Deason et al. discover a novel signaling adapter in the IL-1R pathway in CD4+ T cells that controls the induction of the PI3K–mTOR pathway, downstream of IL-1β, to induce pathogenic Th17 cells involved in the development of autoimmunity.
Abstract
The toll-like receptor (TLR) and interleukin (IL)–1 family of receptors share several signaling components, including the most upstream adapter, MyD88. We previously reported the discovery of B cell adapter for phosphoinositide 3-kinase (BCAP) as a novel toll–IL-1 receptor homology domain–containing adapter that regulates inflammatory responses downstream of TLR signaling. Here we find that BCAP plays a critical role downstream of both IL-1 and IL-18 receptors to regulate T helper (Th) 17 and Th1 cell differentiation, respectively. Absence of T cell intrinsic BCAP did not alter development of naturally arising Th1 and Th17 lineages but led to defects in differentiation to pathogenic Th17 lineage cells. Consequently, mice that lack BCAP in T cells had reduced susceptibility to experimental autoimmune encephalomyelitis. More importantly, we found that BCAP is critical for IL-1R–induced phosphoinositide 3-kinase–Akt–mechanistic target of rapamycin (mTOR) activation, and minimal inhibition of mTOR completely abrogated IL-1β–induced differentiation of pathogenic Th17 cells, mimicking BCAP deficiency. This study establishes BCAP as a critical link between IL-1R and the metabolic status of activated T cells that ultimately regulates the differentiation of inflammatory Th17 cells.
Introduction
TLRs are transmembrane receptors that detect the presence of microbial infections to induce rapid innate immune responses. TLR signaling is initiated by homotypic interactions of the toll–IL-1 receptor (TIR) homology domains found in the cytosolic region of IL-1R/TLR superfamily members. TIR domain interactions further mediate ligand-dependent recruitment of TIR domain–containing signaling adapters (Xu et al., 2000). Myeloid differentiation factor 88 (MyD88) was the first identified TIR domain–containing signaling adapter and is used by all TLRs, apart from TLR4, which signals via MyD88-dependent and MyD88-independent (but TRIF-dependent) pathways, and TLR3, which signals completely independently of MyD88 (Takeuchi and Akira, 2010; Troutman et al., 2012a). TIR domains are also conserved within the family of IL-1 cytokine receptors, which also depend on the recruitment of MyD88 for signal transduction (Garlanda et al., 2013).
Expression of TLRs is restricted to myeloid cells, B cells, and, in some cases, specialized epithelial cells. This localization is thought to restrict the potentially dangerous outcomes of TLR signaling to those cells capable of handling and responding in a manner most beneficial to the host. In contrast, many cell types of the host bear the ability to respond to cytokine cues provided by IL-1 family members (Garlanda et al., 2013). The effect of many IL-1 family members on T cell differentiation and function has been well studied; IL-18 enhances the function of IFN-γ–producing T helper (Th) 1 and cytotoxic CD8+ T cells, IL-1β regulates Th17 cell function and proliferation, and IL-33 heightens Th2 cell responses while also regulating the homeostasis of regulatory T cells in adipose tissues (Han et al., 2015; Kolodin et al., 2015; Vasanthakumar et al., 2015). All three cytokines, IL-1, IL-18, and IL-33, also have critical roles in regulating functions of Group 3, Group 1, and Group 2 innate lymphoid cells, respectively (Garlanda et al., 2013). Inhibiting IL-1 signaling through IL-1R antagonism has proven clinically effective in treating multiple autoimmune diseases. Uncovering the molecular players that control IL-1 receptor family signaling will allow for more complete understanding of the biology of Th cell lineages and innate lymphoid cells and may provide novel therapeutic targets for autoimmune disorders.
We previously identified an obligate role for the signaling adapter B cell adapter for phosphoinositide 3-kinase (BCAP) as a novel TIR domain–containing TLR signaling adapter that mediates activation of the phosphoinositide 3-kinase (PI3K) pathway in macrophages stimulated with TLR ligands (Matsumura et al., 2010; Ni et al., 2012; Troutman et al., 2012b). More importantly, the absence of BCAP led to exaggerated inflammatory responses after TLR activation, demonstrating that BCAP plays a critical role in regulating inflammation (Troutman et al., 2012b). Here we discover that BCAP is an important signaling adapter broadly used by members of the IL-1R/TLR superfamily, including IL-1R and IL-18R. In this capacity, BCAP delivers critical signals downstream of IL-1 and IL-18 receptors in CD4+ T cells during priming to enhance Th17 and Th1 cell responses, respectively. Our results also demonstrate the requirement for BCAP in PI3K-Akt–mechanistic target of rapamycin (mTOR) activation downstream of IL-1β signaling in T cells, including mTOR-induced increases in glycolysis. Consequently, BCAP-deficient T cells are defective in their ability to commit toward pathogenic Th17 effector cells, which has broad implications for the role of IL-1 family members in the generation of autoimmunity.
Results
BCAP is required for efficient T cell priming and effector function
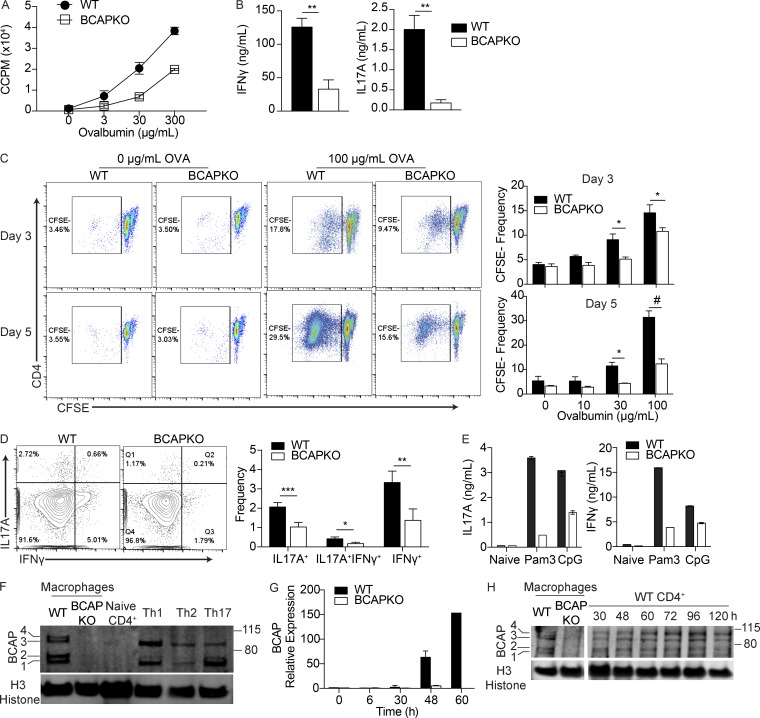
Previously, we described and characterized the adapter BCAP as a TIR domain–containing TLR signaling adapter (Troutman et al., 2012b). Our study demonstrated a crucial role for BCAP in the regulation of inflammation in vivo, whereby BCAP KO (BCAPKO) mice showed enhanced recruitment of inflammatory myeloid cells after infection. In addition, ex vivo priming experiments demonstrated that BCAPKO dendritic cells (DCs) induced robust priming of WT CD4+ T cells with increased production of IFN-γ and IL-17A, suggesting enhanced Th1 and Th17 cell priming (Troutman et al., 2012b). We therefore hypothesized that BCAPKO DCs would be more efficient at priming CD4+ T cells in vivo and predicted that the in vivo Th cell response to immunization would be enhanced in BCAPKO mice. To test this hypothesis, we immunized WT and BCAPKO mice with OVA and LPS emulsified in IFA. After 7 d, we purified CD4+ T cells from the draining popliteal and inguinal lymph nodes and assessed the recall capacity of antigen experienced T cells by restimulation with titrating doses of OVA. To our surprise, we found defective priming and differentiation of antigen-specific CD4+ T cells from BCAPKO mice as revealed by reduced in vitro proliferation and secretion of prototypical Th17 and Th1 cytokines, IL-17A and IFN-γ (Fig. 1, A and B). Using a live-dead stain coupled with CFSE staining, we saw no differences in cell survival (Fig. S1 A) but observed fewer BCAP-deficient CD4+ T cells diluting CFSE (Fig. 1 C). These results argue that generation of fewer OVA-specific T cells in BCAPKO mice during priming in vivo is reflected as reduced clonal expansion in vitro. Intracellular cytokine staining for IL-17A and IFN-γ on the CFSEneg population further showed reduced commitment to Th17 and Th1 lineages by BCAPKO CD4+ T cells (Fig. 1 D). These unexpected results prompted us to further test the possibility that BCAP could be playing a critical role intrinsic to Th cells. To test this hypothesis, we first used an in vitro priming system where BCAP deficiency was restricted to CD4+ T cells. In this system, we cocultured WT or BCAPKO naive CD4+ T cells with WT DCs. By adding low concentrations of anti-CD3 to the coculture, we can mimic TCR signaling through DC interaction with the soluble antibody on the Fc receptors of the DCs (Hu et al., 2011). Further, by provision of TLR ligands in the culture system, we effectively mimic priming conditions in vivo by allowing DCs to produce the necessary cytokines required to promote T cell differentiation into effector T cells. Using this experimental system, we found that BCAP-deficient T cells were highly defective in their ability to secrete IFN-γ and IL-17A, suggesting that BCAP expression in CD4+ T cells is required for optimal Th1 and Th17 cell differentiation (Fig. 1 E).
Figure 1.
BCAP is required for efficient T cell priming and effector function. (A) CD4+ T cells were isolated from popliteal and inguinal lymph nodes of OVA-immunized WT and BCAPKO mice. Purified T cells pooled from three mice were incubated with WT DCs (5:1) and indicated concentrations of OVA. Proliferation was measured by 3H incorporation in the last 16 h of culture. CCPM, cell counts per minute. Shown is the mean ± SD of three technical replicate cultures. Data are representative of three independent experiments. (B) Analysis of IFN-γ and IL-17A in the supernatant of DC–T cell cocultures as in A with 100 µg/ml OVA. Shown is the mean ± SEM; n = 4 mice per group. **, P < 0.01. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (C) Representative flow plots (left) and quantification (right) of CD4+ T cell CFSE dilution after 3 d or 5 d of in vitro restimulation with WT DCs and indicated concentrations of OVA. Shown is the mean ± SEM; n = 4. Statistical analysis was performed with two-tailed unpaired Student’s t test. *, P < 0.05; #, P < 0.0001. (D) Representative flow cytometry (left) and quantification of intracellular cytokine staining (right) of CFSEnegCD4+ T cells after 5 d of DC–T cell coculture with 100 µg/ml OVA. Shown is the mean ± SEM; n = 4 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (E) WT and BCAPKO naive CD4+ T cells pooled from three mice were incubated with WT DCs (5:1) and stimulated with the TLR ligands Pam3CSK4 (100 ng/ml) or CpG (1 µM). After 5 d, supernatant IL-17A and IFN-γ levels were measured by ELISA. Shown is the mean ± SD. Data are representative of three independent experiments. (F) Lysates from WT and BCAPKO bone marrow–derived macrophages and WT CD4+ T cells polarized to Th1, Th2, or Th17 cells were analyzed for BCAP expression by Western blotting. Data are representative of four independent experiments. (G) WT and BCAPKO naive T cells were polarized to Th17 for the indicated time and analyzed for BCAP expression using qPCR. Expression is relative to WT 0 h. Data are representative of three independent experiments. (H) Lysates from WT and BCAPKO bone marrow–derived macrophages and WT CD4+ T cells polarized to Th17 cells for the indicated time points were probed for BCAP expression by Western blotting. Data are representative of three independent experiments.
