Abstract
While guidelines support metformin as a therapeutic option for diabetic patients with mild-to-moderate renal insufficiency, the frequency and outcomes of metformin use in kidney transplant recipients are not well described. We integrated national U.S. transplant registry data with records from a large pharmaceutical claims clearinghouse (2008–2015). Associations (adjusted hazard ratio, 95% LCLaHR95% UCL) of diabetes regimens (with and excluding metformin) in the first year post-transplant with patient and graft survival over the subsequent year were quantified by multivariate Cox regression, adjusted for recipient, donor, and transplant factors and propensity for metformin use. Among 14,144 recipients with pretransplant type 2 diabetes mellitus, 4.7% filled metformin in the first year posttransplant; most also received diabetes comedications. Compared to those who received insulin-based regimens without metformin, patients who received metformin were more likely to be female, have higher estimated glomerular filtration rates, and been transplanted more recently. Metformin-based regimens were associated with significantly lower adjusted all-cause (aHR 0.180.410.91), malignancy-related (aHR 0.450.450.99), and infection-related (aHR 0.120.320.85) mortality, and non-significant trends toward lower cardiovascular mortality, graft failure and acute rejection. No evidence of increased adverse graft or patient outcomes was noted. Use of metformin-based diabetes treatment regimens may be safe in carefully selected kidney transplant recipients.
Keywords: Diabetes mellitus, Kidney transplant, Metformin, Mortality, Pharmacy claims, Risk factors
INTRODUCTION
The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend metformin as first-line therapy for type 2 diabetes1 based on higher efficacy in glucose reduction, favorable effects on lipids, lower risk of side effects such as weight gain and hypoglycemia, and lower cost.2 Large cohort studies have also demonstrated a survival benefit compared with the use of other diabetes treatment agents.3
While data support the benefits of metformin as a first-line agent for diabetes in the general population,1 its safety, efficacy and association with clinical outcomes, are not well described among kidney transplant recipients. In transplant patients, immunosuppressive medications such as corticosteroids,4 calcineurin inhibitors,5 and mammalian target of rapamycin (mTOR) inhibitors6,7 are associated with hyperglycemia that can worsen control of preexisting diabetes or lead to new-onset diabetes after transplant (NODAT).8 In an observational study of kidney transplant recipients linking Scientific Registry of Transplant Recipients (SRTR) data to national pharmacy claims data, Stephen et al. found that of 51,523 recipients from 2001–2012, almost 10% received a metformin as part of their diabetic treatment regimen.9 Metformin use was associated with improved allograft and patient survival.9 A small retrospective single-center united States (U.S.) study examined the efficacy and safety of metformin and thiazolidinediones in kidney transplant recipients with either preexisting diabetes mellitus or NODAT over a mean follow-up of 16.4 months.10 Although a decline in glomerular filtration rate (GFR) was noted in all patients with pre-transplant diabetes mellitus, only 3 of 11 patients had to stop metformin due to drop in GFR. In patients with NODAT, only 2 of 21 stopped metformin during follow-up. Both regimens were equally efficacious in glycemic control. The authors concluded that metformin use may be safe in diabetic kidney transplant recipients.10 In a safety analysis of the Folic Acid for Vascular Outcome Reduction in Transplant (FAVORIT) trial, metformin use in kidney transplant recipients with lower GFR tertile (mean 31, range 9.5–39 ml/min per 1.73 m2) was not associated with more adverse events, based on the Agency for Healthcare Research and Quality (AHRQ) Quality Indicators, compared with use in recipients with upper tertile GFR (mean 69, range 54–132 ml/min per 1.73 m2), although there was a greater association with diabetic ketoacidosis/coma.11
Most of the evidence related to anti-glycemic medication use in transplant recipients comes from NODAT. 12 There is minimal data on patients with preexisting type 2 diabetes mellitus. In NODAT, sulfonylureas13 are often used as the first-line oral agents, while meglitinides14 are recommended as second-line agents due to safe renal profile but greater expense. Among patients that cannot use these two agents, the third-line agents usually include dipeptidyl peptidase-4 inhibitors15 and alpha-glucosidase inhibitors. Thiazolinediones are usually avoided due to concerns about edema and bone loss 16. Although recent data suggest renal benefit associated with sodium–glucose cotransporter 2 (SGLT-2) inhibitors in the general diabetic population with high cardiovascular risk17 and those with early CKD,18 there is minimal data on their use in the transplant population. The concern of lactic acidosis in patients with low GFR prompts many clinicians to avoid prescribing metformin in transplant recipients. This is despite recent data supporting the safety and efficacy of metformin use, in non-transplant patients with mild-to-moderate chronic kidney disease (CKD).19 However, some experts caution against the use of metformin after kidney transplant due to heightened concerns of lactic acidosis as well as the relative inefficiency of metformin alone to provide adequate glycemic control exacerbated by immunosuppression.20 On the other hand metformin has been proposed as a strategy to prevent NODAT by reducing beta-cell stress posttransplant.21 Thus the potential risks and outcomes associated with metformin in kidney transplant recipients remain controversial.
To advance the understanding of the patterns of use, safety and outcomes associated with metformin treatment in kidney transplant recipients with pretransplant diabetes mellitus type 2, we examined a linkage of national transplant registry data with medical fill records from a large pharmaceutical claims clearinghouse. We examined the correlates of metformin use in the first year after transplant, and associations with graft and patient outcomes over the subsequent year.
METHODS
Data Sources
Data for this study was obtained from the SRTR.22 The SRTR registry contains data on all transplant candidates, recipients, and donors in the United States (U.S), provided by the Organ Procurement and Transplantation Network (OPTN).21 The Health Resources and Services Administration (HRSA) and U.S. Department of Health oversee OPTN and SRTR activities. SRTR kidney transplant data include baseline demographics such as recipient age at the time of transplant, sex, and race, as submitted by the hospital to the OPTN. The database also identifies acute rejection, graft failure, and death as reported by the hospital to the OPTN. The SRTR supplements graft failure records with Center for Medicare and Medicaid Studies end-stage renal disease reports and mortality data with the Social Security Death Master File.
