Abstract
Aims/Introduction
Higher hedonic hunger has been observed in obese individuals compared with those without obesity, but little is known about its association with glycemic management. We aimed to examine the association between hedonic hunger and glycemic control in non‐obese and obese patients with type 2 diabetes.
Materials and Methods
Between April and November 2016, a total of 211 type 2 diabetes patients who underwent comprehensive diabetes assessments at a university‐affiliated hospital were recruited into two groups according to body mass index: non‐obese (body mass index 18.5–24.9 kg/m2) and obese (body mass index ≥30 kg/m2) groups. All participants completed the Chinese version of the Power of Food Scale (PFS) for assessment of hedonic hunger. Good glycemic control was defined as glycated hemoglobin <7.0%.
Results
Compared with the non‐obese group, the obese group showed higher PFS aggregated and subscale ‘food available’ scores (both P < 0.05). After adjustment for age, sex, disease duration of diabetes and insulin use, there were positive associations of glycated hemoglobin with PFS aggregated, subscale ‘food available’ and ‘food present’ scores in the obese group (all P for trend <0.05). The PFS aggregated score was negatively associated with good glycemic control in obese type 2 diabetes patients after adjustment using logistic regression analysis (adjusted odds ratio 0.42, 95% confidence interval 0.20–0.91, P = 0.027). By contrast, such associations were not observed in non‐obese type 2 diabetes patients.
Conclusions
Hedonic hunger had an independent and inverse association with good glycemic control in obese Chinese patients with type 2 diabetes, but not in their counterparts without obesity.
Keywords: Hedonic hunger, Obesity, Type 2 diabetes
Introduction
Achieving good glycemic control for obese patients with type 2 diabetes is a prevalent challenge for many clinicians around the world. Owing to a combination of factors including rapid socioeconomic development, genetic predisposition, urbanization and decreased physical activity level, together with an increasing availability of energy dense and palatable foods, the burden of obesity and type 2 diabetes is growing globally, particularly in Asian countries, namely India and China1, 2, 3.
Compared with European people, Asian people tend to have a higher percentage of total body fat and more abdominal obesity at the same level of body mass index (BMI), translating to more insulin resistance and an increased magnitude of inflammation4. Furthermore, commonly used antidiabetic agents, such as sulfonylurea and insulin, are widely known for their potential side‐effect on causing weight gain5. This leads to a vicious cycle with a further increase of insulin resistance when the dosage of antidiabetic medications are escalated to achieve glycemic goals6. Data from the Hong Kong Diabetes Registry, a prospective cohort established since 1995, has shown that obese patients with type 2 diabetes are particularly difficult to treat, with an extremely high risk for future events of diabetes‐related complications7.
Identifying the factors that influence appetite is critical for developing effective interventional strategies in diabetes management, particularly for patients with type 2 diabetes and obesity. The mechanisms controlling food intake are complex, partially modulated by two highly interrelated systems: the homeostatic regulation for the control of energy intake and the hedonic regulation for the control of sensory pleasure in eating8. Hedonic hunger, a relatively new construct, refers to the appetitive drive to eat palatable foods, particularly those high in fat and/or sugar, in the absence of a physiological need (i.e., caloric deficit) in an obesogenic food environment9. Previous studies reported that obese adults expressed higher hedonic hunger than non‐obese controls10, 11. Furthermore, interventions, such as weight loss programs and bariatric surgeries, have shown the impact of these treatment strategies on the change in levels of hedonic hunger in obese individuals. A study examining the changes in hedonic hunger after a 12‐week commercial weight loss program showed that there was a negative association between weight control behaviors and hedonic hunger, with a decrease in hedonic hunger being associated with better weight loss12. Hedonic hunger was also lower in severely obese patients who underwent gastric banding13 and gastric bypass11 as compared with severely obese controls. There was also a marked reduction in hedonic hunger as measured from preoperation Power of Food Scale (PFS) scores, suggesting an improvement in hedonic hunger, in severely obese patients after gastric bypass surgery14.
It is a common clinical observation that obese patients with type 2 diabetes often report their overeating behavior due to an intense desire to consume highly tempting foods, but not the feeling of hunger, suggesting a disturbed regulation of appetite and food intake in these patients. Recognizing the hedonic hunger characteristics of type 2 diabetes patients, as well as the association of hedonic hunger and glycemic control, might help to identify those patients who are most susceptible to overeating behavior and provide early intervention for hedonic hunger control. Studies exploring the clinical profile and their associations with hedonic hunger between type 2 diabetes patients with and without obesity are lacking. Early reported data suggested age and sex would influence appetite rating15. However, whether hedonic drive to eat and its related influential factors affect glycemic control in these patients has not yet been fully investigated. The aim of the present study was to examine the association between hedonic hunger and glycemic control in obese Chinese patients with type 2 diabetes compared with their non‐obese counterparts.
