Abstract
Background
Heart failure (HF) is a severe public health problem because of its high morbidity and mortality and elevated costs, thus requiring better understanding of its course. In its complex and multifactorial pathogenesis, sympathetic hyperactivity plays a relevant role. Considering that sympathetic dysfunction is already present in the initial phases of chronic Chagas cardiomyopathy (CCC) and frequently associated with a worse prognosis, we assumed it could be more severe in CCC than in cardiomyopathies of other etiologies (non-CCC).
Objectives
To assess the cardiac sympathetic dysfunction 123I-MIBG) of HF, comparing individuals with CCC to those with non-CCC, using heart transplant (HT) patients as denervated heart parameters.
Methods
We assessed 76 patients with functional class II-VI HF, being 25 CCC (17 men), 25 non-CCC (14 men) and 26 HT (20 men), by use of cardiac 123I-metaiodobenzylguanidine 123I-MIBG) scintigraphy, estimating the early and late heart-to-mediastinum ratio (HMR) of 123I-MIBG uptake and cardiac washout (WO%). The 5% significance level was adopted in the statistical analysis.
Results
The early and late HMR values were 1.73 ± 0.24 and 1.58 ± 0.27, respectively, in CCC, and 1.62 ± 0.21 and 1.44 ± 0.16 in non-CCC (p = NS), being, however, higher in HT patients (p < 0.001). The WO% values were 41.65 ± 21.4 (CCC), 47.37 ± 14.19% (non-CCC) and 43.29 ± 23.02 (HT), p = 0.057. The late HMR values showed a positive weak correlation with left ventricular ejection fraction (LVEF) in CCC and non-CCC (r = 0.42 and p = 0.045; and r = 0.49 and p = 0.015, respectively).
Conclusion
Sympathetic hyperactivity 123I-MIBG) was evidenced in patients with class II-IV HF, LVEF < 45%, independently of the HF etiology, as compared to HT patients.
Keywords: Heart Failure, Primary Dysautonomies, Chagas Cardiomyopathy, Myocardial/radionuclide imaging, 123 I-metaiodobenzylguanidine 123I-MIBG)
Introduction
Heart failure (HF) currently represents a public health problem because of its epidemic proportions (worldwide prevalence greater than 23 million individuals), its high morbidity and mortality, and, consequently, high health expenditures.1
A large variety of cardiac conditions can result in HF, whose prevalence differs when comparing developed and developing countries.2 In Brazil, the ischemic etiology accounts for 34.1% of the HF cases, being followed by the Chagasic (21.4%) and hypertensive (13.2%) etiologies, the Chagasic being associated with a worse prognosis.3-6
Previous studies have suggested that the pathogenesis of HF is complex and multifactorial,7 sympathetic dysautonomia playing a relevant role in the process.8-11 In the initial phase of HF, the sympathetic nervous system activation would modulate the pump function; however, over time, its action would become deleterious, leading to myocardial remodeling and restructuring, with progressive decline of the cardiac function.10,12,13 In such patients, sympathetic dysfunction would be characterized by a significant reduction in the presynaptic uptake of norepinephrine, with consequent elevation in its serum levels and reduction in the postsynaptic density of b-adrenoreceptors.10,13,14
In chronic Chagas cardiomyopathy (CCC), early impairment of the parasympathetic nervous system has been well established,15,16 and sympathetic dysfunction, although not totally established, is believed to be present in the initial phases of the disease,11,17-19 when the cardiac pump function is preserved and potentially associated with malignant arrhythmias and sudden death.20-22
Because cardiac autonomous dysfunction is present early in CCC and the incidence of malignant arrhythmias and sudden death is elevated in those patients,15,16,20-22 this study was designed aimed at assessing the presence and the magnitude of cardiac sympathetic dysfunction in Chagasic patients with HF. Chagasic versus non-Chagasic patients were compared, and heart transplant (HT) patients were considered as the denervated heart pattern (known to be abnormal).23 Cardiac 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy was used to assess the patients, because it properly evaluates cardiac sympathetic dysfunction,12,13,24,25 providing relevant parameters to understand the progression of HF.12,13,24
Methods
This is a cross-sectional study of 76 patients selected from the Heart Failure and Heart Transplant outpatient clinic of our institution from March 2014 to February 2016.
