Abstract
The effects of intravesical administration of a muscarinic receptor agonist (oxotremorine-M, OXO-M) and antagonist (atropine methyl nitrate, AMN) and of a nicotinic receptor agonist (nicotine) and antagonist (hexamethonium, C6) on reflex bladder activity were investigated in conscious female chronic spinal cord injured (SCI) cats using cystometry. OXO-M (50 μM) decreased bladder capacity (BC) for triggering micturition contractions, increased maximal micturition pressure (MMP), increased frequency and area under the curve of pre-micturition contractions (PMC-AUC). Nicotine (250 μM) decreased BC, increased MMP, but did not alter PMC-AUC. The effects of OXO-M on BC and PMC-AUC were suppressed by intravesical administration of AMN (50–100 μM), and the effects of nicotine were blocked by hexamethonium (1 mM). Antagonists infused intravesically alone did not alter reflex bladder activity. However, AMN (0.2 mg/kg, subcutaneously) decreased PMC-AUC. 8-OHDPAT (0.5 mg/kg, s.c.), a 5-HT1A receptor agonist, suppressed the OXO-M-induced decrease in BC but not the enhancement of PMC-AUC. These results indicate that activation of cholinergic receptors located near the lumenal surface of the bladder modulates two types of reflex bladder activity (i.e., micturition and pre-micturition contractions). The effects may be mediated by activation of receptors on suburothelial afferent nerves or receptors on urothelial cells which release transmitters that can in turn alter afferent excitability. The selective action of nicotine on BC, while OXO-M affects both BC and PMC-AUC, suggests that micturition reflexes and PMCs are activated by different populations of afferent nerves. The selective suppression of the OXO-M effect on BC by 8-OH-DPAT without altering the effect on PMCs supports this hypothesis. The failure of intravesical administration of either AMN or hexamethonium alone to alter bladder activity indicates that cholinergic receptors located near the lumenal surface do not tonically regulate bladder reflex mechanisms in the SCI cat.
Keywords: Bladder reflex, Afferent nerve, Spinal injury, Micturition, Cholinergic receptor, Nicotine, Urothelium
1. Introduction
The normal functions of the urinary bladder to store and eliminate urine are controlled by complex neural pathways in the brain and spinal cord (de Groat et al., 2015). Following spinal cord injury (SCI), a prominent reorganization of the neural pathways occurs in the spinal cord, leading to neurogenic detrusor overactivity (NDO), detrusor–sphincter dyssynergia (DSD), and decreased voiding efficiency accompanied by high voiding pressures (de Groat and Yoshimura, 2006).
Plasticity in peripheral efferent pathways may contribute to NDO. For example, upregulation of post-junctional muscarinic excitatory mechanisms in the bladder smooth muscle (Braverman et al., 1999) and enhancement of pre-junctional muscarinic facilitatory mechanisms in parasympathetic nerves has been observed in the bladders of SCI animals (Somogyi and de Groat, 1999). Thus, antimuscarinic drugs which are used to treat NDO may act by targeting receptors at multiple sites in efferent pathways to the bladder (de Groat et al., 2015).
Cholinergic mechanisms in bladder sensory pathways might also be involved in the initiation of bladder dysfunction after SCI. Activation of cholinergic receptors near the lumenal surface of the bladder by intravesical administration of muscarinic (Kullmann et al., 2008b; Matsumoto et al., 2010) or nicotinic cholinergic receptor agonists (Beckel et al., 2006; Beckel and Birder, 2012; Masuda et al., 2006) modulates reflex bladder activity in spinal cord intact and in chronic spinal cord transected rats (Matsumoto et al., 2012). These effects are attributed to changes in bladder afferent firing. Bladder afferent neurons exhibit multiple subtypes of muscarinic (Nandigama et al., 2010) and nicotinic receptors (Nandigama et al., 2013), and bladder afferent nerves respond to muscarinic (Aizawa et al., 2015; Yu and de Groat, 2010) and nicotinic receptor agonists (Kontani et al., 2009; Yu et al., 2016). Urothelial cells also express muscarinic (Chess-Williams, 2002; Kullmann et al., 2008a; Tyagi et al., 2006) and nicotinic receptors (Beckel et al., 2006; Beckel and Birder, 2012), and chemical or mechanical stimulation of these cells releases neurotransmitters such as acetylcholine (Lips et al., 2007; Hanna-Mitchell et al., 2007; Yoshida et al., 2008), ATP (Ferguson et al., 1997; Kullmann et al., 2008b), and nitric oxide (Birder et al., 1998) that potentially can interact with adjacent afferent nerves to enhance or suppress afferent excitability and in turn influence reflexes to the lower urinary tract (Birder and Andersson, 2013; Cockayne et al., 2000; Kullmann et al., 2008b; Yu and de Groat, 2008).
In the present experiments, we evaluated the role that urothelial or suburothelial cholinergic receptors might play in the initiation of reflex bladder activity after SCI in the cat by examining the effects of intravesical application of a muscarinic (oxotremorine-M, OXO-M) or a nicotinic receptor agonist (nicotine) and the effects of their respective antagonists (atropine methyl nitrate and hexamethonium). It is known that C-fiber bladder afferent nerves are responsible for triggering the micturition reflex in SCI cats (Cheng et al., 1999; de Groat et al., 1981, 1990), while Aδ afferents are essential for triggering micturition in cats with an intact spinal cord (de Groat and Ryall, 1969; de Groat et al., 1981). Thus, these experiments in SCI cats allowed us to examine the role of cholinergic receptors in regulating C-fiber evoked bladder reflexes. In addition, we determined if the effects of muscarinic receptor activation on bladder reflexes are influenced by 8-OH-DPAT, a 5-HT1A receptor agonist, which we showed in a previous study increased the bladder volume threshold for triggering micturition reflex contractions but did not affect pre-micturition contractions (PMC) in SCI cats (Tai et al., 2006). The results of the present study indicate that these two parameters of reflex bladder activity in SCI cats are selectively modulated by two distinct populations of bladder C-fiber afferent nerves that are distinguishable based on their different sensitivities to nicotine and by the sensitivity to 8-OH-DPAT of the spinal interneuronal pathways activated by these afferents. Preliminary reports of these data have appeared in abstracts (Ungerer et al., 2005, 2006).
2. Materials and methods
2.1. Spinal cord transection
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee of the University of Pittsburgh. The spinal cords of seven female cats (3.2–3.8 kg) were transected under isoflurane anesthesia using aseptic surgical techniques. After performing a dorsal laminectomy at T9–T10 vertebral level, a local anesthetic (lidocaine 1%) was applied to the surface of the spinal cord and then injected into the cord through the dura. The dura and spinal cord were then cut completely (T9–T10 spinal segment), and a piece of gel foam was placed between the cut ends (usually a separation of 2–3 mm). The muscle and skin were sutured and after full recovery from anesthesia, the animal was returned to its cage. Following spinal transection, the bladder was emptied by manual expression at least twice daily. If manual expression was not successful, a sterile catheter (3.5F) was inserted through the urethra to empty the bladder. Ketoprofen (2 mg/kg twice a day for 3 days) and an antibiotic (enrofloxacin, 2.5 mg/kg for 7 days) were administered following surgery. Beginning 4–5 weeks following spinal cord transection, animals were used for pharmacological experiments.
2.2. Drugs
Stock solutions of oxotremorine-M (1 mM), nicotine bitartrate (10 mM), hexamethonium chloride (50 mM), atropine methyl nitrate (50 mM), and (±) 8-hydroxy-2-di-N-propylaminotetralin hydrobromide (8-OH-DPAT) were prepared in distilled water and diluted in 0.9% sterile saline prior to administration. All drugs were obtained from Sigma–Aldrich Co. The drugs were infused intravesically in concentrations that were determined in pilot experiments or were selected based on concentrations shown in previous studies in rats to be effective in altering reflex bladder activity (Kullmann et al., 2008b; Masuda et al., 2006; Matsumoto et al., 2010, 2012) or bladder afferent nerve activity (Yu and de Groat, 2010; Yu et al., 2016) after intravesical administration.
2.3. Isovolumetric cystometrograms (CMGs) in conscious SCI cats
A sterile 7 French double lumen balloon catheter (2 cm distance from the catheter tip to the balloon) was inserted through the urethra into the bladder of the chronic SCI cats without anesthesia. The balloon was distended in the bladder by 2 ml of air and then positioned at the bladder neck by gently withdrawing the catheter. The balloon prevented leakage of fluid from the bladder. One lumen of the catheter was connected to a pump to infuse the bladder with sterile saline at a rate of 2–4 ml/min and the other lumen was connected to a pressure transducer that was connected to a computerized data acquisition system running Windaq software (DATAQ Instruments Incorporated, Akron, OH, USA) to record changes in bladder pressure. The rate of filling was usually 2 ml/min but was increased in some experiments to 4 ml/min to reduce filling time if bladder capacity (BC) was very large. In a few experiments in which both filling rates were tested the rate of filling did not markedly affect BC. The catheterized animals rested comfortably in a padded small animal transport carrier during the experiment (usually 5–6 h), and the catheter was withdrawn at the end of the experiment. After each experiment, the cat was given 150 mg/ kg of ampicillin subcutaneously. The cats were used for multiple experiments at 1- to 4-week intervals.