Methods
Participants and setting
Between April and November 2016, we invited adult type 2 diabetes patients who underwent their regular comprehensive assessment for treatment goals and diabetes‐related complications at the Diabetes Center of the Prince of Wales Hospital (PWH) to participate this study. PWH is a university‐affiliated hospital with 1,300 beds and 22 medical clinics serving a population of >1.2 million predominantly of Chinese ethnicity in Hong Kong. All study participants were diagnosed as having type 2 diabetes as defined by the World Health Organization criteria16, and referred from general practitioners, general medical and specialist hospital clinics. The participants were recruited into two groups based on two BMI ranges: non‐obese (body BMI 18.5–24.9 kg/m2) and obese (BMI ≥30 kg/m2) groups. These BMI cut‐offs were based on the World Health Organization international classification of weight 200417. In addition, the participants were considered eligible to enter this study if they met the following inclusion criteria: ages 18–65 years, Chinese ethnicity and a documented diagnosis of type 2 diabetes ≥6 months. Exclusion criteria included type 1 diabetes, pregnancy and lactation, and health conditions that could affect appetite and oral intake including end‐stage renal failure requiring dialysis, chronic kidney disease stage 4 and above, malignancy diagnosed within 3 years and previously underwent bariatric surgery.
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki, and was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong‐ Prince of Wales Hospital (CREC reference number 2016.126). All participants provided informed written consent.
Diabetes‐related metabolic control and complication assessment and laboratory assays
The diabetes‐related metabolic control and complication assessment included an interview with detailed documentation of medical history and current use of medications, anthropometric measurements and biochemical evaluations, and was carried out by qualified diabetes nurses based on the modified European DiabCare protocol18. Anthropometric parameters including bodyweight, body height, waist and hip circumferences, systolic blood pressure, and diastolic blood pressure were measured. Blood samples were collected from participants for biochemical analyses after fasting for at least 8 h. Fasting plasma glucose and lipid profile including total cholesterol, triglyceride and high‐density lipoprotein cholesterol were measured by the Roche Modular Analytics system (Roche Diagnostics GmbH, Mannheim, Germany). Low‐density lipoprotein cholesterol was calculated using the Friedewald formula19. Glycated hemoglobin (HbA1c) was measured by the Cobas Integra 800 System (Roche Diagnostics GmbH). All laboratory assays were carried out within the manufacturer's specifications using standard protocols under the Department of Chemical Pathology at the PWH. The laboratory is accredited by the Australian National Association of Testing Authorities. Good glycemic control was defined as HbA1c <7.0%20.
Assessment of hedonic hunger
All participants were asked to complete the Chinese version of the PFS21. The original PFS is a 15‐item self‐administered questionnaire, which has been developed and validated as a psychometric evaluation to quantify the level of hedonic hunger10, 22. The PFS assesses the motivation to eat highly palatable foods in an obesogenic environment, but not the actual food consumption behavior, in three subscale domains: (i) ‘food available’ as the situation where food is available, but not present; (ii) ‘food present’ as food is present, but not tasted; and (iii) ‘food tasted’ as food first tasted, but not consumed. Examples of PFS items include ‘I find myself thinking about food even when I'm not physically hungry’ for the ‘food available’ domain; ‘If I see or smell a food I like, I get a powerful urge to have some’ for the ‘food present’ domain and ‘When I eat delicious food, I focus a lot on how good it tastes’ for the ‘food tasted’ domain. The patients scored their response for each item on a 5‐point Likert scale from 1 ‘I don't agree at all’ to 5 ‘I strongly agree.’ Hedonic hunger was represented by the PFS aggregated score derived from the average of three subscale scores, rather than a summed score. Each subscale score was the mean score of the items in each domain. The PFS aggregated and subscale scores each range from 1 to 5.
The development of the Chinese version of the PFS involved four main stages: (i) contact with the PFS creator for approval and original questionnaire; (ii) two independent forward and backward translations from the original English version by two native Chinese speakers fluent in English; (iii) content validation of the translated scale by a 10‐content‐expert panel (3 dietitians, 3 diabetologists, 1 bariatric surgeon and 3 diabetes nurses); and (iv) assessment of test–retest reliability within a 2–3‐week interval among Chinese type 2 diabetes patients recruited at the PWH diabetes center21. The overall rating for content validity was high, attaining a mean score of 4.7 ± 0.3 on a 5‐point Likert scale (individual item ratings ranged 4.3–5.0) with minor suggested changes on the fluency of four items. The Chinese version of the PFS was tested in 32 type 2 diabetes patients (68.8% men, mean age 56.5 ± 8.7 years and median diabetes duration 6.0 years [interquartile range 2.0–14.8 years]). The intraclass correlation coefficients for test–retest reliability were satisfactory with 0.95 (95% confidence interval [CI] 0.90–0.98) for PFS aggregated, 0.97 (95% CI 0.93–0.99) for subscale ‘food available,’ 0.92 (95% CI 0.83–0.96) for ‘food present’ and 0.92 (95% CI 0.82–0.96) for ‘food tasted’ scores, respectively.
Statistical analysis
All statistical analysis was carried out by the SPSS version 23 software (2015; SPSS Inc., Chicago, IL, USA). A P‐value <0.05 (two‐tailed) was considered as statistically significant.
We compared the demographic, clinical and biochemical characteristics, as well as hedonic hunger profiles, between the non‐obese and obese groups. We made the further comparison of the participants according to their levels of glycemic control (HbA1c <7.0%, 7.0–8.9% and ≥9.0%). Continuous variables were expressed as mean ± standard deviation for those following normal Gaussian distribution, or median (interquartile range) for those with skewed distribution; whereas categorical variables were expressed as number (percentage). The χ2‐test was used for between‐group comparisons of categorical variables, whereas independent Student's t‐test or one‐way analysis of variance (anova) was used for comparisons of normally distributed continuous variables. The Wilcoxon rank‐sum test was used for comparisons of continuous variables with skewed distribution. Analysis of covariance (ancova) was used for between‐group comparisons for continuous variables, and logistic regression for categorical variables across glycemic status with adjustment for age, sex, disease duration of diabetes and insulin use as covariates where age and sex were reported to be associated with hedonic hunger; and diabetes duration and insulin use were well known to affect bodyweight and appetite. Multiple logistic regression analysis using age, sex, waist circumference, systolic blood pressure, lipid profile (low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and triglyceride), disease duration of diabetes, insulin use and PFS aggregated score as independent variables was carried out to estimate their independent effects on the status of having good glycemic control.