The above data suggest a functional role for BCAP intrinsically regulating T cell differentiation. To investigate if BCAP is equally expressed in all T cell lineages after polarization, we polarized naive WT CD4+ T cells to Th1, Th2, or Th17 lineages. Although we found BCAP expressed in all polarized cells, the expression of the four identified transcript variants (BCAP1-4) varied between lineages (Fig. 1 F). We next determined the kinetics of BCAP expression in activated T cells through quantitative PCR (qPCR) and Western blotting analysis (Fig. 1, G and H). Although BCAP is expressed at minimal levels during the naive state, it is up-regulated after TCR-mediated activation, suggesting that BCAP could play a critical role in signaling events that regulate differentiation of naive T cells into effector lineages.
BCAP functions downstream of IL-1R and IL-18R in CD4 T cells
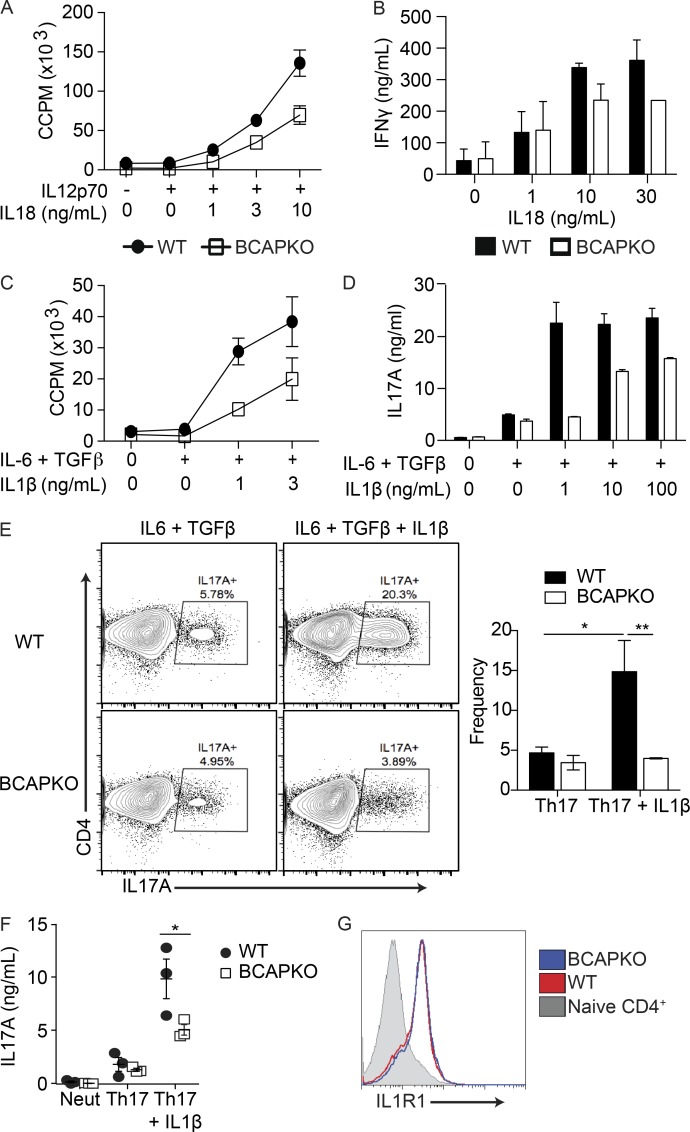
BCAP contains an N-terminal TIR domain that allows for homotypic interaction with the TIR domains of TLRs, MyD88, and TIRAP (TIR domain containing adapter protein; Troutman et al., 2012b; Halabi et al., 2017). The IL-1R/TLR superfamily transmits extracellular signals to the cytosolic environment using shared signaling adapters; as TLRs comprise a subgroup of this broad family, we hypothesized that BCAP could act as a signaling adapter downstream of other members within this superfamily, including IL-1R and IL-18R. Previous studies have reported the importance of the IL-1 family of cytokines in the generation of effector T cells, as well as in the survival, proliferation, and function of T cells (Chung et al., 2009; Hu et al., 2011). IL-1β is particularly well characterized for its role in Th17 cell function, whereas IL-18 has been described to play synonymous roles for Th1 cells (Yoshimoto et al., 1998; Tominaga et al., 2000). To test our hypothesis in depth, we cocultured naive WT or BCAPKO CD4+ T cells with irradiated DCs. In this system, the DCs fail to provide cytokine signals, but can still provide costimulatory signals and TCR stimulation through the addition of soluble αCD3. We used this defect to our advantage by adding exogenous cytokines into the culture system. This approach revealed that BCAP was not required for Th1 cell differentiation in the presence of IL-12; however, when IL-18 was titrated into the culture system, we found that the expected enhancement of IFN-γ secretion and proliferation was dependent on BCAP (Fig. 2, A and B). We performed a similar experiment using the Th17 cell polarizing cytokines IL-6 + TGF-β, which showed no difference in the ability of BCAP-sufficient or -deficient naive CD4+ T cells to differentiate into IL-17A–producing Th17 cells. However, upon addition of IL-1β into the culture system, we found a BCAP-dependent synergistic enhancement of IL-17A production and T cell proliferation (Fig. 2, C and D). There was no significant difference between the proliferation of BCAP-deficient or -sufficient CD4+ T cells in the absence of IL-1β or IL-18 (Fig. S1 B), suggesting a specific role for BCAP downstream of IL-1 and IL-18 receptors. To control for potential unknown DC–T cell interactions, we used plate-bound αCD3 + αCD28 to polarize naive WT and BCAPKO CD4+ T cells to the Th17 lineage (IL-6 + TGF-β) with or without the addition of IL-1β (Fig. 2, E and F). Only the addition of IL-1β revealed a difference in IL-17A production by WT and BCAPKO Th17 polarized cells. There was no difference between RORγt expression by WT or BCAPKO polarized Th17 cells in the presence or absence of IL-1β (Fig. S1 C). BCAP expression also correlated with the expression of IL-1R on T cells, where naive CD4+ T cells have minimal IL-1R expression that is up-regulated after activation (Figs. 1 H and 2 G); importantly, there was no difference in the expression of IL-1R between WT and BCAPKO T cells. These results suggest that BCAP signaling via IL-1R family members is required for optimal differentiation of Th1 and Th17 cells during priming.
Figure 2.
BCAP functions downstream of IL-1R and IL-18R in CD4 T cells. (A and C) Naive WT and BCAPKO CD4+ T cells pooled from three mice were incubated with WT DCs (5:1), 3 ng/ml αCD3, and indicated cytokines. Proliferation was measured by 3H incorporation in the last 16 h of culture. Shown is the mean ± SD of three technical replicate cultures. Data are representative of two independent experiments. (B and D) IL-17A and IFN-γ measured from the supernatant of WT and BCAPKO DC/T cell cocultures (1:5) in the presence of the indicated cytokines. Shown is the mean ± SD of technical replicates. Data are representative of two independent experiments. (E) Representative flow plots (left) and quantification (right) of WT and BCAPKO naive CD4+ T cells polarized to Th17 with or without IL-1β for 5 d. Shown is the mean ± SEM; n = 3. *, P < 0.05, **, P < 0.01. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (F) Naive WT and BCAPKO CD4+ T cells were polarized for 5 d using neutralizing conditions (αIFN-γ + αIL-4) or to Th17 cells (TGF-β + IL-6 + αIFN-γ + αIL-4) with or without IL-1β. After polarization, IL-17A secretion was measured by ELISA. Shown is the mean ± SD; n = 3. *, P < 0.05. (G) Naive WT and BCAPKO CD4+ T cells were polarized using neutralizing conditions; after 24 h, surface expression of IL-1R1 was measured using flow cytometry. Data are representative of three independent experiments.
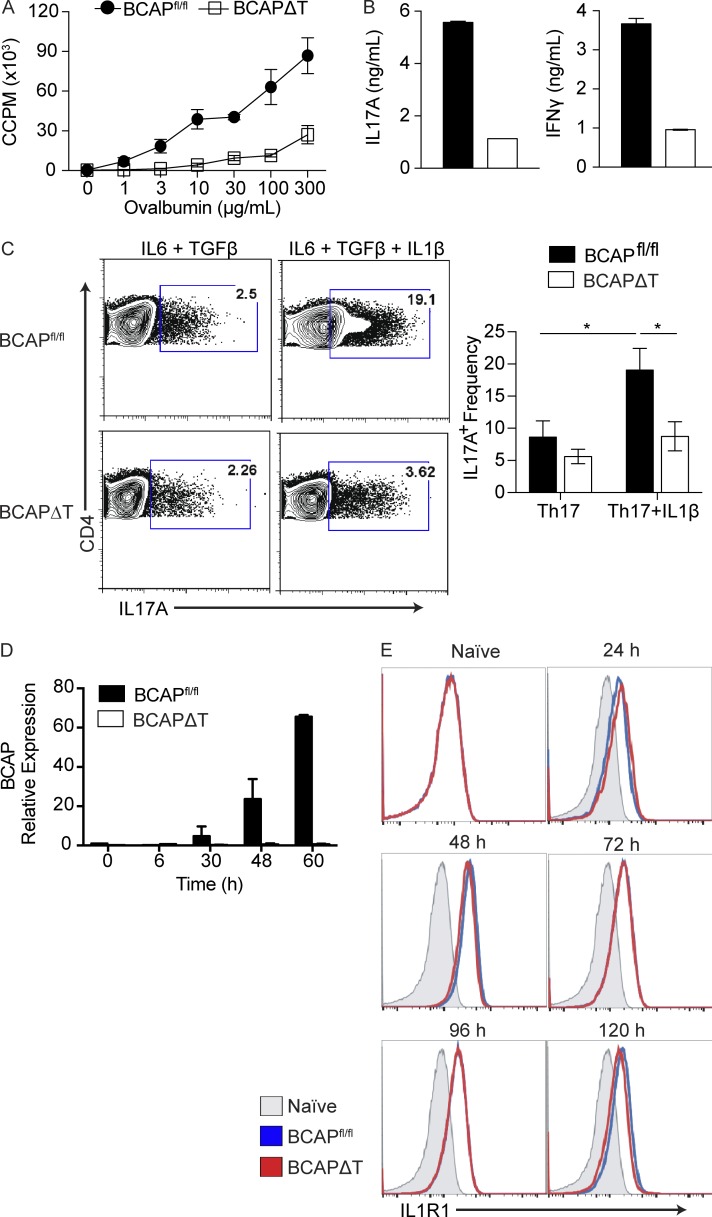
Our results suggest that BCAP functions in vitro through IL-1R family members to promote Th1 and Th17 cell commitment. To confirm that our observations were not an artifact of in vitro culturing, we generated BCAP floxed mice, which permitted the deletion of BCAP specifically in T cells by crossing the BCAPfl/fl mice to LCK.Cre transgenic mice (BCAPΔT). BCAPfl/fl mice and BCAPΔT mice have no obvious developmental abnormalities and reproduce at expected Mendelian ratios (unpublished data). We first repeated the experiments performed using the whole-body BCAPKO mice to confirm that the CD4+ T cell phenotype was specifically a result of BCAP deficiency. We immunized cohorts of BCAPfl/fl and BCAPΔT mice with OVA and LPS emulsified in IFA. After 7 d, we harvested the draining lymph nodes and purified CD4+ T cells, which were subjected to an ex vivo recall assay by coculturing with TLR2/4 double KO DCs and titrating concentrations of OVA. Consistent with the whole-body BCAPKO in vivo priming and in vitro experiments, BCAPΔT T cells showed defects in priming and differentiation to Th1 and Th17 effector lineages (Fig. 3, A and B), as defined by decreased proliferation and cytokine production after in vitro restimulation. The reduced proliferation and cytokine production observed is, again, reflective of reduced in vivo priming of OVA-specific BCAPΔT T cells, which translates to reduced clonal expansion and cytokine production during restimulation in vitro. This is reiterated by the use of a viability dye coupled with CFSE staining, which shows that BCAP deficiency does not affect T cell survival, only clonal expansion during in vivo priming (Fig. S2, A and B). At this point we focused our efforts on the role of BCAP in regulating IL-1β–mediated effects on Th17 cell differentiation. We polarized BCAPΔT and BCAPfl/fl naive CD4+ T cells to Th17 cells using either IL-6 + TGF-β or IL-6 + TGF-β + IL-1β (Fig. 3 C). Although polarization with IL-6 + TGF-β led to similar Th17 lineage commitment, there was a profound defect in BCAPΔT T cell differentiation into IL-17A–producing Th17 cells when IL-1β was present in the culture conditions (Fig. 3 C). Although we found differential expression of IL-17A, there was no difference in the expression of the transcription factor RORγt (Fig. S2 C). BCAPfl/fl naive T cells polarized to Th17 cells up-regulated BCAP similarly to WT T cells (Figs. 1 F and 3 D), whereas BCAPΔT T cells showed no expression of BCAP at any time point (Fig. 3 D). IL-1R expression was up-regulated equally between BCAPfl/fl and BCAPΔT T cells; therefore, the inability of BCAPΔT T cells to properly respond to IL-1β was not a result of a defect in IL-1R expression (Fig. 3 E). Interestingly, IL-1R is highly up-regulated shortly after activation, whereas BCAP expression is slightly delayed in comparison. This may suggest that there is a BCAP-independent IL-1R signaling cascade that occurs, likely through MyD88, before BCAP expression.
