Abstract
Introduction
Thyrotoxic periodic paralysis (TPP) is a rare and potentially lethal complication of hyperthyroidism. It is characterized by sudden onset paralysis associated with hypokalemia. Management includes prompt normalization of potassium, which results in resolution of the paralysis. Definitive treatment of hyperthyroidism resolves TPP completely.
Case presentation
A 23-year-old African American male patient presented to the emergency room at the University of Mississippi Medical Center, USA in November 2016 with sudden onset quadriplegia. He also endorsed a history of weight loss, palpitations, heat intolerance and tremors. The patient reported similar episodes of quadriplegia in the past, which were associated with hypokalemia and resolved with normalization of potassium levels. Physical examination was significant for exophthalmos, smooth goiter with bruit consistent with the diagnosis of Graves’ disease. Laboratory assessment showed severe hypokalemia, hypomagnesemia, suppressed thyroid stimulating hormone (TSH) and high free thyroxine (T4). Urine potassium creatinine ratio was less than one, indicating transcellular shift as the cause of hypokalemia. After normalization of potassium and magnesium, the paralysis resolved in 12 hours. He was started on methimazole. On follow up, the patient was clinically and biochemically euthyroid with no further episodes of paralysis.
Take-away lesson
TPP is a rare and reversible cause of paralysis. Physicians need to be aware of the diagnostic and treatment modalities as delayed recognition in treatment could result in potential harm or unnecessary interventions.
Keywords: Thyrotoxicosis, Hypokalemia, Periodic paralysis, Graves’ disease, Hyperthyroidism
1. Introduction
Thyrotoxic periodic paralysis (TPP) is a rare but serious complication of hyperthyroidism. It has been reported to occur in 1.8–1.9% of thyrotoxic patients in Asia, whereas in North America, it occurs in 0.1–0.2% of thyrotoxic patients (1, 2). However, TPP is increasingly being reported in Caucasians; therefore, early recognition and treatment is of prime importance (3). Unlike hyperthyroidism, which has a strong female preponderance, TPP occurs 22–76 fold more in men as compared to women (4–6). Hypokalemia in the setting of hyperthyroidism is the hallmark feature of this condition. Hypokalemia occurs as a result of transcellular shift of K into the intracellular fluid. This transcellular shift is due to activation of Na-K-ATP pump in thyrotoxicosis. Normalization of potassium resolves the associated paralysis. Definitive treatment of hyperthyroidism results in complete resolution of the paralytic episodes (3). We present a case of TPP who presented with repeated episodes of hypokalemia and quadriplegia.
2. Case presentation
2.1. Demographic information and symptoms
A 23-year-old African American male patient presented the emergency room at the University of Mississippi Medical Center, Jackson, USA in November 2016 with sudden onset of generalized weakness. He had gone to bed 4 hours ago but was unable to move his extremities on waking up.
2.2. Past history
On further questioning, he admitted to a 40 lb. weight loss over the past year, heat intolerance, palpitations and tremors. He had been evaluated in the emergency room 6 months ago for a similar episode, which had lasted for 8 hours. During that visit, he was noted to be severely hypokalemic. He had had several episodes of weakness in the past 6 months mainly at night, which self-resolved. He had no history of alcohol use or laxative or diuretic abuse, and no family history of hypokalemia or paralysis.
2.3. Clinical findings
Vital signs showed a heart rate of 134 bpm, blood pressure of 162/87 mm Hg. Physical exam was significant for bilateral exophthalmos, and a smooth goiter with bruit, which was consistent with Graves’ disease. Neurological exam revealed 1/5 strength and diminished reflexes in all extremities, however cranial nerves and sensation were intact.
2.4. Diagnostic assessment
Laboratory assessment was significant for severe hypokalemia, hypomagnesemia, suppressed thyroid stimulating hormone (TSH), and high free thyroxine (FT4). Urine potassium was low and urinary potassium/creatinine ratio was low as well (Table 1). TPP was suspected based on the history, and laboratory assessment.
Table 1.