To estimate the sample size (n), we have the null hypothesis that hedonic hunger as assessed by the PFS aggregated score has no difference between those with or without good glycemic control (HbA1c <7%). We set a clinically relevant difference (δ) on the PFS score as 10% of the maximal score (i.e., 0.5 point). As the published standard error of the PFS score from the dataset from Cappelleri et al.10 was 0.02, the standard deviation (s) was 0.83. The standardized difference was the ratio of δ and s:
With the standardized difference being 0.6, statistical power at 80% and level of significance at 5%, a minimum sample size (n) = 92 was estimated using the Altman's nomogram for calculating sample size23. In addition, the two groups compared would have unequal sample sizes, as previous reports from the same setting showed that approximately 40% of patients had good glycemic control (HbA1c <7%) and 60% did not have good control22. Hence, the adjusted sample size (n*) was:
where k = 60%/40%.
With an extra 10% added as consideration for potential incomplete data collection after participant recruitment, the sample size (n) = 105 or above was estimated, and was applicable for the two patient groups.
Results
A total of 211 Chinese patients with type 2 diabetes were enrolled in the present study. There were 105 (49.8%) and 106 (50.2%) participants in the non‐obese and obese groups, respectively. The overall mean age ± standard deviation of the study participants was 54.0 ± 8.6 years, with 54.5% being men. The mean disease duration of diabetes was 8.8 ± 7.7 years. Table 1 summarizes the clinical characteristics, biochemical parameters and PFS scores of the study participants. With the exception of age, there were no significant between‐group differences in sociodemographics, disease duration of diabetes and glycemic profiles including fasting plasma glucose, HbA1c and percentage of patients reaching good glycemic control. Compared with their non‐obese counterparts, the obese type 2 diabetes patients had significantly higher diastolic blood pressure, triglyceride, alanine aminotransferase and urate, and lower high‐density lipoprotein cholesterol (all P < 0.05). The PFS aggregated (P = 0.017) and subscale ‘food available’ (P = 0.002) scores were also markly higher in the obese group, whereas the subscale ‘food present’ and ‘food tasted’ scores did not differ significantly between both groups (P = 0.101 and 0.080, respectively).
Table 1.
Clinical, biochemical and hedonic hunger profiles of the study participants
| All (n = 211) | Non‐obese T2D (n = 105) | Obese T2D (n = 106) | P‐value | |
|---|---|---|---|---|
| Clinical profile | ||||
| Age (years) | 54.0 ± 8.6 | 55.8 ± 7.5 | 52.1 ± 9.2 | 0.001 |
| Male | 115 (54.5) | 57 (49.6) | 58 (50.4) | 0.950 |
| Education level | ||||
| Primary or below | 51 (24.2) | 27 (5.7) | 24 (22.6) | 0.116 |
| Secondary | 120 (56.9) | 64 (61.0) | 56 (52.8) | – |
| College or above | 40 (19.0) | 14 (13.3) | 26 (24.5) | – |
| Full/part‐time employment | 136 (64.5) | 66 (62.9) | 70 (66.0) | 0.629 |
| Current and ex‐smoker | 50 (23.7) | 24 (22.9) | 26 (24.5) | 0.692 |
| Current and ex‐drinker | 67 (31.8) | 32 (30.5) | 35 (33.0) | 0.683 |
| Disease duration of diabetes (years) | 8.8 ± 7.7 | 9.1 ± 7.8 | 8.5 ± 7.6 | 0.566 |
| Insulin use | 72 (34.1) | 32 (30.5) | 40 (37.7) | 0.266 |
| Weight (kg) | 78.1 ± 21.1 | 61.2 ± 8.9 | 94.8 ± 15.7 | <0.001 |
| BMI (kg/m2) | 28.7 ± 6.8 | 22.7 ± 1.6 | 34.6 ± 4.3 | <0.001 |
| Waist circumference (cm) | 97.6 ± 16.2 | 84.3 ± 7.4 | 110.7 ± 11.1 | <0.001 |
| Waist‐to‐hip ratio | 0.95 ± 0.07 | 0.91 ± 0.06 | 0.98 ± 0.07 | <0.001 |
| SBP (mmHg) | 131.5 ± 20.3 | 130.1 ± 21.8 | 132.9 ± 18.6 | 0.311 |
| DBP (mmHg) | 74.5 ± 11.7 | 72.0 ± 11.1 | 77.1 ± 11.7 | <0.001 |
| Biochemical profile | ||||
| FPG (mmol/L) | 7.6 ± 2.7 | 7.5 ± 2.2 | 7.7 ± 2.3 | 0.601 |
