Abstract
Aims/Introduction
Recent studies suggest that chronic inflammatory responses are important in the development of diabetic nephropathy (DN). Various inflammatory and angiogenesis molecules affect the pathogenesis and progression of DN. Inflammation damages the microcirculation and causes kidney damage. In the present study, we studied changes in interleukin‐8 (IL‐8) and soluble tumor necrosis factor‐like weak inducer of apoptosis (sTWEAK) levels in patients with DN, and investigated the clinical significance of these two inflammatory factors.
Materials and Methods
Participants were categorized into healthy controls (n = 30) and patients with type 2 diabetes mellitus (n = 124). The type 2 diabetes mellitus group was further subdivided into the normoalbuminuria (n = 34), microalbuminuria (MAU ; n = 46,) and proteinuria (MaAU; n = 44,) groups. Patients with DN were included in the MAU and MaAU groups. Total cholesterol, triglyceride, low‐density lipoprotein cholesterol, glycosylated hemoglobin, fasting blood glucose, 2‐h postprandial blood glucose, blood urea nitrogen, serum creatinine, 24‐h urine microalbumin, IL‐8 and sTWEAK levels were measured. Logistic regression was used to analyze the factors associated with proteinuria.
Results
In the healthy controls, normoalbuminuria, MAU and MaAU groups, we found that IL‐8 levels increased, whereas sTWEAK levels decreased (P < 0.05). IL‐8 might be an independent risk factor and serum sTWEAK a protective factor for MAU and MaAU. Serum levels of sTWEAK, IL‐8 and microalbumin were significantly correlated in the MAU and MaAU groups.
Conclusions
Serum IL‐8 and sTWEAK levels might be markers that can be used for an early diagnosis of DN.
Keywords: Diabetic nephropathy, Interleukin‐8, Soluble tumor necrosis factor‐like weak inducer of apoptosis
Introduction
Diabetic nephropathy (DN) is a severe microvascular complication of diabetes, which has become a common cause of chronic renal failure and a major cause of death due to diabetes. Its pathogenesis has not been completely explained, but is known to be related to multiple factors, including hemodynamic changes, heredity factors, inflammatory mediators, impaired glucose metabolism and cytokines1.
Recently, the role of the inflammatory response in the pathogenesis of DN has been emphasized. The interaction of numerous inflammatory cells, adhesion factors, chemokines and growth regulatory factors promote an inflammatory cascade that ultimately leads to the development of DN. Interleukin‐8 (IL‐8) is a chemokine with extensive sources and diversified biological functions2, which can induce leukocyte (e.g., neutrophil) chemotaxis and activation3. IL‐8 is also involved in the development of complications, such as diabetic retinopathy, DN, cardiovascular disease (CVD) and infections. Serum IL‐8 levels were found to change before a significant decline in renal function among patients with kidney injuries4. Tumor necrosis factor‐like weak inducer of apoptosis (TWEAK) is a glycoprotein of the tumor necrosis factor superfamily that circulates in plasma in the soluble form (sTWEAK)5, 6. TWEAK shows different degrees of expression during organ (including the heart, skeletal muscles and the kidney) damage4, 7. It regulates cell proliferation, death, differentiation and inflammatory responses. Recently, sTWEAK has been found to promote the proliferation of renal parenchymal cells in patients with DN. Furthermore, it causes the fibrosis of renal tubules and increases the exudation of inflammatory cells in the kidneys8, which can gradually decrease sTWEAK levels with a decline in renal function9.
The present study compared the serum levels of IL‐8 and sTWEAK in healthy individuals and type 2 diabetes mellitus patients with normoalbuminuria (NMAU), microalbuminuria (MAU) and proteinuria (MaAU; of these, patients with MAU and MaAU were considered as having DN), and probed the clinical significance of IL‐8 and sTWEAK levels for diagnosing and assessing the progression of DN.
Methods
Study participants
The present study was approved by our hospital's ethics committee (institutional review board number: 20150013), and was carried out in accordance with the Declaration of Helsinki in 1995 (as revised in Fortaleza, Brazil, October 2013). All patients granted written informed consent to participate in the study.
