Abstract
Aims/Introduction
Vascular adhesion protein‐1 (VAP‐1) is a membrane‐bound amine oxidase highly expressed in mature adipocytes and released into the circulation. VAP‐1 has been strongly implicated in several pathological processes, including diabetes, inflammation, hypertension, hepatic steatosis and renal diseases, and is an important disease marker and therapeutic target. Here, we aimed to identify the genetic loci for circulating VAP‐1 levels.
Materials and Methods
We carried out a genomic‐wide linkage scan for the quantitative trait locus of circulating VAP‐1 levels in 1,100 Han Chinese individuals from 398 families in the Stanford Asian Pacific Program for Hypertension and Insulin Resistance study. Regional association fine mapping was carried out using additional single‐nucleotide polymorphisms.
Results
The estimated heritability of circulating VAP‐1 levels is high (h 2 = 69%). The most significant quantitative trait locus for circulating VAP‐1 was located at 38 cM on chromosome 20, with a maximum empirical logarithm of odds score of 4.11 (P = 6.86 × 10−6) in females. Regional single‐nucleotide polymorphism fine mapping within a 1‐unit support region showed the strongest association signals in the MACRO domain containing 2 (MACROD2) gene in females (P = 5.38 × 10−6). Knockdown of MACROD2 significantly suppressed VAP‐1 expression in human adipocytes, as well as the expression of key adipogenic genes. Furthermore, MACROD2 expression was found to be positively associated with VAP‐1 in human visceral adipose tissue.
Conclusion
MACROD2 is a potential genetic determinant of serum VAP‐1 levels, probably through transcriptional regulation of adipogenesis.
Keywords: Linkage analysis, MACRO domain containing 2 gene, Vascular adhesion protein‐1
Introduction
Vascular adhesion protein‐1 (VAP‐1), also known as amine oxidase, copper containing 3, is a membrane‐bound amine oxidase that converts primary amines, such as methylamine and aminoacetone, to aldehydes with the production of hydrogen peroxide and ammonia. Possible substrates of VAP‐1 include cell‐surface molecules containing free NH2 groups1, 2, 3. These endogenous amine substrates have been shown to exert insulin‐like actions in adipocytes4, 5. Deamination of these amines leads to hydrogen peroxide and aldehydes production. Consequently, hydrogen peroxide and aldehydes, which trigger oxidative stress and cross‐linking of biomolecules, respectively, will lead to cellular damage. VAP‐1 expression is strongly increased during adipogenesis, and is highly expressed on the cell surface of mature adipocytes and vascular endothelium6, 7, 8. VAP‐1 is also secreted into the circulation in a soluble form.
VAP‐1 has been implicated in several pathological processes including diabetes, inflammation, and vascular, renal and neurological diseases. Serum VAP‐1 level has been shown to predict mortality in patients with diabetes after 10 years’ follow up9. In case–control analyses, serum VAP‐1 is elevated in patients with hyperglycemia10, hypertension11, chronic kidney disease12, diabetic retinopathy13, hepatic steatosis14 and those who are cigarette smokers15. In addition, inhibition of VAP‐1 suppresses atherosclerosis16, improves prognosis after stroke17, 18, ameliorates renal fibrosis19 and hepatitis20, and also decreases inflammation21. All of these show that VAP‐1 is an important disease marker and therapeutic target22.
In the present study, we sought to identify the genetic determinants of serum VAP‐1 levels. We showed that serum VAP‐1 levels were highly heritable. Through genome‐wide linkage scans and regional association fine mapping in a large family‐based cohort, we identified the strongest association signals in the MACRO domain containing 2 (MACROD2) gene. Knockdown of MACROD2 in human visceral adipocytes significantly reduced VAP‐1 expression and its secretion into the medium, as well as the expression of other key adipogenic genes. Furthermore, a positive correlation between the expression of MACROD2 and VAP‐1 were found in human visceral adipose tissue. These data showed that MACROD2 is the genetic determinant of VAP‐1 expression.
Methods
Stanford Asian Pacific program for hypertension and insulin resistance study cohort
The Stanford Asian Pacific Program for Hypertension and Insulin Resistance (SAPPHIRe) is a collaborative study part of the Family Blood Pressure Program of the National Heart, Lung and Blood Institute of the National Institutes of Health originally designed to investigate the genetic determinants of hypertension and insulin resistance in Chinese and Japanese patients. The study collected >1,300 sib pairs that were either concordant or discordant for high blood pressure. Detailed descriptions of the study cohort can be found in previous studies23. Individuals with chronic illnesses, such as diabetes, cancer, or diseases of the heart, liver or kidney, were excluded. In the present study, 1,100 individuals of Chinese origin from 398 families were recruited for analysis. The numbers of families with one to eight siblings were 57, 139, 112, 45, 29, 10, 4 and 2, respectively. Ethical approval for the present study was obtained from the institutional review board of each participating site. Informed consent was obtained from each participant. For gene expression study of adipose tissue, the study was approved by the National Taiwan University Hospital and Min‐Sheng General Hospital.