Figure 3.
Mice with selective deletion of BCAP in T cells exhibit poor CD4 T cell priming and Th1 and Th17 differentiation. (A) CD4+ T cells were isolated from popliteal and inguinal lymph nodes of OVA-immunized BCAPfl/fl and BCAPΔT mice (three mice per group). Purified CD4+ T cells pooled from all three mice were incubated with WT splenic CD11c+ DCs (5:1) and the indicated concentrations of OVA; proliferation was measured by 3H incorporation in the last 16 h of culture. Shown is the mean ± SEM of three technical replicate cultures. Data are representative of three independent experiments. (B) IL-17A and IFN-γ supernatant levels of DC–T cell cocultures as in A, measured by ELISA. Data are representative of three independent experiments. (C) Representative flow plots (left) and quantification (right) of BCAPfl/fl and BCAPΔT naive CD4+ T cells polarized to Th17 cells with or without IL-1β for 5 d. Shown is the mean ± SEM; n = 4. *, P < 0.05. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (D) BCAPfl/fl and BCAPΔT naive CD4+ T cells were polarized to Th17 cells for the indicated time and analyzed for BCAP expression using qPCR. Expression is relative to BCAPfl/fl 0 h. Data are representative of two independent experiments. (E) BCAPfl/fl and BCAPΔT naive CD4+ T cells were stimulated with plate-bound αCD3 + αCD28. Surface expression of IL-1R1 was measured at the indicated time points by flow cytometry. Data are representative of two independent experiments.
BCAP is required for the generation of pathogenic Th17 cells
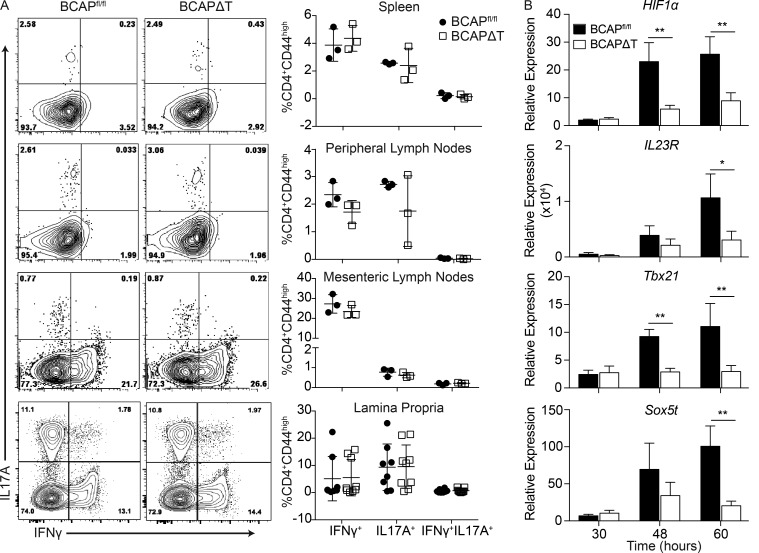
As T cell–intrinsic BCAP appears to be critical for Th1 and Th17 cell differentiation in both in vitro polarization assays and after protein immunization, we were interested in testing the status of steady-state Th1 and Th17 cell differentiation in various lymphoid tissues and barrier sites such as the intestinal lamina propria. We specifically analyzed the cytokine production of steady-state memory CD4+ T cells (CD4+CD44hi) residing in the spleen, peripheral lymph nodes, mesenteric lymph nodes, and lamina propria of BCAPΔT and BCAPfl/fl littermates and found no difference in the proportions of IL-17A or IFN-γ–producing CD4+ T cells in any assessed organs (Fig. 4 A). This agrees with our initial in vitro data, where we found that Th1 and Th17 cell differentiation defects were revealed in BCAP-deficient T cells only when IL-1β or IL-18 was added to the culture conditions. The IL-1 family of cytokines are tightly regulated because of their highly inflammatory potential; in fact, excessive production of these cytokines is linked to many inflammatory and autoimmune disorders (Dinarello, 2009). These data point to the possibility that there are differential signaling requirements for generation of steady-state Th1 and Th17 lineages, presumably driven by the microbiota (Belkaid et al., 2013), and Th1 and Th17 lineages induced after inflammation, such as during active infections or after immunization. We therefore hypothesized that T cell–intrinsic BCAP could be required for inflammation-driven Th17 lineage cells, such as those that arise during autoimmunity.
Figure 4.
BCAP deficiency does not affect steady-state Th1 and Th17 differentiation. (A) Representative flow plots (left) and quantification (right) of IL-17A and IFN-γ in CD4+CD44+ T cells isolated from spleens, peripheral lymph nodes, mesenteric lymph nodes, and lamina propria of BCAPfl/fl and BCAPΔT mice. Shown is the mean ± SEM, n = 3 for spleen, peripheral lymph nodes, and mesenteric lymph nodes, and n = 8 for lamina propria. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (B) qPCR analysis of several genes in BCAPfl/fl and BCAPΔT naive CD4+ T cells polarized for indicated time points to Th17 + IL-1β, relative to their expression in BCAPfl/fl T cells polarized in neutralizing conditions. Shown is the mean ± SD; n = 4. *, P < 0.05; **, P < 0.01. Statistical analysis was performed with the two-tailed unpaired Student’s t test.
Other groups have performed extensive RNA profiling to determine genes associated with Th17 cells that induce high levels of autoimmunity, termed pathogenic Th17 cells. Pathogenic Th17 cells are polarized in the presence of IL-6 + TGF-β + IL-1β ± IL-23, as opposed to nonpathogenic Th17 cells, which are polarized in the presence of IL-6 + TGF-β (Brüstle et al., 2007; Lee et al., 2012; Tanaka et al., 2014; Gaublomme et al., 2015; Meyer Zu Horste et al., 2016; Gibson et al., 2017). Using the gene signatures discovered by these previous groups, we tested whether BCAP was important for the induction of pathogenic Th17 cell–associated genes after polarization in the presence of IL-1β. We tested a few of the key genes shown to be required for Th17 cell pathogenicity: HIF1α, Tbx21, IL23R, and Sox5t. We chose to analyze HIF1α, Tbx21, and Sox5t expression as they are transcription factors that would have broad effects on Th17 cell function, including metabolism and cytokine production (Lee et al., 2012; Tanaka et al., 2014; Zeng and Chi, 2014). We also selected IL23R as increased IL-23R expression has been closely correlated with Th17 cell pathogenicity (Lee et al., 2012). Analysis of the transcript kinetics shows that BCAP is required for IL-1β–mediated differentiation of pathogenic Th17 cells (Fig. 4 B). Based on these data, we hypothesized that mice lacking T cell–intrinsic BCAP would have a defect in the generation of pathogenic Th17 lineage cells and reduced susceptibility to myelin oligodendrocyte glycoprotein (MOG) peptide–induced experimental autoimmune encephalomyelitis (EAE; Okuda et al., 1999; Sutton et al., 2006).
T cell–intrinsic BCAP deficiency modulates EAE disease
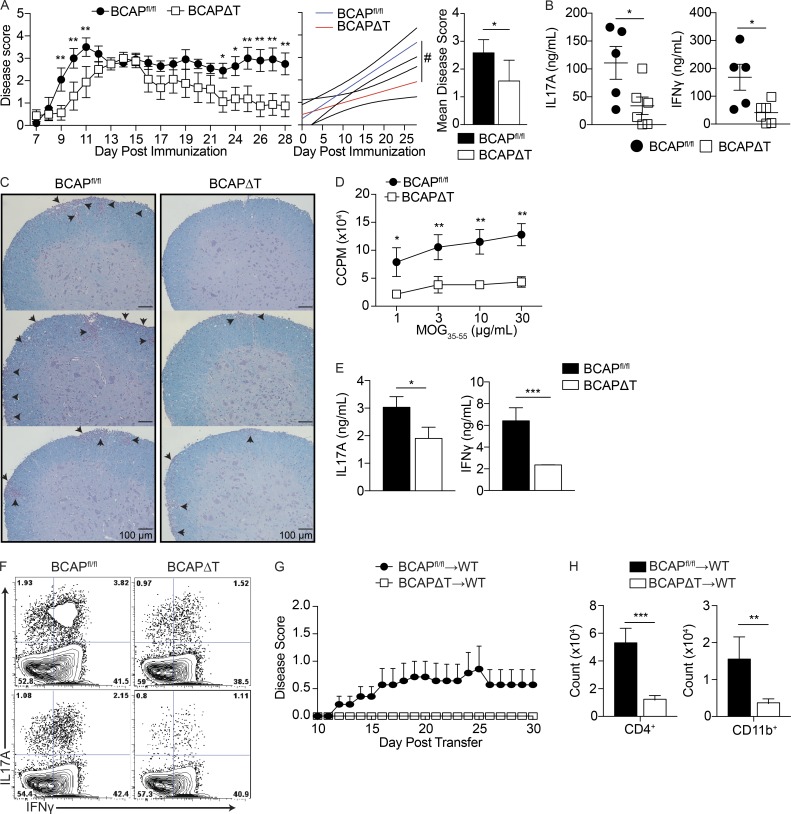
To directly test our hypothesis that T cell–intrinsic BCAP is critical for generation of pathogenic Th17 lineage cells, we used the mouse model EAE, which causes an autoinflammatory disease state whereby pathogenic Th1 and Th17 cells induce demyelination of neurons within the central nervous system, leading to progressive paralysis (Petermann and Korn, 2011).
