Abstract
The present study was designed to assess possible relationships between deterioration of the glycated hemoglobin (HbA1c)‐lowering effects in dipeptidyl peptidase‐4 inhibitor (DPP4i) monotherapy and macronutrient intake among individuals with type 2 diabetes. Type 2 diabetes patients who began and continued DPP4i monotherapy without any prescription change for 1 year were retrospectively stratified into two groups: (i) patients who maintained their HbA1c levels during the 0.5‐ to 1‐year period after DPP4i initiation (group A, ΔHbA1c [1–0.5 year] <0.4%, n = 53); and (ii) those whose HbA1c levels increased [group B, ΔHbA1c (1–0.5 year] ≥0.4%, n = 10). Group B had significantly higher ΔHbA1c (1–0.5 year), Δbodyweight (1–0.5 year) and fat intake, especially of saturated and monounsaturated fats; the carbohydrate and protein intake were similar between groups. Multiple regression analyses showed that fat intake, especially saturated fat intake, was significantly correlated with ΔHbA1c (1–0.5 year). Thus, dietary habits, especially saturated fat intake, might well contribute to deterioration of the HbA1c‐lowering effects in DPP4i monotherapy.
Keywords: Dietary habits, Dipeptidyl peptidase‐4 inhibitors, Fat intake
Introduction
Dipeptidyl peptidase‐4 inhibitors (DPP4is) have been widely used to manage type 2 diabetes, especially in East Asian countries1, 2. Previous studies have shown that DPP4is exert greater glycated hemoglobin (HbA1c)‐lowering effects (HLE) in East Asians3, 4. This enhanced efficacy has been attributed to the unique type 2 diabetes phenotype in East Asians, who tend to be non‐obese and to show β‐cell dysfunction2, 5. However, differences in dietary habits might also affect the HLE of DPP4is, as the glucose‐lowering mechanisms of DPP4i involve the incretins, glucagon‐like peptide‐1 and glucose‐dependent insulinotropic polypeptide (GIP), hormones that are secreted in response to meal ingestion and enhance insulin secretion in a glucose‐dependent manner.6, 7 Indeed, we and others have shown that the HLE of DPP4i in the relatively short‐term are enhanced by fish intake, as estimated by food records and serum levels of eicosapentaenoic acids and docosahexaenoic acids, in type 2 diabetes individuals8, 9. Interestingly, the HLE of DPP4i monotherapy has been shown to deteriorate 3–6 months after DPP4i initiation in some patients10, 11, 12. These patients show a slight, but significant, increase in bodyweight10, 11, 12 and poor adherence to a healthy diet10, 12, suggesting that the deterioration of HLE in DPP4i monotherapy might be caused by unhealthy dietary habits.
In the current study, possible associations between the deterioration of the HLE in DPP4i monotherapy and dietary habits were retrospectively investigated using predefined inclusion and exclusion criteria.
Methods
Study protocol
Medical records of individuals visiting Kansai Electric Power Hospital (Osaka, Japan) during the period between 1 September 2006 and 30 June 30 2017 were retrospectively analyzed (Figure 1). Of the individuals whose records were examined, type 2 diabetes patients who satisfied the predefined inclusion and exclusion criteria (Appendix S1) were stratified into two groups based on changes in HbA1c from 0.5 to 1 year (ΔHbA1c [1–0.5 year]) as previously described11: group A comprised patients whose HbA1c was maintained 1 year after DPP4i initiation (ΔHbA1c [1–0.5 year] <0.4%), and group B comprised patients whose HbA1c decreased (ΔHbA1c [1–0.5 year] ≥0.4%). As in the previous study11, deterioration of the HLE of DPP4i was predefined as ΔHbA1c (1–0.5 year) ≥0.4%, as DPP4i usually reduces HbA1c by 0.7–0.9% at 6 months after administration in Japanese type 2 diabetes patients. HbA1c and bodyweight values at 0, 0.5 and 1 year were calculated as the mean of each value in the periods −2 to 0 months, 5–7 months and 11–12 months, respectively, after initiation of DPP4i monotherapy. Total caloric intake (TCI), intake of carbohydrates, proteins and fats, and intake of saturated fats (SF), monounsaturated fats (MUF) and polyunsaturated fats (PUF) were estimated from self‐administered 3‐day food records taken between 3 months before and 15 months after the initiation of DPP4i monotherapy using Healthy Maker Pro 501 (Mushroom soft Co., Ltd., Okayama, Japan). If there were multiple food records for a participant during that period, the mean of each value was calculated for each individual and then analyzed. The study protocol was approved by the ethics committee of Kansai Electric Power Hospital and registered at UMIN‐CTR (UMIN000027876). Opt‐out recruitment was carried out.