Genotyping and single‐nucleotide polymorphism imputation
Genomic deoxyribonucleic acid was extracted from peripheral leukocytes. Genotyping was carried out using 376 autosomal markers representing short tandem repeat polymorphisms and yielded an average map density of 10 cM. The genotyping concordance rate is 99% based on duplicate samples. An additional 1,231 single‐nucleotide polymorphisms (SNPs) in the 1‐unit support interval (35 cM, 41 cM) of linkage peak on chromosome 20 at 38 cM were further genotyped for regional fine mapping. SNP imputation was carried out using genotype information from the genome‐wide association study from 455 individuals in the Genetics of Insulin Sensitivity consortium. SNPs with a call rate <95%, minor allele frequency (MAF) <5% and deviation from the Hardy–Weinberg equilibrium (P < 10−6) were excluded. A total of 6,098 genotyped and imputed SNPs within the 1‐unit support interval were used for fine mapping.
Individuals for measurement of gene expression in adipose tissue
We recruited 47 non‐diabetic adults undergoing bariatric surgery or elective abdominal surgery, such as cholecystectomy or partial hepatectomy, in Min‐Sheng General Hospital and the Yunlin branch of National Taiwan University Hospital in Taiwan. Abdominal visceral adipose tissues were sampled in a fasting state during surgery, and were placed in liquid nitrogen immediately until processing. The institutional review board of Min‐Sheng General Hospital and National Taiwan University Hospital Yunlin branch approved the study. Written informed consent was also obtained from each patient. All methods were carried out in accordance with the approved guidelines with the tenets of the Declaration of Helsinki.
Human adipocyte culture
Human visceral preadipocytes, preadipocyte growth supplement and preadipocyte differentiation supplement were purchased from ScienCell Research Laboratories (San Diego, California, USA). Fetal calf serum was purchased from Gibco (Carlsbad, California, USA), and penicillin‐streptomycin‐amphotericin B solution was purchased from Biological Industries (Cromwell, Connecticut, USA). Human visceral preadipocytes were maintained in Dulbecco's modified Eagle's medium/F12 medium containing 5% fetal calf serum, preadipocyte growth supplement and penicillin‐streptomycin‐amphotericin B solution according to the manufacturer's protocol. Post‐confluent preadipocytes were replaced with medium containing pre‐adipocyte differentiation supplement and then continued onto differentiation for 5 days. The medium was changed every 3 days. VAP‐1 concentrations in cell culture supernatants were measured using the VAP‐1 human enzyme‐linked immunosorbent assay kit according to the manufacturer's protocol (Abcam, Cambridge, UK). Relative VAP‐1 levels were normalized to total cell counts.
Knockdown of MACROD2 in induced human adipocytes
For the ribonucleic acid (RNA) interference assay, human preadipocytes were transfected with 80 nmol/L of ON‐TARGETplus SMARTpool small interfering RNA targeting MACROD2 (#L‐015258‐00‐0005) or non‐targeting control (#D‐001810‐01‐05; Dharmacon, Lafayette, Colorado, USA) using Dharmafect reagent (Dharmacon) according to the manufacturer's protocol. Then, 24‐h post‐silencing cells were changed to differentiation medium and differentiated for 5 days.
Reverse transcription and quantitative real‐time polymerase chain reaction
Total RNA was isolated using REzol C&T Reagent (Protech, Taipei, Taiwan) and reverse transcribed with Transcriptor Reverse Transcriptase (Roche Applied Science, Basel, Switzerland) according to the manufacturer's protocol. Polymerase chain reaction amplification was carried out using LightCycler FastStart DNA MasterPlus SYBR (Roche Applied Science). Each sample was analyzed and calibrated to the cyclophilin housekeeping gene in duplicates. The relative quantitation method was used to present relative gene expression difference between the two groups. ΔC t was calculated as C t of the target gene − C t of cyclophilin A. Primer information is provided in Table S1.
Statistical analysis
The distribution of serum VAP‐1 levels is skewed, and inverse normal transformation was applied for the linkage and association analyses. The correlations between two gene expressions (ΔC t) or between gene expression and metabolic phenotypes were calculated using Pearson's correlation.
