Abstract
A Japanese woman aged in her late 30s with severe insulin resistance and bodily features including a triangular face, prominent forehead, small chin, large and low‐set ears, and ocular depression was investigated. A similar phenotype was not observed in other family members with the exception of her son, suggesting that the condition was caused by a de novo mutation that was transmitted from mother to son. Exome analysis showed the presence in the proband and her son of a c.1945C>T mutation in PIK3R1, a common mutation associated with SHORT (short stature, hyperextensibility of joints and/or inguinal hernia, ocular depression, Rieger anomaly, and teething delay) syndrome. Administration of a sodium–glucose cotransporter 2 inhibitor lowered the proband's hemoglobin A1c level and allowed a reduction in her insulin dose without treatment‐related adverse events including ketoacidosis, exaggerated loss of body mass or hypoglycemia. Sodium–glucose cotransporter 2 inhibitors might thus offer an additional option for the treatment of genetic syndromes of severe insulin resistance.
Keywords: Insulin resistance, Mutation, PIK3R1
Introduction
Mutations in genes related to insulin action, including those encoding the insulin receptor, a regulatory subunit of phosphatidylinositol 3‐kinase (p85α), and Akt2, cause genetic syndromes of severe insulin resistance.1, 2, 3, 4, 5 Mutations in the gene for p85α (PIK3R1) give rise to severe insulin resistance and characteristic bodily manifestations, including short stature, joint hyperextensibility, deformity of the anterior eye chamber, atrophy of the iris stroma (Rieger anomaly) and a recognizable facial gestalt (triangular face, prominent forehead, small chin and ocular depression).3, 4, 5, 6, 7 SHORT syndrome is characterized by short stature, hyperextensibility of joints and/or inguinal hernia, ocular depression, Rieger anomaly, and teething delay, and mutations of PIK3R1 were first identified in individuals with this condition in 2013.3, 4, 5 More than 30 families with this syndrome and mutations of PIK3R1, all of which (with one African exception) are Caucasian, have been reported to date.3, 4, 5, 6, 7
Here, we report the first case of SHORT syndrome caused by a mutation of PIK3R1 in a Japanese person. We also report the investigative administration of a sodium–glucose cotransporter 2 (SGLT‐2) inhibitor in this case.
Case Report
The study was approved by the ethics committee of Kobe University Graduate School of Medicine (approval no. 160099), and the proband provided written consent to publish data for her case and that of her son. The proband, a Japanese woman aged in her late 30s, was born with a birthweight of 1,800 g at 36 weeks‐of‐gestation, and had started insulin for diabetes at the age of 21 years. Her clinical manifestations in her early 20s were reported previously.8 Despite the absence of an apparent cause of her insulin resistance, she had previously administered >200 U of insulin per day, suggestive of a genetic etiology. Her height was 143 cm, bodyweight 38 kg and body mass index 18.6 kg/m2. Her visceral and subcutaneous fat areas assessed by abdominal magnetic resonance imaging were 57 and 95 cm2, respectively, which were comparable with those observed in non‐obese, young, normoglycemic individuals.9 She had a triangular face, prominent forehead, small chin, large and low‐set ears, and ocular depression (Figure 1), but not hyperextensibility of the joints or Rieger anomaly. Her son, whose birthweight was 1,794 g at 34 weeks‐of‐gestation, had a small chin, large and low‐set ears, hirsutism, and ocular depression (Figure 1). His serum insulin levels were suggestive of the presence of insulin resistance (Table S1). Given the absence of insulin‐resistant diabetes or similar bodily characteristics in family members other than her son (Figure 2), we hypothesized that the proband's condition was caused by a de novo mutation that was transmitted to her son. Exome analysis of the proband, her parents and her son showed that she harbored 45,762 variants with allele frequencies of <0.0001 relative to data in the Exome Aggregation Consortium. A total of 219 of these variants were heterozygous and not present in her parents, 38 of the 219 variants were transmitted to her son and five of the 38 variants altered the amino acid sequence of the encoded proteins. One of the latter variants was c.1945C>T in PIK3R1 (Figure 2), which had previously been identified in individuals with SHORT syndrome.3, 4, 5, 6
Figure 1.