Laboratory assessment
| Sodium (137–145 mEq/lt) | 137 |
| Potassium (3.6–5.0 mEq/lt) | 1.6 |
| Chloride (98–107 mEq/lt) | 102 |
| BUN (9–21 mg/dl) | 8 |
| Creatinine (0.8–1.5 mg/dl) | 0.5 |
| Calcium (8.4–10.2 mg/dl) | 9.1 |
| Magnesium (1.6–2.3 mg/dl) | 1.2 |
| TSH (0.34–4.82 μIU/ml) | 0.01 |
| Free T4 (0.77–1.61 ng/dl) | 4.32 |
| Thyrotropin receptor antibody (0.00–1.74 IU/lt) | 14 |
| Thyroid stimulating immunoglobulin (<1.4 TSI index) | 3.1 |
| Urine potassium (mmol/lt) | 23.8 |
| Urine creatinine (40–278 mg/dl) | 55.8 |
| Urine potassium/creatinine ratio | 0.42 |
| Urine magnesium (28–180 mg/dl) | 34 |
mEq: milliequivalent; lt: litre; mg: milligram; dl: deciliter; μIU: micro international unit; ml: milliliter; ng: nanogram; IU: international unit; mmol: millimole
2.5. Therapeutic intervention
Intravenous potassium chloride (KCl) and Magnesium were initiated to normalize potassium and magnesium levels. Propranolol and methimazole were started for the treatment of hyperthyroidism. The weakness completely resolved in 12 hours. Potassium and magnesium normalized in 24 hours and the patient was discharged on oral potassium and magnesium supplementation.
2.6. Follow-up and Outcomes
Upon follow up at 6 months, the patient experienced resolution of his heat intolerance, palpitations and tremors. He had no further episodes of weakness. Laboratory assessment showed normal levels of TSH, Free T4, potassium, and magnesium (Table 2). The patient refused any definitive therapy for hyperthyroidism and was continued on methimazole.
Table 2.
Trend of potassium, magnesium and thyroid tests
| Variables | Admission | Discharge | 6 month follow up |
|---|---|---|---|
| Potassium(3.6–5.0 mEq/lt) | 1.6 | 3.9 | 3.9 |
| Magnesium(1.6–2.3 mg/dl) | 1.2 | 1.6 | 1.7 |
| TSH (0.34–4.82 μIU/ml) | 0.01 | 1.72 | |
| Free T4(0.77–1.61 ng/dl) | 4.32 | 1.48 |
2.7. Ethics of case report
Written and informed consent from the patient was obtained when he was in the hospital. No identifying information has been used in the manuscript to protect the confidentiality of the patient.
3. Discussion
The majority of TPP is seen in hyperthyroidism due to Graves’ disease, however toxic adenoma, thyroiditis, toxic multinodular goiter, amiodarone induced thyrotoxicosis, levothyroxine intoxication and thyrotropin (TSH) producing pituitary adenoma have all been associated with TPP (7–12). Ragesh et al. described a case of TPP secondary to consumption of nutraceuticals containing triiodothyronine (T3) (13). Our patient had Graves’ disease, which led to the development of TPP in his case.
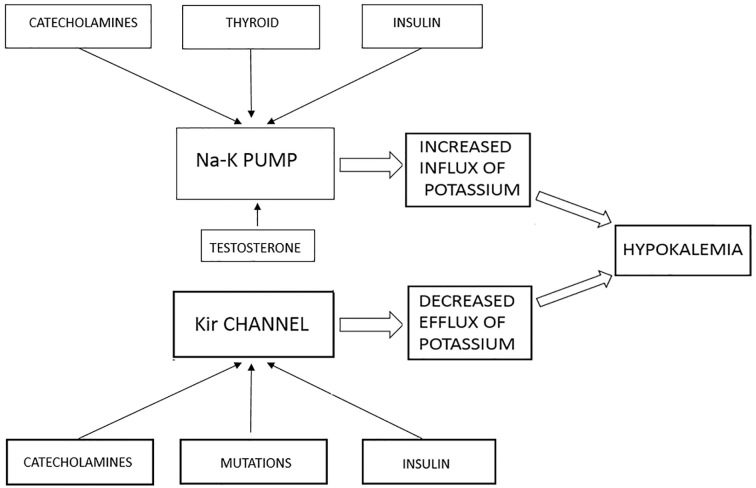
Potassium is the most abundant cation in the intracellular fluid (ICF). It is actively transported into the ICF by the Na-K-ATP pump, which also transports the Na in to the extracellular fluid (ECF). This is expressed in various tissues including the liver, muscle and kidney (14, 15). The Na-K-ATP pump is under the influence of various hormones, which can modulate its activity. The outward flow of potassium is regulated by the inward rectifying K channels (Kir) (4). These two channels working in tandem, keep the potassium levels tightly controlled. Thyroid hormones stimulate the Na-K-ATP pump by binding to the thyroid response elements (TRE) which are upstream of the genes for Na-K pump, and increase its activity by both transcriptional and post transcriptional modification (16, 17). TPP patients have 80% more Na-K pump activity as compared to thyrotoxic patients (18). Catecholamines can also act on stimulating the activity of Na-K