OXO-M, nicotine, hexamethonium, and AMN were administered by intravesical infusion. AMN and 8-OH-DPAT dissolved in 0.5 ml of 0.9% sterile saline were administered by subcutaneous injection. Control saline CMGs were repeated at least 2–3 times to evaluate reproducibility before administering drugs. The bladder was emptied by aspiration before the beginning of each CMG. The infusion pump was usually stopped after the first micturition contraction occurred. A saline rinse (approximately 20 ml infused at 5 ml/min) and a slow-filling saline CMG were performed after each infusion of agonist (OXO-M or nicotine) to determine if the agonist effect could be reversed. Antagonists were administered alone either intravesically or subcutaneously to determine the effect of receptor blockade on reflex activity in untreated bladders or alone prior to infusion of an antagonist–agonist combination in order to demonstrate blockade of agonist evoked responses. In some experiments (n = 5), OXO-M was administered intravesically before and after subcutaneous injection of 8-OH-DPAT (0.5 mg/kg), which was shown in previous experiments to increase BC in chronic SCI cats (Gu et al., 2004; Tai et al., 2006).
2.4. Isovolumetric CMGs in anesthetized SCI cats
Terminal experiments were conducted on three animals anesthetized with α-chloralose (60 mg/kg, i.v., supplemented as needed) following induction with isoflurane (2% in O2). Systemic blood pressure was monitored via a catheter placed in the right carotid artery. A tracheotomy was performed and a cannula was inserted to secure the airway. A catheter for i.v. infusion was introduced into the right cephalic vein. The ureters were cut and drained externally, and a catheter for drug infusion was inserted through the bladder dome and secured with a purse string suture. Drugs were administered intravenously or by intravesical infusion. Bladder activity was recorded with the same methods used in the conscious animals. The temperature of the animal was maintained between 35 and 37 °C using a heating pad. Data were recorded on a computer using the LabVIEW program (National Instruments Corporation, Austin, TX, USA).
2.5. Data analysis
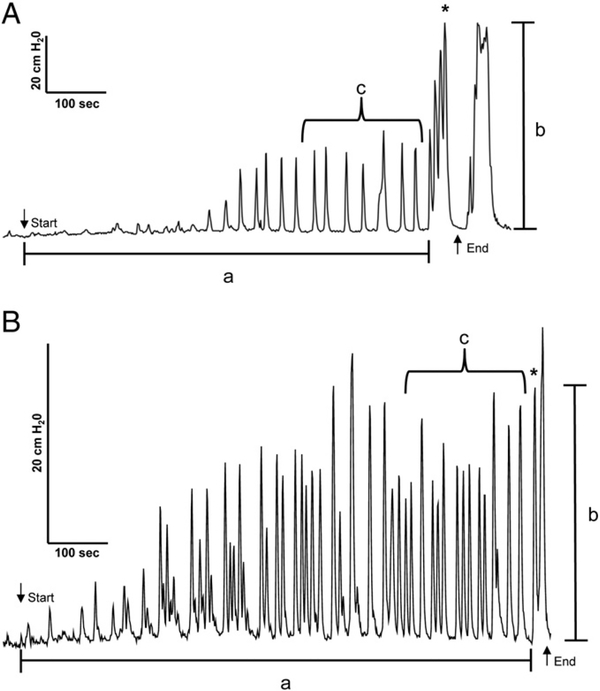
To evaluate the effect of drugs on reflex bladder activity, several parameters (Fig. 1) were measured during CMGs including the bladder volume threshold for triggering a micturition reflex contraction (“a,” BC), maximal micturition pressure (“b,” MMP), and the frequency and area under the curve (cm H2O•sec) of pre-micturition contractions (“c,” PMC-AUC). BC is defined as the bladder volume during a CMG at which the first micturition contraction occurred. The first micturition contraction is defined as a large amplitude (>25 cm H2O), long duration (>20 s) reflex bladder contraction that is accompanied by hind limb stepping movements. Previous studies (Tai et al., 2006; Thor et al., 1983) showed that hind limb movements are a useful surrogate marker for the occurrence of a micturition reflex when CMGs are conducted with a closed urethral outlet that prevents voiding. The rhythmic hind limb movements were also elicited by manual compression of the bladder when attempting to express urine during daily nursing care or during voiding induced by tactile stimulation of the perigenital region. However, the leg movements did not occur during the small amplitude, short duration contractions (i.e., the pre-micturition contractions, PMCs). Because the present experiments were performed with the urethral outlet closed which prevents elimination of the bladder contents, we used the simultaneous occurrence of a large amplitude bladder contraction and hind limb movements as an indicator of the first micturition reflex during the cystometrogram. This method of defining the volume threshold for micturition (i.e., BC) was especially useful in those experiments where pre-micturition contractions gradually increased in amplitude during the cystometrogram (Fig. 1B) in contrast to experiments where the initiation of a micturition reflex was obvious due to an abrupt large increase in the amplitude of the bladder contractions (Fig. 1A). In anesthetized animals, in which the bladder catheter was inserted through the bladder dome, the micturition reflex was indicated by release of fluid from the urethral orifice.
Fig. 1.
A and B. Diagram showing parameters of bladder activity measured during cystometrograms in two conscious chronic spinal cord injured cats. a, Bladder capacity (BC), the volume infused intravesically to induce a micturition reflex contraction that was identified by coincident reflex contractions of the hind legs. b, Maximal micturition pressure (MMP). c, Pre-micturition contraction (PMC) frequency or area under curve (PMC-AUC) measured for a period of 200 s. prior to the micturition reflex contraction. Down arrow indicates start of intravesical infusion. Up arrow indicates end of intravesical infusion. * denotes the first micturition reflex contraction. A. Tracing shows a large difference between the amplitude of PMCs and the first micturition reflex contraction. A second micturition contraction occurred after the end of intravesical infusion. B. Tracing from another experiment shows that the largest amplitude PMCs can be similar in amplitude to that of the first micturition reflex contraction. Both tracings show PMCs gradually increase in amplitude during intravesical infusion.
The small amplitude (8–24 cm H2O) PMCs that occurred during bladder filling prior to the large amplitude micturition contraction were measured for 200 s prior to the micturition contraction. This length of time was chosen because it was the average period during which PMCs occurred under control conditions. Area under the curve for PMCs was chosen because it reflected both the increased amplitude and frequency of the contractions. Each measured parameter after drug administration was normalized to its control value before drug administration in each experimental trial. The normalized parameter was then expressed as a percentage change from control and was averaged across different experiments. Repeated-measures one-way ANOVA followed by Bonferroni multiple comparison test or paired or unpaired t-tests were used to determine statistically significant differences at p b 0.05. Data are reported as the mean ± SEM and were compiled and statistically analyzed using Prism 4 software (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Reflex bladder activity in conscious animals
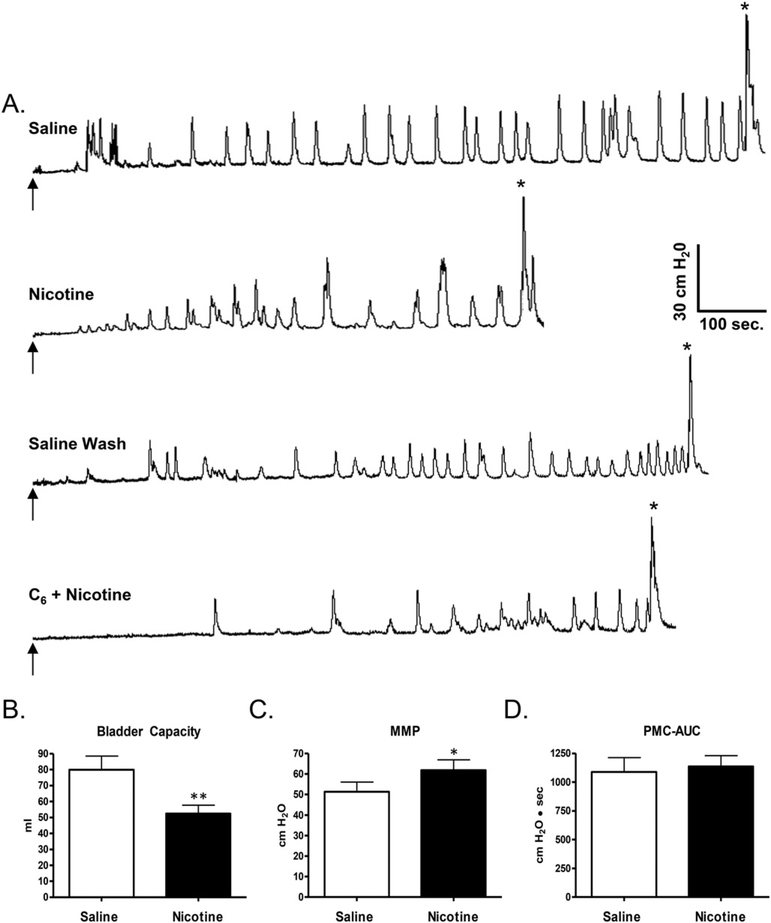
Repeated experiments (total 80) at intervals greater than 1 week were performed in seven conscious adult female cats over periods ranging from 2 to 17 months (mean 10.5 months) after complete transection of the T9–T10 spinal cord. Infusion of saline into the bladder at a rate of 2–4 ml/min when the bladder neck was blocked with a Foley catheter produced an immediate small increase in baseline intravesical pressure (3–8 cm H2O) and three types of phasic bladder activity: (1) low amplitude (8–24 cm H2O), transient (10–20 s duration) increases in intravesical pressure (PMCs) that occurred at frequencies ranging between 0.5 and 3/min during the infusion in the absence of hind limb movements (Fig. 1), (2) large amplitude (25–100 cm H2O), longer duration (20–40 s) increases in pressure (micturition contractions) that usually occurred after infusion of a large volume of saline (25–125 ml) and were accompanied by rhythmic alternating movements of the hind limbs resembling stepping movements and (3) large amplitude rhythmic contractions (40–100 cm H2O in amplitude) that persisted after the bladder infusion was stopped at the end of a cystometrogram (Fig. 1).