Figure 1.
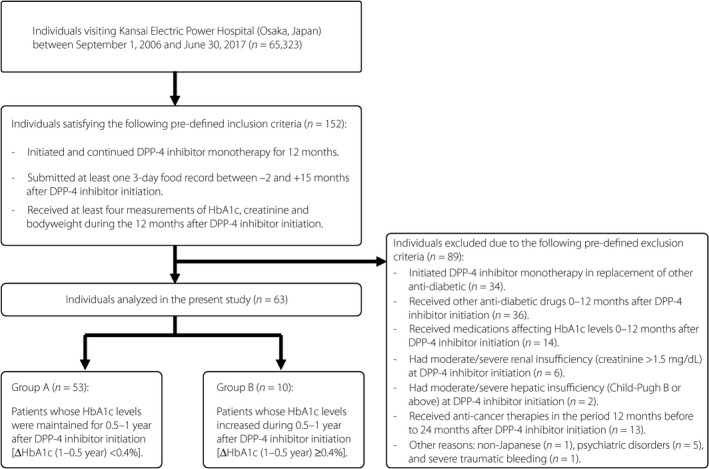
Study diagram. DPP4, dipeptidyl peptidase‐4; HbA1c, glycated hemoglobin.
Study assessment
The primary outcome measures included TCI, and intake of carbohydrate, protein and fat, including intake of SF, MUF and PUF in both groups A and B. Secondary outcome measures included bodyweight changes in groups A and B.
Statistical analysis
All statistical calculations were carried out using SPSS Statistics 22 software (IBM Corp., Armonk, NY, USA). A P‐value of <0.05 was taken to show significant difference. Values are shown as the mean ± standard error of the mean.
Results
The current retrospective analysis of the medical records of 65,323 individuals at a single medical institution in Osaka, Japan, between 1 September 2006 and 30 June 2017 included 152 type 2 diabetes patients in accord with the predefined inclusion criteria who initiated and continued DPP4i monotherapy for 12 months (Figure 1). Of the 152 individuals identified, 89 were excluded due to the predefined exclusion criteria, and the remaining 63 were analyzed. Based on the values of ΔHbA1c (1–0.5 year), as described previously11, the 63 individuals were divided into two groups: group A, which included patients whose HbA1c levels were maintained (ΔHbA1c [1–0.5 year] <0.4%) 1 year after DPP4i monotherapy initiation; and group B, which included patients whose HbA1c levels increased (ΔHbA1c (1–0.5 year) ≥0.4%). Group B was younger, had a higher body mass index and bodyweight, and had a higher baseline HbA1c, none of which differences reached statistical significance, before initiation of DPP4i monotherapy (Table 1). Group B had significantly higher ΔHbA1c (1–0.5 year) and HbA1c at 1 year. Group B had higher bodyweight throughout the observational period, as well as significantly higher Δbodyweight (1–0.5 year) (Table 1). Group B had higher TCI (Table 2). Interestingly, group B also had a significantly higher fat intake; the carbohydrate and protein intake did not differ between the groups (Table 2). From the analysis of various fats consumed, group B showed significantly higher SF and MUF intake (Table 2). Similar findings were also observed when daily intake was normalized by the ideal bodyweight of each patient.
Table 1.