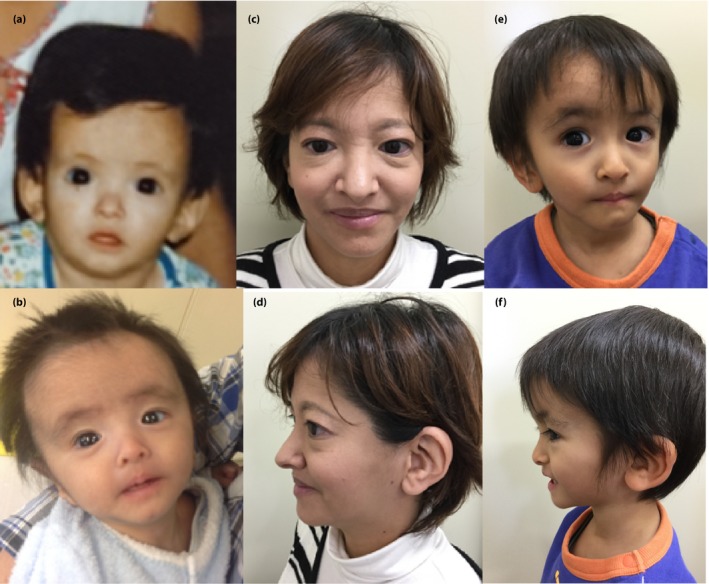
Photos of the (a,c,d) proband and (b,e,f) her son at (a) age 7 months, (b) 4 months, (c,d) late 30s and (e,f) 3 years.
Figure 2.
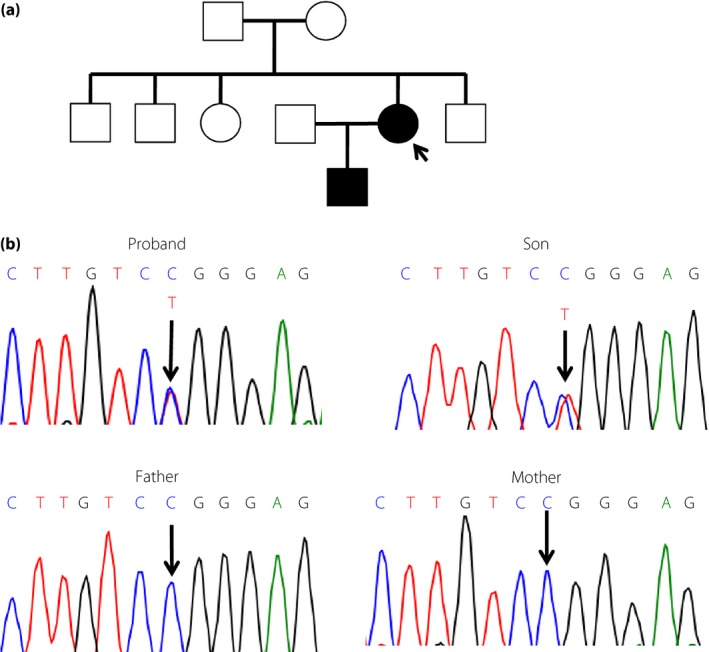
(a) Family pedigree, with the arrow showing the proband, and (b) deoxyribonucleic acid sequencing electrophoretogram traces showing the position of the mutation in PIK3R1.
Treatment
The proband administered >200 U of insulin daily during her late 20s. After the maximum daily dose of metformin was increased from 750 to 2,250 mg in May 2010 in Japan, she began high‐dose metformin therapy. At the current presentation, she administered 2,250 mg of metformin and ~80 U of insulin daily (Figure 3). Her hemoglobin A1c (HbA1c) level did not fall below 7.0%, however. Given that SGLT‐2 inhibitors lower blood glucose through a mechanism independent of insulin action, we hypothesized that such a drug might have a beneficial effect on the proband's glycemia. We initiated treatment with 5 mg of dapagliflozin per day. Within 3 months, her HbA1c level had decreased from 7.5% to 6.5%, and her daily insulin dose had fallen to ~50 U (Figure 3). Five months after the initiation of dapagliflozin, she developed a common cold and subsequently manifested a transient elevation of her HbA1c level. At 14 months, her HbA1c level and daily insulin dose were 6.6% and 50 U, respectively. The proband's body mass declined by 2–3 kg during dapagliflozin treatment, an effect similar to that observed for this drug in the treatment of type 2 diabetes.