The eligibility criteria for individuals with HF were: age over 18 years; left ventricular ejection fraction (LVEF) ≤ 45%; confirmed non-Chagasic or Chagasic etiology (positivity for Chagas disease confirmed by use of two different serological techniques) associated with left ventricular systolic dysfunction;26 and accepting to participate in the study. In addition, for individuals with HF submitted to HT (comparison group or denervated heart model),23 time from HT shorter than 12 months was required. Patients with diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, Parkinson disease, non-sinus heart rhythm or implantable pacemaker were excluded.
The patients were studied prospectively, divided into three groups: CCC group - 25 patients with CCC (mean age, 53.3 ± 9.2 years; 17 males); non-CCC group - 25 patients with heart disease etiologies other than CCC (56% idiopathic, 36% ischemic, and 8% post-partum cardiomyopathy; mean age, 43.3 ± 12 years; 14 males); and HT group - 26 patients previously submitted to HT within less than 12 months (mean, 6.5 ± 3.8 months), with mean age of 47.3 ± 13.1 years, being 20 of the male sex. All patients provided written informed consent, which had been approved by the Ethics Committee of the institution, according to the Declaration of Helsinki. All patients underwent clinical control during the study period.
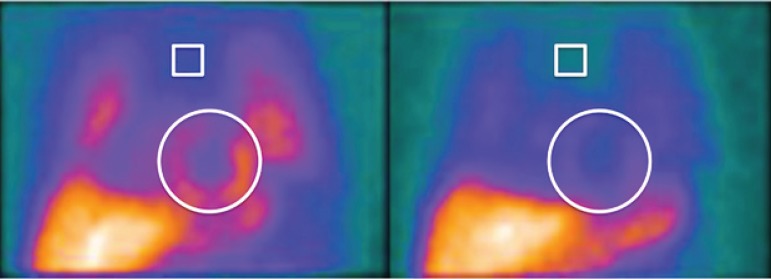
Clinical, electrocardiographic (ECG at rest) and echocardiographic data were collected by the same researcher. Echocardiography was performed using the Phillips iE33® ultrasound device (Phillips Medical, Andover, MA, USA), LVEF being estimated by using Simpson's formula.27 Planar scintigraphy of the myocardial innervation was performed by use of slow intravenous administration of 111 MBq/3 mCi 123I-MIBG (IPEN/CNEN), with anterior image acquisition of the chest after 15 minutes and 180 minutes on a Hawkeye® gamma-camera (GE healthcare, Milwaukie, USA), 10 min/frame, 123I photopeak of 159 KeV, window of 20%, and low-energy high-resolution collimator (LEHR). The heart region of interest (ROI) was drawn encompassing the entire left ventricle, while that of the superior mediastinum encompassed a square ROI of 12x12 pixels. Early and late cardiac uptakes were estimated by use of the ratio between the radioactive counts of the heart and mediastinal ROIs on early and late imaging (early HMR and late HMR, respectively). The cardiac washout rate (WO%) of 123I-MIBG was calculated using the formula: (early heart uptake early mediastinal uptake) (late heart uptake late mediastinal uptake) / (early heart uptake early mediastinal uptake) x 100, without considering radioactive decay, and expressed as percentages28 (Figure 1). Two nuclear physicians analyzed separately the images, with 98% of interobserver agreement, and defined the following as abnormal: WO% > 27% and late HMR ≤ 1.8.29
Figure 1.
Early (15-minute) and late (180-minute) anterior planar imaging of the chest by 123I-MIBG scintigraphy, with regions of interest (ROI) positioned on the superior mediastinum between the pulmonary fields and heart.
The effective radiation dose for the patient, resulting from the administration of 111 MBq/3mCi of 123I-MIBG was estimated as approximately 4.8 mSv, comparable to one of the phases of myocardial perfusion studies with 99mTc-isonitrile.30
Statistical analysis
For this analysis, a sample of 76 patients was calculated to detect a 12% variation in the early or late 123I-MIBG uptake (HMR), with 5% alpha error and 80% power (CI = 95%) for three groups of patients.
To characterize the sample, descriptive analysis of the following variables was initially performed: sex, age, heart rate (HR), LVEF, early HMR, late HMR and WO% of 123I-MIBG expressed according to the distribution of frequency or measures of central tendency and variability. This analysis was stratified per group (CCC, non-CCC and HT). When comparing the three groups, for the categorical variables (sex, HR, NYHA functional class, use of beta-blockers), Pearson chi-square test was performed; for the continuous variables (age, LVEF, WO%), one-factor analysis of variance (ANOVA) was used; and for multiple comparisons, the least significant difference (LSD) test was used. In addition, ANOVA was used to assess the variables (early and late HMR), based, however, on repeated-measures analysis and LSD test for multiple comparisons.