We immunized littermate BCAPΔT and BCAPfl/fl mice using mouse MOG35-55 emulsified in CFA, followed by administration of pertussis toxin day 0 and 2 after immunization (Sutton et al., 2006). BCAPΔT mice had an increased time to disease and an increased remission from disease, which correlates with a decrease in the presence of pathogenic Th17 cells (Fig. 5 A). Serum collected at day 16 after immunization showed significantly decreased IL-17A and IFN-γ from BCAPΔT mice (Fig. 5 B). Histology of BCAPfl/fl and BCAPΔT spinal cords collected at day 16 after immunization showed that BCAPΔT mice were protected from EAE–mediated cellular infiltration and demyelination (Fig. 5 C). We further isolated CD4+ T cells from draining lymph nodes of BCAPΔT and BCAPfl/fl mice immunized for EAE, and performed an ex vivo recall assay to determine the quality of Th cells generated after immunization. Consistent with our previous results using the model antigen OVA, we found defective priming and differentiation of MOG-specific Th1 and Th17 cells in BCAPΔT mice (Fig. 5, D and E). Further, there were fewer IFN-γ–IL-17A double-positive cells, the canonical pathogenic Th17 cells in BCAPΔT mice (Fig. 5 F). Interestingly, we found no difference in the expression of RORγt in the isolated CD4+ T cells, suggesting that the reduced production of IL-17A is not a result of defective expression of RORγt but likely because of other functions of BCAP downstream of the IL-1R (Fig. S3). To confirm that the decreased EAE susceptibility was specifically caused by defective pathogenic Th17 lineage generation in the absence of BCAP, we used the transfer EAE model. BCAPΔT and BCAPfl/fl mice were immunized with MOG35-55 emulsified in CFA, and CD4+ T cells were isolated from draining lymph nodes 10 d later. CD4+ T cells were restimulated in vitro with WT DCs, MOG35-55, IL-1β, and IL-23 for 72 h, after which 5 × 106 CD4+ T cells were transferred to WT recipient mice, which were monitored daily for EAE disease (Fig. 5 G). In concordance with our hypothesis that BCAP is required for the generation of pathogenic Th17 cells, only BCAP-sufficient T cells were able to transfer EAE disease to WT recipients. The reduced pathogenicity of BCAPΔT T cells is also reflected by their reduced ability to induce inflammation within the spinal cord, as seen by fewer infiltrating CD4+ T cells and Ly6C+ monocytes 30 d after transfer (Fig. 5 H).
Figure 5.
BCAP is required for the generation of pathogenic Th17 cells. (A) Mean clinical scores ± SEM (left) of BCAPfl/fl and BCAPΔT mice immunized with MOG35-55 emulsified in CFA, with pertussis toxin given on days 0 and 2. Linear regression analysis of EAE disease progression for BCAPfl/fl (blue line) versus BCAPΔT (red line) mice (center). Mean clinical disease score ± SD (right) was calculated from clinical scores beginning at disease initiation (D7) to experimental end point (D28). *, P < 0.05; **, P < 0.01; #, P < 0.0001. Data shown are for four or five mice per group and are representative of four independent experiments. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (B) Serum cytokine levels of BCAPfl/fl and BCAPΔT mice at day 16 after immunization for EAE were determined by ELISA. Shown is the mean ± SEM, n = 5 for BCAPfl/fl, and n = 6 for BCAPΔT. *, P < 0.05. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (C) Luxol Fast Blue staining of spinal cords (10×) from BCAPfl/fl and BCAPΔT mice at day 16 after immunization for EAE. Images from three mice per genotype are shown. Arrows denote foci of cellular infiltration and/or demyelination. (D) CD4+ T cells were isolated from draining lymph nodes of MOG35-55-immunized BCAPfl/fl and BCAPΔT mice. Purified CD4+ T cells were incubated with WT DCs (10:1) and indicated concentrations of MOG35-55. Proliferation was measured by 3H incorporation in the last 16 h of culture. Shown is the mean ± SD; n = 3. *, P < 0.05; **, P < 0.01. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (E) IL-17A and IFN-γ levels in the supernatant of DC–T cell cocultures as in D with 100 µg/ml MOG35-55, measured by ELISA. Shown is the mean ± SD; n = 3. *, P < 0.05; ***, P < 0.001. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (F) Representative flow plots of CD4+CD44+ T cells from lymph nodes of EAE-immunized BCAPfl/fl and BCAPΔT mice. Shown are two independent mice per genotype. (G) Mean EAE clinical disease scores ± SEM of WT recipients of 5 × 106 BCAPfl/fl and BCAPΔT CD4+ T cells primed in vivo with MOG35-55 emulsified in CFA and restimulated in vitro with WT DCs (1:5), 100 µg/ml MOG35-55, 10 ng/ml IL-1β, and 20 ng/ml IL-23 for 72 h. Data shown are for five to seven mice per group and are representative of two independent experiments. (H) Quantification of infiltrating CD4+ T cells and CD11b+ monocytes in the spinal cord of WT recipients of 5 × 106 BCAPfl/fl and BCAPΔT CD4+ T cells as in G. Shown is the mean ± SEM, n = 7 for BCAPfl/fl and 5 for BCAPΔT. **, P < 0.01; ***, P < 0.001. Statistical analysis was performed with the two-tailed unpaired Student’s t test.
BCAP links IL-1R signaling to PI3K–mTOR pathway and regulates metabolic effects downstream of IL-1R
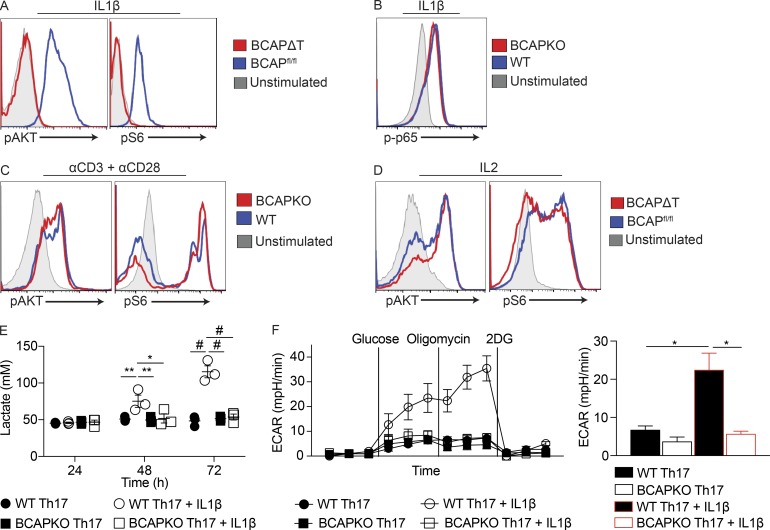
The evidence from our experiments clearly establishes that T cell–intrinsic BCAP regulates the quality of Th17 cells without affecting the expression of the master transcription factory RORγt. BCAP was initially discovered to be an adapter linking the B cell receptor to PI3K activation (Okada et al., 2000), and our earlier work, as well as work from another laboratory, firmly established BCAP to be a critical adapter that links TLR signaling to the PI3K pathway in macrophages (Okada et al., 2000; Ni et al., 2012; Troutman et al., 2012b). Activation of the PI3K pathway downstream of both the TCR and the costimulatory molecule CD28 is critical for regulating the biology of activated T cells (Prasad et al., 1994; Frauwirth et al., 2002). The PI3K pathway activates the mTOR pathway to induce pleiotropic affects, including increasing mRNA translation through the ribosomal protein S6, increasing glycolysis, modifying chromatin through the induction of HDACs, and inducing transcription through the activation of various transcription factors (Chi, 2012). To determine whether BCAP is required for activation of the PI3K pathway downstream of IL-1R, we activated pure CD4+ T cells using plate-bound αCD3 + αCD28 to induce up-regulation of IL-1R, and then stimulated with IL-1β to see PI3K-mediated activation of Akt and S6 ribosomal protein. Using phosphoflow, we probed for the phosphorylation status of S473 on Akt and S240/244 on S6, and found that BCAP is required for IL-1β–mediated activation of the PI3K pathway (Fig. 6 A); however, IL-1β–mediated activation of the MyD88–NF-κB pathway was unperturbed by the loss of BCAP (Fig. 6 B). TCR signaling and IL-2 signaling both activate the PI3K pathway (Frauwirth et al., 2002; Chi, 2012) and are critical for T cell development and function, so we next tested if BCAP is specifically regulating the activation of the PI3K pathway downstream of IL-1R. Our experiments revealed that TCR + αCD28– and IL-2–induced PI3K activation (as revealed by phosphorylation of Akt and S6 ribosomal protein) is not deficient in BCAPΔT T cells (Fig. 6, C and D), clearly suggesting that the functional defects are not a result of a role for BCAP downstream of TCR or CD28 stimulation.
Figure 6.
IL-1β signals through BCAP to activate the mTOR pathway and increase glycolysis. (A–D) BCAP-deficient CD4+ T cells were stimulated with 10 ng/ml IL-1β (A and B), plate-bound αCD3 (5 µg/ml) + αCD28 (5 µg/ml; C), or 20 U/ml IL-2 (D). Cells were immediately fixed to 2% PFA and permeabilized with 100% methanol. After permeabilization, cells were analyzed for pAkt (S473), pS6 (S240/244), and p-p65 (S536). Data are representative of two (B) or three (A, C, and D) experiments. (E) Lactate was measured from the supernatant of naive WT and BCAPKO CD4+ T cells polarized to Th17 cells with irradiated (12 Gy) WT B cells (1:2), 30 ng/ml αCD3, and 10 ng/ml IL-1β as indicated. Shown is the mean ± SEM; n = 3. *, P < 0.05; **, P < 0.01; #, P < 0.0001. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (F) Naive WT and BCAPKO CD4+ T cells were polarized to Th17 cells for 48 h, and then stimulated with 10 ng/ml IL-1β for 12 h as indicated and analyzed using the glycolysis stress test on the Seahorse platform. Left: The experimental time course. Right: The ECAR levels of each sample after the addition of oligomycin. Shown is the mean ± SEM; n = 3. *, P < 0.05. Statistical analysis was performed with the two-tailed unpaired Student’s t test.
