Abstract
Peritoneal surface disease (PSD) has historically been used interchangeably with the term peritoneal carcinomatosis (PC) and has a dismal natural history. A variety of malignant pathologies, including colorectal and appendiceal primary tumors, can disseminate throughout the peritoneal cavity, leading to bowel obstruction and death. In general, peritoneal spread from high-grade appendiceal and colorectal primaries has the potential of hepatic and distant spread and best classified as PC. Low-grade appendiceal tumors are better categorized as PSD, due to low cellularity, high mucin production, and lack of potential spread outside the peritoneal cavity. Growing international experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) over the past 30 years has presented a therapeutic option to patients with PSD from colorectal and appendiceal tumors that can provide significant disease control, as well as potential for previously unattainable long-term survival. The proliferation of HIPEC centers and ongoing prospective trials are helping to standardize HIPEC techniques and patient selection.
Keywords: peritoneal surface disease, peritoneal carcinomatosis, appendiceal cancer, hyperthermic intraperitoneal chemotherapy, colorectal adenocarcinoma, cytoreductive surgery, peritoneal metastasis
Colorectal adenocarcinoma (CRC) most commonly disseminates through lymphatic and hematogenous routes. A small CRC subgroup, however, will spread directly throughout the peritoneal cavity, 1 2 as demonstrated by the presence of exfoliated tumor cells in peritoneal washings at the time of index resection in 3 to 28% of patients, 3 while between 8 and 13% of CRC patients will develop metastasis presenting as peritoneal surface disease (PSD). 4 5 Even though the liver and lung still represent the two most common sites of metastasis from CRC, in up to 25% of patients with metastatic CRC, the peritoneal surfaces are the only site of recurrence. 6 Given that CRC is expected to be the fourth most common malignancy in the United States (excluding nonmelanoma skin cancers) in 2017, 7 according to the National Cancer Institute, isolated peritoneal carcinomatosis (PC) from CRC represents a common indication for regional therapies.
Similar to other primary cancers that metastasize along the peritoneal surfaces, PC from CRC was traditionally seen as a terminal diagnosis. Those patients offered surgical intervention underwent palliative procedures, such as colostomy or bypass. The results from the turn of the century EVOCAPE 1 prospective multicenter trial, which documented the natural history of PSD from nongynecologic sources, showed a dismal 6.9- and 5.2-month mean and median survival from CRC patients with PC treated on a palliative basis. 8 Malignant bowel obstruction is usually the cause of death in these patients. 9 10 Modern systemic chemotherapy regimens offer only a modest improvement in survival for these patients, as these agents do not reach sufficient concentrations within the peritoneal cavity. Franko et al found poorer survival outcomes with chemotherapy with peritoneal metastasis (vs. other sites of disease), with a 12.7-month overall survival (OS) and 5.8-month progression-free survival (PFS) in nearly 2,100 patients with PC from CRC receiving contemporary chemotherapeutic regimens for CRC. 11
Treatment options for patients with PC from CRC have improved greatly with the introduction of oxaliplatin and irinotecan-based regimens, since the end of the 20th century. Further, several centers began to look at a regional approach to malignancies confined to the peritoneal cavity. 12 13 14 This evolved into the current day practice of offering cytoreductive surgery (CRS) to resect macroscopic tumor deposits to selected patients with PC, followed by intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC), with the theoretical intent of eliminating remaining microscopic residual tumor cells. Verwaal et al from the Dutch National Cancer Institute published the results of a prospective randomized trial, comparing CRS and HIPEC with systemic chemotherapy and palliative surgery. The median survival for those patients was 22.3 and 12.6 months, respectively. 15 At 5 years, 45% of the patients in the CRS and HIPEC arm were alive if no macroscopic disease was present after their CRS. 16 Other large, multicenter studies demonstrated approximately 30% 5-year survival in patients with similar resection status. 17 18 Together, the results of these studies demonstrate a chance for long-term survival not previously available to such patients. This has accordingly led to consensus recommendations on the standardized treatment of CRC patients with PC. 19
Low-grade appendiceal (LGA) adenocarcinoma represents an entity entirely different from classic CRC, and will therefore be discussed separately. While primary appendiceal neoplasms (PAN) have a different pathophysiology compared with CRC, the approach to patient selection, preoperative evaluation, and principles of cytoreduction are largely the same.