It is worth noting that the assumptions to use ANOVA were verified and accepted, that is, normally distributed residuals (Kolmogorov-Smirnov test) and constant variances (Levene's test).
To analyze the correlation between the measures of early HMR, late HMR or WO% and LVEF, Pearson correlation test and its respective p value were used. In all analyses, a 5% significance level was considered, and the statistical software SPSS, version 17.0 (SPSS Inc., Illinois, USA), was used.31,32
Results
Table 1 shows the demographic, clinical and echocardiographic data of the patients studied. Those with HF on angiotensin-converting-enzyme inhibitors (ACEI) and beta-blockers maintained their medications. In approximately 70% of the HT patients, HR was maintained over 80 bpm (mean of 90.7 bpm), while in individuals with CCC and non-CCC, the mean HR values were 72.7 bpm and 75.6 bpm, respectively (p = 0.03). No patient was on tricyclic antidepressants.
Table 1.
Demographic, clinical and echocardiographic characteristics of the patients
| CCC | non-CCC | HT | p | |
|---|---|---|---|---|
| Male sex† | 68.0 | 56.0 | 77.0 | 0.281a |
| Age (years)* | 53.3 ± 9.2 | 43.3 ± 12 | 47.3 ± 13.1 | 0.016c |
| HR > 80 bpm† | 30.8 | 33.3 | 69.2 | 0.072a |
| NYHA II-IV† | 62.5 | 92.0 | 0.0 | < 0.001a |
| LVEF % (Echo)* | 30.6 ± 7.8 | 25.9 ± 8.0 | 66.6 ± 8.3 | < 0.001c CCC = non-CCC < HT |
| ACEI† | 91.3 | 88 | 77.3 | 0.394b |
| Beta-blockers† | 91.3 | 100 | 18.2 | < 0.001a CCC = non-CCC > HT |
CCC: chronic Chagas cardiomyopathy; non-CCC: cardiomyopathy other than Chagas disease; HT: heart transplant; HR: heart rate (bpm); NYHA: New York Heart Association (heart failure functional classification); ACEI: angiotensin-converting-enzyme inhibitors; Echo: echocardiography;
expressed as mean and standard deviation;
expressed as percentage. Note: The probability of statistical significance for the comparison of the groups refers to
chi-square test,
Fisher exact test, and
analysis of variance.
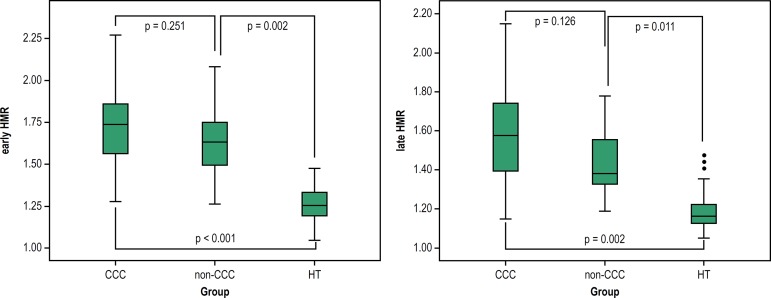
The early and late HMR values were greater than those reported for HT patients (p < 0.001) (Table 2), but did not differ in CCC or non-CCC patients with HF, even when adjusted for age and age group (early HMR: p = 0.251; and late HMR: p = 0.011). The early HMR values of CCC patients were 8.6% higher than those found in non-CCC patients, and 39.7% higher than those found in HT patients. The late HMR values of CCC patients were 9.7% higher than those of non-CCC patients, and 31.7% higher than those of HT patients (Figure 2).
Table 2.
Scintigraphic parameters of myocardial dysfunction (123I-MIBG) in CCC, non-CCC and HT patients
| 123I-MIBG | CCC | non-CCC | HT | p |
|---|---|---|---|---|
| Early HMR* | 1.73 ± 0.24 | 1.62 ± 0.21 | 1.26 ± 0.10 | < 0.001a |
| Late HMR* | 1.58 ± 0.27 | 1.44 ± 0.16 | 1.20 ± 0.12 | < 0.001a |
| WO% | 41.60 ± 21.41 | 47.37 ± 14.19 | 43.29 ± 23.02 | 0.057b |
CCC: chronic Chagas cardiomyopathy; non-CCC: cardiomyopathy other than Chagas disease; HT: heart transplant; early HMR: ratio of the heart/mediastinum radioactive counts estimated on 15-minute images (early uptake); late HMR: ratio of the heart/mediastinum radioactive counts estimated on 180-minute images (late uptake); WO%: cardiac washout of 123I-MIBG, expressed as percentage;
values expressed as mean and standard deviation. Note: The probability of statistical significance refers to analysis of variance based on
repeated measures and
analysis of variance.