| HbA1c (%) | 7.7 ± 1.5 | 7.6 ± 1.6 | 7.7 ± 1.4 | 0.495 |
| Good glycemic control† | 81 (38.4) | 42 (40) | 39 (36.8) | 0.368 |
| TC (mmol/L) | 4.14 ± 0.86 | 4.21 ± 0.85 | 4.07 ± 0.87 | 0.274 |
| LDL‐C (mmol/L) | 2.19 ± 0.74 | 2.23 ± 0.78 | 2.15 ± 0.70 | 0.439 |
| HDL‐C (mmol/L) | 1.25 ± 0.36 | 1.34 ± 0.39 | 1.15 ± 0.30 | <0.001 |
| TG (mmol/L) | 1.33 (0.93–1.97) | 1.12 (0.79–1.77) | 1.42 (1.06–2.08) | 0.033 |
| ALP (mmol/L) | 65.6 ± 17.3 | 63.6 ± 16.9 | 67.6 ± 17.5 | 0.098 |
| ALT (mmol/L) | 29.5 ± 17.4 | 23.7 ± 9.7 | 35.1 ± 21.1 | <0.001 |
| Urate (mmol/L) | 0.36 ± 0.10 | 0.34 ± 0.03 | 0.38 ± 0.10 | 0.002 |
| Hedonic hunger profile | ||||
| PFS aggregated score | 2.17 ± 0.80 | 2.04 ± 0.80 | 2.30 ± 0.79 | 0.017 |
| Subscale ‘food available’ | 2.04 ± 0.84 | 1.87 ± 0.78 | 2.22 ± 0.86 | 0.002 |
| Subscale ‘food present’ | 2.32 ± 0.86 | 2.23 ± 0.86 | 2.42 ± 0.85 | 0.101 |
| Subscale ‘food tasted’ | 2.20 ± 0.88 | 2.10 ± 0.92 | 2.31 ± 0.84 | 0.080 |
Variables are presented as mean ± standard deviation, frequency (%) or median (interquartile range). †Good glycemic control is defined as glycated hemoglobin (HbA1c) <7.0%. ALP, alkaline phosphatase level; ALT, alanine transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PFS, Power of Food Scale (as a measure for hedonic hunger); SBP, systolic blood pressure; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride.
The clinical, biochemical and hedonic hunger profiles according to the glycemic status of the non‐obese and obese type 2 diabetes groups are presented in Tables 2 and 3, respectively. For both groups, the patients with higher HbA1c levels were more likely to have a higher percentage of insulin use and longer disease duration of diabetes than those with lower HbA1c levels. After adjustment for potential confounders, including age, sex, disease duration of diabetes and insulin use, positive linear trends for PFS aggregated (P for trend = 0.017), subscale ‘food available’ (P for trend = 0.019) and ‘food present’ (P for trend = 0.022) scores with HbA1c levels remained to be statistically significant in the obese type 2 diabetes group (Figure 1b). By contrast, no significant difference in PFS scores was observed among the levels of glycemic control in the non‐obese type 2 diabetes group (Figure 1a).
Table 2.
Clinical, biochemical and hedonic hunger profiles of non‐obese type 2 diabetes patients stratified according to glycemic status
| HbA1c <7.0% (n = 42) | HbA1c 7.0–8.9% (n = 46) | HbA1c ≥9.0% (n = 17) | P‐value for trend | Adjusted P‐value† | |
|---|---|---|---|---|---|
| Clinical profile | |||||
| Age (years) | 54.1 ± 8.2 | 56.8 ± 7.3 | 57.5 ± 5.5 | 0.071 | – |
| Male | 25 (64.1) | 24 (50.0) | 9 (64.7) | 0.580 | – |
| Smoking | 10 (23.8) | 12 (26.1) | 2 (11.8) | 0.457 | 0.441 |
| Alcohol drinking | 13 (31.0) | 12 (26.1) | 7 (41.2) | 0.631 | 0.582 |
| Disease duration of diabetes (years) | 5.95 ± 6.3 | 11.35 ± 7.5 | 10.9 ± 9.6 | 0.003 | – |
| Insulin use | 8 (19.0) | 17 (37.0) | 7 (41.2) | 0.049 | – |
| Weight (kg) | 61.1 ± 9.7 | 60.9 ± 8.5 | 62.2 ± 8.3 | 0.757 | 0.892 |
| BMI (kg/m2) | 22.5 ± 1.8 | 22.8 ± 1.6 | 22.8 ± 1.1 | 0.498 | 0.754 |
| Waist circumference (cm) | 84.0 ± 7.7 | 84.0 ± 7.6 | 86.1 ± 6.1 | 0.435 | 0.851 |
| Waist‐to‐hip ratio | 0.91 ± 0.06 | 0.91 ± 0.06 | 0.92 ± 0.04 | 0.512 | 0.989 |
| SBP (mmHg) | 124.7 ± 24.2 | 133.0 ± 19.5 | 135.4 ± 19.5 | 0.046 | 0.043 |
| DBP (mmHg) | 70.8 ± 11.1 | 72.0 ± 11.2 | 74.8 ± 10.6 | 0.227 | 0.080 |
| Biochemical profile | |||||
| FPG (mmol/L) | 6.4 ± 1.0 | 8.0 ± 2.2 | 9.3 ± 3.0 | <0.001 | <0.001 |
| HbA1c (%) | 6.3 ± 0.5 | 7.7 ± 0.6 | 10.6 ± 2.0 | <0.001 | <0.001 |
| TC (mmol/L) | 4.37 ± 0.86 | 4.03 ± 0.80 | 4.25 ± 0.91 | 0.310 | 0.397 |
| LDL‐C (mmol/L) | 2.33 ± 0.79 | 2.08 ± 0.75 | 2.38 ± 0.82 | 0.758 | 0.442 |
| HDL‐C (mmol/L) | 1.39 ± 0.33 | 1.33 ± 0.46 | 1.30 ± 0.36 | 0.408 | 0.870 |