It is well known that IL-1R and IL-6R signaling have synergistic effects that regulate T cell activation and differentiation (Mizutani et al., 1989; Basu et al., 2015). STAT3 is a critical transcription factor for the development of Th17, and multiple studies have implicated mTOR activation in the phosphorylation of STAT3 as well as the repression of SOCS3, which negatively regulates STAT3 (Chen et al., 2006; Harris et al., 2007; Basu et al., 2015). We hypothesized that IL-1β may be playing a synergistic role with IL-6 to induce the differentiation of pathogenic Th17 cells through BCAP. To test this hypothesis, we stimulated BCAP-sufficient and -deficient CD4+ T cells with IL-6 or IL-1β alone, or IL-6 with IL-1β and analyzed the kinetics of STAT3 activation over a 4-h period. Although both BCAP-sufficient and -deficient T cells equally phosphorylated STAT3 after stimulation with IL-6, only BCAP-sufficient T cells had significantly increased pSTAT3 levels with the addition of IL-1β (Fig. S4). These data corroborate earlier evidence linking IL-1R and IL-6R signaling and establish a critical role for BCAP as a molecular player that enables this synergism (Chung et al., 2009; Basu et al., 2015).
The PI3K-Akt-mTOR pathway is a central regulator of cell metabolism, specifically driving cells to increase glycolysis and the synthesis of lipids and nucleotides, culminating in increased proliferation, cell growth, cell survival, and cytokine production (Zeng and Chi, 2013). As BCAP-deficient T cells failed to activate the PI3K pathway in response to IL-1β, we next assessed whether IL-1β would increase Th17 cell glycolysis, and if this is dependent on BCAP. During glycolysis, glucose is converted to pyruvate to generate 2 ATP, and pyruvate is then reduced to lactate, by lactate dehydrogenase, to generate NAD+. Lactate is then shuttled out of the cell by monocarboxylate transporters (Sola-Penna, 2008). Thus, lactate levels in the supernatant can be measured to determine comparative levels of glycolysis (TeSlaa and Teitell, 2014). We polarized WT and BCAPKO naive T cells to Th17 in the presence or absence of IL-1β and measured the lactate production in the supernatant after polarization. IL-1β significantly increased lactate production from WT T cells polarized to Th17 as compared with BCAPKO T cells (Fig. 6 E). Importantly, there was no difference in the lactate production of WT and BCAPKO Th17 cells polarized without IL-1β. We next confirmed that IL-1β enhances glycolysis in a BCAP-dependent manner by measuring the extracellular acidification rate (ECAR) using the glycolysis stress test on the Seahorse platform. In concordance with the increased lactate production, WT Th17 cells with IL-1β had significantly increased ECAR, whereas BCAPKO Th17 cells were not responsive to the addition of IL-1β (Fig. 6 F). WT T cells polarized to Th17 with IL-1β also had a significantly increased glycolytic rate and glycolytic reserve capacity (Fig. S5).
Minimal inhibition of mTOR and glycolysis prevents IL-1β–mediated generation of pathogenic Th17 cells
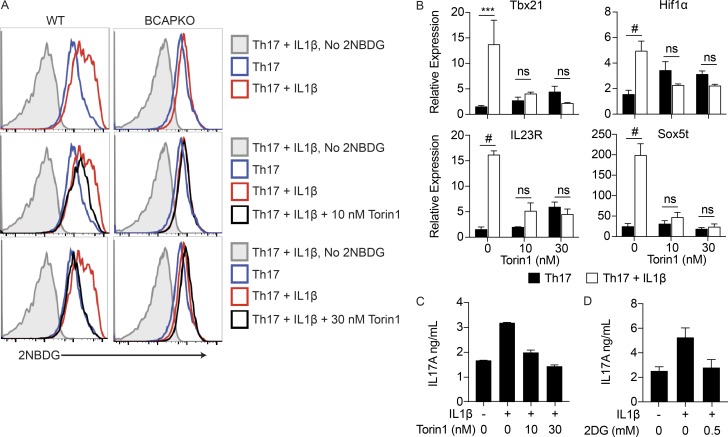
Because increased glycolysis requires increased glucose uptake, we next used the fluorescent glucose analogue 2NBDG to measure glucose uptake in CD4+ T cells polarized to Th17 cells with or without IL-1β. WT Th17 cells polarized in the presence of IL-1β showed increase glucose uptake, as measured by increased 2NBDG uptake, in comparison to WT Th17 cells polarized without IL-1β. Consistent with our previous results, BCAPKO T cells polarized to Th17 cells showed no differences in glucose uptake with or without the addition of IL-1β (Fig. 7 A).
Figure 7.
IL-1β mediated increases in glycolysis, and the generation of pathogenic Th17 cells is completely abrogated by low levels of mTOR and glycolysis inhibition. (A) Naive WT and BCAPKO CD4+ T cells were polarized to Th17 cells for 48 h, and then pretreated with 10 or 30 nM Torin1 for 1 h. After pretreatment, the indicated cells were stimulated with 10 ng/ml IL-1β and assayed with 2NBDG 24 h later to quantify glucose uptake via flow cytometry. Data are representative of three independent experiments. (B) Naive WT CD4+ T cells were polarized to Th17 cells for 48 h, and then pretreated with 10 nM or 30 nM Torin1 for 1 h. After pretreatment, the indicated cells were stimulated with 10 ng/ml IL-1β for 6 h and analyzed for pathogenic Th17 transcripts using qPCR. Shown is the mean ± SEM; n = 2. ***, P < 0.001; #, P < 0.0001. Statistical analysis was performed with the two-tailed unpaired Student’s t test. (C and D) Naive WT CD4+ T cells were polarized as in B, and then pretreated with Torin1 or 2DG for 1 h, and stimulated with 10 ng/ml IL-1β for 6 h. Supernatants were collected to determine levels of IL-17A secretion by ELISA. Shown is the mean ± SD of technical replicates. Data are representative of three independent experiments.
As previously described, activation of the PI3K-Akt-mTOR pathway is critical for T cell activation, differentiation, and function (Chi, 2012). We hypothesized that the enhancement of the PI3K pathway in Th17 lineage cells through IL-1R signaling pushes Th17 cells to increased pathogenicity during the process of differentiation. Thus, minimal and specific inhibition of this pathway should prevent the differentiation of pathogenic Th17 cells. We first assessed whether the addition of low levels of Torin1, an ATP-competitive catalytic inhibitor of mTOR, would prevent the increased glucose uptake specifically after IL-1β addition. Torin1 has an IC50 of ∼10 nM and has previously been used at a minimum concentration of 25 nM, but is typically used at more than 200 nM, and up to 1 µM (Thoreen et al., 2009, 2012; Matsuzawa et al., 2015; Zhao et al., 2015; Mao et al., 2016). We polarized naive WT and BCAPKO CD4+ T cells to the Th17 lineage using IL-6 + TGF-β for 48 h; after 48 h, we pretreated the cells with either 10 nM or 30 nM Torin1 for 1 h, and then added IL-1β. After 24 h of incubation, we analyzed the glucose uptake using 2NBDG. 10 nM Torin1 decreased glucose uptake in WT Th17 cells + IL-1β, and 30 nM Torin1 brought glucose uptake levels to that of WT Th17 cells polarized without IL-1β. Interestingly, the addition of Torin1 did not affect the glucose uptake by BCAPKO Th17 cells, suggesting that the addition of Torin1 is not affecting glycolysis levels determined by initial TCR activation (Fig. 7 A).
Because minimal inhibition of mTOR with low concentrations of Torin1 inhibited the increased glucose uptake after 24 h that was specifically dictated by IL-1β, we next assessed whether this inhibition of mTOR would decrease the pathogenic Th17 cell transcripts seen in WT T cells after the addition of IL-1β. We polarized WT naive CD4+ T cells to Th17 lineage using IL-6 + TGF-β for 48 h, and then pretreated with 10 nM or 30 nM Torin1 for 1 h. After the pretreatment, IL-1β was added to the culture for 6 h, after which the RNA was isolated for qPCR analysis of pathogenic Th17 transcripts. The addition of IL-1β alone significantly increased the transcription of pathogenic Th17 genes, but further addition of 10 nM Torin1 completely abrogated these effects of IL-1β (Fig. 7 B). The addition of Torin1 also returned the enhanced levels of IL-17A secretion to that seen without the addition of IL-1β (Fig. 7 C). Finally, to test if the increased IL-17A secretion is indeed a resultant of increased glycolysis, we used the glucose analogue 2DG, which inhibits hexokinase, thereby inhibiting glycolysis. Again, we polarized WT naive CD4+ T cells to Th17 using IL-6 + TGF-β for 48 h, and then pretreated with 0.5 mM 2DG for 1 h, after which we added IL-1β for 6 h. High levels of 2DG (50 mM) can inhibit T cell proliferation, but levels as low as 1 mM, higher than that used in this study, have been shown to have no significant effect on cell proliferation (Shi et al., 2011). Comparable to the addition of Torin1, 2DG addition completely prevented IL-1β–mediated increases in IL-17A secretion (Fig. 7 D).
Discussion
The IL-1 cytokine family plays a critical role in regulating the activation and differentiation of CD4+ T cells (Garlanda et al., 2013). IL-1R and IL-18R are members of the greater TLR–IL-1R superfamily because of their shared use of TIR domains, as well as TIR domain–containing adapters for downstream signal transduction (Takeuchi and Akira, 2010). The common usage of MyD88 for signal transduction downstream of both TLRs and IL-1R family members places this adapter as a critical node for innate control of adaptive immunity (Takeuchi and Akira, 2010). Previous work from our laboratory identified a cryptic N-terminal TIR domain in the signaling adapter BCAP (Troutman et al., 2012b). We demonstrated that BCAP is critical for TLR-induced activation of the PI3K-Akt pathway, and absence of BCAP not only led to abrogation of PI3K activation downstream of TLRs, but also led to enhanced inflammatory cytokine production by myeloid cells after activation of both plasma membrane and endosomal TLRs. In the current study, our data expand the importance of BCAP and provide critical evidence that establish it as an important signaling adapter in the extended TLR–IL-1R superfamily. Specifically, we discovered that BCAP is required for optimal differentiation of Th1 and Th17 lineage cells and has a very specific and defined role downstream of IL-1 and IL-18 receptors. Our in vitro data demonstrate that when T cells are activated in the presence of canonical Th1 and Th17 polarizing cytokines in the form of IL-12 and IL-6 + TGF-β, respectively, both WT and BCAP-deficient T cells differentiate into Th1 and Th17 lineage cells in a comparable fashion. However, when IL-1β or IL-18 are present in culture conditions, BCAP-deficient T cells fail to increase IFN-γ and IL-17A to levels of WT T cells. Analysis of steady-state Th1 and Th17 lineage cells in mice that specifically lack BCAP in T cells corroborated this finding, where there was no difference in the proportion of Th1 and Th17 lineage cells in various lymphoid organs and tissues between mice that harbored either BCAP-sufficient or BCAP-deficient T cells. This is also supported by previous studies showing normal Th1 and Th17 lineage cell commitment in mice with T cell–specific deletions of IL-1R during the steady-state (Mufazalov et al., 2016). The protein immunization experiments, however, revealed a clear defect in both priming and differentiation of Th1 and Th17 lineage cells. These data point to the fact that immunization with protein + TLR ligands leads to the active production of IL-1 and IL-18, which have important biological effects on CD4+ T cell priming. Although proliferation of BCAP-sufficient and BCAP-deficient T cells is comparable after engagement of TCR and the costimulatory molecule CD28, in vivo data point to the fact that after immunization, there is reduced priming in the absence of BCAP, suggesting a critical role for BCAP downstream of IL-1 and IL-18 receptors in both clonal expansion and effector differentiation. Further, BCAP was required for the generation of pathogenic Th17 cells, which are critical for fulminant autoimmunity, as demonstrated in vivo by decreased active and passive EAE disease development and reduced differentiation of pathogenic Th17 lineage cells in BCAPΔT mice.