Pharmacy fill data were assembled by linking SRTR records for kidney transplant recipients with billing claims from Symphony Health Solutions (SHS), a large U.S. pharmaceutical claims data warehouse that maintains prescription drug fill records including self-paid fills and those reimbursed by private and public payers. SHS comprises National Council for Prescription Drug Program format prescription claims aggregated from multiple sources including claims warehouses, retail pharmacies, and prescription benefit managers for approximately 70% of U.S. retail pharmacy transactions. Individual claim records include the date of a given pharmacy fill with the national drug code identifying agent and dosage. After Institutional Review Board and HRSA approvals, SHS records were linked with SRTR records for kidney transplant recipients. We applied a deterministic de-identification strategy wherein patient identifiers (last name, first name, date of birth, sex, and ZIP code of residence) were transformed before delivery to the Saint Louis University researchers with Health Information Portability and Accountability Act and HITECH-certified encryption technology from SHS. The patient de-identification software employs multiple encryption algorithms in succession to guarantee that the resulting “token” containing encrypted patient identifiers can never be decrypted. However, the algorithm yields the same results for a given set of data elements, such that linkages by unique anonymous tokens are possible.
All direct identifiers were removed before the final dataset was available for analysis. Because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116). The study was approved by the Institutional Review Board (IRB) at Saint Louis University and is in accordance with the Helsinki Declaration of 1975.
Sampling and Exposure Definitions
Patients selected for analysis had SRTR records of kidney-alone transplants between January 1, 2007 and December 31, 2013, were aged 10 years or older (the recommended age limit for metformin use), had type 2 diabetes mellitus as documented at listing or at transplant, had available pharmaceutical fill records for a minimum of 1 year after transplant, and filled a diabetes medication within the first year post-transplant (Figure 1). Patient clinical and demographic characteristics, as well as characteristics of the donated organ and other transplant factors, were extracted from the OPTN Transplant Candidate and Recipient Registration forms (Table 1). The primary exposure of interest was a pharmacy fill for metformin in the year after transplant, regardless of what other medications a patient received; insulin, glitazones, sulfonylureas, or other agents were also extracted. After identifying metformin exposure, we classified the most common regimen, insulin without metformin. We addressed concomitant use of multiple agents by defining mutually exclusive groups based on a hierarchy: metformin-based, insulin-based, glitazone-based, sulfonylurea-based, or other regimens (including dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1)-receptor agonists, meglitinides, alpha-glucosidase inhibitors, amylin analog, and SGLT-2 inhibitors) (Figure 1). In sensitivity analyses, we compared metformin to any non-metformin treatment regimen. The total metformin exposure in the first post-transplant year was quantified based on mg per pill and dispensed pill counts.
Figure 1.
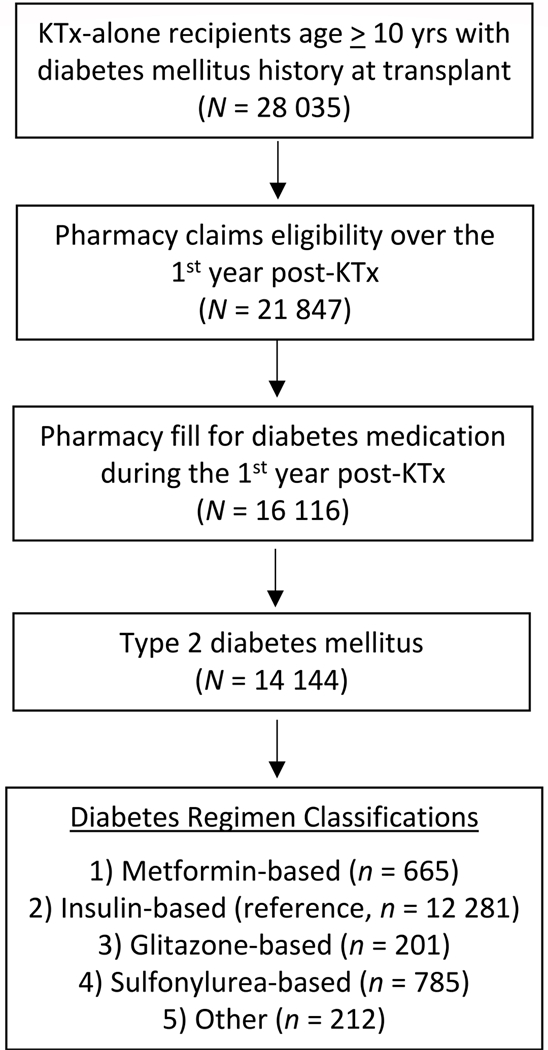
Sampling scheme for mutually exclusive regimens. Metformin-based includes any metformin use, regardless of comedications. Insulin-based includes insulin and any other drug, except metformin. Glitazone-based includes glitazones and any other drug, except metformin and insulin. Sulfonylurea-based includes sulfonylurea and any other drug, except metformin, insulin or glitazones. “Other” diabetes treatment regimens include dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1)- receptor agonists, meglitinides, alpha-glucosidase inhibitors, amylin analog and sodium-glucose co-transporter 2 (SGLT2) inhibitors.
Table 1.
Distributions of clinical traits in the study sample according to diabetes treatment in first year posttransplant. Frequency distributions are compared with insulin-based as the reference. Adjusted odds ratios compare metformin-based versus all other diabetes treatments.
