. A remote loading method using an ammonium sulfate gradient proved to be effective in loading ciprofloxacin into liposomes with high EEs; while the colistin was associated with anionic liposomes via electrostatic attraction. Through optimization in lipid ratios, the liposomal formulations with the size of approximately 100 nm and relatively high EE of ciprofloxacin (89.6 ± 2.7 %) and colistin (67.0 ± 5.2 %) achieved sustained release profiles in vitro. Cytotoxicity studies in the A549 human lung epithelial cells showed the drug-loaded liposomes is safe to be used for pulmonary delivery. The combination of ciprofloxacin and colistin in liposomal formulations may offer a viable alternative treatment option against lung infections caused by MDR P. aeruginosa. Further in vivo studies in our established mouse lung infection models (46) are warranted to determine antimicrobial efficacy.
Table 2.
Effects of ciprofloxacin concentration on encapsulation efficiency of ciprofloxacin and colistin in liposomes (n=3, mean ± SD).
| Ciprofloxacin concentration (mg/mL) |
Cip/lipid ratio (mol/mol) |
EE of ciprofloxacin (%) |
EE of colistin (%) |
|---|---|---|---|
| 2 | 0.17 | 84.5±3.4 | 66.2±3.4 |
| 4 | 0.34 | 87.9±2.3 | 63.5±3.0 |
| 8 | 0.68 | 84.5±2.7 | 63.8±4.2 |
| 12 | 1.02 | 83.6±3.1 | 45.9±3.4 |
DMPG: HSPC=2:3, the concentration of colistin was 2 mg/mL.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI132681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jian Li is an Australian NHMRC Senior Research Fellow. Shaoning Wang and Shihui Yu are supported by the China Scholarship Council. The authors are thankful for the cryo-TEM imaging by Valorie D. Bowman at Purdue Cryo Electron Microscopy Facility.
ABBREVIATIONS
- Cipro
Ciprofloxacin hydrochloride monohydrate
- Col
Colistin sulfate
- NL
Neutral liposomes
- AL
Anionic liposomes
- Cip/Col-NL
Ciprofloxacin/Colistin neutral liposomes
- Cip/Col-AL
Ciprofloxacin/Colistin anionic liposomes
- Cip-AL
Ciprofloxacin anionic liposomes
- Col-AL
Colistin anionic liposomes
- Blank-AL
Blank anionic liposomes
- MWCO
Molecular weight cut off
- cryo-TEM
Cryogenic transmission electron microscopy
- Col-LUVs
Colistin-loaded unilamellar liposomes
- MDR
Multidrug-resistant
REFERENCES
- 1.Stefani S, Campana S, Cariani L, Carnovale V, Colombo C, Lleo MM, Iula VD, Minicucci L, Morelli P, Pizzamiglio G, Taccetti G. Relevance of multidrug-resistant Pseudomonas aeruginosa infections in cystic fibrosis. International journal of medical microbiology : IJMM. 2017;307(6):353–362. [DOI] [PubMed] [Google Scholar]
- 2.Trinh TD, Zasowski EJ, Claeys KC, Lagnf AM, Kidambi S, Davis SL, Rybak MJ. Multidrug-resistant Pseudomonas aeruginosa lower respiratory tract infections in the intensive care unit: Prevalence and risk factors. Diagnostic microbiology and infectious disease. 2017;89(1):61–66. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. The Lancet Infectious Diseases. 2006;6(9):589–601. [DOI] [PubMed] [Google Scholar]
- 4.Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrobial agents and chemotherapy. 2010;54(9):3783–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen HK, Moskowitz SM, Ciofu O, Pressler T, Hoiby N. Spread of colistin resistant non-mucoid Pseudomonas aeruginosa among chronically infected Danish cystic fibrosis patients. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2008;7(5):391–397. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrobial agents and chemotherapy. 2006;50(9):2946–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrobial agents and chemotherapy. 2007;51(9):3413–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, Nation RL, Li J. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. The Journal of antimicrobial chemotherapy. 2008;62(6):1311–1318. [DOI] [PubMed] [Google Scholar]