Figure 3.
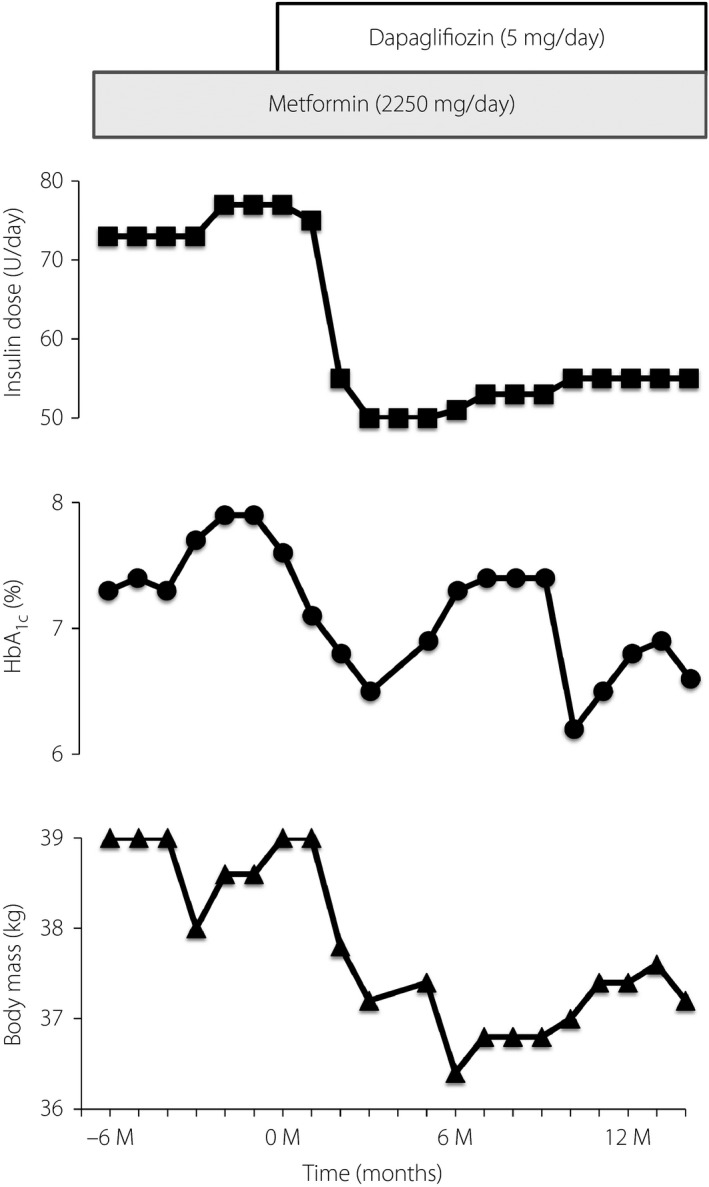
Clinical course for treatment of the proband with dapagliflozin. HbA1c, hemoglobin A1c.
Serum levels of total ketone bodies were not increased during dapagliflozin therapy (Table S2), and hypoglycemia, urinary tract or genital infection, polyuria and thirst were not reported. The hematocrit before and 12 months after the initiation of treatment was 35.5 and 42.0%, respectively.
Discussion
We report the first Japanese case of severe insulin resistance caused by a mutation in PIK3R1. The sporadic insulin resistance syndrome was transmitted from the proband to her son, and exome analysis allowed us to identify the responsible mutation. In retrospect, the facial gestalt of the proband and her son was similar to that described for individuals with mutations in PIK3R1.3, 4, 5, 6, 7 This case thus underscores the importance of recognition of facial gestalt in suspicion of this syndrome.