pump through the beta-adrenergic receptors. Insulin and testosterone can increase the activity of Na-K pump while estrogen decreases it, this may be partly responsible for the male preponderance of TPP (19–21). In addition, insulin and catecholamines can inhibit the Kir channels decreasing the transport of potassium into ECF. Mutations in the KCNJ 18, which encode for the Kir channels, have been found in approximately 33% of patients with TPP (Figure 1) (22). This massive influx of potassium along with decreased outflow leads to hypokalemia and TPP.
Figure 1.
Pathogenesis of Hypokalemia in Thyrotoxicosis.
TPP is characterized by episodes of sudden onset of weakness. Typically, the weakness starts in the proximal muscles of the lower extremities; however, it involves all four extremities in 80% of cases (23). Heavy meals, alcohol, exercise, high salt diet, stress, infections, menstruation and glucocorticoids have all been known to precipitate the paralytic episodes. Most of these attacks happen at night, likely related to prior alcohol or heavy meals and thereby was given the old name “nocturnal palsy.” Manoukian et al. noted that 84% of TPP patients presented to the emergency room between 1 am to 8 am (24). TPP is seen mostly in males aged 20–40 years, although it can be seen in adolescents and children. (1, 25–27) The severity of the episodes can range from weakness to complete paralysis and the duration can range from a few hours to 3 days (28). Ocular, bulbar and respiratory muscle weakness is rare, but has been reported (4, 26). Approximately 75% of patients with TPP have it as the presenting symptom of their hyperthyroidism (3). The diagnosis of TPP is often delayed, possibly due to a lack of awareness and the rarity of the condition; approximately half of the patients have had history of an episode before the diagnosis was made (29). The diagnosis is delayed on average for 14 months (30). Our patient also had repeated attacks for over a year before the diagnosis of TPP was made.
Hypokalemia is the hallmark feature of TPP, most of the time, potassium <3 mmol/lt. As the hypokalemia is the result of transcellular shift, urinary potassium is < 20 mmol/lt and urine potassium creatinine ratio is <2 mmol/lt, consistent with renal conservation of potassium (5, 29). Other electrolyte disorders like hypomagnesemia and hypophosphatemia due to transcellular shift are observed. Urine calcium is high due to increased filtration and decreased reabsorption, whereas urine phosphate excretion is decreased. Lin et al. found that a urine calcium/phosphate ratio of >1.4 detected TPP with a sensitivity of 100% and specificity of 96% (7). TPP occurs in the hyperthyroid state and thyroid studies show a high thyroxine (T4) and suppressed TSH. However, the severity of the paralysis is not directly related to the degree of severity of hyperthyroidism (31). Electrocardiograms (EKG) are abnormal in 83–100% of patients with TPP (29, 32). Besides signs of hypokalemia, (U waves, ST segment depression and T wave flattening), atrioventricular block and arrhythmias (supraventricular arrhythmias, atrial fibrillation and ventricular arrhythmias) have also been reported. TPP needs to be differentiated from familial hypokalemic periodic paralysis (FHPP), which presents with episodic muscular weakness as well. However, it affects Caucasians with equal sex distribution. FHPP is autosomal dominant and a family history of hypokalemic paralysis is often positive (33). Thyroid studies in TPP show hyperthyroidism, whereas in FHPP they are normal.