- 9.Dudhani RV, Turnidge JD, Nation RL, Li J. fAUC/MIC is the most predictive PK/PD index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. Journal of Antimicrobial Chemotherapy. 2010;65(9):1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deryke CA, Crawford AJ, Uddin N, Wallace MR. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrobial agents and chemotherapy. 2010;54(10):4503–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ordooei Javan A, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. European journal of clinical pharmacology. 2015;71(7):801–810. [DOI] [PubMed] [Google Scholar]
- 12.Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clinical Microbiology and Infection. 2008;14(9):816–827. [DOI] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ, Sambatakou H, Galani I, Giamarellou H. In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. Journal of chemotherapy. 2003;15(3):235–238. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Nonejuie P, Munguia J, Hollands A, Olson J, Dam Q, Kumaraswamy M, Rivera H Jr., Corriden R, Rohde M, Hensler ME, Burkart MD, Pogliano J, Sakoulas G, Nizet V. Azithromycin Synergizes with Cationic Antimicrobial Peptides to Exert Bactericidal and Therapeutic Activity Against Highly Multidrug-Resistant Gram-Negative Bacterial Pathogens. EBioMedicine. 2015;2(7):690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangden T, Karvanen M, Friberg LE, Odenholt I, Cars O. Assessment of early combination effects of colistin and meropenem against Pseudomonas aeruginosa and Acinetobacter baumannii in dynamic time-kill experiments. Infectious diseases. 2017;49(7):521–527. [DOI] [PubMed] [Google Scholar]
- 16.Ly NS, Bulitta JB, Rao GG, Landersdorfer CB, Holden PN, Forrest A, Bergen PJ, Nation RL, Li J, Tsuji BT. Colistin and doripenem combinations against Pseudomonas aeruginosa: profiling the time course of synergistic killing and prevention of resistance. J Antimicrob Chemother. 2015;70(5):1434–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buyck JM, Tulkens PM, Van Bambeke F. Activities of antibiotic combinations against resistant strains of Pseudomonas aeruginosa in a model of infected THP-1 monocytes. Antimicrobial agents and chemotherapy. 2015;59(1):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2005;4 Suppl 2:49–54. [DOI] [PubMed] [Google Scholar]
- 19.Zununi Vahed S, Salehi R, Davaran S, Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Materials science & engineering C, Materials for biological applications. 2017;71:1327–1341. [DOI] [PubMed] [Google Scholar]
- 20.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Advanced drug delivery reviews. 2013;65(1):36–48. [DOI] [PubMed] [Google Scholar]
- 21.Cipolla D, Shekunov B, Blanchard J, Hickey A. Lipid-based carriers for pulmonary products: preclinical development and case studies in humans. Advanced drug delivery reviews. 2014;75:53–80. [DOI] [PubMed] [Google Scholar]
- 22.Zhou QT, Leung SSY, Tang P, et al. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Advanced drug delivery reviews, 2015, 85: 83–99. [DOI] [PubMed] [Google Scholar]
- 23.Gubernator J Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opinion on Drug Delivery. 2011;8(5):565–580. [DOI] [PubMed] [Google Scholar]
- 24.Yu Z, Qin W, Lin J, Fang S, Qiu J. Antibacterial mechanisms of polymyxin and bacterial resistance. BioMed research international. 2015;2015:679109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omri A, Suntres ZE, Shek PN. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochemical Pharmacology. 2002;64(9):1407–1413. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Tang C, Zhang E, Yang L. Colistin-entrapped liposomes driven by the electrostatic interaction: Mechanism of drug loading and in vivo characterization. International journal of pharmaceutics. 2016;515(1–2):20–29. [DOI] [PubMed] [Google Scholar]
- 27.Bachar M, Mandelbaum A, Portnaya I, Perlstein H, Even-Chen S, Barenholz Y, Danino D. Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled beta-casein micelles. Journal of controlled release : official journal of the Controlled Release Society. 2012;160(2):164–171. [DOI] [PubMed] [Google Scholar]
- 28.Cipolla D, Wu H, Eastman S, Redelmeier T, Gonda I, Chan HK. Development and Characterization of an In Vitro Release Assay for Liposomal Ciprofloxacin for Inhalation. Journal of Pharmaceutical Sciences. 2014;103(1):314–327. [Google Scholar]