Baseline characteristics and changes in glycated hemoglobin and bodyweight in the study population
| Group A | Group B | P value | |
|---|---|---|---|
| n (male, female) | 53 (13, 40) | 10 (9, 1) | |
| Age (years) | 65.2 ± 1.6 | 58.8 ± 4.2 | 0.127 |
| BMI (kg/m2) | 24.6 ± 0.6 | 25.3 ± 1.2 | 0.609 |
| Duration of the disease | 10.0 ± 0.8 | 7.9 ± 1.6 | 0.280 |
| HbAlc (%) | |||
| 0 year | 7.54 ± 0.13 | 8.22 ± 0.51 | 0.067 |
| 0.5 year | 6.58 ± 0.08 | 6.58 ± 0.20 | 0.967 |
| 1 year | 6.50 ± 0.07 | 7.32 ± 0.34 | 0.000 |
| Δ (1–0.5 year) | −0.09 ± 0.04 | 0.75 ± 0.18 | <0.001 |
| Bodyweight (kg) | |||
| 0 year | 66.0 ± 1.9 | 71.3 ± 3.6 | 0.266 |
| 0.5 year | 64.7 ± 1.9 | 69.6 ± 4.1 | 0.293 |
| 1 year | 64.7 ± 1.9 | 70.9 ± 3.8 | 0.196 |
| Δ (1–0.5 year) | 0.1 ± 0.2 | 1.3 ± 0.5 | 0.043 |
Each value indicates the mean ± standard error of the mean. P values were calculated using a t‐test (Group A vs Group B). BMI, body mass index; HbA1c, glycated hemoglobin.
Table 2.
Dietary habits as assessed by 3‐day food records submitted by the study population
| Dietary intake | Group A | Group B | P value |
|---|---|---|---|
| Total calorie intake | |||
| (kcal/day) | 1,730 ± 32 | 1,960 ± 84 | 0.007 |
| (kcal/day/kg IBW) | 29.4 ± 0.5 | 31.5 ± 1.1 | 0.106 |
| Carbohydrate intake | |||
| (g/day) | 215.8 ± 5.1 | 226.0 ± 11.0 | 0.424 |
| (g/day/kg IBW) | 3.68 ± 0.09 | 3.67 ± 0.23 | 0.965 |
| (% total calorie intake) | 50.1 ± 0.9 | 46.7 ± 2.8 | 0.155 |
| Protein intake | |||
| (g/day) | 69.9 ± 2.0 | 78.5 ± 5.6 | 0.108 |
| (g/day/kg IBW) | 1.19 ± 0.03 | 1.26 ± 0.07 | 0.380 |
| (% total calorie intake) | 16.1 ± 0.3 | 16.1 ± 1.0 | 0.930 |
| Fat intake | |||
| (g/day) | 54.6 ± 1.8 | 68.9 ± 6.6 | 0.016 |
| (g/day/kg IBW) | 0.93 ± 0.03 | 1.1 ± 0.09 | 0.031 |
| (% total calorie intake) | 28.4 ± 0.8 | 31.4 ± 2.0 | 0.159 |
| Saturated fat intake | |||
| (g/day) | 16.2 ± 0.6 | 20.4 ± 2.2 | 0.005 |
| (g/day/kg IBW) | 0.28 ± 0.01 | 0.32 ± 0.03 | 0.081 |
| (% total calorie intake) | 8.5 ± 0.3 | 9.2 ± 0.7 | 0.350 |
| Monounsaturated fat intake | |||
| (g/day) | 19.1 ± 0.7 | 25.9 ± 3.0 | 0.002 |
| (g/day/kg IBW) | 0.32 ± 0.01 | 0.41 ± 0.04 | 0.011 |
| (% total calorie intake) | 10.0 ± 0.3 | 11.6 ± 1.0 | 0.058 |
| Polyunsaturated fat intake | |||
| (g/day) | 12.3 ± 0.4 | 14.3 ± 1.2 | 0.082 |
| (g/day/kg IBW) | 0.21 ± 0.01 | 0.23 ± 0.02 | 0.240 |
| (% total calorie intake) | 6.4 ± 0.2 | 6.6 ± 0.5 | 0.671 |
Each value indicates the mean ± standard error of the mean. P values were calculated using a t‐test (Group A vs Group B). IBW, ideal bodyweight.