The acute management of TPP involves treating the hypokalemia with potassium chloride infusion. In, most instances, less than 50 mmol of KCl is needed; overzealous replacement can result in dangerous hyperkalemia (34). Glucose infusions need to be avoided as they can raise insulin levels and worsen hypokalemia. Prophylactic administration of KCl to prevent TPP is not effective and therefore not recommended (3). Propranolol, by blocking the beta-adrenergic activity, can alleviate TPP. Propranolol 1 mg intravenously every 10 minutes has been used in cases where KCl is not effective (35, 36). Oral propranolol (40 mg qid) is also effective as prophylaxis, preventing further episodes of TPP (37). However, propranolol needs to be administered with caution in case of a heart block, as it can result in severe bradycardia and cardiovascular collapse (38). Definitive treatment of hyperthyroidism, either with radioiodine ablation or with thyroidectomy, results in resolution of TPP. Use of antithyroid medications alone resulted in a relapse attack in 56% patients within 7 months (29).
4. Conclusions
TPP needs to be considered in the differential diagnosis of sudden onset motor weakness with hypokalemia, particularly in a young male patient. These episodes happen most often at night. Proximal muscles are most affected; however, in rare instances, respiratory muscles are also involved. Diagnosis is established when there is concurrent hypokalemia and hyperthyroidism. Acute management involves normalizing potassium and magnesium. Propranolol can be used to treat - as well as prevent further episodes, by decreasing the beta-adrenergic drive. Definitive treatment of hyperthyroidism either with thyroidectomy or radioiodine ablation resolves TPP.
Acknowledgments
The authors acknowledge the Department of Internal Medicine and the Clinical Laboratory at the University of Mississippi Medical Center for supporting this case study. We would also like to acknowledge the patient for consenting to have his case written up.
Footnotes
iThenticate screening: July 11, 2018, English editing: July 21, 2018, Quality control: July 23, 2018
This article has been reviewed/commented by three experts
Note: This case report is prepared using the CARE Checklist (2013) of information to include when writing a case report (https://www.care-statement.org). The CARE guidelines for case reports help reduce bias, increase transparency, and provide early signals of what works, for which patients, and under which circumstance.
Conflict of Interest:
There is no conflict of interest to be declared.
Authors’ contributions:
All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Okinaka S, Shizume K, Iino S, Watanabe A, Irie M, Noguchi A, et al. The association of periodic paralysis and hyperthyroidism in Japan. J Clin Endocrinol Metab. 1957;17(12):1454–9. doi: 10.1210/jcem-17-12-1454. [DOI] [PubMed] [Google Scholar]
- 2.Ober KP. Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of literature. Medicine. 1992;71(3):109–20. doi: 10.1097/00005792-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kung AW. Clinical review: Thyrotoxic periodic paralysis: a diagnostic challenge. J Clin Endocrinol Metab. 2006;91(7):2490–5. doi: 10.1210/jc.2006-0356. [DOI] [PubMed] [Google Scholar]
- 4.Falhammar H, Thorén M, Calissendorff J. Thyrotoxic periodic paralysis: clinical and molecular aspects. Endocrine. 2013;43(2):274–84. doi: 10.1007/s12020-012-9777-x. [DOI] [PubMed] [Google Scholar]