Preoperative Patient Evaluation
Patient Selection
One of the fundamental elements in the successful surgical treatment of PC in CRC is proper patient selection. This is based on a comprehensive preoperative estimation of the patient's intra-abdominal disease burden, as well as the functional reserves of the patient. The preoperative evaluation of the patient being considered for CRS and HIPEC should include a complete history and physical exam, with particular attention paid to the patient's performance status. The patient should have an Eastern Cooperative Oncology Group (ECOG) score less than or equal to 2. Higher ECOG scores are associated with higher rates of morbidity, including anastomotic leak and mortality following these procedures. 20 21 In addition, an evaluation should show adequate renal, hepatic, and hematopoietic function. A colonoscopic examination to rule out synchronous lesions is of value, as second primary lesions are not uncommon. 22
Histologic subtype is important, and has been shown to impact recurrence and survival rates following CRS and HIPEC. In particular, CRC with signet ring features is a poor prognostic indicator for PC patients. 23 Two recent studies from the Netherlands demonstrate worse long-term outcomes for patients with signet ring cells undergoing CRS and HIPEC. Simkens et al observed a median survival of 18 months for patients with signet ring pathology versus 30 months for adenocarcinoma, while van Oudheusden et al observed difference to be 14.1 versus 35.1 months. 24 25 The authors noted that while CRS and HIPEC still afforded some survival benefit to patients with signet ring pathology in their CRC, it was not always superior to treatment with systemic chemotherapy alone. With that in mind, it is important to use pathologic subtype of PC to guide informed decision making, and to strongly consider the risks and benefits of performing CRS and HIPEC in the CRC patient with signet ring cells.
Preoperative Imaging
The patient should also undergo an intravenous contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis to rule out extra-abdominal tumor spread. Magnetic resonance imaging is acceptable as an alternative option but can present false positives around scars from prior abdominal operations. 26 Positron emission tomography (PET) imaging may be useful in some patients to rule out extra-abdominal disease; it may not be sensitive enough, however, to pick up small intra-abdominal tumor deposits. 27 We have not found CT–PET imaging to be helpful for low-grade lesions. 28
Serum Tumor Markers
In addition, the patient should have serum levels of carcinoembryonic antigen (CEA) and CA19-9 drawn prior to resection. Some centers have also found CA-125 levels helpful for appendiceal primaries. These tumor markers are helpful because they can help the surgeon track for potential normalization after surgery and, later, for evidence of recurrence. They are also valuable because higher preoperative levels are negative prognostic indicators for recurrence and survival following CRS and HIPEC. 29 If tumor markers are not elevated preoperatively, however, they need not be followed after surgery.
Quantifying Tumor Burden
This information is crucial in making a preoperative assessment of whether the surgeon expects to be able to achieve complete cytoreduction of all macroscopic disease prior to HIPEC, which is ultimately the goal of the intervention. A major part of this assessment is the tallying of the patient's PC index (PCI) 30 that provides a way to standardize disease burden while acting as a prognostic indicator itself. Briefly, the PCI score is derived by dividing the abdominal cavity into nine regions of equal size. The length of the small bowel is then divided into 4 regions, for a total of 13 regions ( Fig. 1 ). To estimate overall tumor burden and the likelihood of a successful macroscopic resection, each region is given a score of 0 (no tumor present), 1 (up to 0.5 cm tumor), 2 (up to 5 cm of tumor), or 3 (a tumor nodule > 5 cm or confluence of numerous nodules), with a score range of 0 to 39. Indeed, it can be difficult to accurately estimate a patient's PCI score based solely on imaging, and the PCI score is therefore typically calculated at the time of operative exploration. The importance of PCI in the patient's long-term prognosis was first demonstrated by Sugarbaker, who observed a 50% 5-year survival rate in patients with a PCI ≤ 10, which dropped to 20% for scored of 11 to 20. None of these early CRS patients was alive at 5 years if they presented with a PCI >20. 31 Recently, Faron et al studied 180 consecutive patients undergoing CRS and HIPEC for CRC, and observed a linear relationship between PCI and overall survival (OS). They suggested a PCI of 17 as a cutoff for the procedure, as only 7% of patients were alive at 3 years, which was the same for patients undergoing palliative surgery. 32 Shortly thereafter, a meta-analysis of several national and international guidelines identified a range of PCI scores from 20 to 26 as representing a suggested cutoff above which CRS and HIPEC provide marginal value. 33 It is important to note that there is currently no clear consensus on such a cutoff score. It is clear, however, that lower scores are preferable to ensure a higher likelihood of a more complete cytoreduction and better long-term outcomes. Other features that might be considered relative contraindications to CRS include involvement of the porta hepatis, malignant ureteral strictures, multiple points of small bowel obstruction, and/or diffuse small bowel involvement with tumor. These factors make obtaining a complete cytoreduction exceedingly unlikely.