| Diabetes Treatment in First Year after Transplant | Propensity for Metformin Use |
|||||
|---|---|---|---|---|---|---|
| Metformin-based (N=665) |
Insulin-based (N=12281) |
Glitazone-based (N=201) |
Sulfonylurea-based (N=785) |
Other (N=212) |
||
| % | % | % | % | % | Adjusted Odds Ratio (95% CI) | |
| Recipient Factors | ||||||
| Age, yrs. | * | * | ||||
| 10 to 18 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 2.28 (0.10–22.04) |
| 19 to 30 | 0.5 | 0.4 | 0.0 | 0.4 | 0.5 | Reference |
| 31 to 45 | 8.1 | 8.6 | 7.5 | 7.0 | 4.7 | 1.13 (0.39–4.76) |
| 46 to 60 | 39.8 | 42.5 | 40.8 | 36.4 | 35.8 | 1.17 (0.42–4.88) |
| >60 | 51.4 | 48.4 | 51.7 | 56.2 | 59.0 | 1.29 (0.47–5.38) |
| Female | 40.3† | 33.4 | 28.9 | 29.9 | 34.4 | 1.27 (1.07–1.50)* |
| Recipient Race | * | * | † | |||
| White | 45.0 | 43.8 | 41.8 | 43.7 | 49.5 | 1.09 (0.89–1.34) |
| Black | 27.2 | 30.9 | 31.8 | 33.4 | 26.9 | Reference |
| Hispanic | 19.1 | 18.0 | 12.9 | 14.3 | 11.3 | 1.20 (0.94–1.53) |
| Other | 8.7 | 7.3 | 13.4 | 8.8 | 12.7 | 1.26 (0.92–1.71) |
| Body mass index, kg/m2 | * | |||||
| <18.5 | 0.8 | 0.7 | 0.0 | 0.8 | 1.4 | 1.13 (0.39–2.60) |
| 18.5 to 25 | 14.7 | 14.2 | 13.9 | 16.8 | 16.5 | Reference |
| >25 to 30 | 31.3 | 32.5 | 36.3 | 35.0 | 33.0 | 0.91 (0.75–1.10) |
| >30 | 32.6 | 32.0 | 27.4 | 30.2 | 31.6 | 1.00 (0.83–1.22) |
| Missing | 1.5 | 2.4 | 3.5 | 3.1 | 1.4 | 0.62 (0.30–1.12) |
| Cause of ESRD | ‡ | ‡ | ‡ | ‡ | ||
| Diabetes Type 2 | 75.3 | 79.8 | 67.7 | 62.2 | 63.2 | Reference |
| Glomerulonephritis | 6.9 | 3.8 | 6.5 | 10.4 | 9.0 | 1.60 (1.14–2.18)* |
| Hypertension | 17.0 | 15.9 | 22.9 | 26.0 | 24.5 | 1.08 (0.86–1.34) |
| Polycystic kidney disease | 3.0 | 1.9 | 5.0 | 2.2 | 2.4 | 1.54 (0.93–2.42) |
| Other | 2.4 | 3.2 | 5.0 | 6.0 | 5.7 | 0.81 (0.45–1.35) |
| ESRD duration, mos. | * | * | ||||
| None (pre-emptive) | 15.8 | 11.4 | 14.9 | 12.5 | 19.3 | 1.13 (0.88–1.44) |
| >0 to 24 | 31.7 | 29.9 | 27.9 | 28.0 | 32.1 | Reference |
| 25 to 60 | 35.0 | 38.7 | 38.8 | 35.8 | 28.8 | 0.94 (0.76–1.16) |
| >60 | 17.1 | 19.2 | 17.9 | 22.8 | 19.3 | 0.88 (0.67–1.14) |
| Missing | 0.3 | 0.8 | 0.5 | 0.9 | 0.5 | 0.36 (0.06–1.15) |
| Recipient comorbidities | ||||||
| Coronary disease/angina | 12.3 | 11.4 | 10.9 | 12.1 | 9.9 | 1.17 (0.91–1.49) |
| COPD | 0.9 | 1.6 | 1.0 | 3.1* | 1.9 | 0.49 (0.19–1.02) |
|
Hypertension |
86.0* | 82.5 | 84.6 | 84.2 | 84.0 | 1.24 (0.98–1.58) |
| Cerebral vascular disease | 3.9 | 4.2 | 3.5 | 3.8 | 4.2 | 0.93 (0.60–1.38) |
| Peripheral vascular disease | 9.3 | 10.5 | 6.0 | 9.3 | 9.9 | 0.90 (0.68–1.18) |
| Highest Level of Education | * | |||||
| High School or lower | 46.9 | 48.7 | 45.8 | 44.6 | 40.6 | 0.97 (0.82–1.15) |
| College/Graduate School | 45.7 | 44.7 | 48.8 | 47.1 | 50.9 | Reference |
| Unknown | 7.4 | 6.7 | 5.5 | 8.3 | 8.5 | 1.26 (0.91–1.73) |
| Recipient physical capacity | * | * | ||||
| No Limit | 68.0 | 64.1 | 74.6 | 68.2 | 72.6 | Reference |
| Limited | 10.2 | 10.6 | 9.5 | 9.0 | 9.4 | 0.97 (0.74–1.26) |
| Unknown | 21.8 | 25.3 | 15.9 | 22.8 | 17.9 | 0.88 (0.72–1.08) |
| Employment Status | ||||||
| Working | 22.1 | 22.2 | 23.4 | 23.6 | 21.2 | Reference |
| Not Working | 71.9 | 70.6 | 68.2 | 69.2 | 72.6 | 1.08 (0.87–1.33) |
| Unknown | 6.0 | 7.2 | 8.5 | 7.3 | 6.1 | 0.93 (0.63–1.34) |
| Insurance Type | ||||||
| Private | 31.0 | 30.1 | 37.8 | 29.9 | 36.3 | Reference |
| Public | 69.0 | 69.8 | 62.2 | 69.9 | 63.7 | 1.08 (0.88–1.31) |
| Transplant Factors | ||||||
| Previous transplant | 1.4 | 2.4 | 2.5 | 2.4 | 4.7 | 0.54 (0.25–1.06) |
| PRA level (most recent) | * | |||||
| < 10 | 71.1 | 74.1 | 79.1 | 75.8 | 68.4 | Reference |
| 10 to 79 | 17.7 | 14.7 | 11.4 | 14.1 | 15.1 | 1.14 (0.91–1.41) |
| ≥80 | 3.5 | 3.3 | 4.0 | 2.9 | 3.3 | 0.93 (0.58–1.42) |
| Missing | 7.7 | 7.9 | 5.5 | 7.1 | 13.2 | 0.90 (0.66–1.21) |
| HLA mismatches | ||||||
| Zero A, B, and DR | 6.9 | 6.8 | 6.5 | 6.6 | 7.5 | 0.94 (0.67–1.28) |
| Zero DR | 9.8 | 10.9 | 9.0 | 11.2 | 12.3 | 0.81 (0.62–1.05) |
| Other | 83.3 | 82.2 | 84.6 | 82.2 | 80.2 | Reference |
| Donor Type | ‡ | |||||
| Living | 35.9 | 30.1 | 26.9 | 28.9 | 36.3 | 0.95 (0.77–1.17) |
| Standard Criteria Deceased | 42.9 | 43.4 | 44.8 | 41.5 | 38.7 | Reference |
| Expanded Criteria Deceased | 9.6 | 15.9 | 18.9 | 18.6 | 16.5 | 0.64 (0.48–0.84)* |
| Donation after Cardiac Death | 11.6 | 10.6 | 9.5 | 11.0 | 8.5 | 1.32 (1.00–1.72)* |
| Discharge eGFR level | ‡ | |||||
| ≥60 | 30.2 | 19.6 | 17.9 | 20.4 | 23.6 | 1.35 (1.11–1.64)* |
| 30 to 59 | 36.8 | 34.1 | 30.3 | 32.7 | 30.7 | Reference |
| 15 to 29 | 15.5 | 20.6 | 23.4 | 19.0 | 21.2 | 0.69 (0.54–0.88)* |
| < 15 | 17.4 | 25.7 | 28.4 | 27.9 | 24.5 | 0.60 (0.47–0.77)‡ |
| Year of transplant | † | ‡ | * | |||
| 2007–2009 | 23.8 | 30.7 | 55.7 | 32.5 | 21.7 | Reference |
| 2010–2012 | 52.3 | 50.2 | 33.8 | 49.9 | 55.2 | 1.37 (1.13–1.67)* |
| 2013–2015 | 23.9 | 19.1 | 10.4 | 17.6 | 23.1 | 1.69 (1.34–2.13)‡ |
P-values:
p<0.05–0.002
p=0.001–0.0001
p<0.0001
for differences of distributions of clinical traits among patients in a given diabetes treatment group compared with insulin-based reference. COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HLA, human leukocyte antigen; PRA, panel reactive antibody. “Other race” includes Asian, Native American, Pacific Islander and multi-racial.