Healthy adults (n = 30) who underwent a physical examination at the outpatient department of The First Affiliated Hospital of Henan Polytechnic University (Jiaozuo Second People's Hospital), Jiaozuo, China, from October 2015 to May 2016 were enrolled as the NC group. Patients with type 2 diabetes mellitus were further subdivided into those with NMAU (n = 34), MAU (n = 46) and MaAU (n = 44). Patients with NMAU, MAU and MaAU were defined as having a 24‐h urine microalbumin (MAL) level of <30 mg/24 h, 30–300 mg/24 h and >300 mg/24 h, respectively. We excluded patients with infectious diseases, acute infections, heart failure, hyperthyroidism, tumors, immune system diseases, hematological system disorders, and moderate‐to‐severe hepatic and renal insufficiency. The following data were recorded for all participants: sex, age, disease course, disease history, ankle brachial index (ABI) and risk factors for DN (hypertension, hyperlipidemia and obesity).
Data collection
Fasting venous blood samples (4 mL) were collected from the arms of all patients on the morning of the second day after admission. Enzyme‐linked immunosorbent assays were utilized to determine the serum levels of IL‐8 (for optimal IL‐8 ELISA® kit; Dakota Biotechnology Co., Ltd., Wuhan, China) and sTWEAK (soluble tumor necrosis factor micro apoptosis inducers enzyme‐linked immunosorbent assay kit; Huamei Biological Engineering Co., Ltd., Wuhan, China). We also measured total cholesterol, triglyceride (TG), low‐density lipoprotein cholesterol, glycosylated hemoglobin (HbA1c), fasting blood glucose, fasting insulin, 2‐h postprandial blood glucose, blood urea nitrogen, serum creatinine (Scr) and MAL levels. The insulin resistance index (homeostasis model assessment for insulin resistance = FPG × fasting insulin/22.5) was assessed using the homeostasis model assessment steady‐state model. Other relevant auxiliary examinations, such as electrocardiography and liver/kidney color Doppler ultrasonography, were also carried out.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. The χ2‐test was utilized to compare quantitative data between groups. Different statistical methods were used in accordance with the data distribution patterns. For data with normal distribution, the indicators in each group were expressed as mean ± standard deviation. For data with non‐normal distribution, the indicators in each group were expressed as the median (minimum and maximum values). The Kruskal–Wallis test and rank‐sum test were utilized to compare data among the four groups, the Mann–Whitney U‐test was applied for intragroup pairwise comparisons in the case of significant intergroup differences, and Spearman's correlation test was used to analyze the correlations among all indicators. In patients with type 2 diabetes mellitus, a binary logistic regression model was used to investigate the factors associated with MAU and MaAU. P < 0.05 was considered statistically significant.
Results
We found no statistically significant differences for sex, age, body mass index, low‐density lipoprotein cholesterol and blood urea nitrogen levels between the NC, NMAU, MAU and MaAU groups (P > 0.05; Table 1). In contrast, significant differences between the NC and the other three groups were found for the disease course, hypertension, non‐alcoholic fatty liver disease, ABI, homeostasis model assessment for insulin resistance, HbA1c, fasting blood glucose, postprandial blood glucose, total cholesterol, TG, Scr, MAL, IL‐8 and sTWEAK levels (P < 0.05). When comparing the NMAU, MAU and MaAU groups, we detected no statistically significant differences in hypertension, non‐alcoholic fatty liver disease, homeostasis model assessment for insulin resistance, fasting blood glucose, postprandial blood glucose, total cholesterol and TG (P > 0.05). From healthy individuals, to those with diabetes and DN, a decrease in the ABI was noted (P < 0.05). Similarly, the concentrations of IL‐8 and MAL were increased (P < 0.05), whereas the concentration of sTWEAK was decreased (P < 0.05; Figure 1).
Table 1.