Fig. 1.
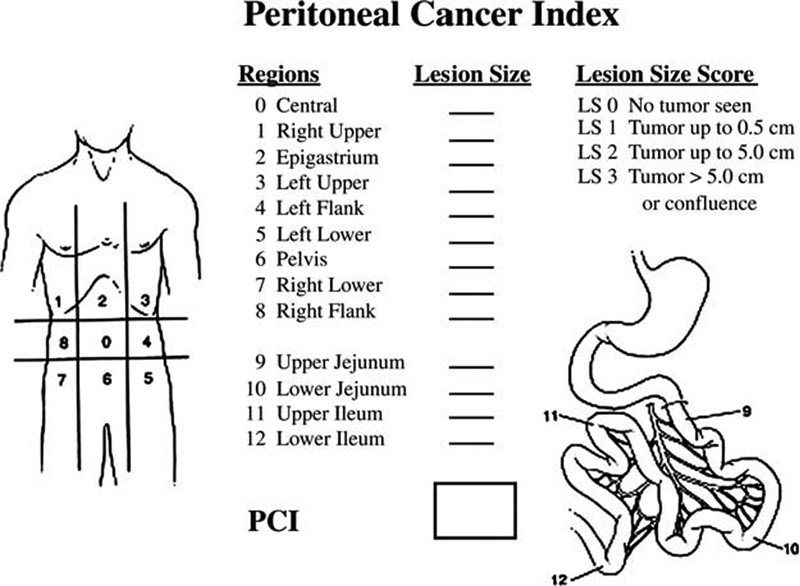
Peritoneal cancer index (PCI).
Cytoreduction
Complete cytoreduction of all gross disease is the goal of the operation. Complete cytoreduction has been consistently demonstrated through multiple studies as the most consistent independent predictor of both PFS and OS in patients with PSD, regardless of the type of primary. 34 35 36 37 38 39 An initial assessment of the organ resections necessary to achieve this must be completed at the outset of the cytoreductive procedure, with specific attention to the extent of small bowel involvement.
Accurate description of completeness of resection status in a reproducible manner is important. Currently, there are two commonly used classification systems for the reporting and clinical documentation of the completeness of cytoreduction (CC) ( Table 1 ). The American Joint Committee on Cancer staging manual utilizes the R status of resection, while many surgeons alternatively utilize the CC score. Complete surgical cytoreduction of all macroscopic disease is designated as CC-0, if using the CC scale, whereas R0 or R1 denote the same extent of resection, using the R scale. The slight difference is that R1 signifies that the final pathology revealed positive margins not appreciated at the time of surgery. If the surgeon scores the cytoreduction as R2 or anything > CC-0, it signifies the acknowledgment of residual macroscopic disease. The CC score and the R system are similar, and both have been shown to be reliable prognostic tools.
Table 1. A comparison of the two widely accepted scoring systems for cytoreductive surgery.
| R status | Diameter of largest remaining tumor deposits | CC score |
|---|---|---|
| – | 0 mm | CC-0N—no visible disease following neoadjuvant chemotherapy |
| R0—clear margins | CC-0S—no visible disease remains | |
| R1—involved margins | ||
| R2a | 2.5 mm | CC-1 |
| 5 mm | CC-2 | |
| R2b | > 5–20 mm | |
| R2c | > 20–25 mm | |
| > 25 mm | CC-3 |
Abbreviation: CC, completion of cytoreduction.
Perfusion
After an adequate cytoreduction has been performed, the patient can proceed to the perfusion stage of the procedure. A choice between the open and closed techniques of perfusion must be made. The closed technique demands that the abdomen is temporarily closed after the transabdominal insertion of inflow and outflow perfusion cannulas. In contrast, some centers still perform the perfusion without closing the abdomen, utilizing what is known as the “coliseum” technique. Neither technique has been shown to have a survival advantage; the open technique, however, has issues with fumes that may contain chemotherapy, which is not allowable in most states. The cannulas are then connected to a perfusion circuit, and the perfusate, based on a crystalloid, solution is warmed to ∼41°C. A heated perfusion circuit is utilized because higher temperatures have been found to provide a synergistic effect with the chemotherapy. 14 Once goal temperature is achieved, the chemotherapeutic agent is added to the system and continuously perfused for 30 to 120 minutes, per institutional protocol. The two most widely used agents for peritoneal colorectal metastases are oxaliplatin and mitomycin C. In brief, these agents are used because they are large molecules that can achieve high intraperitoneal concentrations with minimal ability to cross into the systemic circulation. There is no present consensus favoring one drug over the other. One recently published retrospective study observed a median survival of 56 versus 29 months in 201 CRC patients receiving oxaliplatin versus mitomycin C, respectively, while others have failed to find a difference. 40 41 The prospective, randomized French Prodige 7 trial attempts to answer the question of whether the HIPEC adds significantly to complete cytoreduction.
