Abstract
Appendiceal neoplasms are identified in 0.9 to 1.4% of appendiceal specimens, and the incidence is increasing. It has long been professed that neuroendocrine tumors (formerly carcinoids) are the most common neoplastic process of the appendix; recent data, however, has suggested a shift in epidemiology. Our intent is to distill the complex into an algorithm, and, in doing so, enable the surgeon to seamlessly maneuver through operative decisions, treatment strategies, and patient counseling. The algorithm for evaluation and treatment is complex, often starts from the nonspecific presenting complaint of appendicitis, and relies heavily on often subtle histopathologic differences.
Keywords: appendiceal neoplasms, gastrointestinal neuroendocrine tumors, appendiceal neuroendocrine tumors, pseudomyxoma peritonei, adenoneuroendocrine carcinoma
Disorders of the appendix may be inflammatory or neoplastic, with neoplastic processes hailing from myriad etiologic origins. Our intent is to summarize this complex disease to enable the surgeon to seamlessly maneuver through operative decisions, treatment strategies, and patient counseling.
Historical Perspective
While artistic depictions of the appendix date back to the late 15th century, it was not until 1522 that the appendix was described in the literature by Jacopo Berengario da Carpi. 1 2 Despite a rudimentary knowledge of the existence of this structure, and multiple autopsy reports revealing appendiceal perforations, its role in right lower quadrant inflammation was not fully realized for over three centuries. 2 3 4 The interim years saw the first appendectomy performed by Claudius Amyand in 1735; it was not, however, until 1886 that “appendicitis” gained widespread acceptance as a primary process necessitating surgery. 5 6 Open appendectomy, as we know it today, was popularized by McBurney in 1889. 7
With the acceptance of appendicitis as a primary etiology, a somewhat normalized surgical approach, and improving anesthesia, the late 19th century saw an explosion in the numbers of appendectomies performed. 2 During that time, a limited awareness of appendiceal neoplasia took root. Elting reported a series of 43 cases of appendiceal tumors between 1903 and 1938, of which 23 were true carcinomas of the appendix. 8
Today, nearly 300,000 appendectomies are performed in the United States per year, making it the most common general surgical procedure. 9 10 With these staggering numbers, it stands to reason that a percentage of appendixes will reveal unexpected neoplastic pathology. A majority of these cases are discovered incidentally upon pathological review of the specimen; some, however, may be recognized intraoperatively. 11 Regardless of the modality of identification, the recognition of a neoplastic process inherently changes the treatment algorithm, sometimes drastically. The consequence of ignorance in these cases is particularly grave, as 74% of appendiceal cancers have already spread, and over one-third have regional or distant metastases at the time of diagnosis. 12
Epidemiology
Appendiceal neoplasms are identified in 0.9 to 1.4% of appendiceal specimens, with increasing incidence. 12 13 14 15 It has long been professed that neuroendocrine tumors (formerly carcinoids) are the most common neoplastic process of the appendix; recent data, however, have suggested a shift in epidemiology. A large series from the Surveillance, Epidemiology, and End Results (SEER) database identified neuroendocrine tumor as the etiology in only 28% of appendiceal specimens containing neoplasia. 12 This does not reflect a decrease in incidence of neuroendocrine tumor, as much as it does a relative increase in the frequency of epithelial tumors over the past several decades. 12 15 The SEER data must be interpreted within context, however, as appendiceal neuroendocrine tumors (ANETs) are often considered indolent, and may therefore be excluded from SEER reports.
The appendix is the third most common site for gastrointestinal neuroendocrine tumors, accounting for 16.7% of these cases. The small intestine and rectum make up 44.7 and 19.6%, respectively. 16 ANETs generally occur in young patients, are slightly more common in females, and are diagnosed on average between 32 and 42 years of age. 17 18 This lies in contrast to epithelial tumors of the appendix, which comprise only 0.1% of epithelial malignancies of the colon and rectum, and are usually diagnosed in the seventh decade of life. As with ANETs, epithelial tumors of the appendix have a slight female preponderance, although a hormonal influence has not been proven in either case. 10 11 12 19
Pathology
The appendix itself is a vermiform colonic excrescence. The layered wall is similar to that of the remainder of the colon; immunologic tissue, or gut-associated lymphoid tissue, however, makes up a larger percentage of the submucosal composition. Goblet cells are abundant and secrete approximately 2 to 3 mL of mucin per day. As the appendix has only one luminal communication with the colon, any obstruction results in appendicitis. Consequently, appendiceal tumors generally present as appendicitis, with less than 50% of these lesions being recognized as neoplasms intraoperatively. 11
Appendiceal tumors are broadly categorized as epithelial or nonepithelial. Epithelial tumors include mucinous neoplasms, nonmucinous adenocarcinoma, and signet ring cell tumors, while nonepithelial neoplasms consist of neuroendocrine tumors, lymphoma, and sarcoma. Goblet cell carcinoids are aggressive tumors that, despite their misleading nomenclature, share characteristics of both epithelial and nonepithelial tumors.