Acute Rejection, Graft Failure, and Death
The OPTN queries programs for information on acute rejection according to periods covered by specific reporting forms (0 to 6 months, 7 to 12 months, then annual periods), but dates of acute rejection within reporting periods are not collected. We defined acute rejection from SRTR records according to program reports that an acute rejection event occurred in a reporting period, as per prior methods for identifying acute rejection from U.S. registry data.23,24
Patient death was defined as death from any cause. We examined cause-specific death related to infections, malignancy, cardiovascular/cerebrovascular, and other causes in a secondary analysis. Graft failure was defined as return to maintenance dialysis or “preemptive” re-transplant. To assess the implications of diabetes treatment in the first year posttransplant, we examined clinical outcomes from >1 to 2 years posttransplant. Recipients were censored at the first of outcome of interest, death, date of second transplant anniversary, or end of study period (January 31, 2015).
Statistical Analyses
Pharmacy claims and transplant recipient registry datasets were prepared in SAS 9.4 (SAS Institute, Cary, NC) and analyzed with RStudio version 1.0.143.0 (RStudio Inc., Boston, MA). Distributions of clinical and demographic traits among recipients in each diabetes treatment group were compared by the chi-square test for categorical variables and X test for continuous variables. Propensity models for the likelihood of metformin-based use in the first year posttransplant were constructed by multiple logistic regression. The average amount of metformin filled in the first year was explored in relation to first-anniversary estimated GFR (eGFR) level.
Cumulative patient death and graft failure from >1 to 2 years posttransplant according to diabetes treatment regimen was computed by the Kaplan-Meier method. Cox regression was used to estimate adjusted hazard ratio and define lower and upper confidence limits (95% LCLaHR95% UCL) for associations of diabetes treatments with these events. Multiple logistic regression modeling was used to quantify the adjusted odd ratio (95% LCLaOR95% UCL) of acute rejection from >1 to 2 years. Outcome models were also stratified by propensity for metformin use.
RESULTS
Distributions of clinical traits according to diabetic regimen in first year posttransplant
In the study period, 28,035 kidney-only transplant recipients with pre-transplant diabetes mellitus were recorded in the SRTR database. Of these, 21,847 had linked pharmacy fill activity covering their first year post-transplant, and 16,116 (78%) filled a diabetes medication in that period. The majority of these recipients had type 2 diabetes mellitus (n=14,144) of whom 665 had metformin fills, while the remainder filled non-metformin-based regimens (Table 1). The majority (91.6%) of patients who used metformin also received additional diabetes treatments (insulin 76.6%, sulfonylureas 48.9%, glitazones 12.4%, other diabetic agents 32.4%).
Patients who received metformin were more likely to be female (aOR 1.071.271.50), have hypertension as the cause of end-stage renal disease (aOR 1.141.602.18), have discharge eGFR >60 (vs. 30–60) ml/min per 1.73 m2 (aOR 1.111.351.64), and have undergone transplant in 2010–2012 (aOR 1.131.371.67) or 2013–2015 (aOR 1.341.692.13) compared with 2007–2009 (Table 1). Metformin-treated patients were less likely to have eGFR levels of 15–29 (aOR 0.540.690.88) or <15 (aOR 0.470.600.77) (vs. 30–60) ml/min per 1.73 m2, or to have received expanded criteria deceased donor allografts compared with standard criteria (aOR 0.480.640.84). The proportion of recipients prescribed Metformin decreased as level of eGFR declined (Figure 2).
Figure 2.
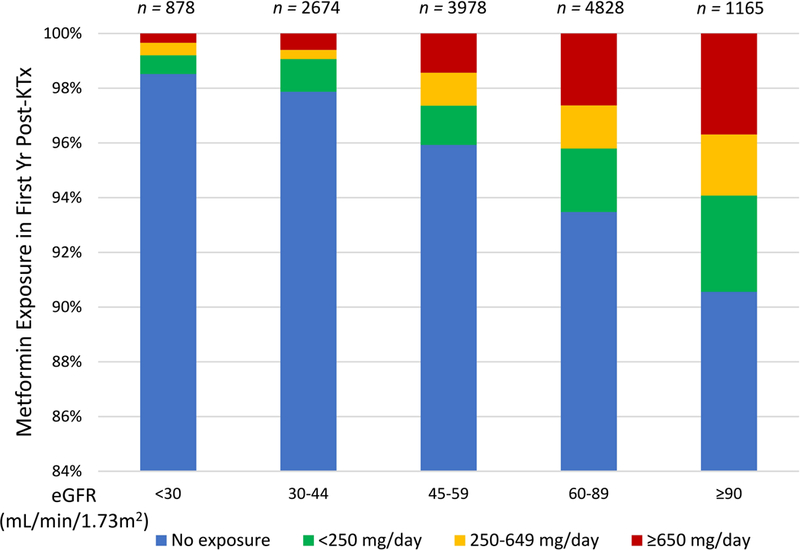
Metformin exposure over the first year post-transplant by eGFR. Compared to eGFR >90ml/min/1.3 m2, use patterns differed at levels <60 ml/min/1.73 m2.