Special Considerations
Synchronous Liver Metastasis
Special considerations must often be taken into account in patients with CRC and HIPEC, including whether or not this procedure in appropriate in the patient with PC and synchronous liver metastasis. It is not unreasonable to question the value of attempting regional control in tumors that show the biologic ability to spread along peritoneal surfaces, as well as into the liver parenchyma. Retrospective analyses of highly selected patients with PC and limited hepatic involvement from CRC have not, however, shown PC to be the contraindication to hepatic resection. Allard et al demonstrated that hepatectomy should still be performed, as long as PC is minimal (patients have no more than two regions involved with PC, as delineated by the PCI system), and complete cytoreduction is attainable. 42 Also arguing in favor of carefully considered hepatic resection and CRS, a recent meta-analysis of patients with CRC, PC, and hepatic metastasis observed a trend toward increased median survival in those who underwent CRS, HIPEC, and hepatic metastasectomy, compared with those receiving only systemic chemotherapy. Recently, our group studied our experience with 32 CRC patients undergoing CRS, HIPEC, and nonanatomic liver resection. 43 We observed that although hepatic metastasis is a negative prognostic indicator of survival, there was no statistical difference in the postoperative morbidity or 30-day mortality of these patients. We found a median OS of 21.2 months in patients who received CRS, HIPEC, and liver resection. It is important to note that these patients already received systemic chemotherapy, and in some cases, second or third line treatment prior to surgery, demonstrating that combined CRS, HIPEC, and liver resection can provide a meaningful survival benefit in properly selected patients. It is clear, however, that complete resection/ablation of all hepatic disease and peritoneal disease is important to achieve a real survival benefit in this setting.
Rectal Cancer
Another area of potential controversy lies in the optimal treatment approach to the patient presenting with PC from true rectal cancer. Colon and rectal cancer are typically lumped together, despite significant differences in anatomy and gene expression. Moreover, patients presenting with a prior rectal resection will have had a considerable area of retroperitoneum dissected and potentially exposed to implantation of tumor around major vascular structures. A retrospective analysis of patients treated for PC from rectal cancer at our institution demonstrated similar rates in the ability to achieve a complete cytoreduction (53.8 vs. 50.5%) and 3-year survival (28.2 vs. 25.1%) in patients undergoing CRS and HIPEC for rectal and colonic cancer, respectively. 44 As long as the same standards for patient selection are followed and a complete cytoreduction of tumor deposits can be achieved, primary rectal adenocarcinoma should not be seen as a contraindication to CRS and HIPEC.
Malignant Ascites
Malignant ascites is a prognostic indicator of inability to achieve a complete CRS. Patients with malignant ascites form colorectal or high-grade appendiceal (HGA) primaries should therefore be referred for upfront chemotherapy as an attempt to downstage the volume of peritoneal disease. In cases where palliation is the only option, HIPEC is effective at controlling ascites, and can be offered in selected patients through a laparoscopic approach. In a study of 299 patients with ascites undergoing CRS and HIPEC for a variety of primary tumors, Randle et al observed that 288 (93%) patients had resolution of their ascites within 3 months of surgery. 45 Moreover, only 15% of this patient population had undergone a complete cytoreduction, underscoring the unique challenges in selecting CRS/HIPEC candidates in the presence of malignant ascites.
Ostomy Creation
An important misconception to dispel about CRS and HIPEC is that patients undergoing colorectal resections require a stoma because of a presumed toxicity or detrimental wound-healing affect caused by the HIPEC itself. Studies, however, have not borne this out, and the existing data suggest that the independent contribution of HIPEC to the morbidity of CRS and HIPEC is minimal. 46 Indeed, in our institutional review of 1,149 patients undergoing CRS and HIPEC, only 1.1% of patients who underwent an anastomosis developed a leak that subsequently required an ostomy. 47 The overall gastrointestinal leak rate for these patients was 4.2%, but this included foregut anastomoses as well. 20 A multivariate logistic regression analysis identified only ECOG status and multiple anastomoses as contributing directly to leaks. It must be noted, however, that anastomotic leaks in this patient population contributes significantly to increased rates of morbidity and mortality, and greatly diminishes long-term survival, even after a complete CRS.