- 29.Storm G, van Bloois L, Brouwer M, Crommelin DJA. The interaction of cytostatic drugs with adsorbents in aqueous media. The potential implications for liposome preparation. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1985;818(3):343–351. [DOI] [PubMed] [Google Scholar]
- 30.Jain PP, Leber R, Nagaraj C, et al. Liposomal nanoparticles encapsulating iloprost exhibit enhanced vasodilation in pulmonary arteries. International journal of nanomedicine, 2014, 9: 3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature protocols, 2008, 3(2): 163. [DOI] [PubMed] [Google Scholar]
- 32.Wallace SJ, Nation RL, Li J, Boyd BJ. Physicochemical aspects of the coformulation of colistin and azithromycin using liposomes for combination antibiotic therapies. J Pharm Sci. 2013;102(5):1578–1587. [DOI] [PubMed] [Google Scholar]
- 33.Cipolla D, Wu H, Gonda I, Eastman S, Redelmeier T, Chan HK. Modifying the release properties of liposomes toward personalized medicine. J Pharm Sci. 2014;103(6):1851–1862. [DOI] [PubMed] [Google Scholar]
- 34.Alinaghi A, Rouini MR, Johari Daha F, Moghimi HR. The influence of lipid composition and surface charge on biodistribution of intact liposomes releasing from hydrogel-embedded vesicles. International journal of pharmaceutics. 2014;459(1–2):30–39. [DOI] [PubMed] [Google Scholar]
- 35.Wallace SJ, Li J, Nation RL, Prankerd RJ, Boyd BJ. Interaction of colistin and colistin methanesulfonate with liposomes: colloidal aspects and implications for formulation. J Pharm Sci. 2012;101(9):3347–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mestres C, Alsina MA, Busquets MA, Murányi I, Reig F. Interaction of colistin with lipids in liposomes and monolayers. International journal of pharmaceutics. 1998;160(1):99–107. [Google Scholar]
- 37.Bearer EL, Friend DS. Anionic lipid domains: correlation with functional topography in a mammalian cell membrane. Proceedings of the National Academy of Sciences. 1980;77(11):6601–6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deris ZZ, Swarbrick JD, Roberts KD, Azad MA, Akter J, Horne AS, Nation RL, Rogers KL, Thompson PE, Velkov T, Li J. Probing the penetration of antimicrobial polymyxin lipopeptides into gram-negative bacteria. Bioconjugate chemistry. 2014;25(4):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cipolla D, Wu H, Eastman S, et al. Tuning Ciprofloxacin Release Profiles from Liposomally Encapsulated Nanocrystalline Drug. Pharmaceutical research, 2016, 33(11): 2748–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidaillac C, Benichou L, Duval RE. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrobial agents and chemotherapy, 2012, 56(9): 4856–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergen PJ, Forrest A, Bulitta JB, et al. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrobial agents and chemotherapy, 2011, 55(11): 5134–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. The Lancet infectious diseases, 2006, 6(9): 589–601. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Poudyal A, Yu H, et al. Targeting multidrug-resistantpseudomonas aeruginosa: pharmacodynamics of the combination of colistin and ciprofloxacin. Clinical Microbiology & Infection, 2010, 16: S469. [Google Scholar]
- 44.Desai TR, Tyrrell GJ, Ng T, et al. In vitro evaluation of nebulization properties, antimicrobial activity, and regional airway surface liquid concentration of liposomal polymyxin B sulfate. Pharmaceutical research, 2003, 20(3): 442–447. [DOI] [PubMed] [Google Scholar]
- 45.Velkov T, Roberts KD, Nation RL, et al. Pharmacology of polymyxins: new insights into an ‘old’class of antibiotics. Future microbiology, 2013, 8(6): 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin YW, Zhou QT, Cheah SE, et al. Pharmacokinetics/pharmacodynamics of pulmonary delivery of colistin against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrobial agents and chemotherapy, 2017, 61(3): e02025–16. [DOI] [PMC free article] [PubMed] [Google Scholar]