- 5.Lin SH. Thyrotoxic periodic paralysis. Mayo Clin Proc. 2005;80(1):99–105. doi: 10.1016/S0025-6196(11)62965-0. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CJ, Lin SH, Lo YF, Yang SS, Hsu YJ, Cannon SC, et al. Identification and functional characterization of Kir2.6 mutations associated with non-familial hypokalemic periodic paralysis. J Biol Chem. 2011;286(31):27425–35. doi: 10.1074/jbc.M111.249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin SH, Chu P, Cheng CJ, Chu SJ, Hung YJ, Lin YF. Early diagnosis of thyrotoxic periodic paralysis: spot urine calcium to phosphate ratio. Crit Care Med. 2006;34(12):2984–9. doi: 10.1097/01.CCM.0000242249.10856.49. [DOI] [PubMed] [Google Scholar]
- 8.Ozaki H, Mori K, Nakagawa Y, Hoshikawa S, Ito S, Yoshida K. Autonomously functioning thyroid nodule associated with thyrotoxic periodic paralysis. Endocr J. 2008;55(1):113–9. doi: 10.1507/endocrj.K07E-017. [DOI] [PubMed] [Google Scholar]
- 9.Alings AM, Fliers E, de Herder WW, Hofland LJ, Sluiter HE, Links TP, et al. A thyrotropin-secreting pituitary adenoma as a cause of thyrotoxic periodic paralysis. J Endocrinol Invest. 1998;21(10):703–6. doi: 10.1007/BF03350802. [DOI] [PubMed] [Google Scholar]
- 10.Lee JI, Sohn TS, Son HS, Oh SJ, Kwon HS, Chang SA, et al. Thyrotoxic periodic paralysis presenting as polymorphic ventricular tachycardia induced by painless thyroiditis. Thyroid. 2009;19(12):1433–4. doi: 10.1089/thy.2009.0253. [DOI] [PubMed] [Google Scholar]
- 11.Hannon MJ, Behan LA, Agha A. Thyrotoxic periodic paralysis due to excessive L-thyroxine replacement in a Caucasian man. Ann Clin Biochem. 2009;46(Pt 5):423–5. doi: 10.1258/acb.2009.009012. [DOI] [PubMed] [Google Scholar]
- 12.Laroia ST, Zaw KM, Ganti AK, Newman W, Akinwande AO. Amiodarone-induced thyrotoxicosis presenting as hypokalemic periodic paralysis. South Med J. 2002;95(11):1326–8. doi: 10.1097/00007611-200295110-00018. [DOI] [PubMed] [Google Scholar]
- 13.Panikkath R, Nugent K. I lost weight, but I became weak and cannot walk—a case of nutraceutical (T3)-induced thyrotoxic periodic paralysis. Am J Ther. 2014;21(6):e211–4. doi: 10.1097/MJT.0b013e318288a460. [DOI] [PubMed] [Google Scholar]
- 14.Lo CS, Edelman IS. Effect of triiodothyronine on the synthesis and degradation of renal cortical (Na+ + k+)-adenosine triphosphatase. J Biol Chem. 1976;251(24):7834–40. [PubMed] [Google Scholar]
- 15.Liberman UA, Asano Y, Lo CS, Edelman IS. Relationship between Na+-dependent respiration and Na+ + K+-adenosine triphosphatase activity in the action of thyroid hormone on rat jejunal mucosa. Biophys J. 1979;27(1):127–44. doi: 10.1016/S0006-3495(79)85207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275(5 Pt 2):F633–50. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 17.Desai-Yajnik V, Zeng J, Omori K, Sherman J, Morimoto T. The effect of thyroid hormone treatment on the gene expression and enzyme activity of rat liver sodium-potassium dependent adenosine triphosphatase. Endocrinology. 1995;136(2):629–39. doi: 10.1210/endo.136.2.7835297. [DOI] [PubMed] [Google Scholar]
- 18.Mulder JE. Thyroid disease in women. Med Clin North Am. 1998;82(1):103–25. doi: 10.1016/S0025-7125(05)70596-4. [DOI] [PubMed] [Google Scholar]
- 19.Chan A, Shinde R, Chow CC, Cockram CS, Swaminathan R. Hyperinsulinaemia and Na+, K+ -ATPase activity in thyrotoxic periodic paralysis. Clinical Endocrinolog. 1994;41:213–6. doi: 10.1111/j.1365-2265.1994.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 20.Azzarolo AM, Mircheff AK, Kaswan RL, Stanczyk FZ, Gentschein E, Becker L, et al. Androgen support of lacrimal gland function. Endocrine. 1997;6(1):39–45. doi: 10.1007/BF02738800. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal R, Chugh P. Thyrotoxic periodic paralysis: a short clinical review. Int J Res Med Sci. 2015;3:539–42. doi: 10.5455/2320-6012.ijrms20150302. [DOI] [Google Scholar]