Linkage analysis
Phenotypic variation of VAP‐1 was decomposed into genetic and environmental components. Broad sense heritability of VAP‐1 refers to the proportion of the phenotypic variance in a trait attributable to the genetic variance accounting for covariates. Maximum likelihood methods implemented in the SOLAR (sequential oligogenic linkage analysis routines) computing program (https://userinfo.surfsara.nl/systems/lisa/software/solar) were used to estimate the phenotypic variance components and heritability24, 25. Multipoint linkage analysis was also carried out using SOLAR. The inverse normal transformation was made to approximate the normal distribution for VAP‐1. The genetic variation was further partitioned into components for a quantitative trait locus and the residuals polygenic component24, 25.
Dividing the likelihood of the estimated variance component due to the quantitative trait locus by the likelihood of this variance component being 0 yields a likelihood ratio. The logarithm to base 10 of the likelihood ratio was calculated as a logarithm of odds (LOD) score. One‐unit LOD support intervals were obtained for maximum LOD scores of ≥3.0. Age, sex and body mass index were adjusted in the analysis. A maximum empirical LOD score ≥2.0, which is regarded as evidence of “suggestive linkage,” was reported26 . Kosambi map function was used for the linkage scan.
Association analysis
Family‐based association analysis was carried out using generalized estimating equations where familial correlations between family members were accounted for. A linear model was used to assess associations between the inverse normal transformed VAP‐1 and individual SNPs adjusting for age, sex and body mass index. The genetic additive mode was assumed for individual SNPs in the regression analysis. P‐values for the estimated regression coefficients for individual SNPs with MAF frequencies ≥0.05 were reported. Imputation of the entire 2.5M HapMap SNP set was carried out using MACH software (http://csg.sph.umich.edu/abecasis/mach/tour/imputation.html)27. A total of 2,419,983 SNPs on autosomes were imputed, and 190,379 SNPs were filtered out due to call rate <0.95, Hardy–Weinberg equilibrium <10−6 or MAF <0.0128.
Results
The demographic and biochemical characteristics according to sex are shown in Table S2. A total of 1,100 siblings from 387 families were recruited (Table S3). The numbers of female–female, male–male and female–male sib pairs were 358, 258 and 516, respectively (Table S4).
Circulating VAP‐1 levels were higher in female participants than in male participants (P = 2.9 × 10−16; Table S2). The heritability (h 2) of circulating VAP‐1 levels was high (69%), especially in female participants (71%) compared with the male participants (54%; Table 1).
Table 1.
Heritability of serum vascular adhesion protein‐1 by sex
| Gender | Heritability | P |
|---|---|---|
| All | 0.69 ± 0.081 | 1.8 × 10−17 |
| Female | 0.71 ± 0.12 | 6.9 × 10−9 |
| Male | 0.54 ± 0.16 | 0.00068 |
Values are presented as estimate ± standard error.
All chromosomal regions with empirical LOD scores >2.00 for circulating VAP‐1 are summarized in Table 2. The highest linkage signal was observed on chromosome 20 at 38 cM (LOD 2.29, P = 5.81 × 10−4; Table 2, Figure 1) near the marker GGAA7E02 with 1‐LOD support interval of 35–41 cM. This linkage signal appeared only in females (LOD 4.10, P = 6.86 × 10−6), but not in males.
Table 2.
Regions with peak logarithm of odds scores ≥2.0
| Chr. | cM | LOD | P‐valuea | Nearest marker | |
|---|---|---|---|---|---|
| All | 20 | 38 | 2.2907 | 5.81 × 10−4 | GGAA7E02 |
| 18 | 77 | 2.1521 | 8.22 × 10−4 | GATA6D09 | |
| Female | 20 | 38 | 4.1057 | 6.86 × 10−6 | GGAA7E02 |
| 10 | 84 | 2.2514 | 6.41 × 10−4 | ATA24F10 | |
| Male | 1 | 258 | 2.3295 | 5.28 × 10−4 | AFM203yg9 |
Adjusted for age, sex and body mass index. Chr., chromosome; cM, centiMorgan; LOD, logarithm of odds.
Figure 1.
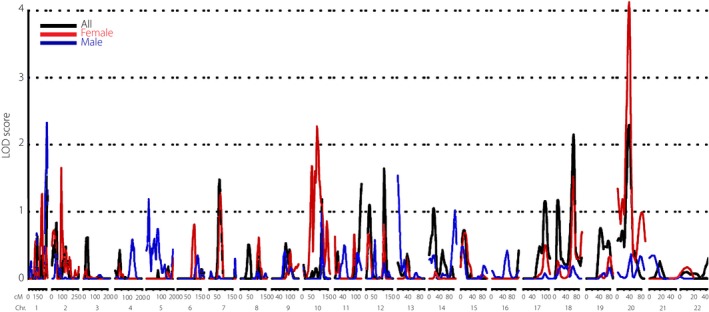
Genome‐wide linkage signals for circulating vascular adhesion protein‐1 levels in the Stanford Asian Pacific Program for Hypertension and Insulin Resistance cohort (SAPPHIRe). Chr., chromosome; cM, centiMorgan; LOD, logarithm of odds.