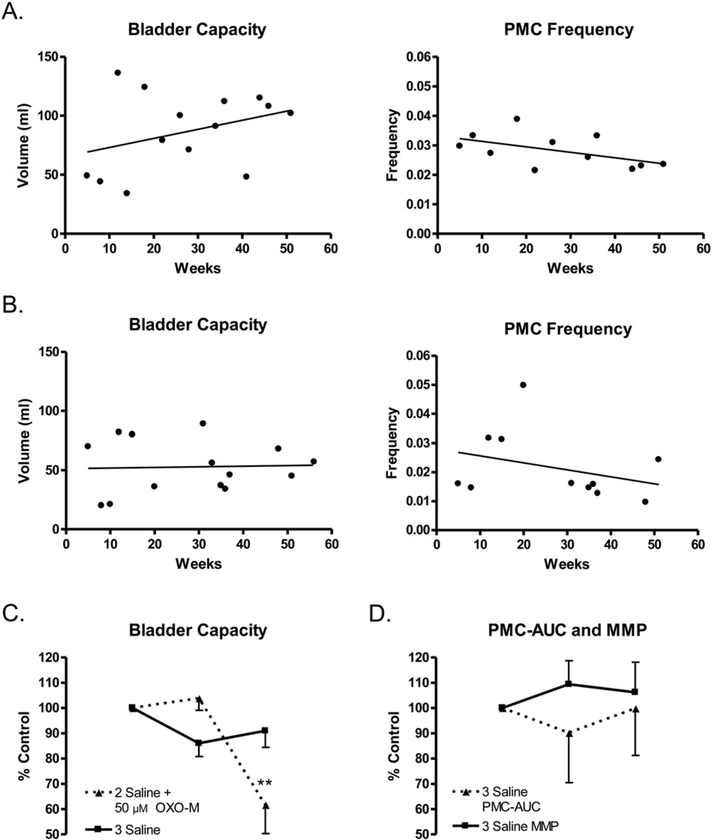
BC and PMC frequency during saline CMGs commonly varied considerably in experiments performed on different days in the same animal (Fig. 2A and B). However, when saline CMGs were repeated at 20– 30 min intervals on the same day bladder capacities were relatively constant varying b15% (Fig. 2C). During repeated experiments performed over the course of 1 year, BC tended to increase and PMC frequency tended to decrease with time in 5 animals (see example for 1 animal in Fig. 2A) but in 1 animal the average BC remained constant during the duration of the study (data for 1 animal shown in Fig. 2B). The PMCs appeared at a bladder volume that ranged from 20 to 75% of the volume threshold for triggering the large amplitude micturition reflex and in most experiments (N80%) gradually increased in amplitude as the bladder was filled, occurring at frequencies ranging between 1 and 3/min (Figs. 1 and 3A). Some animals exhibited many PMCs (maximal 20–30 during filling, Fig. 1) and others had relatively few PMCs (n = 5–10, Fig. 3A). Animals with small bladder capacities commonly had fewer PMCs. Once PMCs began, they continued until a micturition contraction occurred. Bladder capacities (mean, 71.4 ± 7.6 ml, range 16– 125 ml) and maximal micturition contraction pressures (MMP, mean, 40.4 ± 3.9 cm H2O, range 25–100 cm H2O; Table 1) varied between animals.
Fig. 2.
A and B. Variability in measurements of BC and PMC frequency obtained over a period of 5–50 weeks after cord transection during cystometrograms (CMGs) repeated at intervals ranging from 1 to 4 weeks in two conscious chronic spinal cord injured cats. One cat (A) shows a trend of increased BC over time while the other cat (B) does not show this trend. Both cats showed a trend of decreasing PMC frequency over time. Frequency is expressed as events per second. C. Graph (■▬■) showing the variation in BC during three consecutive saline CMGs (infusion rate 2–4 ml/min) repeated at 10–20 min intervals in conscious spinal cord injured cats (n = 9 experiments). Vertical axis represents percentage change of BC between the first CMG and the second and third CMGs. BCs during the three saline CMGs were not significantly different (p N 0.05, repeated-measures ANOVA and Bonferroni’s multiple comparison test). However, intravesial infusion of OXO-M (▲--▲, 50 μM, n = 13 experiments) following two saline CMGs significantly reduced BC (** indicates a significant difference, p < 0.001, repeated-measures ANOVA and Bonferroni’s multiple comparison test). D. Graphs showing that MMP (■▬■) and PMC-AUC (▲--▲) do not significantly change during three consecutive saline CMGs (n = 9 experiments, p > 0.05, repeated-measures ANOVA and Bonferroni’s multiple comparison test).
Fig. 3.
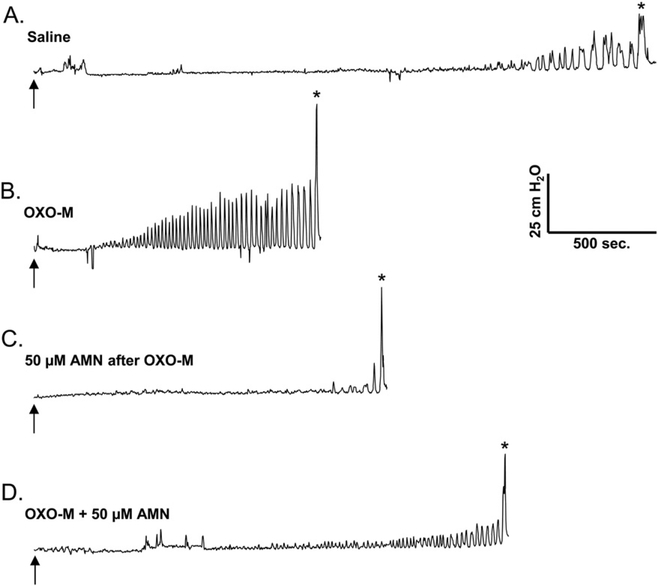
Effect of intravesical infusion of OXO-M (50 μM) on reflex bladder activity in a conscious spinal cord injured cat. A. Control saline CMG. Arrow indicates the start of infusion (2 ml/min). * indicates the first micturition contraction. B. Infusion of OXO-M reduces BC, increases MMP and increases the frequency and amplitude of pre-micturition contractions (PMCs). C. Intravesical infusion of atropine methyl nitrate (AMN, 50 μM) reverses the excitatory effect of OXO-M on PMCs and increases BC. D. Subsequent intravesical infusion of AMN (50 μM) in combination with OXO-M suppresses the facilitatory effect of OXO-M on BC and PMCs. All recordings are from the same experiment.
Table 1.
Effects of intravesical administration of OXO-M on measured voiding parameters in conscious cats.
| Parameters | Saline | Saline | OXO-M (50 μM) |
|---|---|---|---|
| BC (ml) | 71.4 ± 7.56 | 74.1 ± 7.95 | 44.1 ± 5.12** |
| MMP (cm H2O) | 40.4 ± 3.94 | 40.0 ± 4.50 | 56.8 ± 5.83* |
| PMC-AUC (cm H2O·sec) | 998.2 ± 111.1 | 1030 ± 130.0 | 1636 ± 247.2* |
Data are shown as mean ± standard error of the mean. Data are from 13 experiments for bladder capacity (BC) and maximum micturition pressure (MMP) and from 12 experiments for pre-micturition contraction–area under curve (PMC-AUC). Statistical significance was tested using repeated-measures ANOVA followed by Bonferroni’s multiple comparison test. Asterisks represent significant changes (*p < 0.05, **p < 0.001) between the first saline control values and the oxotremorine-M (OXO-M) values. The values for the two saline controls were not significantly different (p > 0.05).
3.2. Effects of OXO-M on reflex bladder activity in conscious animals
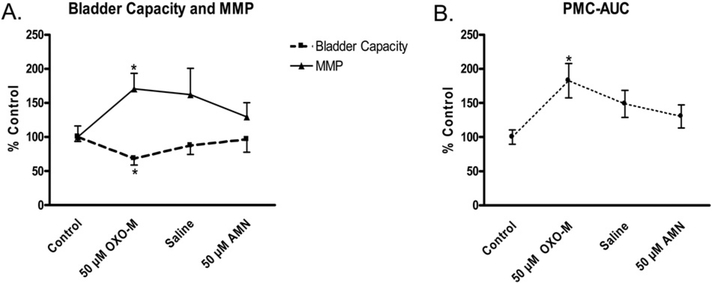
In preliminary experiments, a range of concentrations of OXO-M (10, 25, and 50 μM) were infused intravesically to establish the concentration to be used in this study. OXO-M 50 μM produced the most consistent effects among these three concentrations. This concentration of OXO-M usually did not change baseline pressure throughout the CMG but did alter reflex activity. OXO-M reduced BC (mean, 34.6 ± 6.29%; p b 0.001; n = 13; Figs. 2C, 3B; Table 1) and increased the MMP (mean, 52.7 ± 19.7%; p < 0.05; n = 13; Fig. 3B; Table 1). OXO-M also reduced the volume threshold for inducing PMCs, increased PMC frequency (mean, 89.8 ± 36.2% increase; 11 of 12 experiments; Fig. 3B) and amplitude of PMCs (mean, 80.7 ± 31.2% increase; 7 of 12 experiments; Fig. 3B), and increased the PMC area under the curve (PMC-AUC; mean, 69.4 ± 22.7% increase; p < 0.05; n = 12; Table 1).