Simple liner regression and stepwise multiple regression analyses of ΔHbA1c (1–0.5 year) taking into account age, body mass index, baseline HbA1c, duration of type 2 diabetes, TCI, and carbohydrate, protein and fat intake showed that ΔHbA1c (1–0.5 year) was independently correlated with fat intake and baseline HbA1c (Table 3). A stepwise multiple regression analysis of ΔHbA1c (1–0.5 year) taking into account intake of SF, MUF and PUF showed that ΔHbA1c (1–0.5 year) was independently correlated with SF intake (B = 0.032, standard error [SE] = 0.010, P < 0.01). Simple linear regression analyses showed significant associations of HbA1c (1–0.5 year) with SF (B = 0.0316, SE = 0.0103, P = 0.0033) and MUF (B = 0.0243, SE = 0.0082; P = 0.0044), but not with PUF (B = 0.0194, SE = 0.1676, P = 0.2525).
Table 3.
Simple linear regression and step‐wise multiple linear regression analyses of the associations between changes in glycated hemoglobin levels and various parameters
| Variables | Simple linear regression | Stepwise multiple linear regression | |||||
|---|---|---|---|---|---|---|---|
| B | SE | P value | B | SE | β | P value | |
| Age (years) | −0.0049 | 0.0046 | 0.2904 | ||||
| BMI (kg/m2) | 0.0201 | 0.0138 | 0.1511 | ||||
| Duration of the disease (years) | −0.0076 | 0.0102 | 0.4606 | ||||
| Baseline HbA1c (%) | 0.1270 | 0.0498 | 0.0132 | 0.1088 | 0.0483 | 0.2661 | 0.0280 |
| Total calorie intake (kcal/day) | 0.0005 | 0.0002 | 0.0260 | ||||
| Carbohydrate intake (g/day) | 0.0020 | 0.0015 | 0.1846 | ||||
| Protein intake (g/day) | 0.0025 | 0.0036 | 0.5012 | −0.0064 | 0.0043 | −0.2233 | 0.1406 |
| Fat intake (g/day) | 0.0096 | 0.0035 | 0.0084 | 0.0124 | 0.0044 | 0.4253 | 0.0071 |
Simple linear regression and step‐wise multiple regression analyses of glycated hemoglobin (HbA1c) (1–0.5 year) taking into account age, body mass index (BMI), duration of the disease, baseline HbA1c, and intake of total calories, carbohydrates, proteins and fats in 3‐day food records of 63 patients with type 2 diabetes. Statistical calculations were carried out using SPSS Statistics 22 software (IBM Corp.). B and β denote non‐standardized and standardized regression coefficients, respectively. For the step‐wise multiple regression analysis of changes in HbA1c levels, the correlation coefficient squared (R 2) was 0.203, and the F value with 3 degrees of freedom was 5.0211, resulting in a P value of 0.004.
Discussion
The current study showed that deterioration of the HLE in DPP4i monotherapy is affected by intake of dietary fat, especially saturated fat, in Japanese type 2 diabetes patients. Despite the use of a retrospective cohort study with a limited sample size, these findings are clinically important in two respects: (i) dietary recommendations to consume less SF might prevent deterioration of the HLE in DPP4i monotherapy; and (ii) the differing efficacies of DPP4i monotherapy found among different ethnicities might be due partly to differences in dietary habits.