Epithelial Tumors
The ever-shifting histologic designation of epithelial neoplasms of the appendix may seem confusing. In Table 1 , we list the various descriptors used in the literature to reference these lesions. The simplest breakdown is between mucinous and nonmucinous tumors. 20
Table 1. Epithelial neoplasms of the appendix.
| Mucinous |
|---|
| Mucocele Mucinous cystadenoma Mucinous neoplasm of uncertain malignant potential Borderline appendiceal mucinous tumor Mucinous neoplasm of low malignant potential Low-grade appendiceal mucinous neoplasm Mucinous cystadenocarcinoma Mucinous adenocarcinoma Cystadenocarcinoma Disseminated peritoneal adenomucinosis Peritoneal mucinous carcinomatosis Pseudomyxoma peritonei Pseudomyxoma peritonei syndrome |
| Nonmucinous |
| Appendiceal adenoma Appendiceal adenocarcinoma Appendiceal signet ring cell carcinoma |
Mucinous
Mucinous tumors are classified by grade, with low-grade appendiceal mucinous neoplasms (LAMN) being the generic descriptor for appendiceal adenomas, cystadenomas, borderline tumors, and mucinous tumors of uncertain malignant potential. These are slow-growing, well-differentiated lesions, leading to cystic dilation of the appendix and fibrosis of the appendiceal wall. Microscopic examination reveals replacement of normal mucosa by adenomatous proliferation of villous, papillary, serrated, or flat mucinous lesions. The epithelial lining consists of mucin-laden columnar cells with pencil-shaped, mildly hyperchromatic nuclei with pseudostratification, rare mitoses, and apoptotic nuclear debris. 11 This lining can herniate into the muscularis propria, which may represent the mechanism of perforation and intraperitoneal spread of mucin. 21
High-grade mucinous tumors invade beyond the muscularis mucosa, and are generally referred to as mucinous adenocarcinoma or cystadenocarcinoma. Histologically, these lesions are marked by malignant glandular epithelial cells in strips, clusters, and complex proliferations. Malignant cells are present within infiltrating pools of mucin, and, upon appendiceal rupture, spread along peritoneal surfaces. 11
A rare, though particularly aggressive, variant of mucinous adenocarcinoma is signet ring cell carcinoma. It accounts for an estimated 4% of appendiceal malignancies, and is characterized by mucin-laden malignant cells (signet cells) floating in pools of extracellular mucin. These cancers rapidly disseminate, and may spread covertly beneath normal-appearing mucosa. As a result, up to 60% of these lesions have distant metastases at the time of diagnosis. 11 15 22
Regardless of whether they are low grade or high grade, mucinous neoplasms can lead to the formation of a mucocele, which refers to dilation of the appendix with intraluminal accumulation of mucin. With appendiceal rupture, this mucin is released into the peritoneal cavity. Secondary proliferation of cellular components represents a tremendous clinical dilemma, as it may lead to mucinosis, pseudomyxoma peritonei (PMP), and peritoneal carcinomatosis. The spread of mucin tends to follow the natural clockwise circulation of peritoneal fluid from the right pericolic gutter to the right subdiaphragmatic sulcus, retrohepatic vena cava, splenic hilum, and ligament of Treitz. The falciform ligament directs some flow to the pelvis cul-de-sac, left paracolic sulcus, and the ovaries. Involvement of the small bowel itself is uncommon, supported by the theory that peristalsis impedes the ability of an implant to remain in place. 23 24
Pseudomyxoma Peritonei
Carl Rokitanski first described the phenomenon of PMP in 1842; the term, however, became widely known in 1884. 25 26 Unfortunately, despite knowledge of its existence for more than 150 years, the diagnosis itself remains poorly understood, and is defined as mucinous ascites and mucinous implants diffusely involving peritoneal surfaces. 23 27 As such, the term does not discriminate between mucinous carcinomatosis secondary to appendiceal, colorectal, gastric, urachal, pancreatic, cholecystic, gastric, or ovarian neoplasms. Given the disparate natural histories of PMP, based on the primary tumor site, it is essential that the PMP syndrome secondary to appendiceal dissemination be distinguished from other etiologies. 23
Despite diffuse tumor spread throughout the peritoneum, solid organ and lymphovascular invasion are rare. 28 It ultimately results in death, due to obstruction of abdominal viscera. The histologic constitution of the mucinous implants includes amorphous mucinous material, fibrous tissue, and strips of cytologically bland, noninvasive, mucin-secreting epithelium. Most cases are CK-20 positive, with a minority also staining positive for CK-7. Increased numbers of mucin 2–secreting goblet cells have been associated with accumulation of extracellular mucin. 29 30
Prognosis is determined by the extent to which the spread occurs, as well as the malignant potential of the mucinous tumor cells. The absence of epithelial cells within mucin portends a substantially better prognosis than the converse; epithelial cells, however, can be found in most cases if implants are extensively sampled. 27 PMP resulting from LAMN is often localized to the right lower abdominal quadrant, and is suggested by mucinous implants composed of nonstratified, simple, or focally proliferative columnar and cuboidal epithelium containing uniform, cytologically bland nuclei and few mitotic figures. These lesions have been labeled as disseminated peritoneal adenomucinosis (DPAM) to distinguish them from peritoneal mucinous (adeno-)carcinomatosis (PMAC). 11 20 27
The reason for this distinction cannot be overstated, as response to treatment and overall prognosis are significantly worse in cases of PMAC. 27 Histologic examination of PMAC implants reveals cribriform structures and severe cytologic atypia in the form of enlarged nuclei with prominent nucleoli, similar to intestinal-type mucinous adenocarcinoma. As such, these lesions tend to follow the same behavioral trends as peritoneal carcinomatosis from other gastrointestinal sources, including the penchant for lymphovascular invasion. 29 Simple aspiration of mucin cannot reliably differentiate PMAC from DPAM, although the degree of cytologic atypia seen in peritoneal washings is generally concordant with implant histology. 31
