Abstract
The aim of the present review was to clarify the association between obstructive sleep apnea (OSA) and type 2 diabetes, and discuss the therapeutic role of continuous positive airway pressure (CPAP) in type 2 diabetes. OSA patients are more likely than non‐OSA populations to develop type 2 diabetes, while more than half of type 2 diabetes patients suffer from OSA. Similar to Western countries, in the East Asian population, the association between these two disorders has also been reported. CPAP is the primary treatment for OSA, but the effect of CPAP on comorbid diabetes has not been established. CPAP improved glucose metabolism determined by the oral glucose tolerance test in OSA patients, and several studies have shown that CPAP improves insulin resistance, particularly in obese populations undergoing long‐term CPAP. Diabetes is associated with other sleep‐related manifestations as well, such as snoring and excessive daytime sleepiness. Snoring is associated with the development of diabetes, and excessive daytime sleepiness appears to modify insulin resistance. Well‐designed studies are required to clarify the therapeutic effect of CPAP on diabetes. As both diabetes and OSA lead to cardiovascular disease, clinicians and healthcare professionals should be aware of the association between diabetes and OSA, and should take CPAP and health‐related behaviors into consideration when treating patients with diabetes and/or OSA.
Keywords: Continuous positive airway pressure, Diabetes, Sleep apnea
Introduction
Cardiovascular disease is a shared clinical consequence of sleep‐disordered breathing and type 2 diabetes, and the prevalence of both is higher in older and/or more obese populations1.
Obstructive sleep apnea (OSA) is characterized by repeating partial or complete obstruction of the upper airway during sleep, which leads to cyclic episodes of hypoxemia and normoxemia, as well as reduction in intrathoracic pressure. These repeated episodes lead to various pathophysiological conditions, such as intermittent hypoxia, sleep restriction and sleep fragmentation, resulting in sympathetic neural activation, systemic inflammation, oxidative stress loading and changes in hormonal systems (Figure 1). Changes in hormonal systems include the activation of the hypothalamic–pituitary adrenal axis and changes in adipokine profiles, both of which usually lead to fat accumulation and obesity1. Using animal models, Zhang et al.2 and Xu et al.3 established that intermittent hypoxia causes insulin resistance to deteriorate. Conversely, several reports have shown that diabetes affects central respiratory control, and is thus thought to promote OSA. These mechanisms suggest that sleep‐disordered breathing and type 2 diabetes are associated, independent of aging and obesity.
Figure 1.
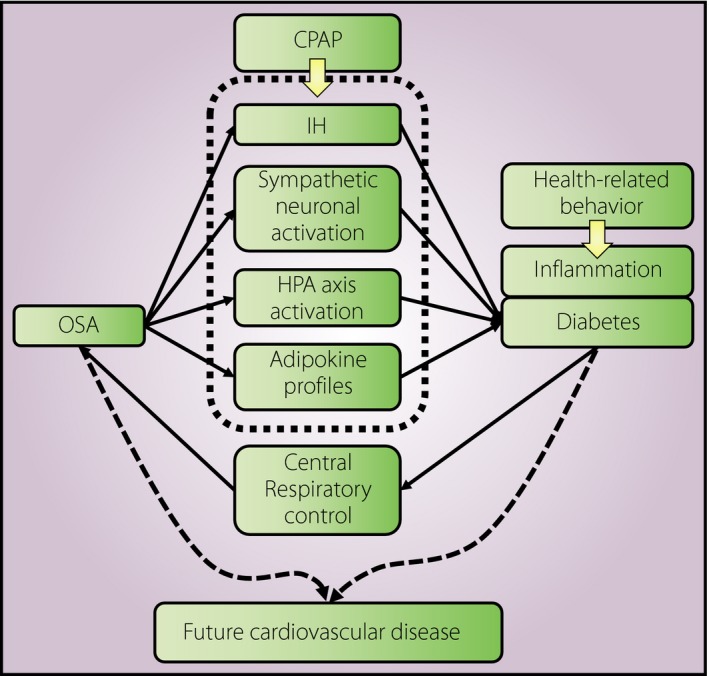
Pathophysiological pathway between obstructive sleep apnea and diabetes. CPAP, continuous positive airway pressure; HPA, hypothalamic–pituitary adrenal axis; IH, intermittent hypoxia; OSA, obstructive sleep apnea.