Another chromosomal region identified as linked with circulating VAP‐1 levels was located on chromosome 18 at 77 cM (LOD 2.15, P = 8.22 × 10−4) near the marker GATA6D09. We also observed suggestive linkage signals at chromosome 10 at 84 cM (LOD 2.25, P = 6.41 × 10−4) near the marker ATA24F10 in female participants as well as another signal on chromosome 1 at 258 cM (LOD 2.32, P = 5.28 × 10−4) near the marker AFM203yg9 in male participants.
We carried out regional association fine mapping for the highest signal at 38 cM on chromosome 20 using additional genotyped and imputed 6,098 SNPs within the 1‐LOD support interval. The strongest association signals were found in the MACROD2 gene, especially in females (Figure 2). An imputed common intronic SNP rs1237183 (MAF 6.7%) was significantly associated with circulating VAP‐1 levels in females (P = 5.38 × 10−6). The study‐wide Bonferroni corrected P‐value threshold was 8.20 × 10−6. The heritability reduced from 69.3% to 66.8% with inclusion of rs1237183 as a covariate. The LOD score at 38 cM reduced from 2.3 to 0.4 after adjusting for rs1237183. Both conditional heritability and linkage analyses suggested that the effect sizes of rs1237183 were substantial.
Figure 2.
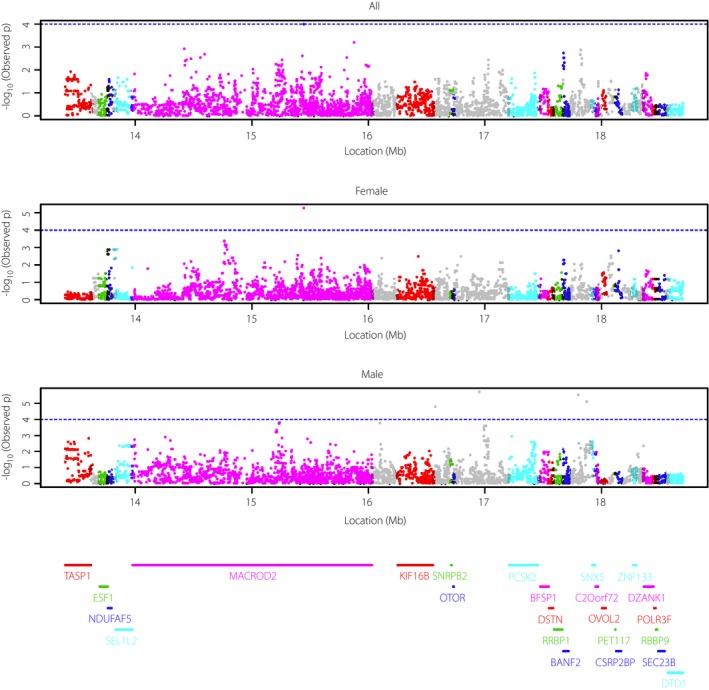
Regional association fine mapping within 1‐unit support region based on the generalized estimating equation approaches. Mb, megabase.
We next examined whether the linkage signal was due to a single sibling pair with phenotypically highly discordant pair without shared alleles at the linkage locus. We defined extreme pairs as their paired VAP‐1 levels were within the (≤5th, ≥95th) or (≥95th, ≤5th) percentiles (Table S5). The two phenotypically highly discordant pairs with VAP‐1 levels within the (≤5th, ≥95th) or (≥95th, ≤5th) percentiles shared one and two identity by descent at the linkage peak, respectively, suggesting this linkage signal was not due to a single extreme sibling pair.
Furthermore, knockdown of MACROD2 (knockdown efficiency 77.4%; Figure 3a) in induced primary human adipocytes significantly reduced VAP‐1 expression (relative expression 0.76 ± 11.1%, P = 0.03; Figure 3b) and its release into the medium (relative concentration 0.73 ± 0.05%, P = 0.01; Figure 3c). As VAP‐1 is a marker of mature adipocytes, we examined the expression of other key adipogenic markers. Interestingly, knockdown of MACROD2 significantly reduced the expression of adipogenic genes including ADIPOQ (P < 0.0001) and CEBPA (P = 0.006; Figure 3d). There were also trends of reduced expression of other adipogenic genes including FABP4 (P = 0.13), PPARG2 (P = 0.12) and SREBP1 (P = 0.06; Figure 3d).