Comparison between the four groups
| Group | NC (n = 30) | NMAU (n = 34) | MAU (n = 46) | MaAU (n = 44) | t‐test/χ2 | P |
|---|---|---|---|---|---|---|
| Sex (male/female) | 15/15 | 15/19 | 23/23 | 18/26 | 6.996 | 0.072 |
| Age (years) | 51.0 ± 11.0 | 52.0 ± 8.0 | 55.0 ± 10.0 | 57.0 ± 12.0 | 2.591 | 0.055 |
| Disease course (years) | ‐ | 6.00 ± 5.40† | 8.00 ± 5.50† , ‡ | 9.0 ± 5.20† , ‡ , § | 6.767 | 0.016 |
| Hypertension, n (%) | 0 (0.0) | 16 (47.1)† | 25 (54.3)† | 26 (59.1)† | 29.83 | <0.001 |
| NAFLD, n (%) | 0 (0.0) | 8 (23.5)† | 9 (19.6)† | 10 (22.7)† | 8.18 | 0.043 |
| BMI (kg/m2) | 23.35 ± 3.88 | 25.05 ± 3.84 | 24.10 ± 3.16 | 23.15 ± 3.37 | 2.166 | 0.094 |
| ABI | 1.22 ± 0.67 | 1.17 ± 0.47 | 0.86 ± 0.39† , ‡ | 0.85 ± 0.47† , ‡ | 5.898 | <0.001 |
| HbA1c (%) | 5.20 (4.70, 6.10) | 10.3 (6.60, 15.0)† | 9.30 (7.00, 15.7)† , ‡ | 10.2 (8.00, 13.2)† , § | 79.676 | <0.001 |
| HOMA‐IR | 1.61 ± 0.65 | 2.05 ± 0.71† | 2.13 ± 0.67† | 2.22 ± 0.59† | 5.735 | <0.001 |
| FBG (mmol/L) | 4.60 (4.20, 5.40) | 9.00 (3.80, 18.9)† | 9.40 (4.20, 20.5)† | 9.61 (6.10, 18.3)† | 59.477 | <0.001 |
| PBG (mmol/L) | 6.20 (5.30, 7.20) | 19.1 (10.6, 30.4)† | 17.0 (12.8, 26.3)† | 18.8 (12.3, 27.8)† | 76.438 | <0.001 |
| CHOL (mmol/L) | 4.20 (3.63, 5.63) | 5.08 (3.00, 6.48)† | 4.75 (2.70, 6.10)† | 5.05 (3.34, 6.29) | 8.829 | 0.032 |
| TG (mmol/L) | 1.20 (0.73, 1.62) | 1.74 (0.38, 3.52)† | 1.76 (0.62, 6.54) | 1.77 (0.41, 3.29)† | 23.573 | <0.001 |
| LDL (mmol/L) | 2.47 (2.02, 3.56) | 2.46 (1.35, 3.19) | 2.55 (1.33, 3.77) | 2.84 (1.14, 3.78) | 2.856 | 0.414 |
| BUN (mmol/L) | 4.67 ± 0.41 | 4.63 ± 1.25 | 4.49 ± 1.25 | 4.45 ± 1.57 | 0.399 | 0.754 |
| Scr (μmol/L) | 55.37 ± 6.57 | 65.59 ± 12.89† | 62.57 ± 11.44† | 72.36 ± 20.08† , ‡ , § | 9.068 | <0.001 |
| MAL (mg/24 h) | 9.00 ± 4.33 | 25.29 ± 5.62† | 147.02 ± 53.53† , ‡ | 855.34 ± 367.07† , ‡ , § | 166.681 | <0.001 |
| sTWEAK (ng/mL) | 241.79 ± 4.43 | 182.86 ± 12.85† | 165.41 ± 10.76† , ‡ | 112.31 ± 15.53† , ‡ , § | 711.933 | <0.001 |
| IL‐8 (ng/mL) | 16.99 ± 0.55 | 26.16 ± 1.63† | 33.96 ± 4.09† , ‡ | 62.94 ± 17.74† , ‡ , § | 160.038 | <0.001 |
For data with non‐normal distribution, the indicators in each group are expressed as the median (minimum and maximum values). For data with normal distribution, the indicators in each group are expressed as mean ± standard deviation. †Compared with the control group (NC) P < 0.05. ‡Compared with the normoalbuminuria group (NMAU) P < 0.05. §Compared with the microalbuminuria group (MAU) P < 0.05. ABI, ankle brachial index; BMI, body mass index; BUN, blood urea nitrogen; CHOL, total cholesterol; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HOMA‐IR, homeostasis model assessment for insulin resistance; IL‐8, interleukin 8; LDL, low‐density lipoprotein cholesterol; MaAU, proteinuria group; MAL, 24‐h urine microalbumin; NAFLD, non‐alcoholic fatty liver disease; NC, control group; PBG, postprandial blood glucose; Scr, serum creatinine; sTWEAK, soluble tumor necrosis factor‐like weak inducer of apoptosis; TG, triglyceride.
Figure 1.