In the current review, we present and discuss the epidemiological data regarding the association between OSA and diabetes, and the effect of continuous positive airway pressure (CPAP) on diabetes.
Association between OSA and type 2 diabetes: evidence from observational studies
OSA and type 2 diabetes
The association between OSA and type 2 diabetes was examined either in clinical settings or in epidemiological cohorts. OSA severity was shown to be positively associated with the incidence of type 2 diabetes, independent of adiposity, during 12.8 years (median) of follow up in a subpopulation (n = 1,453) who were participants of both the Sleep Heart Health Studies (SHHS) and Atherosclerosis Risk in Communities study4. Multivariable adjusted hazard ratios (HR) and 95% confidence intervals (CI) of developing diabetes were 0.94 (0.70–1.27) for mild (5.0 ≤ apnea–hypopnea index [AHI] ≤ 14.9), 1.28 (0.89–1.84) for moderate (15.0 ≤ AHI ≤ 29.9) and 1.71 (1.08–2.71) for severe OSA (AHI ≥ 30), compared with a non‐OSA population (AHI < 5). The latest meta‐analysis of the SHHS–Atherosclerosis Risk in Communities study and nine other studies found that the crude and adjusted pooled relative risks were 1.62 (1.45–1.80) and 1.35 (1.27–1.47), respectively1. These 10 studies present convincing evidence that patients with OSA are more likely to develop type 2 diabetes, regardless of their age and body mass index (BMI) level.
The potential impact of OSA and well‐known risk factors, including adiposity, family history of diabetes and low physical activity, on diabetes risk have been compared, by determination of pooled estimates from different studies: the pooled relative risk of OSA in relation to type 2 diabetes is comparable with that of low physical activity, suggesting that treatment of OSA is crucial for diabetes prevention5.
OSA has also been recognized as a comorbidity of type 2 diabetes, because it is more prevalent in diabetes patients than in non‐diabetics. In the SHHS, a pooled study of multiple population‐based cohorts in the USA, the prevalence of mild OSA (5 ≤ AHI < 15) and moderate‐to‐severe OSA (AHI ≥ 15) was 33.9% and 23.8%, respectively, in patients with self‐reported diabetes, but 27.0% and 15.6% in non‐diabetics, respectively6. Several studies published after the SHHS also confirmed that OSA was more prevalent in diabetes patients, and overall OSA prevalence exceeded 50%. Furthermore, patients with more severe OSA were more likely to have diabetes1. These data clearly show that OSA and diabetes are associated, independent of aging and obesity.
Snoring and type 2 diabetes
Snoring is caused by airflow through a narrowing upper airway, and is considered as a surrogate marker of OSA7. The evidence is increasing that snoring alone is a strong predictor of end‐organ morbidity. In a meta‐analysis of cross‐sectional studies, the odds ratio (OR) and 95% CI of diabetes mellitus in snorers compared with non‐snorers was 1.37 (1.20–1.57), although it is likely there are also sex‐specific differences8.
Although snoring is easy to assess using self‐reports, only two prospective studies have shown an association between snoring and the incidence of type 2 diabetes9, 10. In the Nurses' Health Study of 69,852 USA women, the multivariable‐adjusted HR (95% CI) of developing type 2 diabetes was 1.41 (1.22–1.63) for occasional snorers and 2.03 (1.71–2.40) for habitual snorers9. Among 2,504 Swedish men, obese individuals with or without habitual snoring, but not non‐obese snorers, had a higher risk of type 2 diabetes compared with non‐obese and non‐snoring individuals10. The Cardiovascular Health Study showed that OSA symptoms, including daytime sleepiness, observed apnea and bothersome snoring, were associated with higher levels of fasting glucose, 2‐h glucose and lower insulin sensitivity, depending on the number of symptoms observed11. In addition, the number of OSA symptoms observed was linearly associated with incident type 2 diabetes, independent of known risk factors, such as BMI and waist circumference; the multivariable‐adjusted HRs (95% CI) of developing type 2 diabetes during the 10‐year study period were 1.34 (0.98–1.84) for those with a single OSA symptom, and 2.00 (1.35–2.98) for those with multiple OSA symptoms.