| TG (mmol/L) | 1.20 (0.72–1.89) | 1.15 (0.79–1.78) | 1.12 (0.88–1.73) | 0.970 | 0.648 |
| ALP (mmol/L) | 62.2 ± 17.4 | 63.8 ± 15.5 | 66.6 ± 19.9 | 0.373 | 0.472 |
| ALT (mmol/L) | 21.9 ± 9.1 | 24.6 ± 10.7 | 25.8 ± 8.3 | 0.123 | 0.360 |
| Urate (mmol/L) | 0.35 ± 0.08 | 0.34 ± 0.08 | 0.32 ± 0.09 | 0.333 | 0.449 |
| Hedonic hunger profile | |||||
| PFS aggregated score | 2.11 ± 0.74 | 1.95 ± 0.77 | 2.12 ± 1.01 | 0.775 | 0.731 |
| Subscale ‘food available’ | 1.94 ± 0.73 | 1.78 ± 0.77 | 1.93 ± 0.96 | 0.728 | 0.731 |
| Subscale ‘food present’ | 2.18 ± 0.83 | 2.21 ± 0.79 | 2.40 ± 1.11 | 0.454 | 0.589 |
| Subscale ‘food tasted’ | 2.26 ± 0.86 | 1.95 ± 0.89 | 2.12 ± 1.10 | 0.341 | 0.451 |
Total n = 105. Variables are presented as mean ± standard deviation, frequency (%) or median (interquartile range). † P‐value after adjustment for age, gender, disease duration of diabetes and insulin use. ALP, alkaline phosphatase level; ALT, alanine transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PFS, Power of Food Scale (as a measure for hedonic hunger); SBP, systolic blood pressure; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride.
Table 3.
Clinical, biochemical and hedonic hunger profiles of obese type 2 diabetes patients stratified according to glycemic status
| HbA1c <7.0% (n = 39) | HbA1c 7.0–8.9% (n = 49) | HbA1c ≥9.0% (n = 18) | P‐value for trend | Adjusted P‐value† | |
|---|---|---|---|---|---|
| Clinical profile | |||||
| Age (years) | 49.4 ± 9.5 | 54.1 ± 9.2 | 52.6 ± 7.3 | 0.087 | – |
| Male | 25 (64.1) | 24 (49.0) | 9 (50.0) | 0.215 | – |
| Smoking | 12 (30.8) | 11 (22.4) | 3 (16.7) | 0.221 | 0.287 |
| Alcohol drinking | 13 (33.3) | 15 (30.6) | 7 (38.9) | 0.786 | 0.359 |
| Disease duration of diabetes (years) | 5.3 ± 6.3 | 10.0 ± 7.8 | 11.6 ± 7.5 | 0.001 | – |
| Insulin use (%) | 7 (17.9) | 22 (44.9) | 11 (61.1) | 0.001 | – |
| Weight (kg) | 94.4 ± 14.4 | 94.0 ± 16.1 | 97.5 ± 18.0 | 0.578 | 0.775 |
| BMI (kg/m2) | 34.2 ± 4.4 | 34.4 ± 3.8 | 35.7 ± 5.2 | 0.265 | 0.693 |
| Waist circumference (cm) | 108.2 ± 9.5 | 111.4 ± 11.3 | 113.9 ± 12.9 | 0.055 | 0.217 |
| Waist‐to‐hip ratio | 0.97 ± 0.07 | 0.99 ± 0.07 | 0.99 ± 0.08 | 0.138 | 0.064 |
| SBP (mmHg) | 132.7 ± 19.1 | 134.0 ± 16.6 | 130.2 ± 23.4 | 0.775 | 0.529 |
| DBP (mmHg) | 77.6 ± 11.4 | 75.6 ± 10.1 | 80.2 ± 16.3 | 0.714 | 0.276 |
| Biochemical profile | |||||
| FPG (mmol/L) | 6.2 ± 1.0 | 7.6 ± 1.7 | 10.8 ± 2.6 | <0.001 | <0.001 |
| HbA1c (%) | 6.5 ± 0.4 | 7.7 ± 0.6 | 10.1 ± 1.2 | <0.001 | <0.001 |
| TC (mmol/L) | 4.00 ± 0.94 | 4.06 ± 0.79 | 4.33 ± 0.96 | 0.293 | 0.225 |
| LDL‐C (mmol/L) | 2.14 ± 0.75 | 2.13 ± 0.54 | 2.26 ± 0.85 | 0.652 | 0.350 |
| HDL‐C (mmol/L) | 1.18 ± 0.33 | 1.14 ± 0.29 | 1.11 ± 0.30 | 0.411 | 0.105 |
| TG (mmol/L) | 1.26 (1.00–2.00) | 1.48 (1.16–1.48) | 1.66 (1.13–2.17) | 0.062 | 0.075 |
| ALP (mmol/L) | 64.7 ± 18.3 | 68.1 ± 17.4 | 72.4 ± 15.3 | 0.119 | 0.318 |
| ALT (mmol/L) | 68.1 ± 17.4 | 33.9 ± 15.6 | 40.4 ± 23.3 | 0.401 | 0.324 |
| Urate (mmol/L) | 0.39 ± 0.11 | 0.38 ± 0.10 | 0.35 ± 0.12 | 0.256 | 0.396 |
| Hedonic hunger profile | |||||
| PFS aggregated score | 2.08 ± 0.56 | 2.36 ± 0.83 | 2.63 ± 0.97 | 0.011 | 0.017 |
| Subscale ‘food available’ | 1.96 ± 0.58 | 2.31 ± 0.95 | 2.56 ± 1.02 | 0.010 | 0.019 |
| Subscale ‘food present’ | 2.20 ± 0.68 | 2.46 ± 0.87 | 2.81 ± 0.99 | 0.013 | 0.022 |
| Subscale ‘food tasted’ | 2.13 ± 0.71 | 2.36 ± 0.83 | 2.58 ± 1.10 | 0.051 | 0.067 |
Total n = 106. Variables are presented as mean ± standard deviation, frequency (%) or median (interquartile range). † P‐values after adjustment for age, gender, disease duration of diabetes and insulin use. ALP, alkaline phosphatase level; ALT, alanine transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PFS, Power of Food Scale (as a measure for hedonic hunger); SBP, systolic blood pressure; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride.