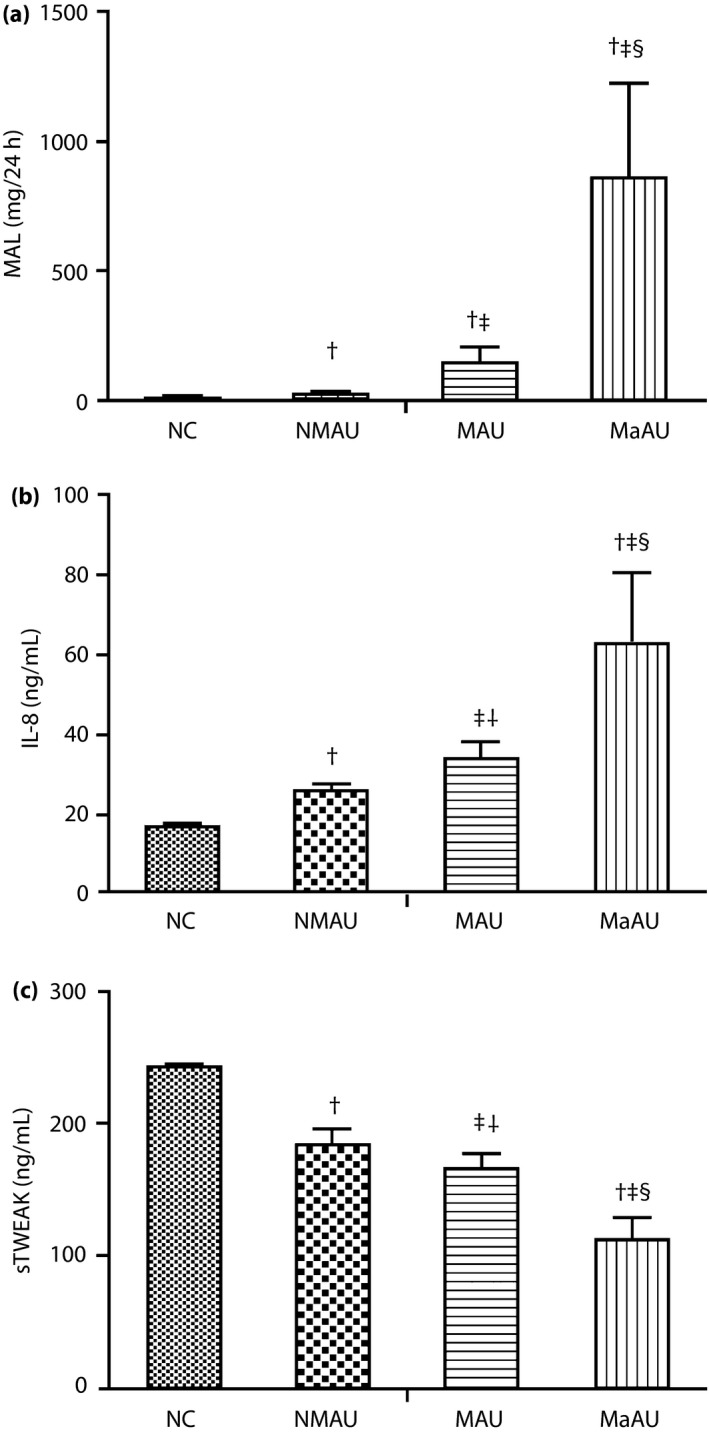
Comparison of 24‐h urine microalbumin (MAL), serum interleukin 8 (IL‐8) and soluble tumor necrosis factor‐like weak inducer of apoptosis (sTWEAK) levels between the control (NC), normoalbuminuria (NMAU), microalbuminuria (MAU) and proteinuria (MaAU) groups. (a) Comparison of MAL levels between the NC, NMAU, MAU and MaAU groups. (b) Comparison of serum IL‐8 levels between the NC, NMAU, MAU and MaAU groups. (c) Comparison of serum sTWEAK levels between the NC, NMAU, MAU and MaAU groups. †Compared with NC: P < 0.05. ‡Compared with NMAU: P < 0.05. §Compared with MAU: P < 0.05.
The factors associated with MAU in patients with type 2 diabetes mellitus were investigated using logistic regression (Table 2). Age, disease course, HbA1c, TG and Scr levels were not significantly associated (P > 0.05), whereas ABI, IL‐8 and sTWEAK were significantly associated (P < 0.05). ABI and sTWEAK were protective factors (relative risk [RR] <1), and IL‐8 was a risk factor (RR > 1).