Questionnaire‐based snoring reports might have some limitations. For example, incomplete or incorrect data might be obtained from individuals who sleep alone, or whose cohabitants do not notice snoring behavior. Therefore, further studies investigating objective indices for snoring and the incidence of diabetes are essential.
Effects of CPAP therapy among non‐diabetic OSA patients: evidence from clinical trials
Glucose metabolism
Chin et al.12 provided the first evidence regarding the effect of CPAP on carbohydrate metabolism. Among 22 obese patients with severe OSA, although 6 months of CPAP treatment did not change fasting glucose levels, glucose levels during the oral glucose tolerance test (OGTT) decreased significantly. However, these changes were only significant in patients who experienced weight loss during the treatment period12, which suggests that the effect of CPAP therapy on glucose metabolism might be dependent on bodyweight. Henley et al.13 also showed that CPAP is effective in treating glucose tolerance, especially in obese patients with severe OSA; in a study comparing the OGTT response in 15 obese men with newly‐diagnosed severe OSA, fasting glucose levels, as well as the corresponding areas under the curve, were significantly reduced after CPAP treatment for 3 months. These data suggest that the therapeutic effects of CPAP on OSA‐accompanied type 2 diabetes is stronger in obese patients.
There is little evidence based on randomized controlled trials (RCTs) regarding the effect of CPAP on the fasting glucose levels of non‐diabetic OSA patients. In a randomized cross‐over trial (n = 50), 8‐week CPAP therapy did not significantly improve fasting and 2‐h post‐prandial glucose levels measured by OGTT14. No effect on fasting glucose levels after 1 week15 or 6 weeks of CPAP treatment16 has been shown. In addition, Sivam et al. found no differences in fasting glucose levels or the amount and distribution of subcutaneous and visceral fat in patients with moderate‐to‐severe OSA (n = 27) who were treated with CPAP for 8 weeks, compared with those who received sham CPAP17. These results suggested that longer CPAP use might be necessary to elicit a response. The effect of CPAP therapy on glucose metabolism appears after long‐term treatment, not during short‐term intervention, which was confirmed in the most recent trial by Hoyos et al.18, reporting that CPAP treatment for 12 weeks failed to improve fasting glucose levels in non‐diabetic men with moderate‐to‐severe OSA who did not receive CPAP (n = 65) compared with the control group.
As blood glucose levels have large within‐day and between‐day variation largely depending on dietary intake, the glycated hemoglobin (HbA1c) level reflecting the mean blood glucose levels in 2–3 months might be better to use as a long‐term and stable measure of glucose metabolism. Nevertheless, there is limited information available regarding the effect of CPAP on HbA1c in non‐diabetic OSA patients. Although some authors did not identify any such effect, other observational studies have detected some promising results. A pre–post comparing study19 showed that CPAP treatment for 3–5 months lowered HbA1c (mean ± standard deviation) from 6.47 ± 0.67% to 6.28 ± 0.51% (P = 0.038) in 12 patients with severe OSA. However, just six patients in that small study had HbA1c levels within the abnormal range, which could lead to underestimation of the true impact of CPAP treatment on such patients. Two further studies, with slightly larger sample sizes and longer follow‐up periods (6 months), confirmed that the effect of CPAP on HbA1c is restricted to patients with good CPAP adherence (≥4 h/night)20, 21.
Insulin resistance and sensitivity
Reducing insulin resistance is essential for improving glucose metabolism, so that glucose homeostasis can be maintained by comparatively lower insulin levels. However, the findings regarding the effect of CPAP were inconsistent on fasting insulin levels alone12, 13, 15, 16, 18, 22. The effect of CPAP on insulin resistance has been evaluated in observational studies using a hyperinsulinemic euglycemic clamp and the homeostatic model assessment (HOMA) index.