The PI3K pathway is important for the survival, activation, and proliferation of all cells, including T cells; its importance in T cells is demonstrated by the convergence of TCR, costimulation, and many cytokine and chemokine receptors on PI3K (Chi, 2012). The multifactorial activation of PI3K leads to the activation of PDK1 and phosphorylation of Akt at T308, the activation of mTORC2, and the phosphorylation of Akt at S473, allowing for complete activation of Akt and the downstream activation of mTORC1. Once active, mTORC1 increases protein synthesis, glycolysis, and proliferation through a multitude of downstream proteins, including S6K, which phosphorylates ribosomal protein S6. IL-1R signaling has long been recognized for its ability to activate the PI3K-Akt pathway, although the signaling adapter responsible for this pathway was not known (Sizemore et al., 1999). In fact, it was previously reported that the IL-1R directly bound the regulatory p85 subunit of PI3K (Davis et al., 2006). Here we demonstrated that the signaling adapter BCAP is specifically required for propagation of PI3K signaling downstream of IL-1R in T cells. T cells lacking BCAP had normal NF-κB activation but were unable to activate the PI3K pathway upon IL-1β stimulation, as demonstrated through deficiencies in both Akt and S6 phosphorylation. Activation of the PI3K-Akt-mTOR pathway with the addition of IL-1β rapidly increased glycolysis and pathogenic Th17 transcripts. Inhibition of this pathway using low levels of Torin1, an ATP-competitive catalytic inhibitor of mTOR, completely abrogated the effects of IL-1β. This suggests that the generation of pathogenic Th17 cells may be an additive result of increased PI3K activation by IL-1R signaling and is likely potentiated by other PI3K activating cytokines, such as IL-23 (Cho et al., 2006).
Overall, these data suggest that the activation of the PI3K-Akt-mTOR pathway by different receptor systems (TCR/CD28 vs. IL-1R) has differential roles in determining the outcome of T cell activation. Although early activation of this pathway by TCR and CD28 is critical for T cell proliferation and survival, activation of the same pathway by IL-1R signaling via BCAP regulates the expression of genes associated with pathogenic effector differentiation. In many of the experiments, we also see a defect in IFN-γ production by BCAP-deficient T cells, likely because of the function of BCAP downstream of the IL-18R; although, it remains to be determined if BCAP has a similar function regulating PI3K-Akt-mTOR activation in response to IL-18. However, we cannot rule out the defect in IFN-γ production in vivo as a reflection of poor development of IL-17A–IFN-γ double-positive T cells and not the canonical Th1 lineage cells by BCAP-deficient T cells. Further work is required to understand the molecular role of BCAP in regulating Th1 cell responses. Inhibition of mTOR, either directly or through the activation of adinosine monophosphate-activated protein kinase, has been a therapeutic target for preventing transplant rejection and suppressing autoimmunity (Perl, 2015; Mangalam et al., 2016). This study provides increased evidence for the role of mTOR in the generation of autoimmunity, while also discovering BCAP as a new potential target for dampening autoimmune or pathogenic Th17 lineage cells in diseases such as multiple sclerosis and inflammatory bowel disease, without impeding the generation of Th17 cells necessary for barrier function. It is critical to note, however, that although targeting BCAP to prevent the generation and expansion of pathogenic Th17 cells is appealing, generic inhibition of BCAP would likely not be beneficial because of its role in controlling inflammation downstream of TLR activation in myeloid cells (Ni et al., 2012; Troutman et al., 2012b). This is an interesting dichotomy in the role of BCAP in immune system function and is a new example of how different arms of the immune system evolve to use similar pathways for opposing effector functions.
Materials and methods
Mice
BCAPKO mice were previously generated (Yamazaki et al., 2002) and were bred and maintained on the C57BL/6 background at the University of Texas Southwestern Medical Center animal facility. C57BL/6 WT control mice for BCAPKO mice were obtained from the University of Texas Southwestern Mouse Breeding Core Facility. Embryonic stem cells with the floxed BCAP locus were obtained from the Knockout Mouse Project repository, and the mice were generated by the University of Texas Southwestern Transgenic and Knockout Core facility. The selection cassette was removed by crossing mice to an Act-Flp recombinase expressing strain. The Act-Flp gene was bred off, and mice were crossed to LCK.Cre transgenic mice to generate mice that specifically lack BCAP in T cells (BCAPΔT mice). BCAPfl/fl littermates were used as controls for all experiments with BCAPΔT mice. Mice used were between 6 and 12 wk of age and were maintained in specific pathogen-free conditions. All mouse experiments were done following approved protocols by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Immunizations
For active EAE, mice were immunized subcutaneously with 200 µg mouse MOG35-55 (CS Bio) emulsified in CFA (Sigma-Aldrich) and were injected intraperitoneally with 200 ng pertussis toxin (List Biologicals) on days 0 and 2 after immunization. For (passive) transfer EAE, CD4+ T cells were isolated from draining lymph nodes of MOG35-55-immunized mice and restimulated with WT DCs (1 DC/5 T cells), 100 µg/ml MOG35-55, 10 ng/ml IL-1β, and 20 ng/ml IL-23 for 72 h, after which 5 × 106 restimulated CD4+ T cells were transferred i.v. into WT recipient mice. Mice were monitored daily, and disease severity was scored as follows: 0 = no clinical signs, 1 = tail paralysis, 2 = tail paralysis and hind limb weakness, 3 = partial hind limb paralysis, 4 = complete hind limb paralysis, 5 = forelimb paralysis or moribund, and 6 = dead. For OVA immunizations, mice were immunized in the footpads with 50 µg OVA (Biovendor) and 5 µg LPS (Invivogen) emulsified in incomplete Freund’s adjuvant (Sigma-Aldrich).
Cell isolation
T cells were purified by negative selection using antibodies against CD8 (TIB-105, TIB-150), MHC II (Y3JP), CD11b (TIB-128), B220 (TIB0146, TIB0164), and NK1.1 (HB191).
Naive T cells were isolated after total CD4+ T cell purification with biotin-conjugated αCD62L (1:200; MEL-14; BioLegend) followed by antibiotin microbeads and then sorted with the AutoMACS sorter (Miltenyi Biotec). Total CD4+ T cells and naive T cells were also isolated from splenocyte or lymph node single cell suspensions by the Mojosort Mouse CD4 Total or Naive T cell Isolation kit (BioLegend). DCs were isolated from spleens of mice previously injected with 5 × 103 B16.FLT3L by biotin-conjugated αCD11c (1:400; N418; BioLegend) followed by antibiotin microbeads (Miltenyi Biotec) and sorting with the AutoMACS sorter (Miltenyi Biotec; Hu et al., 2011). B cells were isolated from spleens of naive WT mice using biotin-conjugated αCD19 (1:400; 1D3; BD Biosciences) followed by antibiotin microbeads (Miltenyi Biotec) and sorting with the AutoMACs sorter (Miltenyi Biotec).
In vitro T cell priming
Purified naive CD4+CD62LhighCD44low T cells (3 × 105) were cultured with splenic CD11c+ DCs (6 × 104) with 3 ng/ml αCD3 (BM10-37; BioLegend) in the presence or absence of TLR ligands (100 ng/ml Pam3CSK4; Invivogen; or 1 µM CpG; Keck Oligos).
T cell polarizations
For polarizations using plate-bound αCD3 + αCD28, tissue culture plates were coated with 5 µg/ml αCD3 (145-2C11; BioLegend) and 5 µg/ml αCD28 (37.51; Tonbo), incubated at 37°C for 4 h, and washed three times immediately before use. For differentiation to Th17 cells, naive CD4+CD62LhighCD44low T cells were incubated with IL-6 (20 ng/ml; Peprotech), hTGF-β (5 ng/ml; Peprotech), αIL-4 (10 µg/ml; 11B11; BioLegend), and αIFN-γ (10 µg/ml; XMG1.2; BioLegend), with or without IL-1β (2–10 ng/ml; Peprotech). For differentiation to Th1 cells, naive CD4+CD62LhighCD44low T cells were incubated with IL-12 (10 ng/ml; Peprotech), IL-2 (50 U/ml; eBioscience), and αIL-4 (10 µg/ml; 11B11; BioLegend), with or without IL-18 (0.3–3 ng/ml; Gibco). Th2 cell differentiation was achieved by incubating naive CD4+CD62LhighCD44low T cells with IL-4 (4 ng/ml; Peprotech), IL-2 (50 U/ml; eBioscience), and αIFN-γ (10 µg/ml; XMG1.2; BioLegend).
T cell antigen recall assays
T cells purified from draining lymph nodes were harvested 7 d after immunization with OVA or 10 d after immunization with MOG35-55. T cells (3 × 105/well) were incubated with DCs (3–6 × 104/well) and titrating doses of antigen for 72 h. Supernatant was collected at 72 h to measure cytokine production. Proliferation of T cells was determined by incorporation of 3H-thymidine for the last 16 h of culture.