Figure 2.
Early and late HMR of 123I-MIBG in CCC or non-CCC or HT patients. Early HMR - ratio of the heart/mediastinum radioactive counts estimated on 15-minute images (early uptake); late HMR: ratio of the heart/mediastinum radioactive counts estimated on 180-minute images (late uptake). Groups: CCC: chronic Chagas cardiomyopathy; non-CCC: cardiomyopathy etiology other than Chagas disease; HT: heart transplant.
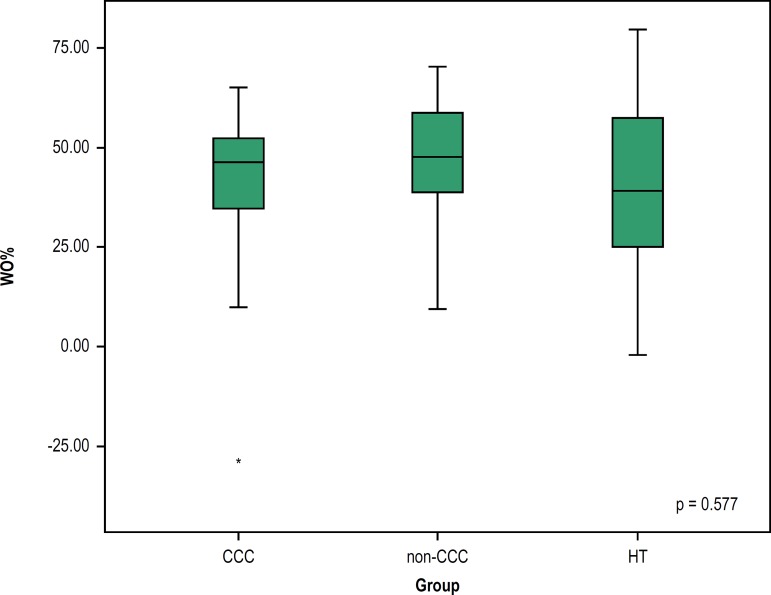
The WO% values showed no statistically significant difference between individuals with HF, and between individuals with HF and those submitted to HT (p = 0.577) (Figure 3).
Figure 3.
Cardiac washout (WO%) of 123I-MIBG in CCC or non-CCC or HT patients. Groups: CCC: chronic Chagas cardiomyopathy; non-CCC: cardiomyopathy etiology other than Chagas disease; HT: heart transplant.
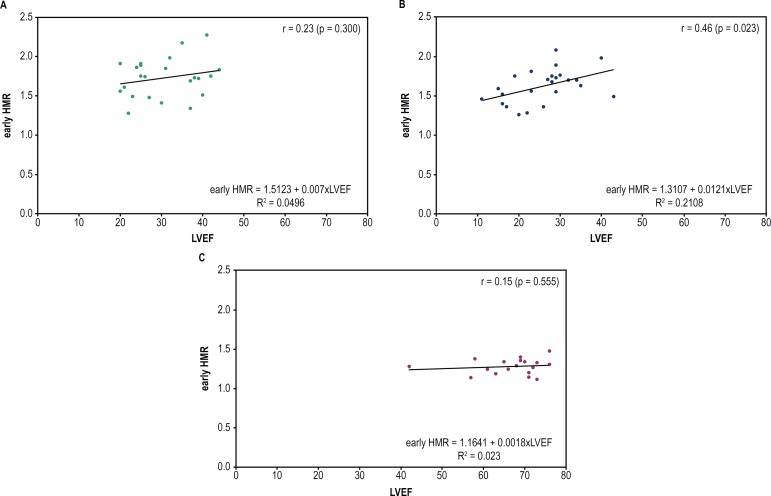
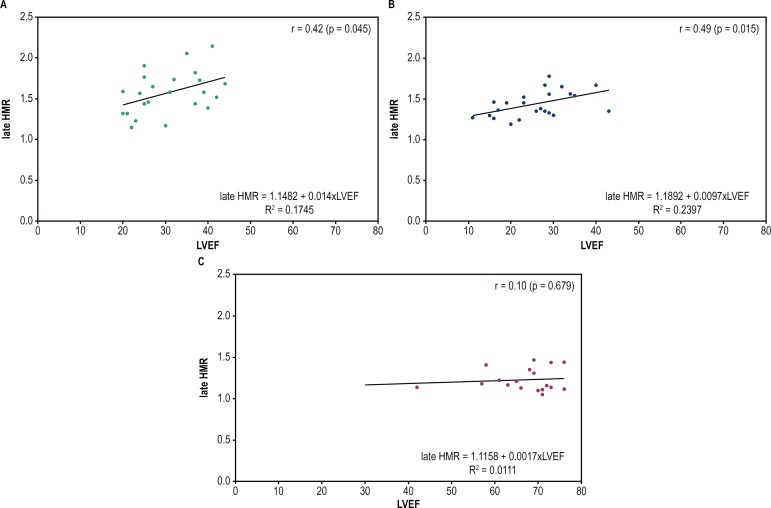
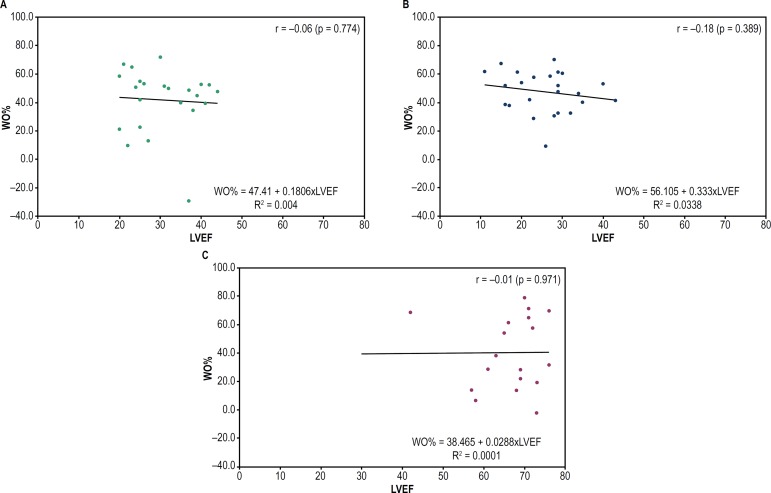
A weak positive correlation was observed between late HMR values and LVEF in CCC patients (r = 0.42; p = 0.045). Regarding the non-CCC patients, a positive correlation was observed both between LVEF and early HMR (r = 0.46; p = 0.023) and between LVEF and late HMR (r = 0.49; p = 0.015). However, none of the groups showed a correlation between LVEF and WO% (Figures 4, 5 and 6).
Figure 4.
Dispersion graph to assess the correlation between LVEF and early HMR in CCC or non-CCC or HT patients. Early HMR: ratio of the heart/mediastinum radioactive counts estimated on 15-minute images (early uptake); LVEF: left ventricular ejection fraction. (A) CCC: chronic Chagas cardiomyopathy; (B) non-CCC: cardiomyopathy etiology other than Chagas disease; (C) HT: heart transplant.
Figure 5.
Dispersion graph to assess the correlation between LVEF and late HMR in CCC or non-CCC or HT patients. Late HMR: ratio of the heart/mediastinum radioactive counts estimated on 180-minute images (late uptake); LVEF: left ventricular ejection fraction. (A) CCC: chronic Chagas cardiomyopathy; (B) non-CCC: cardiomyopathy etiology other than Chagas disease; (C) HT: heart transplant.
Figure 6.
Dispersion graph to assess the correlation between LVEF and WO% of 123I-MIBG in CCC or non-CCC or HT patients. CCC: chronic Chagas cardiomyopathy; LVEF: left ventricular ejection fraction. (A) CCC: chronic Chagas cardiomyopathy; (B) non-CCC: cardiomyopathy etiology other than Chagas disease; (C) HT: heart transplant.