Associations of metformin use in first year after kidney transplant with outcomes >1 to 2 years posttransplant
Incidence of acute rejection >1 to 2 years posttransplant was lower in patients who received metformin-based regimens compared with those who received insulin-based regimens without metformin (1.0% vs. 1.9%, P=0.18), although the trend was not statistically significant after covariate and propensity adjustment (aOR 0.200.591.30) (Figures 3 and 4A; Appendix Table 1). All-cause graft failure >1 to 2 years posttransplant was significantly lower in patients who received metformin-based regimens in the first year compared with those who received insulin-based regimens (1.9% vs 4.2%, P=0.003), but this pattern was not significant after covariate and propensity adjustment (aHR 0.380.651.14). Outcomes did not vary by level of metformin exposure (levels defined per Figure 2). Death-censored graft failure was not different in metformin-based regimens compared with insulin-based regimens. All-cause mortality in >1 to 2 years posttransplant was significantly lower in patients who received metformin-based regimens compared with those who received insulin-based regimens (0.9% vs 2.9%, P=0.002), and this pattern was significant after covariate and propensity adjustment (aHR 0.180.410.91). Compared to insulin-based regimens without metformin, there was no significant difference in outcomes with glitazone-based, sulfonylurea-based, or other regimens excluding metformin (Figure 3). Associations of metformin with study outcomes were similar when compared to any non-metformin treatment regimen. Among metformin users, outcomes did not differ significantly according to whether metformin was received alone or with other classes of agents (P>0.05). Further, in contrast to patterns associated with metformin-based treatment, secondary analysis showed no difference in outcomes in patients who received sulfonylureas compared to any non-sulfonylurea agent.
Figure 3.
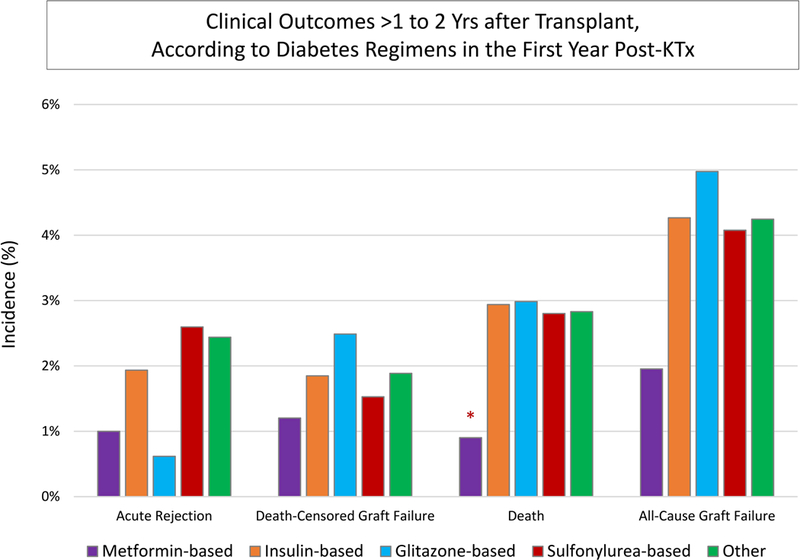
Acute rejection, graft failure, and death after the first transplant anniversary, according to diabetes treatment in first year posttransplant.P-values: * p<0.05for differences in >1 to 2yr outcomes for those in a given diabetes treatment group compared with insulin reference.
Figure 4.
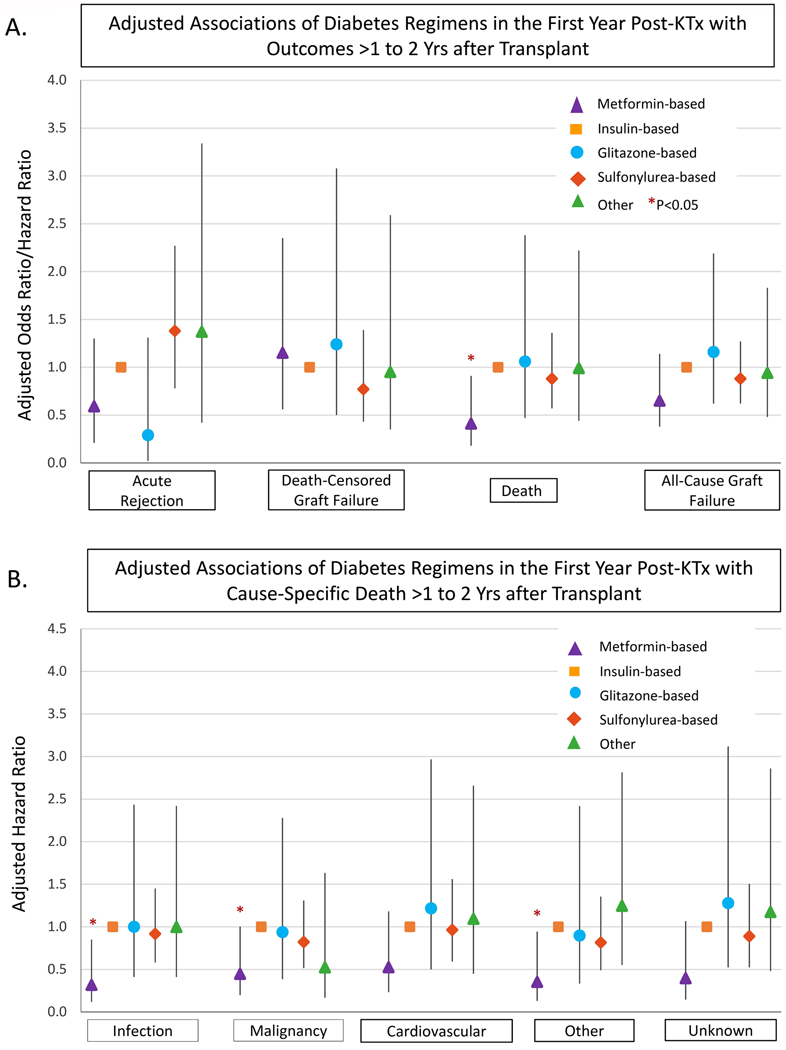
Adjusted associations of diabetes treatment in first year post kidney transplant with subsequent acute rejection, graft failure and death. Adjusted for recipient, donor, and transplant clinical factors as listed in Table 1. ACGF, all-cause graft failure; DCGF, death-censored graft failure; KTx, kidney transplant
Since we noted a significant reduction in all-cause mortality associated with metformin-based treatment in the first year after transplant, we also evaluated associations with causes of death as reported to the registry. After adjusting for baseline factors and propensity for metformin use, statistical significance was noted for malignancy-related death (aHR 0.450.450.99), infection-related death (aHR 0.120.320.85), and death from other causes (aHR 0.130.350.94) (Figure 4B). Trends toward lower mortality were noted for death from cardiovascular disease (aHR 0.230.521.18) and unknown causes of death (aHR 0.150.341.07), but these did not reach statistical significance (Figure 4). Glitazone-based, sulfonylurea-based, and other diabetic regimens were not associated with any differences in cause-specific mortality compared with insulin-based regimens (Figure 4B).