Despite the administration of a large amount of insulin and oral hypoglycemic agents, it is often difficult to achieve desirable glycemic control in individuals with genetic syndromes of severe insulin resistance.1 SGLT‐2 inhibitors might provide an alternative treatment option for such syndromes. Indeed, a case of lipodystrophic diabetes treated successfully with an SGLT‐2 inhibitor was recently reported.10 Although fat tissue was not severely decreased in the present case, SHORT syndrome is sometimes associated with lipodystrophy.3, 4, 5, 6, 7 Metreleptin, which ameliorates various metabolic disorders of lipodystrophy,11 might be a useful option for SHORT syndrome with lipodystrophy. Along with lean body composition, the secession or the reduction of insulin are risk factors for SGLT‐2 inhibitor‐related ketoacidosis.12 It is important to evaluate whether insulin is adequately supplemented to prevent exaggerated catabolism during the treatment with SGLT‐2 inhibitors. In the present case, an increase in the serum ketone bodies was not observed during treatment. Further studies are warranted to confirm the clinical benefits of SGLT‐2 inhibitors for treatment of genetic syndromes of severe insulin resistance.
Disclosure
WO has received a research grant, and WO and YH have received lecture fees from Astra Zeneca. The other authors declare no conflict of interest.
Supporting information
Table S1 | Plasma glucose and serum insulin levels of the proband's son
Table S2 | Fasting serum levels of ketone bodies during treatment
Acknowledgments
This study was supported by a Grant‐in‐Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to YH), by a grant from the Japan Diabetes Foundation (to YH), and by the Practical Research Project for Rare/Intractable Diseases of the Japan Agency for Medical Research and Development (AMED; to WO).
J Diabetes Investig 2018; 9: 1224–1227
References
- 1. Semple RK, Savage DB, Cochran EK, et al Genetic syndromes of severe insulin resistance. Endocr Rev 2011; 32: 498–514. [DOI] [PubMed] [Google Scholar]
- 2. George S, Rochford JJ, Wolfrum C, et al A family with severe insulin resistance and diabetes due to a mutation in AKT2 . Science 2004; 304: 1325–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thauvin‐Robinet C, Auclair M, Duplomb L, et al PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet 2013; 93: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chudasama KK, Winnay J, Johansson S, et al SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. Am J Hum Genet 2013; 93: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dyment DA, Smith AC, Alcantara D, et al Mutations in PIK3R1 cause SHORT syndrome. Am J Hum Genet 2013; 93: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang‐Doran I, Tomlinson P, Payne F, et al Insulin resistance uncoupled from dyslipidemia due to C‐terminal PIK3R1 mutations. JCI Insight 2016; 1: e88766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avila M, Dyment DA, Sagen JV, et al Clinical reappraisal of SHORT syndrome with PIK3R1 mutations: towards recommendation for molecular testing and management. Clin Genet 2016; 89: 501–506. [DOI] [PubMed] [Google Scholar]
- 8. Igaki N, Tanaka M, Goto T. Low birth weight and insulin resistance associated with lean body adiposity in an adolescent onset diabetic patient. Intern Med 2006; 45: 5–9. [DOI] [PubMed] [Google Scholar]
- 9. Komada H, Sakaguchi K, Hirota Y, et al Pancreatic fat content assessed by 1H magnetic resonance spectroscopy is correlated with insulin resistance, but not with insulin secretion, in Japanese individuals with normal glucose tolerance. J Diabetes Investig 2018; 9: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawana Y, Imai J, Sawada S, et al Sodium‐glucose cotransporter 2 inhibitor improves complications of lipodystrophy: a case report. Ann Intern Med 2017; 166: 450–451. [DOI] [PubMed] [Google Scholar]
- 11. Ebihara K, Kusakabe T, Hirata M, et al Efficacy and safety of leptin‐replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 2007; 92: 532–541. [DOI] [PubMed] [Google Scholar]
- 12. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig 2016; 7: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Plasma glucose and serum insulin levels of the proband's son
Table S2 | Fasting serum levels of ketone bodies during treatment