Table 2.
Binary logistic regression analysis of the factors associated with microalbumin in patients with type 2 diabetes mellitus
| Variable | Regression coefficient (B) | Significance level (P) | Relative risk (RR) | 95% CI of the OR | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Age (years) | −0.008 | 0.663 | 0.992 | 0.959 | 1.027 |
| Disease course (years) | 0.075 | 0.082 | 1.078 | 0.990 | 1.172 |
| HbA1c (%) | −0.187 | 0.052 | 0.830 | 0.689 | 0.999 |
| TG (mmol/L) | 0.138 | 0.453 | 1.148 | 0.801 | 1.645 |
| ABI | −0.607 | 0.019 | 1.267 | 1.102 | 1.533 |
| Scr (μmol/L) | 0.007 | 0.579 | 1.007 | 0.982 | 1.033 |
| IL‐8 (pg/mL) | 0.721 | 0.002 | 2.056 | 1.310 | 3.225 |
| sTWEAK (pg/mL) | −0.167 | <0.001 | 0.846 | 0.783 | 0.914 |
Dependent variable, the presence/absence of microalbumin. ABI, ankle brachial index; CI, confidence interval; HbA1c, glycosylated hemoglobin; IL‐8, interleukin 8; OR, odds ratio; Scr, serum creatinine; sTWEAK, soluble tumor necrosis factor‐like weak inducer of apoptosis.
We then investigated the factors associated with MaAU (MAL >300 mg/24 h) in patients with type 2 diabetes mellitus (Table 3). HbA1c, TG and ABI were not significantly associated (P > 0.05). Age, disease course, Scr, IL‐8 and sTWEAK levels were all significantly associated with MaAU (P < 0.05); sTWEAK was a protective factor (RR <1), whereas age, disease course, IL‐8 and Scr were risk factors (RR > 1).
Table 3.
Binary logistic regression analysis of the factors associated with proteinuria in patients with type 2 diabetes mellitus
| Variable | Regression coefficient (B) | Significance level (P) | Relative risk (RR) | 95% CI of the OR | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Age (years) | 0.069 | 0.003 | 1.071 | 1.023 | 1.122 |
| Disease course (years) | 0.197 | <0.001 | 1.218 | 1.100 | 1.349 |
| HbA1c (%) | 0.213 | 0.064 | 1.238 | 0.988 | 1.552 |
| TG (mmol/L) | 0.148 | 0.383 | 1.160 | 0.831 | 1.618 |
| ABI | −0.983 | 0.216 | 1.025 | 0.895 | 1.079 |
| Scr (μmol/L) | 0.038 | 0.008 | 1.039 | 1.010 | 1.069 |
| IL‐8 (pg/mL) | 6.486 | 0.036 | 1.410 | 1.244 | 1.782 |
| sTWEAK (pg/mL) | 5.757 | 0.043 | 0.883 | 0.676 | 0.972 |
Proteinuria, microalbumin >300 mg/24 h. Dependent variable, microalbumin >300 mg/24 h = 1 vs microalbumin ≤300 mg/24 h = 0. ABI, ankle brachial index; CI, confidence interval; HbA1c, glycosylated hemoglobin; IL‐8, interleukin 8; OR, odds ratio; Scr, serum creatinine; sTWEAK, soluble tumor necrosis factor‐like weak inducer of apoptosis.
Correlation analysis showed that in the MAU group, sTWEAK demonstrated a strong negative linear correlation with IL‐8 (r = −0.760) and MAL (r = −0.945); IL‐8 showed a strong positive linear correlation with MAL (r = 0.806). In the MaAU group, TWEAK showed a significant negative linear correlation with IL‐8 (r = −0.651); sTWEAK showed a strong negative linear correlation with MAL (r = −0.820) and IL‐8 showed a strong positive linear correlation with MAL (r = 0.822).