Figure 3.
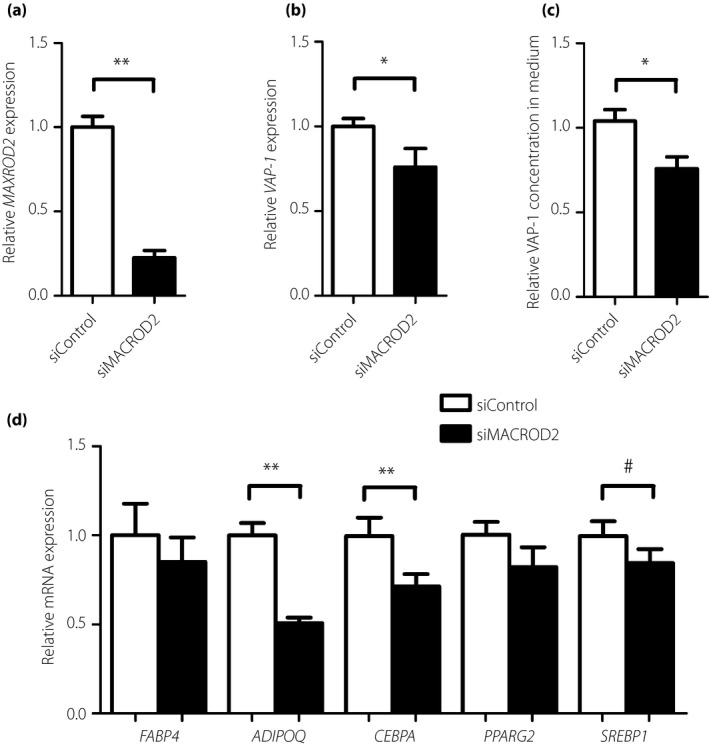
The effect of knockdown of MACRO domain containing 2 (MACROD2; siMACROD2) on (a) MACROD2 and (b) vascular adhesion protein‐1 (VAP‐1) gene expression in human primary preadipocytes (n = 14). (c) VAP‐1 concentration in culture medium (n = 10). (d) Expression of adipogenic genes including FABP4,ADIPOQ,CEBPA, PPARG2 and SREBP1 (n = 14) as compared with control (siControl). Data is expressed as mean ± standard error. *P < 0.05; **P < 0.01; # P < 0.1. mRNA, messenger ribonucleic acid.
We next examined the association between the expression of MACROD2 and VAP‐1 in 47 non‐diabetic human visceral adipose tissues. VAP‐1 expression was associated with MACROD2 expression (r = 0.38, P = 0.01; Figure 4). In addition, VAP‐1 expression levels were also positively associated with body mass index, homeostatic model assessment of insulin resistance and fasting insulin levels. In contrast, VAP‐1 expression levels were negatively associated with high‐density lipoprotein cholesterol levels (Table S6).
Figure 4.
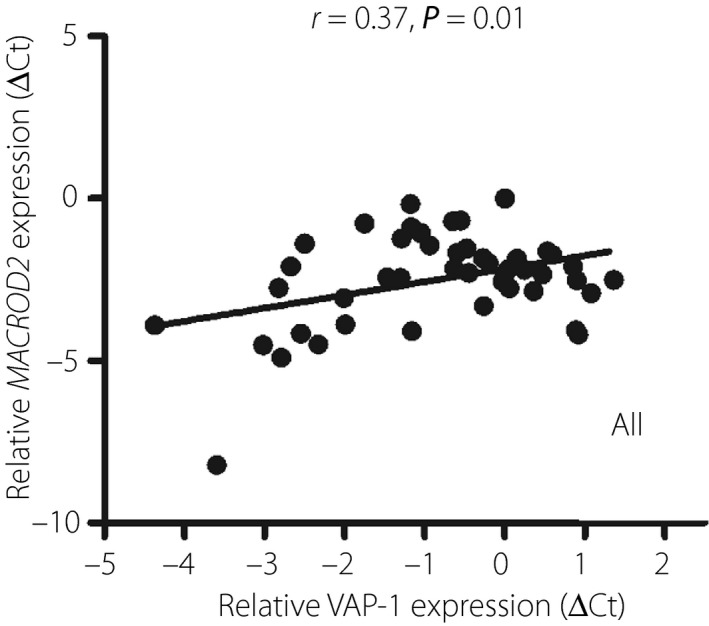
Correlation between vascular adhesion protein‐1 (VAP‐1) and MACRO domain containing 2 (MACROD2) gene expression in human visceral adipose tissue (expressed in ΔC t in relation to C t of cyclophilin A). r, correlation coefficient.