Secondary analyses of all diabetic patients (n=16,116), including those with type 1 diabetes mellitus, showed similar associations of metformin use with subsequent mortality. There was no significant interaction of metformin use with diabetes type in survival analysis.
DISCUSSION
By linking national transplant registry data with pharmacy claims data, we identified, characterized, and studied outcomes associated with the anti-glycemic medication use in the first year after kidney transplant in a large, national, contemporary cohort with preexisting diabetes mellitus. Use of diabetes regimens including metformin was uncommon (4.7%) and inversely correlated with eGFR. Metformin use was not associated with any adverse patient or graft outcomes. In fact, despite prior concerns for safety, metformin-based regimens were associated with 59% lower mortality compared to insulin use without metformin. There were also non-significant trends toward lower risk of acute rejection and all-cause graft failure among patients managed with metformin compared to those who received insulin without metformin. Notably, this survival difference did not occur with other oral hypoglycemic-based regimens excluding metformin (glitazone-based, sulfonylurea-based, or other), consistent with reports in the general population.3 Associations of metformin with study outcomes were similar when compared to any non-metformin treatment regimen.
Our work extends and confirms a prior analysis by Stephen et al.9 examining pharmacy fill records in patients who underwent transplant before recent changes in guidelines for metformin prescribing in CKD.25 The evolution in metformin guidelines motivated our study including more recent data, and argues for continued study over time. Differences in our current study compared with the work of Stephen et al. include our examination of a broader spectrum of outcomes including rejection and cause-specific death, categorization of patients who did not receive metformin into a variety of regimens, and focus on patients with established type 2 diabetes mellitus prior to transplant, whereas Stephen et al included those with both pre-transplant diabetes mellitus and NODAT. We also analyzed metformin use with respect to GFR categories including in patients with eGFR < 30 ml/min per 1.73m2, for whom metformin use is currently contradicted by the FDA.25 Importantly, primary inferences are consistent across both studies despite different samples and data sources.
The main anti-glycemic effect of metformin is inhibiting mitochondrial glycerophosphate dehydrogenase (mGPD), a liver enzyme involved in gluconeogenesis,26 and enhancing insulin-mediated glucose utilization in peripheral tissues.27 Metformin has been associated with lower all-cause mortality compared with insulin and other oral hypoglycemic agents in the general population.3 In an observational study using a United Kingdom database of 78,241 patients treated with metformin, 12,222 matched patients with sulfonylurea, and 90,463 matched subjects without diabetes mellitus, metformin users were noted to have lower mortality compared with diabetic patients using sulfonylureas and with nondiabetic controls.28 Metformin affects various cellular pathways associated with anti-aging, including inflammation, cell survival, autophagy, stress defense, and protein synthesis.29 Observed survival benefits have been attributed to several reasons. Obesity is a risk factor for death,30 and unlike insulin and sulfonylureas, which are associated with weight gain, metformin is associated with weight reduction.31 Dyslipidemia is also a well-known risk factor for mortality, especially cardiovascular death.32 Metformin improves the lipid profile by reducing serum low-density lipoprotein cholesterol concentrations, and increasing serum high-density lipoprotein cholesterol concentrations.33 Metformin has also been associated with lower risk of cancer and reduction in cancer mortality in diabetic patients,34 an effect that may be mediated by regulation of AMP (adenosine monophosphate) -activated protein kinase (AMPK) through the tumor-suppressor liver kinase B135 Metformin is also known to have direct anti-inflammatory effects.36
The most common side effects associated with metformin are gastrointestinal (e.g., nausea, abdominal cramps, and diarrhea).33 Less commonly, metformin has been associated with vitamin B12 deficiency and peripheral neuropathy.37 The most concerning, albeit rare, side effect of metformin is lactic acidosis, which can be fatal.38 Along with effects on gluconeogenesis, inhibition of mGPD also results in decreased conversion of lactate to pyruvate and release of lactate levels into the plasma. Metformin is renally excreted, and thus, despite its benefits, its use is limited in patients with kidney disease. The United States Food and Drug Administration (FDA) issued a black box warning regarding lactic acidosis risk with metformin in patients with GFR <30 ml/min per 1.73 m2. 25 Despite these concerns, recent evidence supports that metformin can be safely used in patients with mild-to-moderate CKD.39 Renal clearance of metformin declines by approximately 75% when the GFR falls below 60 ml/min per 1.73 m2; however, the serum levels remain much lower than those associated with lactic acidosis.40 A meta-analysis of 347 prospective trials and observational studies showed no increase in lactic acid levels or lactic acidosis with metformin use compared with other anti-glycemic regimens.41 In 2012, the Kidney Disease Outcomes Quality Initiative (KDOQI) published an updated Clinical Practice Guideline for Diabetes and CKD42 that questions application of creatinine-based guidelines to metformin use. A recent expert review based on current data recommended that metformin be continued in mild-moderate renal insufficiency with 50% dose reduction when GFR is 30 to 45, but avoided if GFR is <30 ml/min per 1.73m2,43. In the updated metformin labelling in 2016, the FDA advised caution but did not contraindicate use of metformin at GFR levels of 30–60 ml/min per 1.73m2.25
We hypothesized that due to safety concerns, the use of metformin in the kidney transplant population would be low. The observed frequency of 4.7% is substantially lower than use among patients with type 2 diabetes mellitus in the general population, and is consistent with prior studies of transplant recipients.9 Often GFR in transplant recipients is <60 ml/min/1.73 m2, a level associated with the diagnosis of CKD especially when recipient and donor age is > 60 years.44 Not surprisingly, we observed graded declines in metformin use with lower levels of renal function and among recipients of expanded criteria donor kidneys. Moreover, since many type 2 diabetic patients with kidney failure require insulin before transplant, providers might be reluctant to change anti-glycemic therapies after transplant, particularly with the combined impact of higher insulin clearance from improved GFR and the hyperglycemic stresses of immunosuppression. Notably, there was a trend towards more metformin use in more recent years of the study, reflecting the general practices of more common metformin use in patients with mild/moderate kidney disease in recent times based on newer safety data.25
Although our data did not information on lactic acidosis, we examined mortality as a key safety measure. Metformin was not associated with increased risk of any cause of death, but rather was associated with significantly lower all-cause, malignancy-related, infection-related, and “other” deaths, and with trends toward lower risks of unknown and cardiovascular death. While the potential mechanisms of cancer death are noted earlier, the reduction in infection-related death is an interesting finding. It is possible that the anti-inflammatory properties of metformin,36 along with its ability to activate AMPK, a protein that is also involved in the pathogenesis of viral infections,45 play a role. The trends toward reduced cardiovascular death may be a manifestation of the purported benefits on cardiovascular risk noted above, but given the lack of statistical significance, defining impact of this outcome in diabetic transplant recipients requires further study.