While we have not observed colorectal anastomosis to be an independent predictor for anastomotic leaks in CRS/HIPEC patients, we did observe that colorectal primary is a risk factor for ostomy creation at the time of CRS/HIPEC. 47 Other risk factors included increasing PCI scores, increasing ECOG scores, and incomplete cytoreduction. It is our practice to protect rectal anastomoses with a diverting stoma.
The potential need for a stoma is important to keep in mind when evaluating and counseling the patient with PC from a colorectal primary. Moreover, the risks of complications from ostomy reversal following CRS and HIPEC are significant. de Cuba et al observed a 67% complication rate with ostomy reversal for their CRS–HIPEC patients, more than 75% of which were Clavien–Dindo grade III. 48 Fourteen percent of their reversal patients needed a second ostomy created, while 24% of patients who presented for ostomy reversal never regained complete bowel continuity. Similarly, our group has observed a 56% complication rate following ostomy reversal, half of which were Clavien–Dindo grade III or IV. 47 Moreover, we found that only 26% of patients with potentially reversible ostomies underwent reversal, and this is something that should be clearly explained to the patient with CRC who presents for CRS–HIPEC.
Appendiceal Tumors
Appendiceal cancer of epithelial origin is a rare entity that has historically been reported to be present in 1% of all appendectomy specimens. 31 36 49 When treating the patient with PSD from LGA tumors, it is important to recognize that genetically and biologically, these neoplasms are starkly different from colorectal epithelial neoplasms. 50 The natural history of the disease and management can therefore be quite different for these patients. To better understand the role of CRS/HIPEC for PAN, it is further necessary to make distinctions in the complicated nomenclature for describing epithelial tumors of the appendix.
Traditionally, the term “pseudomyxoma peritonei” has been used to describe the disease process caused by mucinous appendiceal neoplasms (MAN) because these tumors are capable of causing high-volume mucinous ascites. Over the years, we have encountered mucinous ascites from a variety of primaries, including ovarian, colon, and pancreatic mucinous tumors. We therefore regard the term pseudomyxoma peritonei, or mucinous ascites, as a clinical sign finding identified during surgical exploration or paracentesis. This term should not be used to describe a clinical syndrome or a distinct disease entity.
Our group found that epithelial appendiceal tumors are currently best described with a two-tier system as being HGA or LGA. 51 LGA tumors can be divided into two histologic entities: diffuse peritoneal adenomucinosis (DPAM) and peritoneal mucinous carcinomatosis with indeterminate or discordant features (PMCA-I/D). On the other end of the pathologic spectrum, high grade includes PMCA and moderately and high-grade primaries, as well as signet ring tumors. These reclassification and simplification were based on the similar 5-year survival rates for DPAM and PMCA-I/D of 68 and 61%, respectively. Patients with PMCA, however, had a much lower 5-year survival rate of 37%. 51 There is a clearly defined role of CRS and HIPEC for both high- and low-grade primaries, but understanding a patient's pathologic subtype is helpful in estimating potential outcomes and surgical planning. For instance, LGAs demonstrate a very low propensity for lymphatic metastasis; therefore, an appendectomy specimen with a negative cecal margin can obviate the need for a right hemicolectomy. Conversely, HGAs can spread lymphatically, as well as throughout the peritoneal cavity; therefore, mesenteric nodal sampling is potentially more valuable, although the ultimate utility of lymphadenectomy in the setting of peritoneal dissemination is unknown. Finally, it is important to note that both LGAs and HGAs confer a better prognosis than nonmucinous appendiceal tumors, such as carcinoids and goblet cell tumors. In our experience, patients with these tumors derived less benefit from CRS and HIPEC, and saw 3-year survival rates of only 15%. 52
The preoperative work-up of the patient with MAN is similar to that of colorectal patients with PC, with a few important additions. These patients should have a CA-125 serum level, checked along with CEA and CA19-9, as normal CA-125 levels have been associated with a 30% absolute 3-year survival benefit, compared with elevated levels. 53 Second, patients with MAN should be offered preoperative colonoscopy because we have observed that 44% of these patients will have synchronous colonic polyps. 54 Considering the good potential for long-term survival of these patients, particularly for patients with low-grade tumors, another colonic malignancy can theoretically be treated at the time of CRS/HIPEC.