Figure 1.
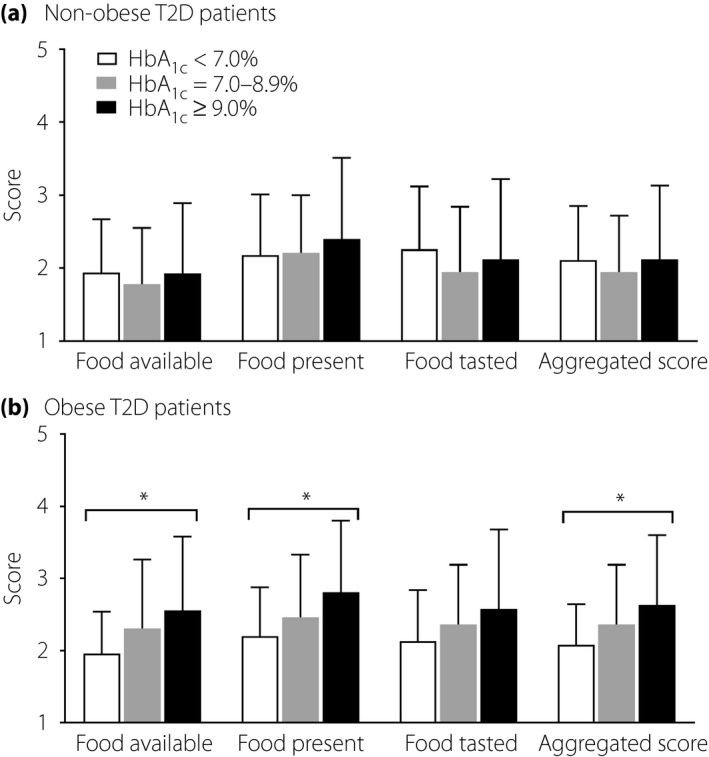
The mean (±standard deviation) Power of Food Scale (PFS) aggregated, subscale ‘food available,’ ‘food present’ and ‘food tasted’ scores for (a) 105 non‐obese and (b) 106 obese type 2 diabetes patients stratified according to glycemic status. *P < 0.05 after adjustment for age, sex, disease duration of diabetes and insulin use.
We analyzed the association of the PFS aggregated score with good glycemic control in the non‐obese and obese type 2 diabetes groups using multiple logistic regression (Table 4). For the obese group, low PFS aggregated score (P = 0.027), young age (P = 0.008), low percentage of insulin use (P = 0.032) and male sex (P = 0.007) were independently associated with good glycemic control (Table 4). The multiple logistic regression analysis for the obese type 2 diabetes group suggested that every 1‐point increase in the PFS aggregated score was associated with a 58% decrease in the likelihood of attaining good glycemic control (95% CI 0.20–0.91, P = 0.027). On the contrary, among the non‐obese type 2 diabetes patients, only short disease duration of diabetes (P = 0.006) was associated with good glycemic control (Table 4).
Table 4.
Multipe regession analysis on the association between the Power of Food Scale aggregated score and good glycemic control† in non‐obese and obese type 2 diabetes patients
| Non‐obese T2D (n = 105) | Obese T2D (n = 106) | |||||
|---|---|---|---|---|---|---|
| β | Odds ratio (95% CI) | P‐value | β | Odds ratio (95% CI) | P‐value | |
| PFS aggregated score | –0.16 | 0.85 (0.46–1.59) | 0.853 | –0.87 | 0.42 (0.19–0.91) | 0.027 |
| Age | –0.04 | 0.96 (0.91–1.02) | 0.962 | –0.09 | 0.91 (0.85–0.98) | 0.008 |
| Male | 0.27 | 1.31 (0.48–3.56) | 0.598 | 1.75 | 5.72 (1.60–20.49) | 0.007 |
| Disease duration of diabetes | –0.11 | 0.89 (0.82–0.97) | 0.006 | –0.08 | 0.93 (0.85–1.02) | 0.103 |
| Insulin use | –0.24 | 0.79 (0.23–2.70) | 0.788 | –1.47 | 0.23 (0.06–0.88) | 0.032 |
| SBP | –0.02 | 0.98 (0.95–1.009) | 0.167 | 0.02 | 1.02 (0.99–1.06) | 0.194 |
| DBP | –0.03 | 0.98 (0.92–1.04) | 0.430 | –0.03 | 0.97 (0.91–1.03) | 0.279 |
| LDL‐C | 0.16 | 1.19 (0.64–2.17) | 0.598 | –0.43 | 0.65 (0.29–1.49) | 0.310 |
| HDL‐C | 0.54 | 1.72 (0.43–6.95) | 0.447 | 1.54 | 4.67 (0.63–34.58) | 0.132 |
| TG | 0.47 | 0.85 (0.46–1.59) | 0.167 | –0.55 | 0.58 (0.30–1.11) | 0.100 |
†Good glycemic control is defined as glycated hemoglobin <7.0%. DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PFS, Power of Food Scale (as a measure for hedonic hunger); SBP, systolic blood pressure; T2D, type 2 diabetes; TG, triglyceride.