Regarding graft outcomes, we observed trends toward lower risk of graft failure and acute rejection with metformin-based regimens, but these patterns were not statistically significant in adjusted analyses. Potential mechanisms of allograft protection would include the ability of metformin to reduce kidney injury (possibly through AMPK activation46,47 and its anti-inflammatory and immune modulatory effects (e.g. via AMPK-mTOR-STAT3 signaling48 and T cell regulation.49 Again, our study does not support definitive conclusions for effects on allograft health, but the absence of increased risk is reassuring for the overall safety profile.
In the general population, metformin is usually titrated to the maximally effective dose of 2000 to 2500 mg per day. In our study, we noted that most (>50%) patients on metformin received a lower dose (average <650 mg/day). This pattern occurred even when the GFR was >60 ml/min, when metformin dose reduction is not recommended, and might reflect the practice of transplant providers to start and maintain lower doses in this population. Interestingly, in our analysis, 1.5% of diabetic transplant recipients with GFR <30 ml/min per 1.73m2 were exposed to metformin in the first year post-transplant; significant portion of these to high doses (≥ 1500 mg/day) (Figure 2). Further study is warranted to determine optimal dosing of metformin in transplant recipients to maximize benefits and reduce risks of those treated with this agent.
Limitations of our study include our inability to compare metformin monotherapy with other monotherapies, primarily due to the small number patients receiving such regimens. Notably, other oral hypoglycemic medication groups (exclusive of metformin) were not associated with any effects on mortality compared with the insulin-based reference group. In a systemic review of 179 trials and 25 observational studies, metformin-based combination therapy had effects similar to those of metformin monotherapy.50 We also could not stratify our analysis of mortality based on GFR subgroups of metformin dose, again due to the low number of metformin-treated patients. Future work should examine whether the mortality benefit of metformin persists in patients on higher doses and with lower GFRs. Although we adjusted for observed recipient, donor, and transplant factors, including the use of the propensity model to limit bias of “confounding by indication” the retrospective design poses inherent risk for residual confounding. It is noted that the metformin group was more likely to be female and have higher discharge/one-year eGFR; and less likely to have received expanded criteria allografts. Hence, it is possible that the despite measures to reduce confounding and bias, the mortality benefit in the metformin group was at least partially related to better overall health status. Findings may not be generalizable to patients who were not included in the linked databases. Further, we lacked laboratory values (e.g., hemoglobin A1c, glucose levels) to adjust for level of glycemic control, and the metformin-treated patients may have had less severe diabetes mellitus. We also lacked granular measures of clinical complications such as lactic acidosis; however, we examined mortality and graft outcomes as safety measures. These observational findings need to be validated in studies using a longer exposure, ideally with prospective designs to minimize confounding. A pilot protocol for a randomized clinical trial (Transdiab), with a 12 month follow-up, designed to study the feasibility, safety, and efficacy of metformin in NODAT, was recently published.51
In conclusion, we found that in a national cohort of diabetic kidney transplant recipients, use of metformin-based diabetes therapy was uncommon, declined with lower levels of allograft function, and was not associated with increased risk of adverse patient or graft outcomes. Rather, metformin exposure was associated with reduced mortality and non-significant trends toward lower rates of rejection and graft loss. While these findings warrant replication in additional studies, examination of linked national registry and pharmacy fill records is an efficient strategy for identifying uncommon treatments in transplant recipients that can confirm, extend, and provide hypotheses for gold standard, but costly, clinical trials.
Supplementary Material
ACKNOWLEDGEMENTS
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing.
DISCLOSURES
This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981.
Abbreviations
- ADA
American Diabetes Association
- aHR
adjusted hazard ratio
- AHRQ
Agency for Healthcare Research and Quality
- aOR
adjusted odds ratio
- CKD
chronic kidney disease
- DPP-4
dipeptidyl peptidase-4
- eGFR
estimated glomerular filtration rate
- EASD
European Association for the Study of Diabetes
- FAVORIT
Folic Acid for Vascular Outcome Reduction in Transplant
- FDA
Food and Drug Administration
- GFR
glomerular filtration rate
- HRSA
Health Resources and Services Administration
- GLP-1
glucagon-like peptide-1
- KDOQI
Kidney Disease Outcomes Quality Initiative
- mGPD
mitochondrial glycerophosphate dehydrogenase
- mTOR
mammalian target of rapamycin
- NODAT
new-onset diabetes after transplantation
- OPTN
Organ Procurement and Transplantation Network
- SGLT-2
sodium–glucose cotransporter 2
- SHS
Symphony Health Solutions
- SRTR
Scientific Registry of Transplant Recipients
- U.S.
United States
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Participated in study design, data analysis, interpretation, and writing of the paper
Participated in study design, interpretation, and writing of the paper.