Discussion
This study investigated the presence and magnitude of cardiac dysautonomia in patients with HF and LVEF ≤ 45% by use of 123I-MIBG scintigraphy. Patients were divided into three groups, CCC, non-CCC and HT, the latter, by representing the denervated heart model, served as the abnormality pattern.23
There was scintigraphic evidence of sympathetic hyperactivity, based on the findings of low 123I-MIBG uptake (early and late HMR) by the presynaptic endings in the three groups studied, which is aligned with the literature.13,23,24,28 The low 123I-MIBG uptake indicates dysfunction of the receptors and integrity loss of the presynaptic sympathetic fibers, reinforcing the theory of sympathetic hyperactivity in the pathogenesis of HF.8-10,12,24
Scintigraphy is the only noninvasive and safe method, sufficiently sensitive to assess the autonomic sympathetic nervous system,12,24 that can provide parameters known for their accuracy and reproducibility to estimate the efficacy of clinical treatment13 and the prognosis of patients with HF.13,24,25,33 However, the lack of standardization in the scintigraphic imaging acquisition and processing hinders the incorporation of the method into clinical practice, because there is no well-defined reference value.28,29 In a meta-analysis of seven studies with 96 healthy individuals, Patel and Iskandrian have reported HMR of 2.13 ± 0.3 and WO% of 20 ± 10% (ranging from 10 ± 6% to 37 ± 5%) for healthy individuals.29
The literature on HF has reported reduced late HMR values (1.80),24 with a correlation between a reduction in uptake and worse prognosis, expressed as a higher number of cardiac events and higher mortality.13,24,25 The late HMR values found for our patients with HF (Table 2) were lower than those adopted by different authors using cutoff point values < 1.75 (sensitivity of 84% and specificity of 60%),13 < 1.68,25 or even, more restrictive, < 1.60.24
The HMR values found for HT patients (1.20 ± 0.12) were lower than those found for individuals with HF, with statistical significance (p<0.001), which is aligned with that reported in the literature for patients within the first post-HT year, specially individuals with idiopathic heart disease.23,29
It is worth noting that the patients of this study were on regular use of beta-blockers and ACEI, which do not interfere directly in the uptake of noradrenaline. However, by improving the cardiac performance, and, thus, the sympathetic tone, they increment the 123I-MIBG uptake.34 Thus, supposedly, the HMR values of our patients are overestimated, reinforcing their sympathetic dysfunction degree.
Considering that the late HMR values of individuals with CCC (1.58 ± 0.27) are overestimated, and that Gadioli et al.21 have reported a significant correlation between late HMR values of 1.68 ± 0.19 and severe ventricular arrhythmias, we assumed that the sympathetic dysfunction was more severe in the CCC group because of its arrhythmic findings as compared to the non-CCC group.20,22 However, in our study, those values did not differ significantly from those of the non-CCC group (1.44 ± 0.16), even when statistically adjusted to age and LVEF (p = 0.111).
This fact might be explained by the advanced HF stage of our patients, when sustained autonomic sympathetic dysfunction represents a deleterious mechanism in the pathogenesis of HF itself,8-10,13,24,25,33 independently of etiology, which is aligned with the report by other authors.13
On the other hand, the importance of sympathetic dysfunction in CCC has been questioned by different authors because of: variation in the intensity of denervation; absence of correlation between parasympathetic denervation and myocardial dysfunction extent;19 presence of autonomic dysfunction in the early stages of Chagas disease;4,15,19 correlation between persistence of the myocardial inflammatory process and those patients' morbidity and mortality,35 despite the serum levels of catecholamines.
The estimated WO% values were elevated and abnormal in the three groups studied, being compatible with sympathetic dysfunction in patients with HF and not different from those of HT (p = 0.577). The sympathetic tone, translated by WO%,13,28 might be altered earlier and more markedly than late HMR, and, thus, could be a more sensitive parameter for prognostic assessment, as described.33
Finally, a positive, although weak, but statistically significant correlation between HMR and LVEF was observed in individuals with HF. The LVEF is routinely used for the prognostic assessment of HF.22,36 Thus, that weak correlation can indicate that this scintigraphic parameter is more accurate and earlier altered, as reported by Ogita et al., suggesting it is a better predictor of prognosis than LVEF.33 In addition, it is worth noting that the correlation of early and late HMR with LVEF in CCC patients identified in this study has not been reported in the literature.
Study limitations
This is a cross-sectional nonrandomized study of patients with advanced HF (NYHA functional class II-IV), thus its findings cannot be generalized to all individuals with CCC. There are no strictly established reference values to quantify the scintigraphic parameters because different methodologies have been used (decay factor, correction of septal penetration of iodine).28,29 The maintenance of the medication by the patients (ACEI and beta-blockers) might have led to overestimation of the HMR values and influenced our results, although the number of patients on medications did not differ between the CCC and non-CCC groups, and most studies in the literature have been performed considering the severity of HF.24,25,33
Conclusion
This study evidenced the presence of cardiac sympathetic autonomic dysfunction on myocardial 123I-MIBG scintigraphy, regardless of the HF etiology, and its magnitude was equal in individuals with CCC and non-CCC as compared to HT patients.