After emptying the bladder and flushing the bladder with saline, a control saline CMG was performed to determine if the effects of OXOM were rapidly reversible (Figs. 4, 7–10 experiments). BC (Fig. 4A), MMP (Fig. 4A), and the PMC-AUC (Fig. 4B) were not significantly lower (p > 0.05) than the measurements during OXO-M application.
Fig. 4.
Changes in BC, maximal micturition pressure (MMP), and pre-micturition contraction–area under the curve (PMC-AUC) during a series of cystometrograms (2 ml/min, infusion rate) consisting of initial saline control followed by OXO-M (50 μM), second saline control and AMN (50 μM). OXO-M (50 μM) produced a statistically significant change in the three parameters compared to the initial saline controls (p < 0.05, repeated-measures ANOVA and Bonferroni’s multiple comparison test, n = 7–10 experiments). However, the measurements during second saline and AMN cystometrograms were not significantly different (p > 0.05) from those during OXO-M infusion
Fig. 7.
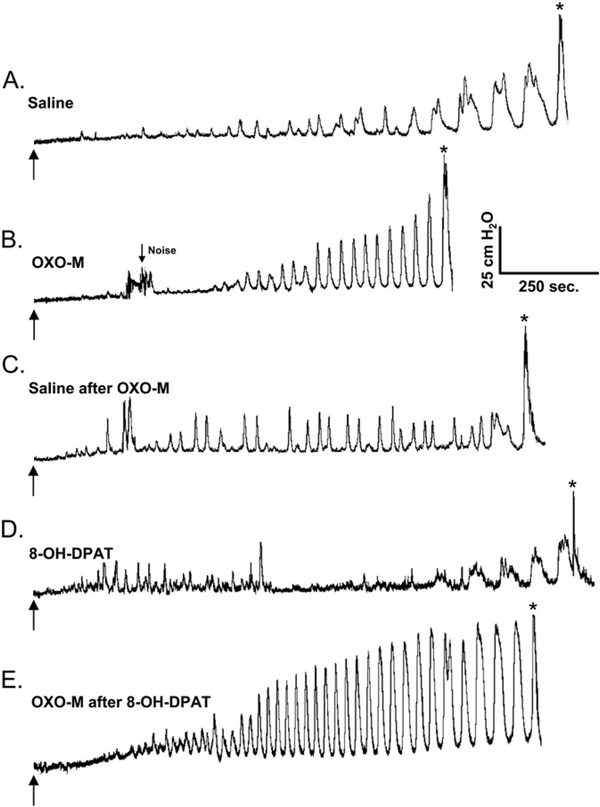
The influence of subcutaneous administration of 8-OH-DPAT (0.5 mg/kg,) on the effects of intravesical OXO-M (50 μM) on reflex bladder activity in a conscious SCI cat. A. Control saline CMG. Arrow indicates start of infusion (2 ml/min). * indicates the first micturition contraction. B. Infusion of OXO-M reduces BC and increases the frequency of PMCs. C. Saline infusion partially reverses the effects of OXO-M. D. Administration of 8-OH-DPAT increases BC and normalizes PMCs. E. Infusion of OXO-M after administration of 8-OH-DPAT increases the frequency and amplitude of PMCs but has a minimal effect on BC. All recordings are from the same experiment.
Fig. 10.
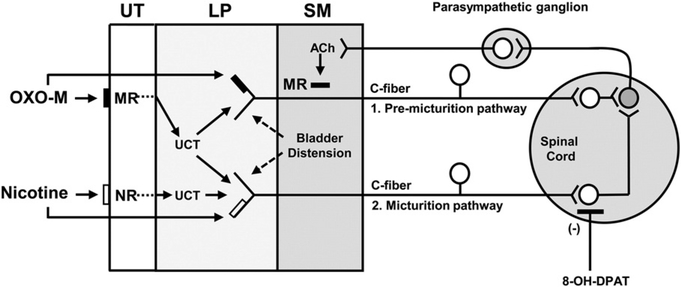
Diagram showing possible C-fiber primary afferent pathways controlling bladder reflexes in the spinal cord injured cat and their modulation by activation of muscarinic, nicotinic, and serotonergic receptors. 1. Population of afferents responsible for eliciting pre-micturition contractions. 2. Population of afferents responsible for eliciting micturition contractions. Primary afferents synapse with spinal excitatory interneurons (white) which have excitatory synaptic connections with a parasympathetic preganglionic neuron (shaded) which in turn projects to a peripheral parasympathetic ganglion. Note that OXO-M stimulates both types of afferents either by directly activating muscarinic receptors (▬) on the afferent nerves or indirectly by activating muscarinic receptors (▬) on urothelial cells which in turn release urothelial cell transmitters (UCT) that stimulate afferent nerves. Nicotine reduces BC without affecting PMC activity by directly stimulating nicotinic receptors (□) on type 2 afferent nerves or stimulating receptors (□) on the urothelial cells which in turn release urothelial cell transmitters (UCT) that stimulate type 2 afferent nerves to reduce BC without affecting PMC activity. 8-OH-DPAT selectively targets spinal interneuronal circuitry (white neuron) activated by type 2 afferents to suppress type 2 afferent input to the preganglionic neuron and thereby suppresses the OXO-M-induced reduction in BC without affecting the OXO-M-induced changes in PMC activity. UT = urothelium, LP = lamina propria, SM = smooth muscle, MR = muscarinic receptor, NR = nicotinic receptor, UCT = urothelial cell transmitter.
3.3. Effect of a muscarinic receptor antagonist on the responses to OXO-M
The effect of AMN, a muscarinic receptor antagonist, on the response to OXO-M (50 μM) was examined in two types of experiments: (1) by administering AMN (50 μM) following OXO-M (Figs. 3C and 4) and (2) by administering OXO-M before and after two concentrations of AMN (50 μM and 100 μM, Fig. 5). In the first set of experiments, the effect of AMN (50 μM) was variable, ranging from no effect to a reduction or complete reversal (Fig. 3C) of the effect of OXO-M on PMC-AUC (10 experiments). The mean reduction in the OXO-M effect was 28.6% (Fig. 4B); however, this change was not statistically significant (p > 0.05). In these experiments, AMN also produced a non-significant (p > 0.05) decrease in the effects of OXO-M on BC and MMP (41.1% increase and 24.1% decrease, respectively, Figs. 3C, 4A).
Fig. 5.
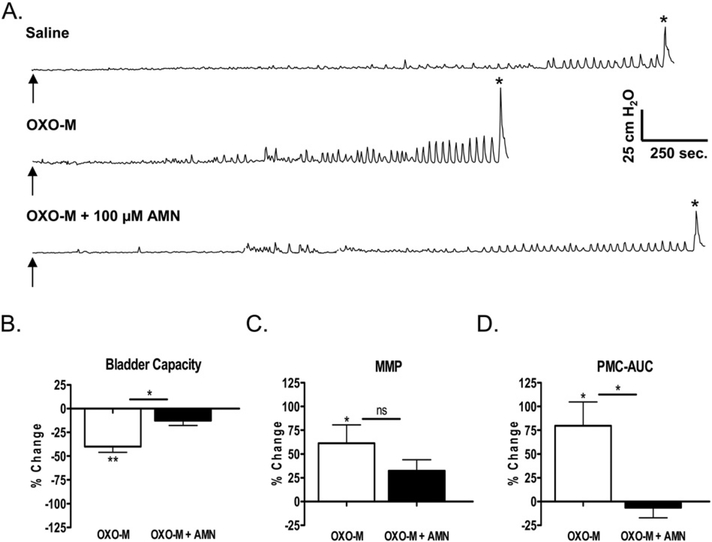
Effect of intravesical infusion of OXO-M (50 μM) and AMN on reflex bladder activity in conscious spinal cord injured cats. A. Top tracing, control saline CMG. Arrow indicates the start of infusion (2 ml/min). * indicates the first micturition contraction. Middle tracing, infusion of OXO-M reduces BC, increases MMP, and increases the amplitude of pre-micturition contractions (PMCs). Bottom tracing, infusion of AMN (100 μM) in combination with OXO-M completely blocks the effects of OXO-M. All recordings are from the same experiment. B, C, and D. Graphs summarizing the effects of AMN (50 μM or 100 μM) on the changes in CMG parameters elicited by intravesical infusion of OXO-M (50 μM). The effects of the two AMN concentrations were not significantly different; therefore, for statistical analyses, we combined the data from experiments in which either concentration of AMN was tested. Effects of OXO-M in untreated preparations (white bars) or when administered in combination with AMN (black bars) represent the percentage change from the measurements during an initial saline control CMG (n = 12 experiments in B and C, 11 experiments in D). The differences between the effects of saline and OXO-M before and after AMN were compared using repeated-measures ANOVA and Bonferroni’s multiple comparison test. Compared to saline control, OXO-M significantly decreased BC (p < 0.001) and significantly increased MMP and PMC-AUC (p < 0.05). AMN treatment significantly (p < 0.05) reduced the effects of OXO-M on BC and PMC-AUC but did not significantly suppress the effect on MMP.