Nonmucinous
Nonmucinous adenomatous lesions include adenocarcinomas of the appendix. They are analogous to other primary colorectal adenomatous neoplasia. As with adenocarcinoma of the colon, appendiceal nonmucinous adenocarcinoma invades locally, and metastasizes to regional lymph nodes and the liver. 21 Microscopic analysis often reveals malignant glandular formations with relative cellular disorganization and increased stratification; cuboidal glandular epithelium with modest amounts of extracellular mucin may, however, also be identified. 11 29
Nonepithelial Tumors
Neuroendocrine
Formerly referred to as carcinoids, ANETs arise from neuroendocrine progenitor cells in the appendiceal lamina propria and submucosa. 32 33 A majority (75%) of these tumors are located at the appendiceal tip, a phenomenon likely explained by the relative abundance of subepithelial neuroendocrine cells at the apex of the appendix. 33 In contrast to foregut and hindgut neuroendocrine tumors, midgut carcinoids are frequently hormonally active. Secreted hormones include growth hormone (GH), GH-releasing hormone, gastrin, calcitonin, substance P, insulin, and neurotensin, as well as serotonin. These hormones undergo first-pass metabolism by the liver, which prevents clinical manifestations, such as serotonin syndrome until liver metastases allow for entry into the systemic circulation. Byproducts of hepatic metabolism of serotonin include 5-hydroxyindoleacetic acid (5-HIAA), which is secreted in the urine and may function as a tumor marker. 11
Neuroendocrine tumors are well differentiated, and thus are relatively indolent processes with only a small fraction progressing to extensive disease. 10 15 29 Although the terminology is similar, these tumors must be differentiated from neuroendocrine carcinoma, which is the representative term for poorly differentiated small cell and large cell variants. 34 These more aggressive variants are quite rare, and are outside the scope of this article, and will not be discussed further. Neuroendocrine tumors appear grossly as yellow-tan, firm nodules, and a majority of which them are less than 1 cm at the time of diagnosis. 34 Microscopic analysis reveals submucosal uniform cell conglomerates with a nested or insular pattern. The cytoplasm has a modestly eosinophilic, fine granularity, and the nuclei show the classic endocrine “salt-and-pepper” chromatin pattern. Immunohistochemical staining is positive for neuroendocrine tissue markers, to include neuron-specific enolase, synaptophysin, and chromogranin A. 11 21 35 Chromogranin A is a widely used biomarker, as it may predict relapse even prior to radiographic evidence of such. 35 The K i -67 protein is also useful as a predictor of proliferative capacity and tumor grade. 34 36
Lymphoma/Sarcoma
While rare by comparison to other appendiceal neoplasms, given the high percentage of appendiceal lymphoid tissue present in the appendix, it should not be surprising that lymphoma may manifest in this location. Appendiceal lymphoma is slightly more prevalent in men (1.5:1), with a mean age of 18 years at diagnosis. The etiology is almost exclusively attributed to Burkitt's lymphoma; older patients, however, may develop diffuse, large B cell lymphoma. 21 37 Other rare solid appendiceal tumors include neuromas, stromal tumors, and Kaposi's sarcoma. These tumors will not be discussed in further detail here.
Goblet Cell
Another point of confusion with regard to histologic classification is the goblet cell “carcinoid.” Previously thought of as an aggressive variant of neuroendocrine tumor, it is now known to have features of both neuroendocrine and epithelial neoplasms. In fact, a more accurate and increasingly popular term is mucinous adenoneuroendocrine carcinoma. 38 39 40 These tumors most commonly present in the sixth decade, occur predominantly in females (4:1), and have a far worse stage-for-stage prognosis than neuroendocrine tumors. 39 40 41
Presentation
The clinical presentation of appendiceal tumors is variable and nonspecific. Distention of the appendix with accumulation of intraluminal mucin may cause vague lower abdominal symptoms, due to the stretching of the visceral peritoneum, although this may be a late finding. Even PMP may be asymptomatic until the end stages of the disease, when abdominal distention becomes the presenting sign. 23 Similarly, nonmucinous neoplasms are either asymptomatic or associated with ambiguous symptoms, such as weight loss, or chronic iron deficiency anemia. Unfortunately, diagnostic colonoscopy to evaluate these clinical findings will almost invariably fail to identify appendiceal tumors, unless they cause extrinsic compression, or involve the appendiceal base and cecum. 42 Even hormonally active neuroendocrine tumors can be surreptitious, as serotonin syndrome manifests in less than 5% of these patients, and is generally due to widespread liver metastases and bypass of first-pass metabolism. 11
Regardless of the pathologic etiology, the most acute manifestation of appendiceal neoplasia is appendicitis. In this setting, neoplastic obstruction of the appendiceal lumen leads to distention, venous engorgement, swelling, and eventually infection, with characteristic right lower quadrant abdominal pain and appendiceal dilation and inflammation on imaging. In order for this to occur, the lesion must be in the body or base of the appendix, as tumors at the “tip” will not lead to luminal obstruction. In cases of appendicitis, a neoplastic etiology is rarely suspected intraoperatively, and often does not come to light until the pathologic exam is completed. Patient factors that should raise the surgeon's clinical suspicion for a neoplastic process include age older than 50 years, a family history of colon cancer or inflammatory bowel disease, “chronic appendicitis,” or unexplained anemia. 11 A dilated appendix in the absence of appendiceal wall thickening (i.e., < 6 mm), or adjacent fat stranding should also raise suspicion for a neoplastic process. Clearly, these factors are somewhat nonspecific, highlighting the importance of maintaining a broad differential diagnosis in all cases of appendicitis.