Footnotes
Sources of Funding
This study was funded by FAPEMIG.
Study Association
This article is part of the thesis of Post-Doctoral submitted by Viviane Santuari Parisotto Marino, from Faculdade de Medicina da Universidade Federal de Minas Gerais.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Universidade Federal de Minas Gerais - Comitê de ética em Pesquisa (COEP) under the protocol number ETIC 0116.0.203.000-11. All the procedures in this study were in accordance with the 1975 Helsinki Declaration, updated in 2013. Informed consent was obtained from all participants included in the study.
Author contributions
Conception and design of the research: Marino VSP, Dumont SM, Mota LG, Moreira MCV; Acquisition of data: Marino VSP, Mota LG, Braga DS, Moreira MCV; Analysis and interpretation of the data: Marino VSP, Dumont SM, Moreira MCV; Statistical analysis: Marino VSP, Moreira MCV; Obtaining financing: Moreira MCV; Writing of the manuscript: Marino VSP, Dumont SM, Freitas SS, Moreira MCV; Critical revision of the manuscript for intellectual content: Marino VSP, Dumont SM, Mota LG, Braga DS, Freitas SS, Moreira MCV.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocchi EA. Heart failure in South America. Curr Cardiol Rev. 2013;9(2):147–156. doi: 10.2174/1573403X11309020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freitas HF, Chizzola PR, Paes AT, Lima AC, Mansur AJ. Risk stratification in a Brazilian hospital-based cohort of 1220 outpatients with heart failure: role of Chagas' heart disease. Int J Cardiol. 2005;102(2):239–247. doi: 10.1016/j.ijcard.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Bestetti RB, Muccillo G. Clinical course of Chagas' heart disease: a comparison with dilated cardiomyopathy. Int J Cardiol. 1997;60(2):187–193. doi: 10.1016/s0167-5273(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 5.Vilas Boas LG, Bestetti RB, Otaviano AP, Cardinalli-Neto A, Nogueira PR. Outcome of Chagas cardiomyopathy in comparison to ischemic cardiomyopathy. Int J Cardiol. 167(2):486–490. doi: 10.1016/j.ijcard.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa AP, Cardinalli Neto A, Otaviano AP, Rocha BF, Bestetti RB. Comparison of outcome between Chagas cardiomyopathy and idiopathic dilated cardiomyopathy. Arq Bras Cardiol. 2011;97(6):517–525. doi: 10.1590/s0066-782x2011005000112. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR. Why does the myocardium fail? Insights from basic science. Lancet. 1998 Aug;352(Suppl 1):Si8–S14. doi: 10.1016/s0140-6736(98)90311-7. [DOI] [PubMed] [Google Scholar]
- 8.Meredith IT, Eisenhofer G, Lambert GW, Dewar EM, Jennings GL, Esler MD. Cardiac sympathetic nervous activity in congestive heart failure. Evidence for increased neuronal norepinephrine release and preserved neuronal uptake. Circulation. 1993;88(1):136–145. doi: 10.1161/01.cir.88.1.136. [DOI] [PubMed] [Google Scholar]
- 9.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87(2):454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 10.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Bestetti RB, Coutinho-Netto J, Staibano L, Pinto LZ, Muccillo G, Oliveira JS. Peripheral and coronary sinus catecholamine levels in patients with severe congestive heart failure due to Chagas' disease. Cardiology. 1995;86(3):202–206. doi: 10.1159/000176874. [DOI] [PubMed] [Google Scholar]
- 12.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54(5):375–385. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 13.Carrio I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc Imaging. 2010;3(1):92–100. doi: 10.1016/j.jcmg.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Vasudevan NT, Mohan ML, Goswami SK, Prasad SV. Regulation of β-adrenergic receptor function: an emphasis on receptor resensitization. Cell Cycle. 2011;10(21):3684–3691. doi: 10.4161/cc.10.21.18042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro AL, Moraes RS, Ribeiro JP, Ferlin EL, Torres RM, Oliveira E, et al. Parasympathetic dysautonomia precedes left ventricular systolic dysfunction in Chagas disease. Am Heart J. 2001;141(2):260–265. doi: 10.1067/mhj.2001.111406. [DOI] [PubMed] [Google Scholar]