In the second set of experiments (n = 11 or 12) when OXO-M was administered in combination with either 50 μM (Fig. 3D) or 100 μM AMN (Fig. 5A, lower tracing), the effects of OXO-M were reduced compared to the effects of the initial application of OXO-M. Because the effects of the two concentrations of AMN were similar, the data from these experiments were combined for statistical analysis. As shown in Fig. 5B, C, and D, AMN significantly reduced (p < 0.05) the effect of the second application of OXO-M on BC and PMC-AUC and produced a non-significant (p > 0.05) decrease in the effect on MMP.
3.4. Effect of intravesical and subcutaneous administration of a muscarinic receptor antagonist on reflex bladder activity
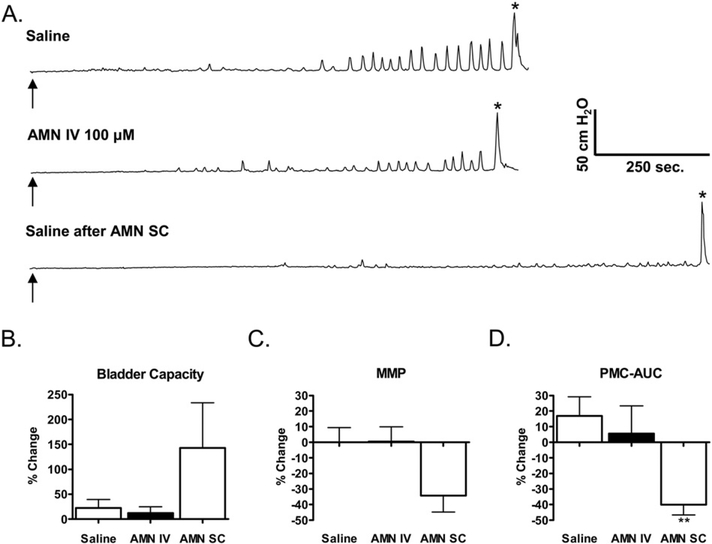
AMN (100 μM) administered intravesically alone (n = 5 experiments) did not alter BC, MMP, or PMC-AUC (Fig. 6A-D). AMN also did not cause any detectable systemic effects such as pupillary dilation. In contrast, subcutaneous injection of AMN (0.2 mg/kg, n = 11 experiments) which elicited pupillary dilation within 5 min after administration, significantly reduced the PMC-AUC (mean, 40.0 ± 6.6% decrease; p < 0.05; n = 11, Fig. 6A, D) and produced non-significant increases in BC (142.7 ± 90.7% increase, n = 9) and decreases in MMP (34.3 ± 10.5% decrease, n = 8, Fig. 6A–C) during saline CMGs. The effects of subcutaneous AMN were long lasting, persisting for the duration of the experiment (4–5 h). When infused after subcutaneous injection of AMN, intravesical administration of OXO-M (n = 7) had no detectable effect on either PMCs or BC, but decreased MMP (59.9 ± 9.7% decrease, p < 0.05; data not shown).
Fig. 6.
The effects of atropine methyl nitrate (AMN) on reflex bladder activity following sequential intravesical (IV) and subcutaneous (SC) administration in the same experiments. A. Representative CMGs in a conscious SCI cat. Top tracing, control saline CMG. Arrow indicates the start of infusion. * indicates the first micturition contraction. Middle tracing, intravesical infusion of AMN (100 μM). Bottom tracing, saline infusion following AMN (0.2 mg/kg SC). Arrows indicate start of infusions. * denotes first micturition reflex. B, C, and D. Summaries of the effects of IV and SC AMN on three CMG parameters: BC, maximal micturition pressure (MMP) and PMC-AUC. Data are plotted as the percentage change in these parameters compared to measurements in the first control saline CMG before administration of the drug. The saline bar shows the difference between the first and second saline control CMGs. Numbers of experiments range from 5 to 11. IV AMN did not significantly change any parameter (unpaired t-test). SC AMN only significantly reduced PMC-AUC. ** indicates a significant difference (p b 0.006, paired t-test) between the SC administration of AMN and the saline control CMGs.
3.5. Interactions between OXO-M and 8-OH-DPAT
OXO-M (50 μM) was administered intravesically before and after the subcutaneous injection of 8-OH-DPAT in 5 experiments. The systemic effects of subcutaneous injection of 8-OH-DPAT which were observed within 10 min of injection included pupillary dilation and increased alertness. Saline CMGs performed 15–20 min after injection of 8-OHDPAT were characterized by a significant (p < 0.01) increase in BC (Fig. 7D), a significant decrease in MMP (p < 0.05), and a non-significant decrease in the PMC-AUC (Table 2). When OXO-M was administered intravesically after subcutaneous injection of 8-OH-DPAT (Fig. 7E), the PMC-AUC and MMP significantly increased (p < 0.05, Table 2), while the change in BC was not significant (p > 0.05).
Table 2.
The effect of 8-OH-DPAT on the responses to intravesical administration of OXO-M.
| Parameters | Saline | OXO-M (50 μM) | 8-OH-DPAT (0.5 mg/kg) | OXO-M (50 μM) |
|---|---|---|---|---|
| BC (ml) | 68.2 ± 7.66 | 38.6 ± 6.5 | 87.4 ± 15.0a | 53.8 ± 9.79 |
| MMP (cm H2O) | 52.7 ± 7.92 | 72.8 ± 9.85b,d | 53.0 ± 6.17 | 75.8 ± 8.01c,d |
| PMC-AUC (cm H2O·sec) | 1620 ± 564.3 | 2218 ± 450.4 | 1409 ± 68.8 | 3774 ± 383.3c,e,f |
Data are shown as mean ± standard error of the mean. Data are from 5 experiments for bladder capacity (BC), maximum micturition pressure (MMP), and pre-micturition contraction– area under curve (PMC-AUC). Statistical significance was tested using repeated-measures ANOVA followed by Bonferroni’s multiple comparison test.
indicates significant difference (p < 0.01) from first OXO-M value.
indicates significant difference (p < 0.05) from saline.
indicates significant difference (p < 0.01) from saline.
indicates significant difference (p < 0.05) from 8-OH-DPAT.
indicates significant difference (p < 0.05) from first OXO-M.
indicates significant difference (p < 0.01) from 8-OH-DPAT.
3.6. Effects of nicotine and hexamethonium on reflex bladder activity in conscious animals
Intravesical administration of nicotine (n = 16 experiments) in a concentration of 250 μM significantly (p < 0.001) reduced BC by 31.8 ± 4.2% (from a control of capacity of 79.9 ml; Fig. 8A and B). The effect of nicotine on BC occurred in every experiment and was partially reversed (28% increase in capacity, n = 14 experiments) by flushing the bladder with saline (Fig. 8A). Nicotine also significantly increased MMP Fig. 8C; mean, 24.3 ± 6.4% increase; p < 0.05) but did not significantly change PMC-AUC (Fig. 8D; mean, 19.9 ± 13.5% increase; p > 0.05). Nicotine did not significantly change (p > 0.05) BC, MMP, or PMC-AUC after intravesical administration of a nicotinic receptor antagonist, hexamethonium (1 mM, n = 4–6 experiments, Fig. 8A). Hexamethonium alone did not alter baseline cystometric parameters.
Fig. 8.
A. Effect of intravesical infusion of nicotine (250 μM) on reflex bladder activity in a conscious spinal cord injured cat. Top tracing, control saline CMG. Arrow indicates the start of infusion (2 ml/min).* indicates the first micturition contraction. Second tracing, infusion of nicotine reduces BC but does not influence the frequency or amplitude of pre-micturition contractions. Third tracing, intravesical infusion of saline reverses the effect of nicotine. Bottom tracing, intravesical infusion of hexamethonium (C6, 1 mM) in combination with nicotine suppresses the nicotine-induced reduction in BC. All recordings are from the same experiment. B, C, and D. Graphs summarizing the effects of nicotine (250 μM) on the CMG parameters (n = 16). Intravesical infusion of nicotine significantly reduced bladder capacity (B, p < 0.001, paired t-test), significantly increased MMP (C, p < 0.05, paired t-test) but did not significantly change PMC-AUC (D, p > 0.05, paired t-test).