Diagnosis/Staging
Clinical work-up of these lesions depends in part on where in the algorithm the patient presents. When the presenting complaint is appendicitis, a preoperative computed tomography (CT) scan has often been performed, and there an appendix specimen is taken, allowing for pathologic diagnosis. In other settings, the diagnosis is less clear, and the suspicion may simply be due to incidentally found appendiceal dilation on imaging performed for an unrelated complaint. Regardless of the diagnostic modality or malignant etiology, cross-sectional imaging (either CT or magnetic resonance imaging [MRI]) of the abdomen is necessary. The only exception is small (< 1 cm) neuroendocrine tumors without high-risk features, for which appendectomy alone is considered curative. Imaging studies are performed not only for characterization of the primary lesion but also to rule out metastatic disease. In cases of adenocarcinoma, it is important to obtain a CT of the chest as well to evaluate for pulmonary metastases. Baseline carcinoembryonic antigen (CEA) and chromogranin A may be measured for adenocarcinoma and neuroendocrine tumors, respectively. Urine 5-HIAA may also be obtained in cases of neuroendocrine tumor. Additional reflex testing should include complete colonoscopy, as both epithelial and neuroendocrine tumors have a 10 to 20% incidence of metachronous colonic lesions. 43
On cross-sectional imaging, mucinous tumors are characterized by cystic dilation of the appendix generally more than 15 mm, but may also be represented by a moderately enhancing soft tissue mass. 44 The lesions are well encapsulated, with smooth, nonthickened walls, often displaying punctate, curvilinear calcifications. 11 45 Larger mucoceles may displace or compress the small bowel in the right lower quadrant. Cases of appendicitis secondary to neoplasia present with classic findings of a thickened, dilated appendix with a hyperemic wall, and surrounding inflammatory changes, often without signs of an underlying neoplastic process.
Preoperative suspicion of PMP is based on physical examination and CT or MRI. The patient's abdomen is distended, dull to percussion, and nontender. Imaging reveals hypoattenuating ascites with secondary signs of extrinsic compression, such as scalloping of the liver, peritoneal reflection, or pouch of Douglas. 11
Other imaging modalities may be utilized for ruling out metastatic disease in cases where malignancy is highly suspected, or has already been diagnosed. CT or MRI chest is necessary for ruling out lung metastases. Positron emission tomography (PET) may be a reasonable adjunct in cases with suspicious lesions on more conventional imaging. Single-photon emission CT/CT (somatostatin receptor PET with 3-phase CT) may be considered for further metastatic evaluation in cases ofANET. 11
Surgical Management
Surgery is the mainstay of treatment for nearly all appendiceal neoplasia. Notable exceptions include lymphoma, advanced PMP (peritoneal carcinomatosis index [PCI] > 22), neuroendocrine tumors with diffuse systemic metastases, or adenocarcinoma with both peritoneal disease and either supradiaphragmatic or multiple solid organ metastases. As previously alluded to, many appendiceal neoplasms are not identified until pathologic analysis is performed. As such, the index operation is frequently complete by the time a diagnosis is established. In cases where appendectomy alone is adequate therapy, all that is needed is patient counseling and appropriate surveillance. In other cases, however, additional procedures may be necessary for completion of staging and curative intent. If suspicion is high during appendectomy, it is certainly reasonable to send a frozen section and proceed with a right hemicolectomy and mesocolic lymph node dissection if malignancy (with the exception of lymphoma or small neuroendocrine tumor) is detected. Nevertheless, if the base of the appendix is free of tumor, it is never wrong to think of appendectomy as a diagnostic excisional biopsy, terminate the case, and formulate further plans according to the definitive pathologic diagnosis. If the base of the appendix/cecum is involved, it is prudent to be prepared for an oncologic colectomy. A complete algorithm based on surgical or pathologic findings can be seen in Fig. 1 .
Fig. 1.
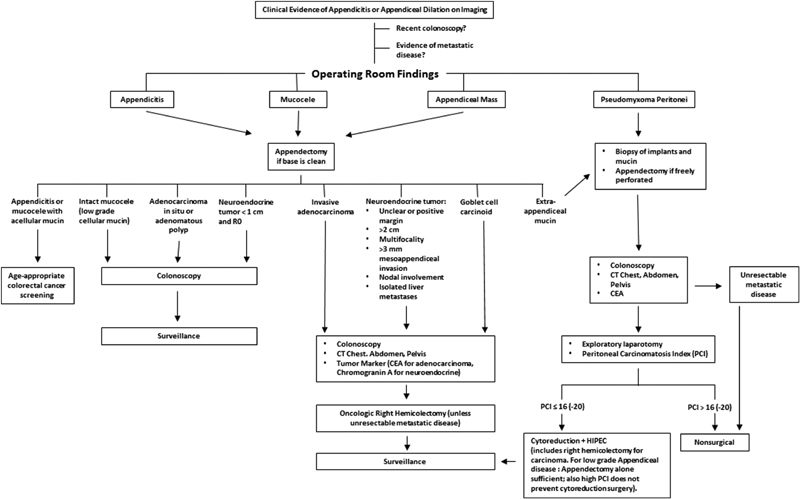
Algorithm for management of appendiceal neoplasia. CEA, carcinoembryonic antigen; CT, computed tomography; HIPEC, hyperthermic intraperitoneal chemotherapy; PCI, Peritoneal Carcinomatosis Index.
Appendectomy
In the setting of appendiceal neoplasia, appendectomy alone should be thought of as a diagnostic biopsy. For premalignant lesions, such as adenomatous polyps, adenocarcinoma in situ, or nonperforated low-grade mucinous tumors with R0 margins, appendectomy alone is sufficient. Small (< 1 cm), isolated neuroendocrine tumors with an R0 margin are also appropriately treated with appendectomy alone. Larger (1–2 cm) neuroendocrine tumors may be definitively treated with an appendectomy, assuming the margin is clear, there is less than 3 mm of mesoappendiceal invasion, multifocality is not present, and there is no regional nodal or isolated liver involvement. 36 46 It is important that the patients are counseled according to definitive pathology, and that screening and/or surveillance colonoscopy is performed in the case of pre or early malignancy.