Discussion
Diabetic nephropathy is a common and severe microangiopathy that can occur in patients with diabetes10; its major pathological changes include glomerular hypertrophy, mesangial matrix expansion and obvious thickening of the basilar membrane, resulting in nodular or diffuse glomerulosclerosis11. Most cases of DN show no obvious clinical symptoms early in the disease. Although renal puncture is an important criterion for the diagnosis of DN, patients are reluctant to undergo this procedure; therefore, it cannot be routinely used for patients with type 2 diabetes mellitus. The clinical test with the highest sensitivity and the best predictive value for an early diagnosis of DN is the measurement of albuminuria in urinary tests12; measuring MAL is considered the ‘gold standard’13. Although, clinically, MAL is used for an early diagnosis of DN, it is affected by a variety of factors, such as systemic or urinary tract infections, bleeding, vigorous exercise, or kidney damage related to drugs14. Therefore, MAL alone cannot provide a timely and accurately diagnosis DN. This necessitates the development of more accurate and specific diagnostic markers for DN.
Pickup et al.15 first reported in 1997 that inflammatory cytokines and oxidative stress played important roles in the pathogenesis and progression of DN. DN is associated with high glucose levels and hemodynamic disturbance, which can lead to intrinsic renal cell injury, secretion of pre‐inflammatory mediators, migration of white blood cells to the injured site, secretion of additional chemokines and stimulation of renal mesangial cells to secrete collagen fiber, laminin and fibronectin, thus leading to glomerulosclerosis. Renal tubular epithelial cells secrete immune mediators and cytokines, which allow aggregation of the renal interstitial mononuclear cells/macrophages, leading to renal interstitial inflammatory fibrosis16. Macrophages, adipocytes and endothelial cell‐derived inflammatory cytokines can all participate in the pathogenesis and progression of DN17. Some researchers have hypothesized that a long‐term micro‐inflammatory state and immune factors might related to the pathogenesis of DN16, whereas others have proposed that inflammation is the key factor in the development of DN18.
IL‐8, which can be expressed in vascular endothelial cells, fibroblasts, monocytes and epithelial cells, can mediate a series of cascade reactions stimulating the migration of white blood cells, the formation of neutrophil peroxide, as well as lysosome release, activation and chemotaxis. Thus, IL‐8 is thought to be involved in the pathogenesis and progression of complications, such as diabetic retinopathy, DN, CVD and infections. IL‐8 was found to stimulate neutrophils19, leading to a significant promotion of human renal mesangial cell proliferation. Furthermore, IL‐8 can induce oxidative stress20, changes in vascular permeability, increased endothelial coagulation ability and reduced diastolic function, resulting in abnormal blood flow regulation that contributes to the development of DN.
Tumor necrosis factor‐like weak inducer of apoptosis is a tumor necrosis factor superfamily cytokine, consisting of 249 amino acids, that has a molecular weight of 27 kD. As for most tumor necrosis factor members, the TWEAK protein is present as a membrane‐bound (mTWEAK) and soluble (sTWEAK) form after furin protease proteolytic cleavage. Both forms are biologically active and can bind to their only signal transduction receptor, fibroblast growth factor‐induced 14 (Fn14)21. In vitro studies have shown that mTWEAK, which can act as a collocation signal molecule, and sTWEAK through Fn1422, 23, lead to qualitatively different activity states. A combination of the two factors is involved in cell proliferation, apoptosis, cytokine production, angiogenesis and mediated immune injury during organ damage during the pathophysiology of immune system‐mediated tissue damage and disease24.
Some authors have observed a low level of sTWEAK in patients with atherosclerosis, and a negative correlation between sTWEAK levels and endometrial/medial thickness in asymptomatic patients; furthermore, sTWEAK levels were identified as a CVD indicator25. Therefore, sTWEAK can be considered as a potential biomarker of CVD26. Urbonaviciene et al.27 found that, in patients with severe ischemic intermittent claudication, sTWEAK levels were significantly reduced. However, they also found that sTWEAK levels and atherosclerosis in the lower extremities showed a positive correlation. According to a recent prospective case–control study28, low serum sTWEAK levels can be used to predict type 2 diabetes mellitus. In that study, a lower sTWEAK serum level was found in the type 2 diabetes mellitus group when compared with the NC group, suggesting a link between sTWEAK concentration and type 2 diabetes mellitus.28 Although low sTWEAK levels were shown to have a protective effect on vascular diseases, the mechanism of this association has not yet been fully elucidated, and several hypotheses have been proposed. One hypothesis for the decrease in sTWEAK levels is the uptake of sTWEAK by Fn14 receptors29. Fn14 is expressed in renal innate cells, including mesangial cells, podocytes and endothelial cells. Fn14 expression is significantly increased during acute and chronic inflammation kidney damage; an increase in the sTWEAK ligand can lead to a decrease in peripheral serum sTWEAK levels30. Recently, sTWEAK has been found to promote the proliferation of renal parenchymal cells, the fibrosis of renal tubules and an increase in the exudation of inflammatory cells in patients with DN8; sTWEAK levels might gradually decrease with a decline in renal function9. These findings show the value of sTWEAK in diagnosing DN.