Glucose‐dependent insulinotropic polypeptide facilitates energy storage into adipose tissues in mice on high‐fat, but not on a regular, diet, despite the beneficial effects exerted by GIP on glucose metabolism through enhanced insulin secretion13, 14, 15, 16. Furthermore, foods rich in SF and MUF, but not in PUF, strongly enhance GIP secretion in humans17, 18. Thus, it is possible that DPP4i monotherapy, which increases the levels of biologically intact GIP, would exacerbate insulin resistance in type 2 diabetes patients who consume diets high in SF and MUF; and thereby lead to deterioration of the HLE DPP4i monotherapy. In fact, the individuals with HbA1c levels that decreased 1 year after DPP4i initiation in group B had a significantly higher SF and MUF intake, and significantly more bodyweight gain (Table 1). The current findings do not conflict with our previous observations that the HLE of DPP4i monotherapy in the relative short term is enhanced by fish intake, as estimated by food records, and serum eicosapentaenoic acids and docosahexaenoic acids levels in type 2 diabetes patietns,8, 9 as fish has less SF and MUF than other meats. Critical remaining questions include whether the association between the HLE of DPP4i and SF intake is specific to DPP4i monotherapy. A stepwise multiple regression analysis of ΔHbA1c (1–0.5 year) taking into account SF, MUF and PUF intake showed that ΔHbA1c (1–0.5 year) was independently correlated with SF intake (B = 0.032, SE = 0.010, P < 0.01). A recent study comparing the long‐term HLE of the DPP4i, sitagliptin, with those of the sodium–glucose cotransporter 2 inhibitor, canagliflozin, carried out in the USA showed that, as in group B in the current study, the HLE decreased with a slight bodyweight increase in type 2 diabetes patients who received sitagliptin, but not in those who received canagliflozin19. Thus, it would be of interest to compare the effects of dietary habits on the long‐term efficacy of antidiabetic drugs in randomized, controlled trials.
Limitations of the current study include the following: (i) there were no predefined rules for dealing with changing prescriptions for antidiabetic drugs among the physicians in charge, which might have affected the current study population due to the retrospective nature of the study; (ii) dietary habits were assessed by 3‐day food records that might not fully represent the food intake in the 1‐year study period, although similar assessments were previously used to evaluate dietary habits8, 20; (iii) exercise and other lifestyle habits, and the effects of lifestyle modification education were not assessed because the relevant information could not be retrieved from the medical records examined in the current study; and (iv) there was an imbalance in the two groups, presumably due to our dietitians' encouragement of patients taking DPP4is to consume PUF rather than SF based on our previous reports8, 9.
In conclusion, the current findings show a novel association between deterioration of the HLE in DPP4i monotherapy and dietary SF intake in type 2 diabetes patients, suggesting that dietary recommendations to avoid SF‐rich foods can help maintain the long‐term efficacy of DPP4i therapy.
Disclosure
Daisuke Yabe received consulting or speaker fees from MSD K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Company Limited, and Taisho Toyama Pharmaceutical Co. Ltd. Daisuke Yabe also received clinical commissioned/joint research grants from Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly and Company, Taisho Toyama Pharmaceutical Co. Ltd., MSD K.K., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., Arklay Co. Ltd., and Takeda Pharmaceutical Company Limited. Yoshiyuki Hamamoto received consulting or speaker fees from Novo Nordisk Pharma Ltd. Takeshi Kurose received consulting or speaker fees from Sanofi K.K. Takeshi Kurose also received clinical commissioned/joint research grants from the Japan Vascular Disease Research Foundation. Yutaka Seino received consulting or speaker fees from Eli Lilly Japan K.K., Sanofi K.K., Novo Nordisk Pharma Inc., Glaxo‐Smith‐Kline, Taisho Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Astellas Pharma Inc., BD, Nippon Boehringer Ingelheim Co., Ltd., Johnson & Johnson, and Takeda Pharmaceutical Company Limited. Yutaka Seino also received clinical commissioned/joint research grants from Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly and Company, Taisho Toyama Pharmaceutical Co. Ltd., MSD K.K., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., and Arklay Co. Ltd. Saki Okamoto, Hitoshi Kuwata, Kenta Murotani, Yusuke Seino, Hisato Tatsuoka and Ryota Usui declare no conflict of interest.