Discussion
In the present study, we found that genetic loci near the MACROD2 gene were associated with circulating VAP‐1 levels in females. Knockdown of MACROD2 reduced VAP‐1 expression in induced human primary visceral adipocytes and its release into the culture medium. Knockdown of MACROD2 also significantly suppressed the expression of several other key adipogenic genes. MACROD2 expression level was found to be positively correlated with VAP‐1 expression in human visceral adipose tissue. These data indicate MACROD2 as genetic loci regulating VAP‐1 expression, probably through modulation of adipogenesis.
Adenosine diphosphate (ADP)‐ribosylation is a post‐translational modification catalyzed by an enzyme family of ADP‐ribosyl transferases, which uses NAD+ as a cofactor to transfer single or multiple ADP‐ribose onto target proteins. This process can be reversely regulated by macrodomain‐containing hydrolase, MACROD2, by removing mono‐ADP‐ribosyl moiety from proteins28, 29. ADP‐ribosylation is involved in diverse biological processes, such as immunity, insulin secretion, and regulation of chromatin structure, transcription, deoxyribonucleic acid repair and RNA metabolism, especially in response to different forms of cellular stress30, 31, 32, 33. It is possible that MACROD2 removes the ADP‐ribosyl moiety from transcription factors or chromatin‐modifying enzymes associated with the VAP‐1 gene expression, thereby modulating its transcription.
Genome‐wide genetic scans showed that genetic polymorphisms in the MACROD2 gene are associated with hypertension34, pulmonary function test parameters35, Crohn's disease36, neuropsychiatric disorders including autism spectrum disorder37 and attention deficit hyperactivity disorder38, severity of dementia39, and liver cancer40, 41. In our present study, genetic variants within the MACROD2 region were associated with circulating VAP‐1 levels. Our results suggest that MACROD2 positively regulates VAP‐1 gene transcription in human primary visceral adipocytes and human visceral adipose tissue. Collectively, human genetic studies show the pleiotropic effects of MACROD2 for a variety of diseases or traits, possibly mediated through the regulation of gene expression.
We also observed a higher heritability of VAP‐1 and a stronger association signaling in females (71%) than in males (54%). The reason is unclear, but environmental factors, including higher frequency of smoking in males, might interfere with the heritability difference associated with sex.
The present study is important in the aspects of the family‐based design to control for population stratification, which is the most common reason for false positive findings in genetic association studies, the completeness of clinical data from the SAPPHIRe cohort and the relatively large sample size. Although the present study provides these novel findings, some limitations of this study should be recognized. First, no replication study was carried out. However, this study is currently the only study to identify the genetic determinant of serum VAP‐1 levels. Second, the probands recruited in this study were hypertension patients. Therefore, the magnitude of SNP association with VAP‐1 might not be representative of the general population, but for families suffering from hypertension.
In summary, we provide the first evidence for the association of variants in the MACROD2 gene, a mono‐ADP‐ribosyl hydrolase, with the circulating VAP‐1 levels. We also found a possible correlation between MACROD2 and VAP‐1 genetic expression in human adipocytes and adipose tissue. The association of MACROD2 genetic variations with several human diseases and traits in genome‐wide analyses strongly suggests its pleiotropic roles.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Primers used for quantitative real‐time polymerase chain reaction.
Table S2 | Baseline characteristics and serum vascular adhesion protein‐1 level by sex.
Table S3 | Number of families by sibship size.
Table S4 | Numbers of female–female, male–male and female–male sib pairs.
Table S5 | Distribution of identity by descent at the linkage peak between phenotypically extremely discordant sibling pairs.
Table S6 | Association between serum vascular adhesion protein‐1 gene expression in human adipose tissue and metabolic phenotypes.
Acknowledgments
The authors thank all participants in this study. We also thank Ms Chun‐Yi Lee for her computing support. This work was supported in part by grants from the Ministry of Science and Technology (MOST), Taiwan (MOST 97–2314‐B‐002‐047‐MY3 and 101‐2314‐B‐002‐069‐MY3 for Dr. Lee‐Ming Chuang; and 05D2‐PHMOST07 for Dr. Yen‐Feng Chiu), and NHRI‐PH‐PP03 and NHRI‐PH‐PP04 from the National Health Research Institutes (NHRI), Taiwan.
J Diabetes Investig 2018; 9: 1067–1074
[Correction added on 7 March 2018, after first online publication: The author affiliations for Hung‐Yuan Li and Tien‐Jyun Chang have been corrected.].