Discussion
To our knowledge, this is the first study to show distinct differences in hedonic hunger level as well as an association between hedonic hunger and glycemic control in type 2 diabetes patients with and without obesity. The obese type 2 diabetes group did not only have higher hedonic hunger than the non‐obese type 2 diabetes group, but also a significant and positive linear association between hedonic hunger and glycemic control. Furthermore, a low PFS aggregated score remained associated with achieving good glycemic control in the obese type 2 diabetes group after adjusting for potential confounders. The present findings are consistent with previous studies that hedonic hunger is associated with obesity, even among individuals with type 2 diabetes, as shown in our patients. Interestingly, these associations were not observed in the non‐obese type 2 diabetes group. One reason might be that the degree of hedonic hunger in this group of patients is not particularly strong and with our relatively small sample size, the clinical significance cannot be shown in this cohort. In addition, other potential factors might be involved that require further and bigger studies for a better understanding.
Our finding of higher PFS aggregated, subscale ‘food available’ and ‘food present’ scores in the obese type 2 diabetes patients when compared with the non‐obese counterparts is in keeping with the previous reports of a positive relationship between BMI and PFS scores in severely obese individuals who received bariatric‐surgery11 or attended a clinical weight‐loss treatment program12. This observation suggests that hedonic eating drives are significantly increased in these severe obese patients, and might be an important driver in the pathogenesis of obesity and its related diseases. Among the PFS subscale scores, the ‘food tasted’ domain was the only exception that did not reach statistical difference between the non‐obese and obese groups, as well as across glycemic levels, suggesting that obesity and glycemic control might be relatively less susceptible to the food taste‐related pleasure in these patients. Although insulin is notorious for its potential adverse effects on increasing the risk of hypoglycemia, feeling of physical hunger and weight gain5, we did not observe a significant difference in the percentage of insulin use between the non‐obese and obese groups. In addition, the independent linear trend of PFS scores with HbA1c level remained significant in the obese group after adjusting for potential confounders including insulin use. Thus, it is reasonable to believe that the high hedonic hunger in the obese patients, as well as the linear relationship with poor glycemic control, was unlikely to be related to insulin use.
The strength of the present study was the assessment of hedonic hunger with a Chinese content‐validated questionnaire together with a detailed documentation of medication use including insulin and the comprehensive clinical and metabolic profile of the participants. As all participants were part of the Hong Kong Diabetes Registry7, 24, there is a potential in the future for us to explore the secular trend and associations between the hedonic drive and diabetes‐related outcomes in a prospective follow up of the study participants.
We acknowledge that the present study had some limitations. First, the determination of hedonic hunger by using a self‐reported questionnaire could lead to potential subjective bias. Neuroimaging has emerged as an objective and non‐invasive recording of brain activity to assess the neural control of human appetite, but it is expensive and not readily available in routine clinical settings25. A previous study on healthy, normal‐weight men has shown a positive correlation between the intensity of magnetoencephalography responses, a form of neuroimaging, to viewing food images with the PFS aggregated, ‘food available’ and ‘food present’ subscale scores26. This supports using PFS as a practical alternative measure in evaluating appetite control. Second, because of the cross‐sectional nature of the present study, it precludes the examination of a causal relationship between hedonic hunger and glycemic control. Based on a study of healthy individuals recruited from the community, higher PFS scores were associated with more daily snacking on average than those with lower PFS scores, and more average daily snacking was associated with higher BMI than their counterparts with less snacking habit27. From another study involving obese patients who had received a Roux‐en‐Y gastric bypass, there was a significant reduction in the hedonic drive to consume palatable food, as well as beneficial changes in dietary behaviors characterized by an increased intake of protein‐rich foods and vegetables, and a reduced consumption of sugary snacks and beverages after surgery, when compared with their preoperative assessment14. Hence, future studies examining both dietary intake and quality in relation to hedonic drive specifically in the type 2 diabetes population will be useful to better elucidate the mechanism among actual food consumption, hedonic hunger and glycemic management. Finally, the participant recruitment based on two distinct BMI ranges for the non‐obese and obese groups was intentional to allow potentially larger between‐group contrast. A larger sample size with a continuum of BMI for different weight classifications might be considered in future studies. In conclusion, the present study provides clinical evidence that low hedonic hunger, as measured by PFS scores, is associated with good glycemic control in obese Chinese patients with type 2 diabetes. Such an association was not observed in the non‐obese type 2 diabetes patients. The findings from the present study highlight the importance of assessing hedonic hunger in type 2 diabetes patients, especially those with obesity. PFS is a relatively convenient tool to identify those type 2 diabetes patients with high hedonic hunger in a daily clinical setting for further management. Cognitive behavioral strategies, such as mindful eating, have been shown to help individuals cultivate awareness of eating stimuli and regulating the quantity of food intake through physiological cues of hunger and satiety28. The provision of more clinical attention and support from multidisciplinary healthcare teams to implement effective strategies in addressing hedonic hunger control is warranted to improve glycemic control in this population.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank Dr Michael Lowe from Drexel University for providing the Power of Food Scale. The authors are also grateful to the participants for their contributions. This study was supported by the Hong Kong Association for the Study of Obesity (HKASO) research grant. This study was selected as a poster presentation at the 9th Asian Association for the Study of Diabetes (AASD) Scientific Meeting, Nagoya, Japan, 17–20 May 2017.