The results of the present study found that, with DN progression, serum IL‐8 levels gradually increased and sTWEAK levels gradually decreased. Logistic regression analysis showed that serum IL‐8 levels were an independent risk factor for type 2 diabetes mellitus patients with MAU and MaAU. Serum IL‐8 levels were an independent risk factor for MAU and MaAU, whereas serum sTWEAK levels were an independent protective factor for MAU and MaAU in patients with type 2 diabetes mellitus. The concentration of IL‐8 increased with an increase in renal damage. Furthermore, serum IL‐8 concentration was highly correlated with MAL in the MAU and MaAU groups, and sTWEAK concentration showed a negative correlation with IL‐8 and MAL in patients with DN. These findings suggest that serum IL‐8 and sTWEAK have important roles in the development of DN. We suggest that IL‐8 and sTWEAK levels could be used for an early assessment of DN damage and as indicators of type 2 diabetes mellitus progression.
We also found that ABI was a protective factor for DN; however, we detected no difference in ABI between the NC and the NMAU groups or between the MAU and MaAU groups. Our data only showed that the ABI level in the MAU and MaAU groups were lower than those in the NC and MAU groups. This relationship will require further analysis in future studies.
In conclusion, serum IL‐8 and sTWEAK levels might be sensitive markers for diagnosing DN, as they play important roles in the occurrence and progression of DN. The limitations of the present study include its small sample size and that all participants were residents of Henan Province, China. As this was a cross‐sectional study, no causal relationship between IL‐8/sTWEAK levels and DN could be established. However, as we found an RR < 1 for sTWEAK and >1 for IL‐8, we hypothesize that sTWEAK might be a protective factor for DN, whereas IL‐8 might be a risk factor for DN. Further prospective cohort studies with larger sample sizes are required to explore the causal relationship between these two indicators and DN.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
J Diabetes Investig 2018; 9: 1182–1188
References
- 1. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013; 124: 139–152. [DOI] [PubMed] [Google Scholar]
- 2. Baggiolini M, Walz A, Kunkel SL. Neutrophil‐activating peptide‐l/interleukin8, a novel cytokine that activates neutrophils. J Clin Invest 1989; 84: 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith WB, Gamble JR, Clark‐Lewis L, et al Interleukin‐8 induces neutrophil transendothelial migration. Immunology 1991; 72: 65–72. [PMC free article] [PubMed] [Google Scholar]
- 4. Perlman AS, Chevalier JM, Wilkinson P, et al Serum Inflammatory and Immune Mediators Are Elevated in Early Stage Diabetic Nephropathy. Ann Clin Lab Sci 2015; 45: 256–263. [PubMed] [Google Scholar]
- 5. Winkles JA. The TWEAK‐Fn14 cytokine‐receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov 2008; 7: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiley SR, Winkles JA. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev 2003; 14: 241–249. [DOI] [PubMed] [Google Scholar]
- 7. Desplat‐Jego S, Varriale S, Creidy R, et al TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol 2002; 133: 116–123. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz N, Rubinstein T, Burkly LC, et al Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther 2009; 11: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carrero JJ, Ortiz A, Qureshi AR, et al Additive effects of soluble TWEAK and in flammation on droded in hemodialysis patients. Clin J Am Soc Nephrol 2009; 1: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamano K, Nakadaira I, Suzuki J, et al N‐terminal fragment of probrain natriuretic peptide is associated with diabetes microvascular complications in type 2 diabetes. Vasc Health Risk Manag 2014; 10: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang GM, Huang KY, Lee TY, et al An interpretable rule‐based diagnostic classification of diabetic nephropathy among type 2 diabetes patients. BMC Bioinformatics 2015; 16: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narita T, Hosoba M, Kakei M, et al Increased urinary excretions of immunoglobuling, ceruloplasmin, and transferrin predict development of microalbuminuria in patients with type 2 diabetes. Diabetes Care 2006; 29: 142–144. [DOI] [PubMed] [Google Scholar]