Right Hemicolectomy
Oncologic resection is the standard of care for malignancy. Not only is lymph node harvest essential for staging of adenocarcinoma but also it is critical in cases of large neuroendocrine tumors, as effective chemotherapy does not exist. While small (≤ 1 cm) neuroendocrine tumors are fairly indolent, rarely spreading to lymph nodes, those > 2 cm have a 20 to 30% risk of regional spread. 21 34 47 Therefore, any ANET with a size > 2 cm, a questionable margin, or with any of the other aforementioned high-risk features warrants a right hemicolectomy. 47 48
Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
Evidence of extra-appendiceal spread of mucin, whether it be due to the pathophysiology of disease or iatrogenic rupture of the appendix during appendectomy, necessitates consultation to a hyperthermic intraperitoneal chemotherapy (HIPEC) center. 47 An appendiceal mucinous neoplasm should not be considered a benign occurrence, as PMP is common after resection, especially in the case of perforation, and may occur as late as 10 years after the initial appendectomy. If either localized or disseminated PMP is identified, it is important to remember the Hippocratic oath and “first do no harm.” Multiple biopsies of peritoneal implants are critical for further strategizing and treatment, as accurate diagnosis correlates with the number of biopsies. 27 An appendectomy may be necessary for control of contamination in the setting of perforation, but it is overall unwise to manipulate the abdominal viscera, as it may compromise the subsequent cytoreductive surgery. While thorough discussion of cytoreduction and HIPEC are outside the scope of this article, it is important to understand that the goal after complete staging and colonoscopy is complete surgical removal of all visible tumor, leaving HIPEC to address remaining microscopic disease. Prognosis and treatment decisions are heavily reliant on the PCI, which is calculated by the HIPEC surgeon at the time of exploratory laparotomy. The score itself has a maximum of 39 points from nine abdominal squares and four small bowel segments, with each area being scored between 0 and 3, when deposits are > 5 cm. 48 A score > 16 generally signifies inoperable disease; HIPEC, however, may be employed with scores as high as 22 in certain instances.
Unresectable Disease
Unresectable cases include adenocarcinoma with diffuse systemic metastases, PMP from adenocarcinoma with a PCI > 18, PMP with simultaneous systemic metastases, and neuroendocrine tumors with diffuse metastatic disease beyond one organ. 11 Patients burdened by these circumstances must rely on palliative medicine alone for symptom relief and prolonged survival.
Medical Management
As with colon adenocarcinomas, appendiceal epithelial tumors with nodal invasion and distant metastases may benefit from adjuvant chemotherapy. Treatment is generally 5-fluourouracil based, although additional regimens utilizing capecitabine are increasingly being utilized. Bevacizumab plays a role in cases of adenocarcinoma with distant metastases in which excision of the primary tumor is not eminent. 49 Neuroendocrine tumors do not have an effective chemotherapeutic agent, although somatostatin may be used for palliation in metastatic or symptomatic cases.
Outcomes
Outcomes in patients with appendiceal neoplasia are variable, and correspond to the etiology and stage of the tumor. The 5-year disease-specific survival ranges from 27 to 93%, depending on histological type, with signet ring cell tumors portending the worst prognosis and neuroendocrine tumors portending the most favorable. 50
Recent staging algorithms have classified appendiceal epithelial neoplasia as a distinct entity separate from colorectal malignancy ( Table 2 ). 11 51 Interestingly, epithelial tumors of the appendix are often biologically indolent in comparison to their colorectal counterparts. Aggressive histology is seen much less frequently, and regional or distant spread is less common at the time of diagnosis. Despite these favorable features, however, 5-year survival is significantly lower in appendiceal adenocarcinoma. This is likely secondary to the relatively high percentage of mucinous tumors, signet ring cell histology, and PMP ( Table 3 ). 23 These worse outcomes are independent of stage and grade, which also contribute to the ultimate prognosis. 28 52 With serial laparotomy and aggressive debulking, survival rates range from 50 to 85% at 5 years, but decline to approximately 20% at 10 years in cases of disseminated mucinosis. Mucinous carcinomatosis confers a particularly grim prognosis, with 5-year survival rates of less than 10%. 27 The combination of cytoreductive surgery and intraperitoneal chemotherapy is thought to improve survival in select patient populations with PMP with overall 5- and 10-year survival rates of 72% and 54%, respectively. 53 Unfortunately, comparative data analyzing standard care (resection and chemotherapy) versus cytoreduction and early postoperative intraperitoneal chemotherapy or HIPEC are lacking, and the only randomized comparison was heavily criticized, due to bias in the form of superior tumor debulking in the experimental group. 54 As of the writing of this article, there is no clear consensus on which patients benefit from HIPEC.
Table 2. TNM staging by AJCC for appendiceal adenocarcinoma 11 50 .
| Stage | T | N | M |
|---|---|---|---|
| X | Primary tumor not determined, or any T | Regional lymph nodes not determined, or any N | Metastatic disease not determined, or any M |
| 0 | No evidence of primary tumor | No regional lymph node metastasis | No distant metastasis |
| Is | Carcinoma in situ: intraepithelial or invasion of lamina propria | – | – |
| I | Tumor invades submucosa | Metastasis in 1–3 regional lymph nodes | Ia: Intraperitoneal metastasis beyond the right lower quadrant, including pseudomyxoma peritonei IIb: Nonperitoneal metastases |
| II | Tumor invades muscularis propria | Metastasis in four or more regional lymph nodes | |
| III | Tumor invades through muscularis propria into subserosa or into mesoappendix | ||
| IV | IVa: Tumor penetrates visceral peritoneum, including mucinous peritoneal tumor within the right lower quadrant IVb: Tumor directly invades other organs or structures |
Abbreviation: AJCC, American Joint Committee on Cancer.