J Diabetes Investig 2018; 9: 1135–1143
References
- 1. Yoon KH, Lee JH, Kim JW, et al Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–1688. [DOI] [PubMed] [Google Scholar]
- 2. Ma RCW, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol 2014; 2: 980–991. [DOI] [PubMed] [Google Scholar]
- 3. Yang W, Lu J, Weng J, et al Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 4. Nazare JA, Smith JD, Borel AL, et al Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra‐Abdominal Adiposity and Its Relationship with Cardiometabolic Risk/Intra‐Abdominal Adiposity. Am J Clin Nutr 2012; 96: 714–726. [DOI] [PubMed] [Google Scholar]
- 5. McFalane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep 2009; 9: 249–254. [DOI] [PubMed] [Google Scholar]
- 6. Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care 2015; 38: 1161–1172. [DOI] [PubMed] [Google Scholar]
- 7. Luk AO, So WY, Ma RC, et al, et al Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5‐year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care 2008; 31: 2357–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 2009; 139: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav 2007; 91: 432–439. [DOI] [PubMed] [Google Scholar]
- 10. Cappelleri JC, Bushmakin AG, Gerber RA, et al Evaluating the power of food scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes 2009; 33: 913–922. [DOI] [PubMed] [Google Scholar]
- 11. Schultes B, Ernst B, Wilms B, et al Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr 2010; 92: 277–283. [DOI] [PubMed] [Google Scholar]
- 12. O'Neil PM, Theim KR, Boeka A, et al Changes in weight control behaviors and hedonic hunger during a 12‐week commercial weight loss program. Eat Behav 2012; 13: 354–360. [DOI] [PubMed] [Google Scholar]
- 13. Ullrich J, Ernst B, Wilms B, et al The hedonic drive to consume palatable foods appears to be lower in gastric band carriers than in severely obese patients who have not undergone a bariatric surgery. Obes Surg 2013; 23: 474–479. [DOI] [PubMed] [Google Scholar]
- 14. Ullrich J, Ernst B, Wilms B, et al Roux‐en Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes Surg 2013; 23: 50–55. [DOI] [PubMed] [Google Scholar]
- 15. Gregersen NT, Moller BK, Raben A, et al Determinants of appetite ratings: the role of age, gender, BMI, physical activity, smoking habits, and diet/weight concern. Food Nutr Res 2011; 55: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 17. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 18. Piwernetz K, Home PD, Snorgaard O, et al Monitoring the targets of the St. Vincent Declaration and the implementation of quality management in diabetes care: the DiabCare initiative. Diabet Med 1993; 10: 371–377. [DOI] [PubMed] [Google Scholar]
- 19. Friedewald WT, Levy RI, Fredrickason DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 20. American Diabetes Association . Glycemic targets. Sec. 6. In Standards of Medical Care in Diabetes 2017. Diabetes Care 2017; 40(Suppl. 1): S48–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheung LTF, Ko GTC, Chow FCC, et al Development and validation of the Chinese version of the Power of Food Scale for type 2 diabetic patients. Presented at the 19th Diabetes & Cardiovascular Risk Factors East Meets West Symposium, Hong Kong, Sep 30–Oct 1, 2017.
- 22. Lowe MR, Butryn ML, Didie ER, et al The power of food scale. A new measure of the psychological influence of the food environment. Appetite 2009; 53: 114–118. [DOI] [PubMed] [Google Scholar]
- 23. Altman DG. How large a sample? In: Gore SM, Altman DG. (eds). Statistics in Practice. British Medical Association, London, 1982; 6–8. [Google Scholar]
- 24. Kong AP, Yang X, Luk A, et al Severe hypoglycemia identifies vulnerable patients with type 2 diabetes at risk for premature death and all‐site cancer: the Hong Kong diabetes registry. Diabetes Care 2014; 37: 1024–1031. [DOI] [PubMed] [Google Scholar]
- 25. Dagher A. Functional brain imaging of appetite. Trends Endocrinol Metab 2012; 23: 250–260. [DOI] [PubMed] [Google Scholar]
- 26. Yoshikawa T, Tanaka M, Ishii A, et al Immediate neural responses of appetitive motives and its relationship with hedonic appetite and body weight as revealed by magnetoencephalography. Med Sci Monit 2013; 19: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schüz B, Schüz N, Ferguson SG. It's the power of food: individual differences in food cue responsiveness and snacking in everyday life. Int J Behav Nutr Phys Act 2015; 12: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller CK. Mindful eating with diabetes. Diabetes Spectr 2017; 30: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