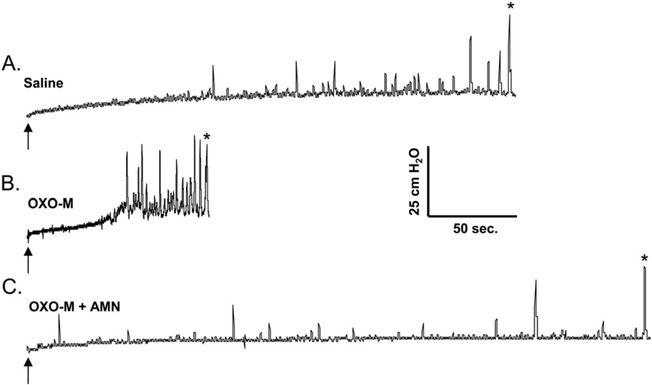
3.7. Effects of OXO-M and AMN on reflex bladder activity in anesthetized animals
Anesthetized animals had smaller bladder capacities (mean, 12.7 ± 2.6 ml; n = 3) than conscious animals (mean, 71.4 ± 7.6 ml). OXO-M (50 μM) reduced BC (77% decrease), increased PMC amplitude, reduced the volume threshold for inducing PMCs, and increased PMC-AUC (58% increase), while not altering MMP (Fig. 9B). The effects on BC and PMCs were suppressed by intravesical administration of 50 μM AMN in combination with OXO-M (Fig. 9C, n = 2). Mean blood pressure was not changed by intravesical application of AMN or OXO-M; however, when administered intravenously in one experiment, 10 μg/kg of OXO-M significantly reduced systolic blood pressure (from 120 to 60 mmHg).
Fig. 9.
OXO-M stimulates reflex bladder activity in the anesthetized spinal cord injured cat. A. Control saline CMG. Arrow indicates the start of infusion (2 ml/min). * indicates the first micturition contraction. B. Infusion of OXO-M (50 μM) reduces BC and increases the frequency and amplitude of PMCs. C. Intravesical infusion of AMN (100 μM) in combination with OXO-M suppresses the facilitatory effect of OXO-M on BC and PMCs. All recordings are from the same experiment.
4. Discussion
The present experiments revealed that intravesical administration of cholinergic receptor agonists in conscious chronic spinal cord injured (SCI) cats modulates two types of reflex bladder activity: i.e., PMCs and micturition contractions. Oxotremorine (OXO-M), a muscarinic receptor agonist, enhances PMCs and reduces the BC for eliciting a micturition reflex, while nicotine, a nicotinic receptor agonist, selectively reduces BC. Both agents also enhance the MMPs. These effects are blocked, respectively, by intravesical administration of antagonists for muscarinic (AMN) and nicotinic receptors (hexamethonium). Because the antagonists are hydrophilic quaternary compounds that would be expected to pass poorly through the urothelial barrier into the bladder wall, it is reasonable to conclude that the effects of the agonists on reflex bladder activity are due to actions on receptors located in proximity to the bladder lumen, i.e. either in the urothelium or in adjacent suburothelial afferent nerves (Fig. 10). Activation of the receptors could lead to enhanced afferent input to the spinal cord and modulation of reflex bladder activity. This putative mechanism is supported by studies in rats showing that intravesical administration of muscarinic (Yu and de Groat, 2010) or nicotinic receptor agonists (Yu et al., 2016) enhance the firing of bladder mechano-sensitive afferent nerves.
4.1. Two types of bladder afferents
It is probable that at least two types of afferent nerves are involved in initiating bladder reflexes in SCI cats (see Fig. 10) because OXO-M reduces BC and enhances PMC-AUC, while nicotine selectively reduces BC without affecting PMC-AUC. This suggests that activation of muscarinic receptors modulates a bladder afferent pathway (Type 1) and spinal circuitry controlling PMCs, while activation of either nicotinic or muscarinic receptors modulates a bladder afferent pathway (Type 2) and spinal circuitry controlling BC. Both pathways must consist of unmyelinated C-fiber afferents because Aδ bladder afferents do not contribute to reflex bladder activity after spinal cord injury in cats (Cheng et al., 1999).
4.2. Interactions between 8-OH-DPAT and OXO-M
The effects of 8-OH-DPAT, a 5HT1A serotonin receptor agonist, on reflex bladder activity and on the excitatory effects of OXO-M on bladder activity provide further support for the proposed dual sensory pathway hypothesis. Previous studies (Tai et al., 2006) revealed that 8-OH-DPAT increases BC in SCI cats without altering the frequency of PMCs, raising the possibility that these two parameters of bladder function in SCI cats are controlled by different reflex circuits. Tai et al. (2006) also provided evidence that 8-OH-DPAT targets interneuronal circuitry in the spinal micturition reflex pathway and does not affect the efferent limb of this pathway, i.e., the preganglionic parasympathetic neurons. Thus, it was proposed that 8-OH-DPAT increases BC by targeting interneuronal circuitry in the spinal cord. The ability of 8-OH-DPAT in the present experiments to suppress the facilitatory effect of OXO-M on BC without altering the facilitatory effect of OXO-M on PMCs suggests that Type 1 and Type 2 bladder afferents activate separate populations of interneurons which then synapse with a common population of preganglionic parasympathetic neurons (Fig. 10).
A selective action of 8-OH-DPAT on different bladder reflexes was also observed by Thor et al., 2002 in chloralose anesthetized cats with an intact spinal cord. The drug did not alter BC during saline CMGs but increased BC when the bladder was irritated by infusion of dilute acetic acid which activates C-fiber bladder afferents. These findings are consistent with the view that C-fiber evoked bladder reflexes even in spinal cord intact animals are sensitive to serotonergic modulation but also indicate the existence of a third bladder sensory pathway that is only functional in cats with an intact spinal cord and which consists of Aδ bladder afferents and spinal interneurons that are resistant to 8-OHDPAT. However, in chronic SCI cats, 8-OH-DPAT increases BC during saline CMGs (Gu et al., 2004) as well as acetic acid CMGs (Gu et al., 2007). These findings are consistent with the view that C-fiber bladder afferents which are mechano-insensitive in spinal intact cats (Häbler et al., 1990) become mechano-sensitive after SCI. This plasticity may not occur in all C-fiber afferents because pharmacological experiments in SCI cats comparing the effects of 8-OH-DPAT and GR-46611, a 5HT1A/B/1D agonist, on BC suggest that two distinct populations of bladder C-fiber afferents (chemo-sensitive and mechano-sensitive) can initiate spinal bladder reflexes (Gu et al., 2007).
4.3. Bladder reflexes evoked by two types of bladder afferents
The initiation of PMCs and micturition contractions in the present experiments by bladder distension and the facilitation of these contractions by stimulation of cholinergic receptors near the bladder lumen clearly demonstrate that two reflex responses are activated by bladder afferents that are sensitive to chemical as well as mechanical stimuli in the bladder of SCI cats. Furthermore, because our experiments indicate that the two reflexes are activated by separate populations of C-fiber bladder afferents (Types 1 and 2, Fig. 10), both types must be mechano-sensitive as well as chemo-sensitive in chronic SCI cats.
On the other hand, in acute SCI cats bladder distension alone does not induce reflex bladder activity, but chemical sensitization of afferents located in proximity to the bladder lumen by intravesical infusion of dilute acetic acid unmasks short duration rhythmic reflex bladder contractions that are similar in duration and frequency to PMCs in chronic SCI cats (Reese et al., 2015; Rogers et al., 2015; Xiao et al., 2014). However, the larger amplitude, longer duration micturition contractions do not occur in acute SCI cats even after treatment with acetic acid. Thus, the spinal circuitry for generating PMCs seems to exist in the acute spinal animal, but the C-fiber afferent input necessary to activate that circuitry is mechano-insensitive and requires chemical stimulation. Conversely, the micturition reflex pathway is inactive and unresponsive to mechanical or chemical stimulation indicating that the emergence of this pathway after SCI requires remodeling of spinal circuitry and/or plasticity of the Type 2 population of bladder C-fiber afferents.
4.4. Mechanisms underlying the activation of bladder afferents by cholinergic agonists
Although the detailed mechanisms underlying the effects of cholinergic agonists on bladder sensory pathways in the SCI cat are uncertain, it is possible as shown in Fig. 10 that when administered intravesically, these agents act directly on afferent nerves and indirectly by evoking the release of transmitters from urothelial cells. Furthermore, if urothelial cells can release inhibitory as well as excitatory transmitters (Birder and Andersson, 2013; Kullmann et al., 2008b) and if bladder afferent neurons and urothelial cells in the cat express multiple types of muscarinic and nicotinic receptors as previously demonstrated in rodents (Beckel et al., 2006; Kullmann et al., 2008a; Nandigama et al., 2010, 2013), then the effects of cholinergic agonists on bladder reflexes in the SCI cat may be complex and reflect the summation of inhibitory and facilitatory responses depending on the conditions of the experiment. For example, low concentrations of OXO-M infused intravesically in the spinal intact rat suppress reflex bladder activity via the release nitric oxide (Kullmann et al., 2008b) that has an inhibitory effect on bladder afferent neurons (Yoshimura et al., 2001), while high concentrations of OXO-M enhance reflex bladder activity in part via the release of ATP (Kullmann et al., 2008b). Furthermore, activation of α3 containing heteromeric subtypes of nicotinic receptors in urothelial cells releases ATP from urothelial cells and enhances reflex bladder activity, whereas activation of the α7 homomeric nicotinic receptor subtype in urothelial cells suppresses ATP release (Beckel and Birder, 2012). In the rat nicotinic receptor, agonists excite one population of bladder afferents and inhibit or facilitate the responses induced in other afferents by bladder distension, suggesting that three populations of bladder afferents can be identified in the rat based on responses to nicotinic agonists (Yu et al., 2016). Although inhibitory responses to cholinergic agonists were not obvious in the present experiments, these effects might only be unmasked by varying the concentrations of agonists or using antagonists selective for certain subtypes of receptors or by targeting receptors at certain sites.