- 16.Gerbi FC, Takahashi JT, Cardinalli-Neto A, Nogueira PR, Bestetti RB. Heart rate variability in the frequency domain in chronic Chagas disease: Correlation of autonomic dysfunction with variables of daily clinical practice. Int J Cardiol. 150(3):357–358. doi: 10.1016/j.ijcard.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Marin-Neto JA, Bromberg-Marin G, Pazin-Filho A, Simões MV, Maciel BC. Cardiac autonomic impairment and early myocardial damage involving the right ventricle are independent phenomena in Chagas' disease. Int J Cardiol. 1998;65(3):261–269. doi: 10.1016/s0167-5273(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 18.Landesmann MC, da Fonseca LM, et al. de B Pereira B, do Nascimento EM, Rosado-de-Castro PH, de Souza SA. Iodine-123 metaiodobenzylguanidine cardiac imaging as a method to detect early sympathetic neuronal dysfunction in chagasic patients with normal or borderline electrocardiogram and preserved ventricular function. Clin Nucl Med. 2011;36(9):757–761. doi: 10.1097/RLU.0b013e31821772a9. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115(9):1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 20.Miranda CH, Figueiredo AB, Maciel BC, Marin-Neto JA, Simoes MV. Sustained ventricular tachycardia is associated with regional myocardial sympathetic denervation assessed with 123I-metaiodobenzylguanidine in chronic Chagas cardiomyopathy. J Nucl Med. 2011;52(4):504–510. doi: 10.2967/jnumed.110.082032. [DOI] [PubMed] [Google Scholar]
- 21.Gadioli LP, Miranda CH, Pintya AO, de Figueiredo AB, Schmidt A, Maciel BC, et al. The severity of ventricular arrhythmia correlates with the extent of myocardial sympathetic denervation, but not with myocardial fibrosis extent in chronic Chagas cardiomyopathy: Chagas disease, denervation and arrhythmia. J Nucl Cardiol. 2018;25(1):75–83. doi: 10.1007/s12350-016-0556-6. [DOI] [PubMed] [Google Scholar]
- 22.Rassi Jr A, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101–1108. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 23.Estorch M, Camprecios M, Flotats A, Mari C, Berna L, Catafau AM, et al. Sympathetic reinnervation of cardiac allografts evaluated by 123I-MIBG imaging. J Nucl Med. 1999;40(6):911–916. [PubMed] [Google Scholar]
- 24.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. ADMIRE-HF Investigators Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55(20):2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013;6(7):772–784. doi: 10.1016/j.jcmg.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Ministério da Saúde. Secretaria de Vigilância em Saúde Brazilian Consensus on Chagas Disease. Rev Soc Bras Med Trop. 2005;38(Suppl. 3):7–29. [PubMed] [Google Scholar]
- 27.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. Erratum in Eur Heart J Cardiovasc Imaging. 2016;17(4):412. [DOI] [PubMed] [Google Scholar]
- 28.Morozumi T, Kusuoka H, Fukuchi K, Tani A, Uehara T, Matsuda S, et al. Myocardial iodine-123-metaiodobenzylguanidine images and autonomic nerve activity in normal subjects. J Nucl Med. 1997;38(1):49–52. [PubMed] [Google Scholar]
- 29.Patel AD, Iskandrian AE. MIBG imaging. J Nucl Cardiol. 2002;9(1):75–94. doi: 10.1067/mnc.2002.121471. [DOI] [PubMed] [Google Scholar]
- 30.Perkins A. Nuclear medicine:science and safety: London: John Libbey Company; 1995. [Google Scholar]
- 31.Montgomery DC. Design and Analysis of Experiments. New York: John Wiley & Sons; 1991. [Google Scholar]
- 32.Johnson RA, Bhattacharyya G. Statistics: principles and methods: New York: John Wiley & Sons; 1987. [Google Scholar]
- 33.Ogita H, Shimonagata T, Fukunami M, Kumagai K, Yamada T, Asano Y, et al. Prognostic significance of cardiac (123)I metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: a prospective study. Heart. 2001;86(6):656–660. doi: 10.1136/heart.86.6.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanelli A, Treglia G, Bruno I, Rufini V, Giordano A. Pharmacological interference with 123I-metaiodobenzylguanidine: a limitation to developing cardiac innervation imaging in clinical practice? Eur Rev Med Pharmacol Sci. 2013;17(10):1326–1333. [PubMed] [Google Scholar]
- 35.Machado FS, Jelicks LA, Kirchhoff LV, Shirani J, Nagajyothi F, Mukherjee S, et al. Chagas heart disease: report on recent developments. Cardiol Rev. 2012;20(2):53–65. doi: 10.1097/CRD.0b013e31823efde2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rassi Jr A, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, et al. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]