Flow cytometry
For surface staining, cells were first blocked with purified αCD16/CD32 (1:1,000; 2,4G2; Tonbo) for 15 min, and then stained with relevant antibodies for 30 min on ice. For intracellular cytokine staining, cells were first stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 1 µM ionomycin (Sigma-Aldrich) in the presence of brefeldin A (BioLegend) for 5 h, followed by surface staining. Cells were then fixed with 4% paraformaldehyde for 15 min at room temperature, permeabilized with 0.3% saponin, and then stained for intracellular cytokines. For transcription factor staining, cells were stained, fixed, and permeabilized using the Transcription Factor Staining Buffer Set (eBioscience). For pSTAT3 analysis, total lymphocytes were isolated and stimulated with IL-6 (20 ng/ml; Peprotech) and IL-1β (10 ng/ml; Peprotech), alone or together, for 30, 60, 240, or 360 min. Immediately after stimulation, cells were fixed to 2% PFA for 10 min at 37°C, washed, permeabilized with 100% ice-cold methanol, and chilled to −20°C for a minimum of 30 min. For pAkt and pS6 analysis, total CD4+ T cells were isolated from lymph nodes and spleen and activated for 30–72 h. After activation, cells were washed extensively and incubated in 1% FCS-RPMI for 16 h. Cells were stimulated with IL-1β (10 ng/ml; Peprotech), IL-2 (20 U/ml; BioLegend), or plate-bound 5 µg/ml αCD3 (BM10-37; BioLegend) and 5 µg/ml αCD28 (37.51; Tonbo). For p-p65 analysis, cells were stimulated directly in 10% FCS-RPMI media. After stimulation, cells were immediately fixed to 2% PFA for 10 min at 37°C, washed, permeabilized with 100% ice-cold methanol, and chilled to −20°C for a minimum of 30 min. After permeabilization, cells were blocked with purified αCD16/CD32 (1:1,000; 2.4G2; Tonbo) for 15 min and stained with relevant antibodies for 1 h at room temperature. For CFSE labeling, equal numbers of cells were washed in PBS and stained with 5 µM CFSE (BioLegend) in PBS for 7 min at room temperature. After incubation, cells were immediately washed in serum containing media and recounted for plating. For secondary antibody conjugation, cells were washed to remove primary antibody and stained for another 20 min with the appropriate antibody. Antibodies used were as follows: from BioLegend, CD4 (1:400; RM4-5), CD44 (1:800; IM7), CD62L (1:600; DREG-56), CD90.2 (1:1,000; 30-H12), IL-17A (1:200; TC11-18H10.1), IFN-γ (1:300; XMG1.2), Zombie Yellow (1:500), and CD121a (1:200; JAMA-147); from BD Biosciences, pSTAT3 (1:1,000; 4/P-STAT3); from Cell Signaling Technologies, pAkt (1:50; D9E), pS6 (1:1,000; D68F8), and p-p65 (1:50; 93H1); from eBiosciences, RORγt (1:100; AFKJS-9); and from Tonbo, FcBlock (1:1,000; 2.4G2).
Western blotting
Purified T cells were stimulated with plate-bound 5 µg/ml αCD3 (145-2C11; BioLegend) and 5 µg/ml αCD28 (37.51; Tonbo), polarized to the indicated lineages, and harvested at the designated time points. Cells were washed twice with PBS and lysed with RIPA buffer. Protein concentrations were quantified using the Bradford Assay, and equal amounts of protein were resolved on 4–12% Bis-Tris Plus gels (Thermo Fisher Scientific), transferred to PVDF membrane (EMD Millipore), and blotted for BCAP (1 µg/ml; 501813; R&D Systems) or H3 histone (1:3,000; Cell Signaling). Blots were then probed with appropriate secondary antibodies conjugated to fluorophores (IRDye 800CW goat anti–mouse, IRDye 680RD goat anti–rabbit), and membranes were imaged using LI-COR Odyssey CLx.
Isolation of lamina propria lymphocytes
Lamina propria lymphocytes were isolated as previously described (Ivanov et al., 2006; Hu et al., 2011). In brief, intestines were carefully removed from mice and extensively washed using ice-cold PBS. Intestinal tissue was cut longitudinally, and then into ∼1-inch pieces. Tissues were incubated in 1 mM EDTA with rotation for removal of the epithelial cell layer. Tissues were further digested three times using Collagenase Type IV (Sigma-Aldrich) and DNase1 (Sigma-Aldrich), passed through a 40-µm filter, and subjected to a 40–70% Percoll gradient. Lymphocytes were collected from the Percoll gradient and used for further analysis.
Quantitative real-time PCR
Purified naive CD4+CD62LhighCD44low T cells were activated with plate-bound 5 µg/ml αCD3 (145-2C11; BioLegend) and 5 µg/ml αCD28 (37.51; Tonbo), and were polarized to Th17 cells with IL-6 (20 ng/ml; Peprotech), hTGF-β (5 ng/ml; Peprotech), αIL-4 (10 µg/ml; 11B11; BioLegend), and αIFN-γ (10 µg/ml; XMG1.2; BioLegend), with or without IL-1β (10 ng/ml, Peprotech), for 6, 30, 48, or 60 h. For the experiments with Torin1 (Cell Signaling Technologies), cells were polarized to Th17 cells without IL-1β for 48 h, and then pretreated with 10 or 30 nM Torin1 for 1 h. After pretreatment, cells were treated with IL-1β as indicated for 6 h, after which supernatant was collected for cytokine quantification by ELISA, and cells were isolated for qPCR. After cells were collected and washed with PBS, cells were lysed with TRIzol Reagent (Invitrogen), RNA was isolated, and cDNA was synthesized using M-MLV Reverse transcription (Invitrogen). The QuantStudio 7 Flex Real-Time PCR System (Thermo Fisher Scientific) was used to measure SyBR green (Thermo Fisher Scientific) incorporation. Relative amounts of mRNA were normalized to 18S RNA levels within each sample. Oligonucleotides used were the following: Tbx21: forward, 5′-GGAGCCCACAAGCCATTACA-3′, reverse, 5′-ACATATAAGCGGTTCCCTGGC-3′; Hif1α: forward, 5′-GGGTACAAGAAACCACCCAT-3′, R-GAGGCTGTGTCGACTGAGAA-3′; Sox5t: forward, 5′-ACATCGGGAAGTAGGAGAGACTGA-3′, reverse, 5′-TACCTCTCCATCTGTCTCCCCATA-3′; IL-23R: forward, 5′-CTTCCCAGACAGTTTCCCAGG-3′, reverse, 5′-CCAAGAAGACCATTCCCGACA-3′; and BCAP: forward, 5′-TATCGCCTGGGAACCTGATG-3′, reverse, 5′-CCTTCTCGTCCAGCTTGCAT-3′.
Metabolic assays
For the Seahorse Assay, purified naive CD4+CD62LhighCD44low T cells were activated with plate-bound 5 µg/ml αCD3 (145-2C11; BioLegend) and 5 µg/ml αCD28 (37.51; Tonbo), and were polarized to Th17 cells with IL-6 (20 ng/ml; Peprotech), hTGF-β (5 ng/ml; Peprotech), αIL-4 (10 µg/ml; 11B11; BioLegend), and αIFN-γ (10 µg/ml; XMG1.2; BioLegend). After 48 h of polarization, IL-1β (10 ng/ml; Peprotech) was added to the appropriate wells. At 60 h, cells were transferred to Cell-Tak (Corning)–coated XF24 assay plates at 106 cells per well and analyzed using the glycolysis stress test (20 mM glucose, 1 µM oligomycin, and 100 mM 2DG) by the Seahorse Extracellular Flux Analyzer (Agilent) in the University of Texas Southwestern Metabolic Phenotyping Core. To determine lactate production, naive T cells were polarized with irradiated (12 Gy) B cells (1:2 ratio), and IL-6 (20 ng/ml; Peprotech), hTGF-β (5 ng/ml; Peprotech), αIL-4 (10 µg/ml; 11B11; BioLegend), αIFN-γ (10 µg/ml; XMG1.2; BioLegend), and IL-1β (10 ng/ml; Peprotech), as described. Supernatants were collected at the indicated time points, and lactate was measured using the BioVision Lactate Assay kit II. For glucose uptake, naive T cells were polarized to Th17 cells using plate-bound 5 µg/ml αCD3 (145-2C11; BioLegend) and 5 µg/ml αCD28 (37.51; Tonbo) for 48 h, pretreated with 10 nM or 30 nM Torin1, and then treated with IL-1β (10 ng/ml; Peprotech) for 24 h. At 72 h, cells were washed with PBS twice, incubated with glucose-free media for 1 h, and treated with 2NBDG (30 µM; Cayman Chemical) for 30 min. Cells were immediately stained and run for FACS analysis using NovoCyte (Acea Biosciences).
ELISA
Primary antibodies (αIFN-γ; R4-6A2; BioLegend; αIL-17A; TC11-18H10.1; BioLegend) were diluted in ELISA Coating Buffer (BioLegend) and coated on ELISA plates overnight at 4°C. Coated plates were blocked with PBS containing 1% BSA for 2 h. Then samples and appropriate standards were loaded in duplicate, diluted in blocking buffer, and incubated overnight. Detection antibodies (αIFN-γ; XMG1.2; BioLegend; αIL-17A; TC11-8H4; BioLegend) were used according to manufacturers’ instructions. Plates were washed extensively in between steps with PBS and 0.05% Tween-20, and were developed using the o-Phenylenediamine colorimetric assay and read at 490 nm using a iMark microplate reader (Bio-Rad).
Histology
On day 16 after immunization, mice were sacrificed and perfused through the heart with 10 ml of ice-cold PBS. Spinal cords were collected and fixed in 10% neutral-buffered formalin for 36 h, and then submitted to the University of Texas Southwestern Molecular Pathology Core for tissue embedding and Luxol Fast Blue staining. Slides were imaged at 10× using a DM2000 Compound Research Photomicroscope (Leica).
Isolation of lymphocytes from spinal cord
On day 30 after transfer, mice were sacrificed and perfused through the heart with 10 ml of ice-cold PBS. Spinal cords were collected in 2 ml PBS and digested using 2 mg/ml collagenase D and 28 U/ml DNase I for 30 min at 37°C. After digestion, cells were washed with PBS containing 2% FCS and 2 mM EDTA and passed through a 100-µm filter, and then subjected to a 40–70% Percoll gradient. Lymphocytes were collected from the Percoll gradient and used for further analysis.
Statistical analysis
Data are presented as means ± SD or means ± SEM, and comparison of datasets was performed with the two-tailed unpaired Student’s t test unless otherwise noted in the figure legend. Significance was considered at P < 0.05. *, P < 0.05; **, P < 0.01; ***, P < 0.001; and #, P < 0.0001. All statistical analysis was performed using Prism (GraphPad).
Online supplemental information
Fig. S1 shows that BCAPKO T cells have deficient priming in vivo but proliferate normally in response to TCR and CD28 stimulation and have equal expression of RORγt. Fig. S2 shows equal viability but decreased antigen-specific proliferation by BCAPΔT CD4+ T cells as well as normal expression of RORγt in BCAP-deficient T cells polarized to Th17 lineage with or without IL-1β. Fig. S3 shows that RORγt is comparable in CD4+ T cells isolated from lymph nodes of EAE-induced BCAPfl/fl and BCAPΔT mice. Fig. S4 shows defects in sustained STAT3 phosphorylation in CD4+ T cells that lack BCAP after IL-6 + IL-1β stimulation. Fig. S5 demonstrates defective glycolytic capacity of BCAP-deficient CD4+ T cells in response to IL-1β stimulation.
Supplementary Material
Acknowledgments
We would like to thank all members of the Pasare laboratory for helpful discussions and critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (AI113125 and AI123176) to C. Pasare. K. Deason is supported by American Heart Association grant 17PRE33410075.
The authors declare no competing financial interests.
Author contributions: K. Deason and T.D. Troutman designed experiments, performed experiments, analyzed results, and wrote the manuscript. A. Jain, D.K. Challa, R. Mandraju, and T. Brewer performed experiments. E.S. Ward designed experiments and reviewed the manuscript. C. Pasare designed experiments, analyzed results, and wrote the manuscript.