Note : Stage I: T1–2 N0 M0; stage II: T3–4 N0 M0; stage III: Tx N1–2 M0; and stage IV: Tx Nx M1.
Table 3. Contrast of the clinical and pathological features of colorectal cancer and appendiceal neoplasms 23 .
| Feature (%) | Colon | Appendix |
|---|---|---|
| Peritoneal dissemination at disease onset | 10 | 85 |
| Adenocarcinoma histology | 85 | 10 |
| Mucinous histology | 10–15 | 90 |
| Minimally invasive | 1 | 75 |
| Signet ring cell histology | 1/1,000 | 1/10 |
| Adenocarcinoid | 0 | 2.5 |
| Well differentiated | 10 | 80 |
| Moderately differentiated | 80 | 10 |
| Poorly differentiated | 10 | 10 |
Size of tumor is a significant prognostic indicator for neuroendocrine tumors of the appendix, as this parameter is a more reliable indicator of regional and distant spread than its depth of invasion. Subcentimeter tumors have rarely spread at the time of diagnosis, and, accordingly, have 5-year survival rates approaching 100%. Tumors more than 2 cm in size, however, have a 20 to 30% rate of nodal or distant spread, and an average 5-year survival rate of 31%. 21 34 46 55 Both the American Joint Committee on Cancer and European Neuroendocrine Tumor Society rely heavily on tumor size for determining disease stage ( Table 4 ). 11 36
Table 4. TNM staging (by AJCC and ENETS) for neuroendocrine appendiceal tumors.
| Stage | T (AJCC) | T (ENETS) | N (AJCC/ENETS) | M (AJCC/ENETS) |
|---|---|---|---|---|
| X | Primary tumor not determined, or any T | Primary tumor not determined, or any T | Lymph nodes not determined, or any N | Metastatic disease not determined, or any M |
| 0 | No evidence of primary tumor | No evidence of primary tumor | No lymph node metastasis | No distant metastasis |
| I | Ia: Tumor ≤1 cm Ib: Tumor 1–2 cm |
T1 Tumor ≤1 cm invading submucosa and muscularis propria | Lymph node metastasis | Distant metastasis |
| II | Tumor 2–4 cm or with extension to the cecum | Tumor ≤2 cm with invasion of submucosa or muscularis propria, and/or minimal invasion (up to 3 mm) of subserosa/mesoappendix | ||
| III | Tumor >4 cm or with extension to the ileum | Tumor >2 cm and/or extensive invasion (>3 mm) of subserosa/mesoappendix | ||
| IV | Tumor directly invades other adjacent organs or structures, e.g., abdominal wall and skeletal muscle a | Tumor invades peritoneum/other organs |
Abbreviations: AJCC, American Joint Committee on Cancer; ENETS, European Neuroendocrine Tumor Society.
Note : Stage I: T1 N0 M0; stage II: T2–3 N0 M0; stage III: T4 N0 M0 or Tx N1 M0; and stage IV: Tx Nx M1.
Tumor adherent to other organs or structures grossly classified as cT4 but if microscopically negative adhesion as pT1–3 depending on depth of wall invasion.
Goblet cell carcinoids confer a grim prognosis, as at least 10% of these tumors have widespread metastases at the time of diagnosis. The 5-year survival rates are 100, 76, 22, and 14% for stages I, II, III, and IV disease, respectively. 38 39 Based on these data, it is clear that early presentation/diagnosis is paramount for long-term survival.
Surveillance
Postoperative surveillance algorithms vary, based on disease severity and type. Adenocarcinoma surveillance should follow colorectal cancer protocols, regardless of whether or not the tumor is mucinous or nonmucinous. The follow-up strategy should include serial exams and CEA levels every 3 months for 2 years and every 6 months for the following 3 years. CT of the chest, abdomen, and pelvis should be performed biannually for 5 years, and colonoscopy should be performed at years 1 and 3, and then every 5 years, depending on findings. 56
Surveillance after complete resection of a nonperforated mucocele with low-grade cellular mucin is controversial, but, in general, should follow a similar algorithm to that for adenomatous polyps. Surveillance colonoscopy should therefore be performed at 3 to 5 years postappendectomy. 57
Follow-up of neuroendocrine tumors is somewhat different, based on disease behavior. Tumors less than 2 cm in size without high-risk features do not require specific surveillance, as an R0 resection is essentially curative. Tumors more than 2 cm or exhibiting high-risk features require clinical exam, CT abdomen and pelvis, and consideration of tumor marker levels every 6 to 12 months for at least 7 years after surgery. 58
Conclusion
Neoplastic processes of the appendix arise from a wide array of pathologic etiologies, each with its own idiosyncratic tendencies. The algorithm for evaluation and treatment is complex, often starts from the nonspecific presenting complaint of appendicitis, and relies heavily on often subtle histopathologic differences. Given the relative frequency of appendix surgery, it is easy to take this diminutive structure for granted. It is clear from this review, however, that appendiceal pathology is a grandiose topic with significant nuance that impacts prognosis.