Participated in study design, acquisition of data and regulatory approvals, data analysis, and writing of the paper. Support provided to the author’s institution by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Participated in study design, interpretation, and writing of the paper. Support provided to the author’s institution by the NIH/NIDDK
REFERENCES
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care . 2015;38(1):140–149. [DOI] [PubMed] [Google Scholar]
- 2.Qaseem A, Barry MJ, Humphrey LL, Forciea MA, Clinical Guidelines Committee of the American College of P. Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med . 2017;166(4):279–290. [DOI] [PubMed] [Google Scholar]
- 3.Ekstrom N, Schioler L, Svensson AM, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open . 2012;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjelmesaeth J, Hartmann A, Kofstad J, et al. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation . 1997;64(7):979–983. [DOI] [PubMed] [Google Scholar]
- 5.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant . 2004;4(4):583–595. [DOI] [PubMed] [Google Scholar]
- 6.Johnston O, Rose CL, Webster AC, Gill JS. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol . 2008;19(7):1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ . 1995;310(6972):83–88. [PMC free article] [PubMed] [Google Scholar]
- 8.Sulanc E, Lane JT, Puumala SE, Groggel GC, Wrenshall LE, Stevens RB. New-onset diabetes after kidney transplantation: an application of 2003 International Guidelines. Transplantation . 2005;80(7):945–952. [DOI] [PubMed] [Google Scholar]
- 9.Stephen J, Anderson-Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK. Metformin use in kidney transplant recipients in the United States: an observational study. Am J Nephrol . 2014;40(6):546–553. [DOI] [PubMed] [Google Scholar]
- 10.Kurian B, Joshi R, Helmuth A. Effectiveness and long-term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr Pract . 2008;14(8):979–984. [DOI] [PubMed] [Google Scholar]
- 11.Weir MR, Gravens-Muller L, Costa N, et al. Safety events in kidney transplant recipients: results from the folic Acid for vascular outcome reduction in transplant trial. Transplantation . 2015;99(5):1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant . 2014;14(9):1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasayama S, Tanaka T, Hashimoto K, Koga M, Kawase I. Efficacy of glimepiride for the treatment of diabetes occurring during glucocorticoid therapy. Diabetes Care . 2002;25(12):2359–2360. [DOI] [PubMed] [Google Scholar]
- 14.Turk T, Pietruck F, Dolff S, et al. Repaglinide in the management of new-onset diabetes mellitus after renal transplantation. Am J Transplant . 2006;6(4):842–846. [DOI] [PubMed] [Google Scholar]
- 15.Strom Halden TA, Asberg A, Vik K, Hartmann A, Jenssen T. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant . 2014;29(4):926–933. [DOI] [PubMed] [Google Scholar]
- 16.Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin Drug Saf . 2010;9(2):347–354. [DOI] [PubMed] [Google Scholar]
- 17.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med . 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 18.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. The lancet Diabetes & endocrinology . 2014;2(5):369–384. [DOI] [PubMed] [Google Scholar]
- 19.Sharif A Should metformin be our antiglycemic agent of choice post-transplantation? Am J Transplant . 2011;11(7):1376–1381. [DOI] [PubMed] [Google Scholar]
- 20.Larsen JL. Potential risks of metformin in transplant patients. Am J Transplant . 2012;12(3):795; author reply 796. [DOI] [PubMed] [Google Scholar]
- 21.Lalau JD, Arnouts P, Sharif A, De Broe ME. Metformin and other antidiabetic agents in renal failure patients. Kidney Int . 2015;87(2):308–322. [DOI] [PubMed] [Google Scholar]
- 22.Scientific Registry of Transplant Recipients. The Living Donor Collective: SRTR to Launch a Pilot Project to Create a Registry of Living Donors. https://www.srtr.org/news-publications/news/news-items/news/ Accessed February 8, 2017.
- 23.Lentine KL, Gheorghian A, Axelrod D, Kalsekar A, L'Italien G, Schnitzler MA. The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation . 2012;94(4):369–376. [DOI] [PubMed] [Google Scholar]
- 24.Gheorghian A, Schnitzler MA, Axelrod DA, Kalsekar A, L'Italien G, Lentine KL. The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation . 2012;94(3):241–249. [DOI] [PubMed] [Google Scholar]
- 25.Communication FDS. FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. 2017. [Google Scholar]
- 26.Madiraju AK, Erion DM, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature . 2014;510(7506):542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med . 1995;333(9):550–554. [DOI] [PubMed] [Google Scholar]
- 28.Bannister CA, Holden SE, Jenkins-Jones S, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab . 2014;16(11):1165–1173. [DOI] [PubMed] [Google Scholar]
- 29.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab . 2016;23(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA . 2007;298(17):2028–2037. [DOI] [PubMed] [Google Scholar]
- 31.Madhavan S, Stockwell D, Cohen H, Alderman MH. Renal function during antihypertensive treatment. Lancet . 1995;345(8952):749–751. [DOI] [PubMed] [Google Scholar]
- 32.Goldschmid MG, Barrett-Connor E, Edelstein SL, Wingard DL, Cohn BA, Herman WH. Dyslipidemia and ischemic heart disease mortality among men and women with diabetes. Circulation . 1994;89(3):991–997. [DOI] [PubMed] [Google Scholar]
- 33.Bailey CJ, Turner RC. Metformin. N Engl J Med . 1996;334(9):574–579. [DOI] [PubMed] [Google Scholar]
- 34.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One . 2012;7(3):e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science . 2005;310(5754):1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saisho Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr Metab Immune Disord Drug Targets . 2015;15(3):196–205. [DOI] [PubMed] [Google Scholar]
- 37.Bell DS. Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy. South Med J . 2010;103(3):265–267. [DOI] [PubMed] [Google Scholar]
- 38.Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care . 1999;22(6):925–927. [DOI] [PubMed] [Google Scholar]
- 39.Herrington WG, Levy JB. Metformin: effective and safe in renal disease? Int Urol Nephrol. 2008;40(2):411–417. [DOI] [PubMed] [Google Scholar]
- 40.Sambol NC, Chiang J, Lin ET, et al. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol . 1995;35(11):1094–1102. [DOI] [PubMed] [Google Scholar]
- 41.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev . 2010(4):CD002967. [DOI] [PubMed] [Google Scholar]
- 42.National Kidney F. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis . 2012;60(5):850–886. [DOI] [PubMed] [Google Scholar]
- 43.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care . 2011;34(6):1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gourishankar S, Hunsicker LG, Jhangri GS, Cockfield SM, Halloran PF. The stability of the glomerular filtration rate after renal transplantation is improving. J Am Soc Nephrol . 2003;14(9):2387–2394. [DOI] [PubMed] [Google Scholar]
- 45.Hardie DG. Adenosine monophosphate-activated protein kinase: a central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb Symp Quant Biol . 2011;76:155–164. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Gui Y, Ren J, et al. Metformin Protects Against Cisplatin-Induced Tubular Cell Apoptosis and Acute Kidney Injury via AMPKalpha-regulated Autophagy Induction. Sci Rep . 2016;6:23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cavaglieri RC, Day RT, Feliers D, Abboud HE. Metformin prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. Mol Cell Endocrinol . 2015;412:116–122. [DOI] [PubMed] [Google Scholar]
- 48.Lee SY, Moon SJ, Kim EK, et al. Metformin Suppresses Systemic Autoimmunity in Roquin(san/san) Mice through Inhibiting B Cell Differentiation into Plasma Cells via Regulation of AMPK/mTOR/STAT3. J Immunol . 2017;198(7):2661–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarrinpar A, Bensinger SJ. The Therapeutic Potential of T Cell Metabolism. Am J Transplant . 2017;17(7):1705–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maruthur NM, Tseng E, Hutfless S, et al. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann Intern Med . 2016;164(11):740–751. [DOI] [PubMed] [Google Scholar]
- 51.Alnasrallah B, Pilmore H, Manley P. Protocol for a pilot randomised controlled trial of metformin in pre-diabetes after kidney transplantation: the Transplantation and Diabetes (Transdiab) study. BMJ Open . 2017;7(8):e016813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