References
- Basu R., Whitley S.K., Bhaumik S., Zindl C.L., Schoeb T.R., Benveniste E.N., Pear W.S., Hatton R.D., and Weaver C.T.. 2015. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nat. Immunol. 16:286–295. 10.1038/ni.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Bouladoux N., and Hand T.W.. 2013. Effector and memory T cell responses to commensal bacteria. Trends Immunol. 34:299–306. 10.1016/j.it.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüstle A., Heink S., Huber M., Rosenplänter C., Stadelmann C., Yu P., Arpaia E., Mak T.W., Kamradt T., and Lohoff M.. 2007. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8:958–966. 10.1038/ni1500 [DOI] [PubMed] [Google Scholar]
- Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., and O’Shea J.J.. 2006. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. USA. 103:8137–8142. 10.1073/pnas.0600666103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. 2012. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12:325–338. 10.1038/nri3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M.L., Kang J.W., Moon Y.M., Nam H.J., Jhun J.Y., Heo S.B., Jin H.T., Min S.Y., Ju J.H., Park K.S., et al. . 2006. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J. Immunol. 176:5652–5661. 10.4049/jimmunol.176.9.5652 [DOI] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., and Dong C.. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587. 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.N., Mann E., Behrens M.M., Gaidarova S., Rebek M., Rebek J. Jr., and Bartfai T.. 2006. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc. Natl. Acad. Sci. USA. 103:2953–2958. 10.1073/pnas.0510802103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519–550. 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., and Thompson C.B.. 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity. 16:769–777. 10.1016/S1074-7613(02)00323-0 [DOI] [PubMed] [Google Scholar]
- Garlanda C., Dinarello C.A., and Mantovani A.. 2013. The interleukin-1 family: back to the future. Immunity. 39:1003–1018. 10.1016/j.immuni.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaublomme J.T., Yosef N., Lee Y., Gertner R.S., Yang L.V., Wu C., Pandolfi P.P., Mak T., Satija R., Shalek A.K., et al. . 2015. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell. 163:1400–1412. 10.1016/j.cell.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S.A., Yang W., Yan Z., Liu Y., Rowse A.L., Weinmann A.S., Qin H., and Benveniste E.N.. 2017. Protein Kinase CK2 Controls the Fate between Th17 Cell and Regulatory T Cell Differentiation. J. Immunol. 198:4244–4254. 10.4049/jimmunol.1601912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi S., Sekine E., Verstak B., Gay N.J., and Moncrieffe M.C.. 2017. Structure of the Toll/Interleukin-1 Receptor (TIR) Domain of the B-cell Adaptor That Links Phosphoinositide Metabolism with the Negative Regulation of the Toll-like Receptor (TLR) Signalosome. J. Biol. Chem. 292:652–660. 10.1074/jbc.M116.761528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.M., Wu D., Denroche H.C., Yao Y., Verchere C.B., and Levings M.K.. 2015. IL-33 Reverses an Obesity-Induced Deficit in Visceral Adipose Tissue ST2+ T Regulatory Cells and Ameliorates Adipose Tissue Inflammation and Insulin Resistance. J. Immunol. 194:4777–4783. 10.4049/jimmunol.1500020 [DOI] [PubMed] [Google Scholar]
- Harris T.J., Grosso J.F., Yen H.R., Xin H., Kortylewski M., Albesiano E., Hipkiss E.L., Getnet D., Goldberg M.V., Maris C.H., et al. . 2007. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179:4313–4317. 10.4049/jimmunol.179.7.4313 [DOI] [PubMed] [Google Scholar]
- Hu W., Troutman T.D., Edukulla R., and Pasare C.. 2011. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity. 35:1010–1022. 10.1016/j.immuni.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., and Littman D.R.. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Kolodin D., van Panhuys N., Li C., Magnuson A.M., Cipolletta D., Miller C.M., Wagers A., Germain R.N., Benoist C., and Mathis D.. 2015. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21:543–557. 10.1016/j.cmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A., Wu C., Kleinewietfeld M., Kunder S., Hafler D.A., et al. . 2012. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13:991–999. 10.1038/ni.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam A.K., Rattan R., Suhail H., Singh J., Hoda M.N., Deshpande M., Fulzele S., Denic A., Shridhar V., Kumar A., et al. . 2016. AMP-Activated Protein Kinase Suppresses Autoimmune Central Nervous System Disease by Regulating M1-Type Macrophage-Th17 Axis. J. Immunol. 197:747–760. 10.4049/jimmunol.1501549 [DOI] [PubMed] [Google Scholar]
- Mao Y., van Hoef V., Zhang X., Wennerberg E., Lorent J., Witt K., Masvidal L., Liang S., Murray S., Larsson O., et al. . 2016. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood. 128:1475–1489. 10.1182/blood-2016-02-698027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T., Oyama M., Kozuka-Hata H., Ishikawa K., Inoue T., Muta T., Semba K., and Inoue J.. 2010. Identification of BCAP-(L) as a negative regulator of the TLR signaling-induced production of IL-6 and IL-10 in macrophages by tyrosine phosphoproteomics. Biochem. Biophys. Res. Commun. 400:265–270. 10.1016/j.bbrc.2010.08.055 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Oshima S., Takahara M., Maeyashiki C., Nemoto Y., Kobayashi M., Nibe Y., Nozaki K., Nagaishi T., Okamoto R., et al. . 2015. TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy. Autophagy. 11:1052–1062. 10.1080/15548627.2015.1055439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Zu Horste G., Wu C., Wang C., Cong L., Pawlak M., Lee Y., Elyaman W., Xiao S., Regev A., and Kuchroo V.K.. 2016. RBPJ Controls Development of Pathogenic Th17 Cells by Regulating IL-23 Receptor Expression. Cell Reports. 16:392–404. 10.1016/j.celrep.2016.05.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani H., May L.T., Sehgal P.B., and Kupper T.S.. 1989. Synergistic interactions of IL-1 and IL-6 in T cell activation. Mitogen but not antigen receptor-induced proliferation of a cloned T helper cell line is enhanced by exogenous IL-6. J. Immunol. 143:896–901. [PubMed] [Google Scholar]
- Mufazalov I.A., Regen T., Schelmbauer C., Kuschmann J., Muratova A.M., Nikolaev A., Müller W., Pinteaux E., and Waisman A.. 2016. Generation of a Novel T Cell Specific Interleukin-1 Receptor Type 1 Conditional Knock Out Mouse Reveals Intrinsic Defects in Survival, Expansion and Cytokine Production of CD4 T Cells. PLoS One. 11:e0161505 10.1371/journal.pone.0161505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M., MacFarlane A.W. IV, Toft M., Lowell C.A., Campbell K.S., and Hamerman J.A.. 2012. B-cell adaptor for PI3K (BCAP) negatively regulates Toll-like receptor signaling through activation of PI3K. Proc. Natl. Acad. Sci. USA. 109:267–272. 10.1073/pnas.1111957108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Maeda A., Iwamatsu A., Gotoh K., and Kurosaki T.. 2000. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 13:817–827. 10.1016/S1074-7613(00)00079-0 [DOI] [PubMed] [Google Scholar]
- Okuda Y., Sakoda S., Fujimura H., Saeki Y., Kishimoto T., and Yanagihara T.. 1999. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35-55 induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 101:188–196. 10.1016/S0165-5728(99)00139-3 [DOI] [PubMed] [Google Scholar]
- Perl A. 2015. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann. N. Y. Acad. Sci. 1346:33–44. 10.1111/nyas.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F., and Korn T.. 2011. Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett. 585:3747–3757. 10.1016/j.febslet.2011.03.064 [DOI] [PubMed] [Google Scholar]
- Prasad K.V., Cai Y.C., Raab M., Duckworth B., Cantley L., Shoelson S.E., and Rudd C.E.. 1994. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc. Natl. Acad. Sci. USA. 91:2834–2838. 10.1073/pnas.91.7.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R., and Chi H.. 2011. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208:1367–1376. 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore N., Leung S., and Stark G.R.. 1999. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol. Cell. Biol. 19:4798–4805. 10.1128/MCB.19.7.4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola-Penna M. 2008. Metabolic regulation by lactate. IUBMB Life. 60:605–608. 10.1002/iub.97 [DOI] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., and Lavelle E.C.. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691. 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., and Akira S.. 2010. Pattern recognition receptors and inflammation. Cell. 140:805–820. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Tanaka S., Suto A., Iwamoto T., Kashiwakuma D., Kagami S., Suzuki K., Takatori H., Tamachi T., Hirose K., Onodera A., et al. . 2014. Sox5 and c-Maf cooperatively induce Th17 cell differentiation via RORγt induction as downstream targets of Stat3. J. Exp. Med. 211:1857–1874. 10.1084/jem.20130791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeSlaa T., and Teitell M.A.. 2014. Techniques to monitor glycolysis. Methods Enzymol. 542:91–114. 10.1016/B978-0-12-416618-9.00005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen C.C., Kang S.A., Chang J.W., Liu Q., Zhang J., Gao Y., Reichling L.J., Sim T., Sabatini D.M., and Gray N.S.. 2009. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284:8023–8032. 10.1074/jbc.M900301200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen C.C., Chantranupong L., Keys H.R., Wang T., Gray N.S., and Sabatini D.M.. 2012. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 485:109–113. 10.1038/nature11083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K., Yoshimoto T., Torigoe K., Kurimoto M., Matsui K., Hada T., Okamura H., and Nakanishi K.. 2000. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int. Immunol. 12:151–160. 10.1093/intimm/12.2.151 [DOI] [PubMed] [Google Scholar]
- Troutman T.D., Bazan J.F., and Pasare C.. 2012a Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 11:3559–3567. 10.4161/cc.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutman T.D., Hu W., Fulenchek S., Yamazaki T., Kurosaki T., Bazan J.F., and Pasare C.. 2012b Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc. Natl. Acad. Sci. USA. 109:273–278. 10.1073/pnas.1118579109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar A., Moro K., Xin A., Liao Y., Gloury R., Kawamoto S., Fagarasan S., Mielke L.A., Afshar-Sterle S., Masters S.L., et al. . 2015. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16:276–285. 10.1038/ni.3085 [DOI] [PubMed] [Google Scholar]
- Xu Y., Tao X., Shen B., Horng T., Medzhitov R., Manley J.L., and Tong L.. 2000. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 408:111–115. 10.1038/35040600 [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Takeda K., Gotoh K., Takeshima H., Akira S., and Kurosaki T.. 2002. Essential immunoregulatory role for BCAP in B cell development and function. J. Exp. Med. 195:535–545. 10.1084/jem.20011751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Takeda K., Tanaka T., Ohkusu K., Kashiwamura S., Okamura H., Akira S., and Nakanishi K.. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161:3400–3407. [PubMed] [Google Scholar]
- Zeng H., and Chi H.. 2013. mTOR and lymphocyte metabolism. Curr. Opin. Immunol. 25:347–355. 10.1016/j.coi.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., and Chi H.. 2014. mTOR signaling and transcriptional regulation in T lymphocytes. Transcription. 5:e28263 10.4161/trns.28263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhai B., Gygi S.P., and Goldberg A.L.. 2015. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc. Natl. Acad. Sci. USA. 112:15790–15797. 10.1073/pnas.1521919112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.