References
- 1. Smith DJ, Salmi M, Bono P, et al Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J Exp Med 1998; 188: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bono P, Salmi M, Smith DJ, et al Cloning and characterization of mouse vascular adhesion protein‐1 reveals a novel molecule with enzymatic activity. J Immunol 1998; 160: 5563–5571. [PubMed] [Google Scholar]
- 3. Lyles GA. Mammalian plasma and tissue‐bound semicarbazide‐sensitive amine oxidases: biochemical, pharmacological and toxicological aspects. Int J Biochem Cell Biol 1996; 28: 259–274. [DOI] [PubMed] [Google Scholar]
- 4. Mercader J, Iffiu‐Soltesz Z, Brenachot X, et al SSAO substrates exhibiting insulin‐like effects in adipocytes as a promising treatment option for metabolic disorders. Future Med Chem 2010; 2: 1735–1749. [DOI] [PubMed] [Google Scholar]
- 5. Karim S, Liaskou E, Fear J, et al Dysregulated hepatic expression of glucose transporters in chronic disease: contribution of semicarbazide‐sensitive amine oxidase to hepatic glucose uptake. Am J Physiol Gastrointest Liver Physiol 2014; 307: G1180–G1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ambele MA, Dessels C, Durandt C, et al Genome‐wide analysis of gene expression during adipogenesis in human adipose‐derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res 2016; 16: 725–734. [DOI] [PubMed] [Google Scholar]
- 7. Bour S, Daviaud D, Gres S, et al Adipogenesis‐related increase of semicarbazide‐sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie 2007; 89: 916–925. [DOI] [PubMed] [Google Scholar]
- 8. Sole M, Unzeta M. Vascular cell lines expressing SSAO/VAP‐1: a new experimental tool to study its involvement in vascular diseases. Biol Cell 2011; 103: 543–557. [DOI] [PubMed] [Google Scholar]
- 9. Li HY, Jiang YD, Chang TJ, et al Serum vascular adhesion protein‐1 predicts 10‐year cardiovascular and cancer mortality in individuals with type 2 diabetes. Diabetes 2011; 60: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li HY, Wei JN, Lin MS, et al Serum vascular adhesion protein‐1 is increased in acute and chronic hyperglycemia. Clin Chim Acta 2009; 404: 149–153. [DOI] [PubMed] [Google Scholar]
- 11. Maciorkowska D, Zbroch E, Malyszko J. Circulating renalase, catecholamines, and vascular adhesion protein 1 in hypertensive patients. J Am Soc Hypertens 2015; 9: 855–864. [DOI] [PubMed] [Google Scholar]
- 12. Lin MS, Li HY, Wei JN, et al Serum vascular adhesion protein‐1 is higher in subjects with early stages of chronic kidney disease. Clin Biochem 2008; 41: 1362–1367. [DOI] [PubMed] [Google Scholar]
- 13. Murata M, Noda K, Fukuhara J, et al Soluble vascular adhesion protein‐1 accumulates in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2012; 53: 4055–4062. [DOI] [PubMed] [Google Scholar]
- 14. Weston CJ, Shepherd EL, Claridge LC, et al Vascular adhesion protein‐1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest 2015; 125: 501–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang YC, Li HY, Wei JN, et al Serum vascular adhesion protein‐1 level is higher in smokers than non‐smokers. Ann Hum Biol 2013; 40: 413–418. [DOI] [PubMed] [Google Scholar]
- 16. Anger T, Pohle FK, Kandler L, et al VAP‐1, Eotaxin3 and MIG as potential atherosclerotic triggers of severe calcified and stenotic human aortic valves: effects of statins. Exp Mol Pathol 2007; 83: 435–442. [DOI] [PubMed] [Google Scholar]
- 17. Xu HL, Garcia M, Testai F, et al Pharmacologic blockade of vascular adhesion protein‐1 lessens neurologic dysfunction in rats subjected to subarachnoid hemorrhage. Brain Res 2014; 1586: 83–89. [DOI] [PubMed] [Google Scholar]
- 18. Sun P, Sole M, Unzeta M. Involvement of SSAO/VAP‐1 in oxygen‐glucose deprivation‐mediated damage using the endothelial hSSAO/VAP‐1‐expressing cells as experimental model of cerebral ischemia. Cerebrovasc Dis 2014; 37: 171–180. [DOI] [PubMed] [Google Scholar]
- 19. Katagiri D, Hamasaki Y, Doi K, et al Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide‐sensitive amine oxidase inhibition. Kidney Int 2016; 89: 374–385. [DOI] [PubMed] [Google Scholar]
- 20. Lee WY, Salmi M, Kelly MM, et al Therapeutic advantage of anti‐VAP‐1 over anti‐alpha4 integrin antibody in concanavalin a‐induced hepatitis. Hepatology 2013; 58: 1413–1423. [DOI] [PubMed] [Google Scholar]
- 21. Foot JS, Yow TT, Schilter H, et al PXS‐4681A, a potent and selective mechanism‐based inhibitor of SSAO/VAP‐1 with anti‐inflammatory effects in vivo . J Pharmacol Exp Ther 2013; 347: 365–374. [DOI] [PubMed] [Google Scholar]