Another factor influencing the effect of intravesically administered cholinergic agonists is the permeability of the urothelium. Although the normal urothelium is thought to be relatively impermeable to hydrophilic substances (Negrete et al., 1996) such as OXO-M, AMN, and hexamethonium, it is possible that that urothelial permeability is increased by bladder overdistension and other pathological changes that occur in the bladder after SCI (Apodaca et al., 2003). Thus, diffusion of drugs through the urothelial barrier cannot be completely discounted. If this occurs, then it is possible that intravesically administered muscarinic agonists could not only target urothelial cells and afferent nerves but also muscarinic receptors in bladder smooth muscle. However, the probability of this happening is low because the drugs would also have to diffuse across the highly vascularized lamina propria, thereby exposing the drugs to uptake by capillaries leading to systemic effects such as increased salivation and pupillary constriction with OXO-M or pupillary dilation with AMN. These effects were not observed in either conscious or anesthetized animals after intravesical administration but were observed after subcutaneous administration of AMN in conscious animals or after intravenous administration of OXO-M in anesthetized animals. In addition, direct stimulation of muscarinic receptors in the detrusor smooth muscle by diffusion of OXO-M through the bladder wall would be expected to markedly increase baseline intravesical pressure, an effect that was only rarely observed during the experiments (Fig. 7). Thus, the urothelium seems to be intact in SCI cats and to be an effective barrier to diffusion of hydrophilic substances into the muscle layers of the bladder.
4.5. Physiological role of cholinergic mechanisms in the activation of bladder afferents
Although the results of this study indicate that activation of urothelial and/or suburothelial cholinergic receptors by intravesical infusion of a muscarinic or a nicotinic agonist can enhance reflex bladder activity after spinal cord injury and that these effects can be blocked by intravesical administration of hydrophilic cholinergic receptor antagonists, AMN, or hexamethonium, the failure of the antagonists administered alone intravesically to affect either PMCs or micturition contractions indicates that the cholinergic receptors accessible to the antagonists are not tonically activated by endogenous acetylcholine and that the neurogenic bladder overactivity following spinal cord injury is due to mechanisms other than cholinergic modulation of urothelial cell-afferent nerve communication. It should be noted, however, that intravesical antimuscarinic therapy has been successful in managing bladder hyperactivity in patients refractory to oral antimuscarinics (Lose and Norgaard, 2001) or in patients with spinal cord injury (Glickman et al., 1995) or multiple sclerosis (Deaney et al., 1998) and has shown efficacy equal to that of oral administration of the antimuscarinic drug, oxybutynin (Fader et al., 2007). The discrepancy between these clinical findings and our data may be due, in part, to the fact that the agents used in the latter studies have greater lipid solubility than the AMN used in our experiments or that the urothelial barrier is more permeable in SCI patients than in SCI cats. Therefore, activation of cholinergic receptors on urothelial cells by endogenously released acetylcholine may not contribute significantly to neurogenic reflex bladder activity; however, cholinergic receptors on afferent nerves beneath the urothelium may be important therapeutic targets for treatment of bladder hyperactivity following spinal cord injury.
4.6. Characteristics of the cat chronic spinal cord injury model
The animal model used in our experiments is unusual in regard to species, duration of the study, and cystometric methods. Studies of SCI are commonly performed in rodents and are usually of relatively short duration (4–8 weeks after SCI). To our knowledge, our study is longest evaluation of neurogenic bladder dysfunction in a non-rodent animal species. Multiple CMGs were performed in the same group of conscious animals over periods ranging as long as 17 months (mean, 10.5 months in 7 female cats). In individual animals, CMG parameters such as BC and PMC frequency measured at weekly or biweekly intervals could exhibit considerable variation, and in most animals, there was a general long-term trend of increased BC and reduced PMC frequency, although two animals showed relatively consistent BC over time. On the other hand, during repeated CMGs on the same day, the measurements including BC (Fig. 2C), MMP, and PMC-AUC (Fig. 2D) were relatively constant. Because spinal transection removes the modulatory influences of the brain on voiding function and therefore eliminates the impact of changes in the arousal state of the animal and the influence of external, potentially stressful stimuli related to the lab environment, the observed wide variations in BC were unexpected. Several factors might have contributed to the variations. For example, in the cat mechanical stimulation of the distal bowel suppresses reflex bladder activity (de Groat, 1971). Thus, feces in the distal bowel could increase BC. Variations in hormone levels in female cats could also influence reflex bladder activity as could subclinical bladder infections, although bladder infections were relatively rare in this group of animals.
Cystometry was performed with a closed urethral outlet to prevent voiding, and the micturition reflex was identified by the amplitude and duration of the bladder contractions and occurrence of a hind limb reflex (“leg kick”) that is associated with voiding in SCI cats (Thor et al., 1983; Tai et al., 2006). Thus, voiding efficiency, an important parameter of lower urinary tract function after SCI, was not evaluated in these experiments. In addition, the impact of subjecting the bladders to repeated high pressures by evoking multiple micturition reflexes with a closed outlet is unknown. This experimental design may have contributed to the gradual long-term changes in CMG parameters noted in most animals. Despite these deficiencies, the animal model developed in this study which utilized weekly/biweekly urethral catheterization in place of indwelling catheters to monitor bladder function in conscious animals should be useful for examining the efficacy of long-term drug treatments, neuromodulation (de Groat and Tai, 2015), intraspinal therapies (Lee et al., 2013), or peripheral nerve re-routing (Gomez-Amaya et al., 2015) in reversing bladder dysfunction after SCI in a non-rodent, larger animal model.
5. Conclusions
Pharmacological experiments in conscious SCI cats indicate the existence of two distinct C-fiber bladder afferent pathways that project to two spinal interneuronal pathways that in turn converge onto the parasympathetic preganglionic neurons that comprise the efferent limb of the bladder reflex circuit (Fig. 10). These two primary afferent-spinal interneuronal pathways generate two types of reflex bladder activity: (1) PMCs and (2) micturition contractions.
It is tempting to speculate about the physiological functions of these two reflex pathways in spinal cord intact animals and how these functions might change after SCI. Because both reflex mechanisms are activated by C-fiber bladder afferents, it is likely that the afferents which are mechano-insensitive but chemo-sensitive in spinal cord intact animals (Häbler et al., 1990) undergo plasticity after SCI leading to the emergence of mechano-sensitivity (de Groat et al., 2015). Thus, if these two reflex mechanisms exist in spinal cord intact animals, one would predict that they function as nociceptive reflexes to promote bladder emptying in response to infection or chemical irritation of the bladder. The spinal reflexes could act synergistically with the supraspinal mechanisms and voluntary control of voiding that are dependent on the Aδ bladder afferent pathway that signals bladder filling.
However, it is not certain that both spinal reflexes exist in spinal cord intact animals because in acute SCI cats chemical irritation of the bladder with dilute acetic acid only activates reflexes that resemble PMCs (Rogers et al., 2015; Xiao et al., 2014), while larger amplitude, longer duration reflex micturition contractions are absent. Thus, following SCI, the micturition contractions activated by Type 2 afferents may be due to a new reflex mechanism that only appears after remodeling/reorganization of the spinal reflex circuitry.
Alternatively, it is possible that this reflex pathway is part of the spinal reflex mechanisms that control involuntary voiding in neonatal animals prior to the development of supraspinal control and then are downregulated during maturation of the nervous system (de Groat, 2002; de Groat et al., 2015). For example, voiding in the neonatal cat and many other species is triggered when the mother licks the perineum of the neonate and activates cutaneous afferents in the pudendal nerve which then evoke a somato-bladder reflex and a large amplitude bladder contraction (de Groat et al., 1975). This reflex is downregulated during postnatal development as the brain assumes control of the lower urinary tract but reappears after SCI when brain control is lost.
A neonatal bladder-to-bladder reflex which induces voiding in humans and cats in response to infusion of cold water into the bladder undergoes a similar downregulation during postnatal development and reemergence after SCI (Geirsson et al., 1999). This reflex is mediated by a population of C-fiber bladder afferents and therefore may be a component of the Type 2 afferent population shown in Fig. 10 which is sensitive to both nicotine and OXO-M and triggers large amplitude micturition contractions. Other than generating cold-evoked bladder reflexes in neonates and in people with neurogenic bladder disorders, the physiological functions of these afferents and the associated spinal reflex pathway are unknown.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-091253 and DK-093424.