Though rare as mentioned, appendiceal neoplasms have provided the classic indication for CRS and HIPEC, and are well studied. In our review of 481 patients undergoing CRS and HIPEC, 77% of which were for low-grade tumors and 23% for high-grade tumors, patients undergoing an R0/R1 resection demonstrated a median survival of 175 months. 55 This dropped to 73 months for patients with an R2a resection. These results were noted with 30-day morbidity and mortality rates of 27.8 and 2.7%, respectively, and seem to be decreasing over time. 32 The most important predictors of long-term survival were resection status, performance status, and lymph node status, regardless of tumor grade. Indeed, patients with low-grade MAN and positive nodes had worse survival rates than patients with negative nodes and high-grade primary ( Fig. 2 ). This held true, even when patients had a complete cytoreduction. Even though a CC1 (or R0/R1) resection is often considered complete CRS for LGAs, this is not the case for HGA primaries.
Fig. 2.
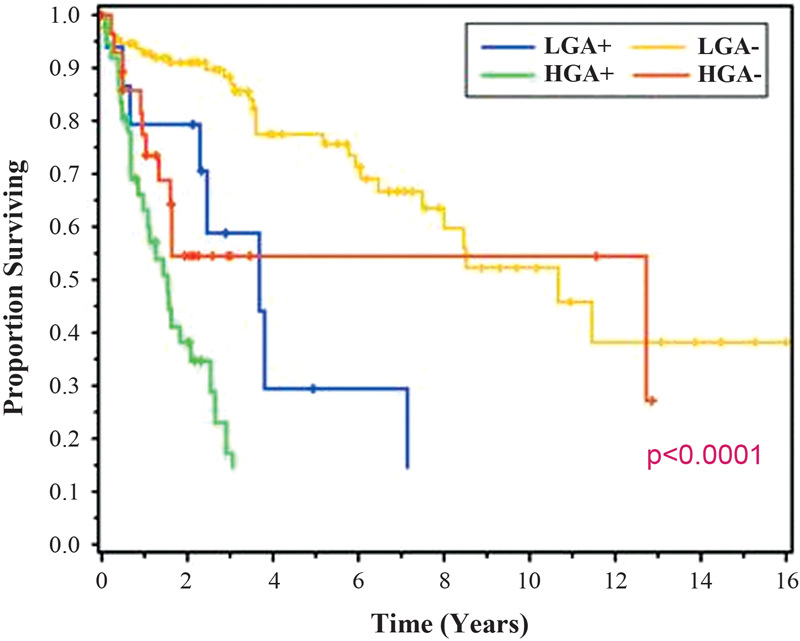
Survival by appendiceal tumor grade and nodal status.
Another factor negatively effecting survival was treatment with preoperative systemic chemotherapy, which we postulate is owing to a chemotherapy-related drop in ECOG score/functional performance status. Unlike CRC, systemic chemotherapy likely has little benefit in node negative patients, as it poorly penetrates the abdominal cavity. The role of preoperative (or any) systemic chemotherapy remains controversial, and we typically reserve this treatment for patients with high-grade tumors and distribution or volume of disease not amenable to complete CRS.
Future Considerations
Current research efforts are focusing on patients' quality of life following these physically and emotionally demanding procedures. Initial results suggest that patients can expect to return to their preoperative quality of life within 6 months of CRS and HIPEC. 56 This information will potentially play a role in keeping patients better informed and in guiding their expectations, as well as those of their caregivers. Several European centers have begun investigating pressurized intraperitoneal aerosolized chemotherapy in an effort to increase the depth of penetration of the chemotherapy into the tissue. While promising, this is currently an experimental approach.