Supporting information
Appendix S1. Supplemental materials and methods.
Acknowledgments
The authors are grateful to H Abe of Kansai Electric Power Medical Research Institute and M Yamane of Kansai Electric Power Hospital for secretarial assistance. This study was funded by Grant‐in‐Aids for Scientific Research (C) from the Japan Society for the Promotion of Science, Grants for Young Researchers from the Japan Association for Diabetes Education and Care, and grants from the Japan Vascular Disease Research Foundation.
J Diabetes Investig 2018; 9: 1084–1090
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000027876
Contributor Information
Yutaka Seino, Email: seino.yutaka@e2.kepco.co.jp.
Daisuke Yabe, Email: ydaisuke-kyoto@umin.ac.jp.
References
- 1. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016; 7(Suppl 1): 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig 2015; 6: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim YG, Hahn S, Oh TJ, et al Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia 2013; 56: 696–708. [DOI] [PubMed] [Google Scholar]
- 4. Park H, Park C, Kim Y, et al Efficacy and safety of dipeptidyl peptidase‐4 inhibitors in type 2 diabetes: meta‐analysis. Ann Pharmacother 2012; 46: 1453–1469. [DOI] [PubMed] [Google Scholar]
- 5. Yabe D, Seino Y, Fukushima M, et al beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr DiabRep 2015; 15: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig 2010; 1: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drucker DJ. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes 2015; 64: 317–326. [DOI] [PubMed] [Google Scholar]
- 8. Iwasaki M, Hoshian F, Tsuji T, et al Predicting efficacy of DPP‐4 inhibitors in patients with type 2 diabetes: association of HbA1c reduction with serum eicosapentaenoic acid and docosahexaenoic acid levels. J Diabet Investig. 2012; 3: 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Senmaru T, Fukui M, Kobayashi K, et al Dipeptidyl‐peptidase IV inhibitor is effective in patients with type 2 diabetes with high serum eicosapentaenoic acid concentrations. J Diabet Investig. 2012; 3: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tajiri Y, Tsuruta M, Ohki T, et al Long‐term efficacy of sitagliptin for the treatment of type 2 diabetic patients in Japan. Endocr J 2012; 59: 197–204. [DOI] [PubMed] [Google Scholar]
- 11. Kubota A, Yabe D, Kanamori A, et al Factors influencing the durability of the glucose‐lowering effect of sitagliptin combined with a sulfonylurea. J Diabetes Investig 2014; 5: 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanamori A, Matsuba I. Factors associated with reduced efficacy of sitagliptin therapy: analysis of 93 patients with type 2 diabetes treated for 1.5 years or longer. J Clin Med Res 2013; 5: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seino Y, Yabe D. Glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1: incretin actions beyond the pancreas. J Diabetes Investig 2013; 4: 108–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nasteska D, Harada N, Suzuki K, et al Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high‐fat diet conditions. Diabetes 2014; 63: 2332–2343. [DOI] [PubMed] [Google Scholar]
- 15. Miyawaki K, Yamada Y, Ban N, et al Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002; 8: 738–742. [DOI] [PubMed] [Google Scholar]
- 16. Miyawaki K, Yamada Y, Yano H, et al Glucose intolerance caused by a defect in the entero‐insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 1999; 96: 14843–14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lardinois CK, Starich GH, Mazzaferri EL. The postprandial response of gastric inhibitory polypeptide to various dietary fats in man. J Am Coll Nutr 1988; 7: 241–247. [DOI] [PubMed] [Google Scholar]
- 18. Kuwata H, Iwasaki M, Shimizu S, et al Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: a randomised, controlled crossover, exploratory trial. Diabetologia 2016; 59: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schernthaner G, Gross JL, Rosenstock J, et al Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care 2013; 36: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eloranta AM, Lindi V, Schwab U, et al Dietary factors associated with overweight and body adiposity in Finnish children aged 6‐8 years: the PANIC Study. Int J Obes (Lond) 2012; 36: 950–955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental materials and methods.