References
- 1.Deaver J B. Philadelphia: P Blakiston's Son & Co.; 1905. Appendicitis, 3rd ed. [Google Scholar]
- 2.Williams G R. Presidential address: a history of appendicitis. With anecdotes illustrating its importance. Ann Surg. 1983;197(05):495–506. doi: 10.1097/00000658-198305000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cope Z. London: Oxford University Press; 1965. A History of the Acute Abdomen. [Google Scholar]
- 4.Major R H. Springfield: Charles C Thomas; 1945. Classic Descriptions of Disease, 3rd ed. [Google Scholar]
- 5.Shepherd J A.Acute appendicitis: a historical survey Lancet 1954267(6833):299–302. [DOI] [PubMed] [Google Scholar]
- 6.Fitz R H. Perforating inflammation of the appendix; with special reference to its early diagnosis and treatment. Am J Med Sci. 1886;92:321–346. [Google Scholar]
- 7.McBurney C. Experience with early operative interference in cases of disease of the vermiform appendix. NY Med J. 1889;50:676–684. [Google Scholar]
- 8.Elting A W. IX. Primary carcinoma of the vermiform appendix, with a report of three cases. Ann Surg. 1903;37(04):549–574. [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett M L, Hines A L, Andrews R M.Trends in rates of perforated appendix, 2001–2010: statistical brief #159. 2006Available at:http:// www.ncbi.nlm.nih.gov/pubmed/24199256. Accessed January 22, 2016
- 10.McCusker M E, Coté T R, Clegg L X, Sobin L H. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94(12):3307–3312. doi: 10.1002/cncr.10589. [DOI] [PubMed] [Google Scholar]
- 11.Spanos C P, Kaiser A M. New York, NY: Springer; 2016. Appendiceal neoplasms; pp. 617–629. [Google Scholar]
- 12.Marmor S, Portschy P R, Tuttle T M, Virnig B A. The rise in appendiceal cancer incidence: 2000-2009. J Gastrointest Surg. 2015;19(04):743–750. doi: 10.1007/s11605-014-2726-7. [DOI] [PubMed] [Google Scholar]
- 13.Whitfield C G, Amin S N, Garner J P. Surgical management of primary appendiceal malignancy. Colorectal Dis. 2012;14(12):1507–1511. doi: 10.1111/j.1463-1318.2012.03052.x. [DOI] [PubMed] [Google Scholar]
- 14.Overman M J, Fournier K, Hu C Y et al. Improving the AJCC/TNM staging for adenocarcinomas of the appendix: the prognostic impact of histological grade. Ann Surg. 2013;257(06):1072–1078. doi: 10.1097/SLA.0b013e318269d680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGory M L, Maggard M A, Kang H, O'Connell J B, Ko C Y. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48(12):2264–2271. doi: 10.1007/s10350-005-0196-4. [DOI] [PubMed] [Google Scholar]
- 16.Maggard M A, O'Connell J B, Ko C Y. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240(01):117–122. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaib W, Krishna K, Kim S et al. Appendiceal neuroendocrine, goblet and signet-ring cell tumors: a spectrum of diseases with different patterns of presentation and outcome. Cancer Res Treat. 2016;48(02):596–604. doi: 10.4143/crt.2015.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandraki K I, Kaltsas G A, Grozinsky-Glasberg S, Chatzellis E, Grossman A B. Appendiceal neuroendocrine neoplasms: diagnosis and management. Endocr Relat Cancer. 2016;23(01):R27–R41. doi: 10.1530/ERC-15-0310. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz M, Akbulut S, Kutluturk K et al. Unusual histopathological findings in appendectomy specimens from patients with suspected acute appendicitis. World J Gastroenterol. 2013;19(25):4015–4022. doi: 10.3748/wjg.v19.i25.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr N J, Sobin L H. Lyon: World Health Organization; 2010. Adenocarcinoma of the appendix; pp. 122–125. [Google Scholar]
- 21.Misdraji J. London: Wiley; 2013. Tumors of the appendix; pp. 490–498. [Google Scholar]
- 22.Ruoff C, Hanna L, Zhi W, Shahzad G, Gotlieb V, Saif M W. Cancers of the appendix: review of the literatures. ISRN Oncol. 2011;2011:728579. doi: 10.5402/2011/728579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugarbaker P H. The natural history, gross pathology, and histopathology of appendiceal epithelial neoplasms. Eur J Surg Oncol. 2006;32(06):644–647. doi: 10.1016/j.ejso.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Sugarbaker P H. Epithelial appendiceal neoplasms. Cancer J. 2009;15(03):225–235. doi: 10.1097/PPO.0b013e3181a9c781. [DOI] [PubMed] [Google Scholar]
- 25.Weaver C H. Mucocele of appendix with pseudomucinous degeneration. Am J Surg. 1937;36:347–351. [Google Scholar]
- 26.Werth R. Pseudomyxoma peritonei. Arch Gynecol. 1884;24:100–118. [Google Scholar]
- 27.Ronnett B M, Zahn C M, Kurman R J, Kass M E, Sugarbaker P H, Shmookler B M. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19(12):1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Compton C, Fenoglio-Preiser C M, Pettigrew N, Fielding L P. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88(07):1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Pai R K, Longacre T A. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol. 2005;12(06):291–311. doi: 10.1097/01.pap.0000194625.05137.51. [DOI] [PubMed] [Google Scholar]
- 30.O'Connell J T, Tomlinson J S, Roberts A A, McGonigle K F, Barsky S H. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol. 2002;161(02):551–564. doi: 10.1016/S0002-9440(10)64211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson S L, Fleming R A, Loggie B W, Geisinger K R. Gelatinous ascites: a cytohistologic study of pseudomyxoma peritonei in 67 patients. Mod Pathol. 2001;14(07):664–671. doi: 10.1038/modpathol.3880370. [DOI] [PubMed] [Google Scholar]