- 13. Dyer AR, Greenland P, Elliott P, et al Evaluation of measures of measures of urinary albumin excretion in epidemiologic studies. Am J Epidemiol 2004; 160: 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu D, Xu P, Yuan Y, et al Albuminuria is Suggested as a Potential Health Screening Biomarker for Senior Citizens and General Population with Hypertension or Diabetes in China. Clin Lab 2016; 62: 2267–2269. [DOI] [PubMed] [Google Scholar]
- 15. Pickup JC, Mattock MB, Chusney GD, et al NIDDM as a disease of the innate immune system: association of acute‐phase reactants and interleukin‐6 with metabolic syndrome X. Diabetologia 1997; 40: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 16. Chow FY, Nikolie‐Paterson DJ, Ozols E, et al Monocyte chemoattractant protein‐1 promotes the development of diabetic renal injury in streptozotocin‐treated mice. Kidney Int 2006; 69: 73–80. [DOI] [PubMed] [Google Scholar]
- 17. Chen Y, Ding H, Wang L, et al Effects of Ad‐FLT‐1/PC on the expression of inflammatory factors in rats with diabetic nephropathy atherosclerosis. Zhonghua Yi Xue Za Zhi 2015; 95: 1961–1965 (Chinese). [PubMed] [Google Scholar]
- 18. Utimura R, Fujihara CK, Mattar AL. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int 2003; 63: 209–216. [DOI] [PubMed] [Google Scholar]
- 19. Hidalgo MA, Carretta MD, Teuber SE, et al fMLP‐Induced IL‐8 Release Is Dependent on NADPH Oxidase in Human Neutrophils. J Immunol Res 2015; 2015: 120348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mojiminiyi OA, Abdella N, Moussa MA, et al Association of C‐reactive protein with coronary heart disease risk factors in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2002; 58: 37–44. [DOI] [PubMed] [Google Scholar]
- 21. Browns SA, Ghosh A, Winkles JA. Full‐length, membrane‐anchored TWEAK can function as a juxtacrine signaling molecule and activate the NF‐kappaB pathway. J Biol Chem 2010; 285: 17432–17441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roos C, Wicovsky A, Müller N, et al Soluble and transmembrane TNF‐like weak inducer of apoptosis differentially activate the classical and noncanonical NF‐kappa B pathway. J Immunol 2010; 185: 1593–1605. [DOI] [PubMed] [Google Scholar]
- 23. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: optimized mammalian biology. Cell 2001; 104: 487–501. [DOI] [PubMed] [Google Scholar]
- 24. Kaplan MJ, Lewis EE, Shelden EA, et al The Apoptotic Ligands TRAIL, TWEAK, and Fas Ligand Mediate Monocyte Death Induced by Autologous Lupus T Cells. J Immunol 2002; 169: 6020–6029. [DOI] [PubMed] [Google Scholar]
- 25. Chorianopoulos E, Rosenberg M, Zugck C, et al Decreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failure. Eur J Heart Fail 2009; 11: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 26. Blanco‐Colio LM, Martín‐Ventura JL, Muñóz‐García B, et al Identification of soluble tumor necrosis factor‐like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 2007; 27: 916–922. [DOI] [PubMed] [Google Scholar]
- 27. Urbonaviciene G, Martin‐Ventura JL, Lindholt JS, et al Impact of soluble TWEAK and CD163/TWEAK ratio on long‐term cardiovascular mortality in patients with peripheral arterial disease. Atherosclerosis 2011; 16: 1–8. [DOI] [PubMed] [Google Scholar]
- 28. Dyaz‐López A, Chacón MR, Bulló M, et al Serum sTWEAK concentration and risk of developing type 2 diabetes in a high tube risk population: a nested case. J Clin Endocrinol Metab 2013; 98: 3482–3489. [DOI] [PubMed] [Google Scholar]
- 29. Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 2002; 106: 640–642. [DOI] [PubMed] [Google Scholar]
- 30. Valdivielso JM, Coll B, Martín‐Ventura JL, et al Soluble TWEAK is associated with atherosclerotic burden in patients with chronic kidney disease. J Nephrol 2013; 26: 1105–1113. [DOI] [PubMed] [Google Scholar]