Abbreviations:
- AMN
Atropine methyl nitrate
- ATP
Adenosine triphosphate
- 5-HT1A
Serotonergic receptor1A
- 8-OH-DPAT
(±) 8-hydroxy-2-di-N-propylaminotetralin hydrobromide
- BC
Bladder capacity
- CMGs
Cystometrograms
- MMP
Maximal micturition pressure
- OXO-M
Oxotremorine methiodide
- PMCs
Pre-micturition contractions
- PMC-AUC
Pre-micturition contraction–area under curve
- SCI
Spinal cord injury
References
- Aizawa N, Ito H, Sugiyama R, Fujimura T, Suzuki M, Fukuhara H, Homma Y, Igawa Y, 2015. Selective inhibitory effect of imidafenacin and 5-hydroxymethyl tolterodine on capsaicin sensitive C fibers of the primary bladder mechanosensitive afferent nerves in the rat. J. Urol 193, 1423–1432. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L, 2003. Disruption of bladder epithelium barrier function after spinal cord injury. Am. J. Physiol. Renal Physiol 284, F966–F976. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Birder LA, 2012. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J. Physiol 590, 1465–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA, 2006. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am. J. Physiol. Renal Physiol 290, F103–F110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder L, Andersson KE, 2013. Urothelial signaling. Physiol. Rev 93, 653–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Apodaca G, de Groat WC, Kanai AJ, 1998. Adrenergic- and capsaicinevoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am. J. Physiol 275, F226–F229. [DOI] [PubMed] [Google Scholar]
- Braverman A, Legos J, Young W, Luthin G, Ruggieri M, 1999. M2 receptors in genitourinary smooth muscle pathology. Life Sci. 64, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC, 1999. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol 277, R786–R794. [DOI] [PubMed] [Google Scholar]
- Chess-Williams R, 2002. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton. Autocoid Pharmacol 22, 133–145. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP, 2000. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407, 1011–1015. [DOI] [PubMed] [Google Scholar]
- de Groat WC, 1971. Inhibition and excitation of sacral parasympathetic neurons by visceral and cutaneous stimuli in the cat. Brain Res. 33, 499–503. [DOI] [PubMed] [Google Scholar]
- de Groat WC, 2002. Plasticity of bladder reflex pathways during postnatal development. Physiol. Behav 77, 689–692. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Douglas JW, Glass J, Simonds W, Weimer B, Werner P, 1975. Changes in somato-vesical reflexes during postnatal development in the kitten. Brain Res. 94, 150–154. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Griffiths D, Yoshimura N, 2015. Neural control of the lower urinary tract. Compr. Physiol 5, 327–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR, 1990. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst S71–S77. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K, 1981. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J. Auton. Nerv. Syst 3, 135–160. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW, 1969. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J. Physiol 200, 87–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Tai C, 2015. Impact of bioelectronic medicine on the neural regulation of pelvic visceral function. Bioelectron. Med 25–36. [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N, 2006. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res 152, 59–84. [DOI] [PubMed] [Google Scholar]
- Deaney C, Glickman S, Gluck T, Malone-Lee JG, 1998. Intravesical atropine suppression of detrusor hyperreflexia in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 65, 957–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader M, Glickman S, Haggar V, Barton R, Brooks R, Malone-Lee J, 2007. Intravesical atropine compared to oral oxybutynin for neurogenic detrusor overactivity: a double-blind, randomized crossover trial. J. Urol 177, 208–213. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ, 1997. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J. Physiol 505, 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirsson G, Lindström S, Fall M, 1999. The bladder cooling reflex and the use of cooling as stimulus to the lower urinary tract. J. Urol 162, 1890–1896. [DOI] [PubMed] [Google Scholar]
- Glickman S, Tsokkos N, Shah PJ, 1995. Intravesical atropine and suppression of detrusor hypercontractility in the neuropathic bladder. A preliminary study. Paraplegia 33, 36–39. [DOI] [PubMed] [Google Scholar]
- Gomez-Amaya SM, Barbe MF, de Groat WC, Brown JM, Tuite GF, Corcos J, Fecho SB, Braverman AS, Ruggieri MR Sr., 2015. Neural reconstruction methods of restoring bladder function. Nat. Rev. Urol 12, 100–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Olejar KJ, Reiter JP, Thor KB, Dolber PC, 2004. Inhibition of bladder activity by 5-hydroxytryptamine1 serotonin receptor agonists in cats with chronic spinal cord injury. J. Pharmacol. Exp. Ther 310, 1266–1272. [DOI] [PubMed] [Google Scholar]
- Gu B, Thor KB, Reiter JP, Dolber PC, 2007. Effect of 5-hydroxytryptamine1 serotonin receptor agonists on noxiously stimulated micturition in cats with chronic spinal cord injury. J. Urol 177, 2381–2385. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M, 1990. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J. Physiol 425, 545–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA, 2007. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 80, 2298–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontani H, Okamura T, Kimura S, Ishida K, Takeno S, 2009. Effect of nicotine on the pelvic afferent nerve activity and bladder pressure in rats. Int. J. Urol 16, 692–698. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Beckel JM, Barrick S, de Groat WC, Birder LA, 2008a. Heterogeneity of muscarinic receptor mediated Ca2+ responses in cultured urothelial cells from rat. Am. J. Physiol 294, F971–F981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, de Groat WC, 2008b. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J. Neurosci 28, 1977–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lin CY, Jiang HH, Depaul M, Lin VW, Silver J, 2013. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci 33, 10591–10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W, 2007. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur. Urol 51, 1042–1053. [DOI] [PubMed] [Google Scholar]
- Lose G, Norgaard JP, 2001. Intravesical oxybutynin for treating incontinence resulting from an overactive detrusor. BJ. Int 87, 767–773. [DOI] [PubMed] [Google Scholar]
- Masuda H, Hayashi Y, Chancellor MB, Kihara K, de Groat WC, de Miguel F, Yoshimura N, 2006. Roles of peripheral and central nicotinic receptors in the micturition reflex in rats. J. Urol 176, 374–379. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Miyazato M, Furuta A, Torimoto K, Hirao Y, Chancellor MB, Yoshimura N, 2010. Differential roles of M2 and M3 muscarinic receptor subtypes in modulation of bladder afferent activity in rats. Urology 75, 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Miyazato M, Yokoyama H, Kita M, Hirao Y, Chancellor MB, Yoshimura N, 2012. Role of M2 and M3 muscarinic acetylcholine receptor subtypes in activation of bladder afferent pathways in spinal cord injured rats. Urology 1184, e1115–e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandigama R, Bonitz M, Papadakis T, Schwantes U, Bschleipfer T, Kummer W, 2010. Muscarinic acetylcholine receptor subtypes expressed by mouse bladder afferent neurons. Neuroscience 168, 842–850. [DOI] [PubMed] [Google Scholar]
- Nandigama R, Ibañez-Tallon I, Lips KS, Schwantes U, Kummer W, Bschleipfer T, 2013. Expression of nicotinic acetylcholine receptor subunit mRNA in mouse bladder afferent neurons. Neuroscience 229, 27–35. [DOI] [PubMed] [Google Scholar]
- Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML, 1996. Permeability properties of the intact mammalian bladder epithelium. Am. J. Physiol 271, F886–F894. [DOI] [PubMed] [Google Scholar]
- Reese JN, Rogers MJ, Xiao Z, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C, 2015. Role of spinal metabotropic glutamate receptor 5 in pudendal inhibition of the nociceptive bladder reflex in cats. Am. J. Physiol. Renal Physiol 308, F832–F838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MJ, Xiao Z, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C, 2015. Propranolol, but not naloxone, enhances spinal reflex bladder activity and reduces pudendal inhibition in cats. Am. J. Physiol. Regul. Integr. Comp. Physiol 308, R42–R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi GT, de Groat WC, 1999. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sci 64, 411–418. [DOI] [PubMed] [Google Scholar]
- Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC, 2006. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5HT1A receptors. Exp. Neurol 199, 427–437. [DOI] [PubMed] [Google Scholar]
- Thor KB, Katofiasc MA, Danuser H, Springer J, Schaus JM, 2002. The role of 5HT1A receptors in control of lower urinary tract function in cats. Brain Res. 946, 290–297. [DOI] [PubMed] [Google Scholar]
- Thor KB, Roppolo JR, de Groat WC, 1983. Naloxone induced micturition in unanesthetized paraplegic cats. J. Urol 129, 202–205. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Tyagi P, Van-le S, Yoshimura N, Chancellor MB, de Miguel F, 2006. Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J. Urol 176, 1673–1678. [DOI] [PubMed] [Google Scholar]
- Ungerer T, Roppolo JR, Tai C, de Groat WC, 2005. Influence of urothelial and suburothelial muscarinic receptors on voiding in chronic spinal cord injured cats. Neuroscience Abstract Viewer, 1047. [Google Scholar]
- Ungerer T, Roppolo JR, Tai C, de Groat WC, 2006. Mechanisms underlying modulation of bladder reflexes in spinal cord injured (SCI) cats by intravesical administration of muscarinic and nicotinic receptor agonists. Neuroscience Abstract Viewer, 58816. [Google Scholar]
- Xiao Z, Rogers MJ, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C, 2014. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. Am. J. Physiol. Renal Physiol 307, F673–F679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Masunaga K, Satoji Y, Maeda Y, Nagata T, Inadome A, 2008. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in urothelium and its clinical significance. J. Pharmacol. Sci 106, 193–198. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, de Groat WC, 2001. Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons innervating rat urinary bladder. J. Neurophysiol 86, 304–311. [DOI] [PubMed] [Google Scholar]
- Yu Y, de Groat WC, 2008. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am. J. Physiol. Renal Physiol 294, F1146–F1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, de Groat WC, 2010. Effects of stimulation of muscarinic receptors on bladder afferent nerves in the in vitro bladder-pelvic afferent nerve preparation of the rat. Brain Res. 1361, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Daugherty S, de Groat WC, 2016. Effects of nicotinic receptor agonists on bladder afferent nerve activity in an in vitro bladder-pelvic nerve preparation. Brain Res. 1637, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]