References
- 1.Jayne D. Molecular biology of peritoneal carcinomatosis. Cancer Treat Res. 2007;134:21–33. doi: 10.1007/978-0-387-48993-3_2. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker P H. Peritoneum as the first-line of defense in carcinomatosis. J Surg Oncol. 2007;95(02):93–96. doi: 10.1002/jso.20676. [DOI] [PubMed] [Google Scholar]
- 3.Koppe M J, Boerman O C, Oyen W J, Bleichrodt R P. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(02):212–222. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayne D G, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 5.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99(05):699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro J F, Chase J L, Wolff R A et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer. 2010;116(02):316–322. doi: 10.1002/cncr.24715. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society.Cancer Facts and Figures 2017 Atlanta, GA: American Cancer Society; 2017 [Google Scholar]
- 8.Sadeghi B, Arvieux C, Glehen O et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(02):358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Paul Olson T J, Pinkerton C, Brasel K J, Schwarze M L. Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg. 2014;149(04):383–392. doi: 10.1001/jamasurg.2013.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shariat-Madar B, Jayakrishnan T T, Gamblin T C, Turaga K K. Surgical management of bowel obstruction in patients with peritoneal carcinomatosis. J Surg Oncol. 2014;110(06):666–669. doi: 10.1002/jso.23707. [DOI] [PubMed] [Google Scholar]
- 11.Franko J, Shi Q, Goldman C D et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(03):263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugarbaker P H. Patient selection and treatment of peritoneal carcinomatosis from colorectal and appendiceal cancer. World J Surg. 1995;19(02):235–240. doi: 10.1007/BF00308632. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker P H. Peritonectomy procedures. Ann Surg. 1995;221(01):29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugarbaker P H. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14(03):254–261. doi: 10.1002/(sici)1098-2388(199804/05)14:3<254::aid-ssu10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Verwaal V J, van Ruth S, de Bree E et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 16.Verwaal V J, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(09):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 17.Elias D, Gilly F, Boutitie F et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(01):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 18.Glehen O, Kwiatkowski F, Sugarbaker P H et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Turaga K, Levine E, Barone R et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21(05):1501–1505. doi: 10.1245/s10434-013-3061-z. [DOI] [PubMed] [Google Scholar]
- 20.Chouliaras K, Levine E A, Fino N, Shen P, Votanopoulos K I. Prognostic factors and significance of gastrointestinal leak after cytoreductive surgery (CRS) with heated intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2017;24(04):890–897. doi: 10.1245/s10434-016-5738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwakman R, Schrama A M, van Olmen J P et al. Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: a meta-analysis. Ann Surg. 2016;263(06):1102–1111. doi: 10.1097/SLA.0000000000001593. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi A N, Levine E A, Mishra G. Adenocarcinoma of the appendix is rarely detected by colonoscopy. J Gastrointest Surg. 2009;13(04):668–675. doi: 10.1007/s11605-008-0774-6. [DOI] [PubMed] [Google Scholar]
- 23.Winer J, Zenati M, Ramalingam L et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2014;21(05):1456–1462. doi: 10.1245/s10434-013-3328-4. [DOI] [PubMed] [Google Scholar]
- 24.van Oudheusden T R, Braam H J, Nienhuijs S W et al. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J Surg Oncol. 2015;111(02):237–242. doi: 10.1002/jso.23784. [DOI] [PubMed] [Google Scholar]
- 25.Simkens G A, Razenberg L G, Lemmens V E, Rutten H J, Creemers G J, de Hingh I H. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. Eur J Surg Oncol. 2016;42(06):794–800. doi: 10.1016/j.ejso.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Randle R W, Votanopoulos K I, Levine E A, Shen P, Stewart J H. Philadelphia: Lippincott Williams and Wilkins; 2015. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Surface Dissemination of Colorectal Cancer. Operative Techniques in Colon and Rectal Surgery. [Google Scholar]
- 27.Patel C M, Sahdev A, Reznek R HCT. CT, MRI and PET imaging in peritoneal malignancy. Cancer Imaging. 2011;11:123–139. doi: 10.1102/1470-7330.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohani P, Scotti S D, Shen P et al. Use of FDG-PET imaging for patients with disseminated cancer of the appendix. Am Surg. 2010;76(12):1338–1344. [PubMed] [Google Scholar]
- 29.Taflampas P, Dayal S, Chandrakumaran K, Mohamed F, Cecil T D, Moran B J. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal pseudomyxoma peritonei: analysis of 519 patients. Eur J Surg Oncol. 2014;40(05):515–520. doi: 10.1016/j.ejso.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Jacquet P, Sugarbaker P H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 31.Sugarbaker P H.Successful management of microscopic residual disease in large bowel cancer Cancer Chemother Pharmacol 199943(Suppl):S15–S25. [DOI] [PubMed] [Google Scholar]
- 32.Faron M, Macovei R, Goéré D, Honoré C, Benhaim L, Elias D. Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann Surg Oncol. 2016;23(01):114–119. doi: 10.1245/s10434-015-4627-8. [DOI] [PubMed] [Google Scholar]