- 32.Lundqvist M, Wilander E. Subepithelial neuroendocrine cells and carcinoid tumours of the human small intestine and appendix. A comparative immunohistochemical study with regard to serotonin, neuron-specific enolase and S-100 protein reactivity. J Pathol. 1986;148(02):141–147. doi: 10.1002/path.1711480204. [DOI] [PubMed] [Google Scholar]
- 33.Shaw P A. The topographical and age distributions of neuroendocrine cells in the normal human appendix. J Pathol. 1991;164(03):235–239. doi: 10.1002/path.1711640308. [DOI] [PubMed] [Google Scholar]
- 34.Komminoth P, Arnold R, Capella C . Lyon: World Health Organization; 2010. Neuroendocrine neoplasms of the appendix. [Google Scholar]
- 35.Modlin I M, Kidd M, Latich I et al. Genetic differentiation of appendiceal tumor malignancy: a guide for the perplexed. Ann Surg. 2006;244(01):52–60. doi: 10.1097/01.sla.0000217617.06782.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pape U F, Perren A, Niederle B et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012;95(02):135–156. doi: 10.1159/000335629. [DOI] [PubMed] [Google Scholar]
- 37.Stewart R J, Mirakhur M. Primary malignant lymphoma of the appendix. Ulster Med J. 1986;55(02):187–189. [PMC free article] [PubMed] [Google Scholar]
- 38.Roy P, Chetty R. Goblet cell carcinoid tumors of the appendix: an overview. World J Gastrointest Oncol. 2010;2(06):251–258. doi: 10.4251/wjgo.v2.i6.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham T H, Wolff B, Abraham S C, Drelichman E. Surgical and chemotherapy treatment outcomes of goblet cell carcinoid: a tertiary cancer center experience. Ann Surg Oncol. 2006;13(03):370–376. doi: 10.1245/ASO.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Tang L H, Shia J, Soslow R A et al. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008;32(10):1429–1443. doi: 10.1097/PAS.0b013e31817f1816. [DOI] [PubMed] [Google Scholar]
- 41.Reid M D, Basturk O, Shaib W L et al. Adenocarcinoma ex-goblet cell carcinoid (appendiceal-type crypt cell adenocarcinoma) is a morphologically distinct entity with highly aggressive behavior and frequent association with peritoneal/intra-abdominal dissemination: an analysis of 77 cases. Mod Pathol. 2016;29(10):1243–1253. doi: 10.1038/modpathol.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trivedi A N, Levine E A, Mishra G. Adenocarcinoma of the appendix is rarely detected by colonoscopy. J Gastrointest Surg. 2009;13(04):668–675. doi: 10.1007/s11605-008-0774-6. [DOI] [PubMed] [Google Scholar]
- 43.Connor S J, Hanna G B, Frizelle F A. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. 1998;41(01):75–80. doi: 10.1007/BF02236899. [DOI] [PubMed] [Google Scholar]
- 44.Madwed D, Mindelzun R, Jeffrey R B., Jr Mucocele of the appendix: imaging findings. AJR Am J Roentgenol. 1992;159(01):69–72. doi: 10.2214/ajr.159.1.1609724. [DOI] [PubMed] [Google Scholar]
- 45.Puvaneswary M, Proietto A. Mucocele of the appendix with magnetic resonance imaging findings. Australas Radiol. 2006;50(01):71–74. doi: 10.1111/j.1440-1673.2005.01530.x. [DOI] [PubMed] [Google Scholar]
- 46.Mullen J T, Savarese D M. Carcinoid tumors of the appendix: a population-based study. J Surg Oncol. 2011;104(01):41–44. doi: 10.1002/jso.21888. [DOI] [PubMed] [Google Scholar]
- 47.Kusamura S, Moran B J, Sugarbaker P H et al. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br J Surg. 2014;101(13):1758–1765. doi: 10.1002/bjs.9674. [DOI] [PubMed] [Google Scholar]
- 48.Esquivel J, Chua T C, Stojadinovic A et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol. 2010;102(06):565–570. doi: 10.1002/jso.21601. [DOI] [PubMed] [Google Scholar]
- 49.Franko J, Shi Q, Goldman C D et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(03):263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turaga K K, Pappas S G, Gamblin T. Importance of histologic subtype in the staging of appendiceal tumors. Ann Surg Oncol. 2012;19(05):1379–1385. doi: 10.1245/s10434-012-2238-1. [DOI] [PubMed] [Google Scholar]
- 51.Edge S B, Byrd D R, Compton C C . New York: Springer; 2010. AJCC (American Joint Committee on Cancer) Cancer Staging Manual, 7th ed; p. 133. [Google Scholar]
- 52.Sugarbaker P H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(01):69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 53.González-Moreno S, Sugarbaker P H. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91(03):304–311. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 54.Verwaal V J, van Ruth S, de Bree E et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 55.Stinner B, Kisker O, Zielke A, Rothmund M. Surgical management for carcinoid tumors of small bowel, appendix, colon, and rectum. World J Surg. 1996;20(02):183–188. doi: 10.1007/s002689900028. [DOI] [PubMed] [Google Scholar]
- 56.Anthony T, Simmang C, Hyman N et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47(06):807–817. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 57.Lieberman D A, Rex D K, Winawer S J, Giardiello F M, Johnson D A, Levin T R. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(03):844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Boudreaux J P, Klimstra D S, Hassan M M et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the jejunum, ileum, appendix, and cecum. Pancreas. 2010;39(06):753–766. doi: 10.1097/MPA.0b013e3181ebb2a5. [DOI] [PubMed] [Google Scholar]