- 22.Lin SH, Huang CL. Mechanism of Thyrotoxic Periodic Paralysis. J Am Soc Nephrol. 2012;23:985–8. doi: 10.1681/ASN.2012010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh MJ, Lyu RK, Chang WN, Chang KH, Chen CM, Chang HS, et al. Hypokalemic thyrotoxic periodic paralysis: clinical characteristics and predictors of recurrent paralytic attacks. Eur J Neurol. 2008;15(6):559–64. doi: 10.1111/j.1468-1331.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 24.Manoukian MA, Foote JA, Crapo LM. Clinical and metabolic features of thyrotoxic periodic paralysis in 24 episodes. Arch Intern Med. 1999;159(6):601–6. doi: 10.1001/archinte.159.6.601. [DOI] [PubMed] [Google Scholar]
- 25.McFadzean AJ, Yeung R. Periodic paralysis complicating thyrotoxicosis in Chinese. Br Med J. 1967;1(5538):451–5. doi: 10.1136/bmj.1.5538.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satam N, More V, Shanbag P, Kalgutkar A. Fatal thyrotoxic periodic paralysis with normokalemia. Indian J Pediatr. 2007;74(11):1041–3. doi: 10.1007/s12098-007-0194-8. [DOI] [PubMed] [Google Scholar]
- 27.Wong GW, Leung TF, Lo AF, Ahuja AT, Cheng PS. Thyrotoxic periodic paralysis in a 14-year-old boy. Eur J Pediatr. 2000;159(12):934. doi: 10.1007/PL00008375. [DOI] [PubMed] [Google Scholar]
- 28.Shizume K, Shishiba Y, Kuma K, Noguchi S, Tajiri J, Ito K, Noh JY. Comparison of the incidence of association of periodic paralysis and hyperthyroidism in Japan in 1957 and 1991. Endocrinol Jpn. 1992;39(3):315–8. doi: 10.1507/endocrj1954.39.315. [DOI] [PubMed] [Google Scholar]
- 29.Abbas MT, Khan FY, Errayes M, Baidaa AD, Haleem AH. Thyrotoxic periodic paralysis admitted to the medical department in Qatar. Neth J Med. 2008;66(9):384–8. [PubMed] [Google Scholar]
- 30.Maciel RM, Lindsey SC, Dias da Silva MR. Novel etiopathophysiological aspects of thyrotoxic periodic paralysis. Nat Rev Endocrinol. 2011;7(11):657–67. doi: 10.1038/nrendo.2011.58. [DOI] [PubMed] [Google Scholar]
- 31.Nellen H, Mercado M, Mendoza V, Villanueva S, Pérez M, Hernández A, et al. Thyrotoxic periodic paralysis in Mexican mestizo patients: a clinical, biochemical and HLA-serological study. Arch Med Res. 1999;30(1):74–6. doi: 10.1016/S0188-0128(98)00014-1. [DOI] [PubMed] [Google Scholar]
- 32.Elston MS, Orr-Walker BJ, Dissanayake AM, Conaglen JV. Thyrotoxic hypokalaemic periodic paralysis: Polynesians, an ethnic group at risk. Intern Med J. 2007;37(5):303–7. doi: 10.1111/j.1445-5994.2007.01313.x. [DOI] [PubMed] [Google Scholar]
- 33.Lapie P, Lory P, Fontaine B. Hypokalemic periodic paralysis: an autosomal dominant muscle disorder caused by mutations in a voltage-gated calcium channel. Neuromuscul Disord. 1997;7(4):234–40. doi: 10.1016/S0960-8966(97)00435-5. [DOI] [PubMed] [Google Scholar]
- 34.Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544–7. doi: 10.1016/j.ajem.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199–202. doi: 10.1016/S0736-4679(99)00194-8. [DOI] [PubMed] [Google Scholar]
- 36.Shayne P, Hart A. Thyrotoxic periodic paralysis terminated with intravenous propranolol. Ann Emerg Med. 1994;24(4):736–40. doi: 10.1016/S0196-0644(94)70286-1. [DOI] [PubMed] [Google Scholar]
- 37.Yeung RT, Tse TF. Thyrotoxic periodic paralysis. Effect of propranolol. Am J Med. 1974;57(4):584–90. doi: 10.1016/0002-9343(74)90010-2. [DOI] [PubMed] [Google Scholar]
- 38.Dalan R, Leow MK. Cardiovascular collapse associated with beta blockade in thyroid storm. Exp Clin Endocrinol Diabetes. 2007;115(6):392–6. doi: 10.1055/s-2007-971065. [DOI] [PubMed] [Google Scholar]