- 33.Klaver C E, Groenen H, Morton D G, Laurberg S, Bemelman W A, Tanis P J; research committee of the European Society of Coloproctology.Recommendations and consensus on the treatment of peritoneal metastases of colorectal origin: a systematic review of national and international guidelines Colorectal Dis 20171903224–236. [DOI] [PubMed] [Google Scholar]
- 34.Chua T C, Moran B J, Sugarbaker P H et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 35.Levine E A, Stewart J H, IV, Russell G B, Geisinger K R, Loggie B L, Shen P.Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures J Am Coll Surg 200720405943–953., discussion 953–955 [DOI] [PubMed] [Google Scholar]
- 36.Levine E A, Stewart J H, IV, Shen P, Russell G B, Loggie B L, Votanopoulos K I. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218(04):573–585. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen P, Levine E A, Hall J et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138(01):26–33. doi: 10.1001/archsurg.138.1.26. [DOI] [PubMed] [Google Scholar]
- 38.Spiliotis J, Vaxevanidou A, Sergouniotis F, Lambropoulou E, Datsis A, Christopoulou A. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of recurrent advanced ovarian cancer: a prospective study. J BUON. 2011;16(01):74–79. [PubMed] [Google Scholar]
- 39.Van Sweringen H L, Hanseman D J, Ahmad S A, Edwards M J, Sussman J J.Predictors of survival in patients with high-grade peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy Surgery 201215204617–624., discussion 624–625 [DOI] [PubMed] [Google Scholar]
- 40.Leung V, Huo Y R, Liauw W, Morris D L. Oxaliplatin versus mitomycin C for HIPEC in colorectal cancer peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43(01):144–149. doi: 10.1016/j.ejso.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Lambert L A. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65(04):284–298. doi: 10.3322/caac.21277. [DOI] [PubMed] [Google Scholar]
- 42.Allard M A, Adam R, Ruiz A et al. Is unexpected peritoneal carcinomatosis still a contraindication for resection of colorectal liver metastases? Combined resection of colorectal liver metastases with peritoneal deposits discovered intra-operatively. Eur J Surg Oncol. 2013;39(09):981–987. doi: 10.1016/j.ejso.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Randle R W, Doud A N, Levine E A et al. Peritoneal surface disease with synchronous hepatic involvement treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2015;22(05):1634–1638. doi: 10.1245/s10434-014-3987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Votanopoulos K I, Swett K, Blackham A U et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from rectal cancer. Ann Surg Oncol. 2013;20(04):1088–1092. doi: 10.1245/s10434-012-2787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randle R W, Swett K R, Swords D S et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol. 2014;21(05):1474–1479. doi: 10.1245/s10434-013-3224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton A D, Bartlett E K, Karakousis G C. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality. J Gastrointest Oncol. 2016;7(01):99–111. doi: 10.3978/j.issn.2078-6891.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doud A N, Levine E A, Fino N F, Stewart J H, Shen P, Votanopoulos K I. Stoma creation and reversal after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(02):503–510. doi: 10.1245/s10434-015-4674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Cuba E M, Verwaal V J, de Hingh I H et al. Morbidity associated with colostomy reversal after cytoreductive surgery and HIPEC. Ann Surg Oncol. 2014;21(03):883–890. doi: 10.1245/s10434-013-3370-2. [DOI] [PubMed] [Google Scholar]
- 49.Collins D C. 71,000 Human appendix specimens. A final report, summarizing forty years' study. Am J Proctol. 1963;14:265–281. [PubMed] [Google Scholar]
- 50.Levine E A, Blazer D G, III, Kim M Ket al. Gene expression profiling of peritoneal metastases from appendiceal and colon cancer demonstrates unique biologic signatures and predicts patient outcomes J Am Coll Surg 201221404599–606., discussion 606–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley R F, Stewart J H, IV, Russell G B, Levine E A, Geisinger K R. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30(05):551–559. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 52.Stewart J H, IV, Shen P, Russell G B et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13(05):624–634. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 53.Ross A, Sardi A, Nieroda C, Merriman B, Gushchin V. Clinical utility of elevated tumor markers in patients with disseminated appendiceal malignancies treated by cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2010;36(08):772–776. doi: 10.1016/j.ejso.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 54.Votanopoulos K I, Shen P, Stewart J H, IV, Levine E A. Current status and future directions in appendiceal cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012;21(04):599–609. doi: 10.1016/j.soc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 55.Votanopoulos K I, Russell G, Randle R W, Shen P, Stewart J H, Levine E A. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol. 2015;22(04):1274–1279. doi: 10.1245/s10434-014-4147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dodson R M, McQuellon R P, Mogal H D et al. Quality-of-life evaluation after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23 05:772–783. doi: 10.1245/s10434-016-5547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