- 22. Dunkel P, Gelain A, Barlocco D, et al Semicarbazide‐sensitive amine oxidase/vascular adhesion protein 1: recent developments concerning substrates and inhibitors of a promising therapeutic target. Curr Med Chem 2008; 15: 1827–1839. [DOI] [PubMed] [Google Scholar]
- 23. Chiu YF, Chuang LM, Hsiao CF, et al An autosomal genome‐wide scan for loci linked to pre‐diabetic phenotypes in nondiabetic Chinese subjects from the Stanford Asia‐Pacific Program of Hypertension and Insulin Resistance Family Study. Diabetes 2005; 54: 1200–1206. [DOI] [PubMed] [Google Scholar]
- 24. Almasy L, Blangero J. Multipoint quantitative‐trait linkage analysis in general pedigrees. Am J Hum Genet 1998; 62: 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet 2000; 66: 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 1995; 11: 241–247. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Willer CJ, Ding J, et al MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 2010; 34: 816–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Y, Gao H, Li H, et al A meta‐analysis of genome‐wide association studies for adiponectin levels in East Asians identifies a novel locus near WDR11‐FGFR2. Hum Mol Genet 2014; 23: 1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenthal F, Feijs KL, Frugier E, et al Macrodomain‐containing proteins are new mono‐ADP‐ribosylhydrolases. Nat Struct Mol Biol 2013; 20: 502–507. [DOI] [PubMed] [Google Scholar]
- 30. Jankevicius G, Hassler M, Golia B, et al A family of macrodomain proteins reverses cellular mono‐ADP‐ribosylation. Nat Struct Mol Biol 2013; 20: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryu KW, Kim DS, Kraus WL. New facets in the regulation of gene expression by ADP‐ribosylation and poly(ADP‐ribose) polymerases. Chem Rev 2015; 115: 2453–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feijs KL, Verheugd P, Luscher B. Expanding functions of intracellular resident mono‐ADP‐ribosylation in cell physiology. FEBS J 2013; 280: 3519–3529. [DOI] [PubMed] [Google Scholar]
- 33. Butepage M, Eckei L, Verheugd P, et al Intracellular Mono‐ADP‐ribosylation in signaling and disease. Cells 2015; 4: 569–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feijs KL, Forst AH, Verheugd P, et al Macrodomain‐containing proteins: regulating new intracellular functions of mono(ADP‐ribosyl)ation. Nat Rev Mol Cell Biol 2013; 14: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slavin TP, Feng T, Schnell A, et al Two‐marker association tests yield new disease associations for coronary artery disease and hypertension. Hum Genet 2011; 130: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Obeidat M, Wain LV, Shrine N, et al A comprehensive evaluation of potential lung function associated genes in the SpiroMeta general population sample. PLoS One 2011; 6: e19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Julia A, Domenech E, Ricart E, et al A genome‐wide association study on a southern European population identifies a new Crohn's disease susceptibility locus at RBX1‐EP300. Gut 2013; 62: 1440–1445. [DOI] [PubMed] [Google Scholar]
- 38. Anney R, Klei L, Pinto D, et al A genome‐wide scan for common alleles affecting risk for autism. Hum Mol Genet 2010; 19: 4072–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lionel AC, Crosbie J, Barbosa N, et al Rare copy number variation discovery and cross‐disorder comparisons identify risk genes for ADHD. Sci Transl Med 2011; 3: 95ra75. [DOI] [PubMed] [Google Scholar]
- 40. Jahanshad N, Rajagopalan P, Hua X, et al Genome‐wide scan of healthy human connectome discovers SPON1 gene variant influencing dementia severity. Proc Natl Acad Sci USA 2013; 110: 4768–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujimoto A, Furuta M, Totoki Y, et al Whole‐genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet 2016; 48: 500–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Primers used for quantitative real‐time polymerase chain reaction.
Table S2 | Baseline characteristics and serum vascular adhesion protein‐1 level by sex.
Table S3 | Number of families by sibship size.
Table S4 | Numbers of female–female, male–male and female–male sib pairs.
Table S5 | Distribution of identity by descent at the linkage peak between phenotypically extremely discordant sibling pairs.
Table S6 | Association between serum vascular adhesion protein‐1 gene expression in human adipose tissue and metabolic phenotypes.