Although several observational studies aimed at examining the non‐glucose response, such as fat accumulation, endothelial function, lipid metabolism and appetite‐regulating hormones through several months of CPAP in OSA patients, none of these studies detected an effect of CPAP on insulin resistance23, 24, 25, 26. In a pre–post comparison within an individuals through 6 months of CPAP treatment, improvements in insulin resistance were not found, as well as changes in serum levels of leptin, adiponectin and resistin, but change in bodyweight was the main determinant of insulin resistance in obese OSA patients27. As weight gain during intervention was reported by some patients with increasing insulin resistance in that study, minimizing the impact of confounding factors is very important to examine the effect of CPAP therapy on insulin resistance.
Although these negative results existed, Harsch et al.28 first reported the beneficial effect of CPAP treatment on insulin sensitivity measured with the hyperinsulinemic euglycemic clamp, which is considered as the gold standard for the measurement of insulin sensitivity. In patients with moderate‐to‐severe OSA (n = 40), insulin sensitivity increased significantly after 2 days, and remained stable after 3 months of CPAP treatment28. In addition, a smaller improvement in insulin sensitivity was found among obese patients than among non‐obese patients, suggesting that obesity is also an important determinant of insulin resistance, similar to the previous studies with negative findings. In a recent study carried out by De Lima et al.29 using the HOMA index as an indicator of insulin resistance, CPAP treatment for 2 months reduced insulin resistance in moderate‐to‐severe OSA patients. Furthermore, they observed that the HOMA index was positively associated with AHI and superoxide production, and inversely associated with nitrite and nitrate levels, thus corroborating the role of oxidative stress in OSA‐induced increases in insulin resistance.
Other observational studies estimated the changes in insulin resistance in response to CPAP therapy and identified related factors. A population‐based investigation by Lindberg et al.22 of less‐symptomatic participants with an AHI of 10/h showed that the HOMA index was reduced after CPAP treatment for 3 weeks. Patruno et al.30 also emphasized the importance of optimal apnea–hypopnea reversion in improving insulin resistance: patients treated with the standard CPAP titration for 3 months not only achieved better levels of correction of OSA (as evaluated using the AHI) and oxygen desaturation index (ODI), but also showed a greater reduction in the HOMA index, than patients treated with auto‐adjusting CPAP.
Excessive daytime sleepiness (EDS) is an important symptom of OSA through sleep fragmentation, and might modify the impact of CPAP therapy on insulin resistance31, 32. Barceló et al.31 reported that CPAP treatment for 3 months reduced the HOMA index in OSA patients with EDS, but did not in those without. This finding possibly corroborates the importance of sleep fragmentation in the development of insulin resistance in OSA patients. In addition, sleep fragmentation is also associated with EDS32. Another case–control study concurs with these results33, but some cross‐sectional studies did not detect differences in metabolic abnormalities between patients with and without EDS34, 35. Therefore, further investigations are required to determine whether EDS itself is associated with insulin resistance independently of OSA.
Due to the well‐known limitations of observational studies, the effect of CPAP on insulin resistance in OSA patients without diabetes has also been investigated in RCTs. In a trial involving 34 patients with moderate‐to‐severe OSA treated for 6 weeks with therapeutic or sham CPAP16, the HOMA index did not significantly change in the therapeutic group (P = 0.08). The negative findings could be caused by the small sample size, the moderate level of CPAP adherence (3.9 h/night) and the low sensitivity to change of the HOMA index. Later, Lam et al.15 carried out a similar RCT with 61 men with moderate‐to‐severe OSA and no significant comorbidities who were given therapeutic or sham CPAP treatment. They found that good CPAP adherence (6.2 h/night) for a week improved insulin sensitivity, as evaluated using the short insulin tolerance test15. Furthermore, this effect persisted until 3 months later in patients with a BMI of 25–34.9 kg/m2.
Hoyos et al.18 reaffirmed that prolonged treatment was required to achieve an effect on glucose metabolism. No effect of CPAP on the OGTT‐evaluated insulin sensitivity of moderate‐to‐severe OSA patients was detected after treatment for 12 weeks, whereas patients who continued treatment for another 3 months experienced improved OGTT‐evaluated insulin sensitivity, but no improvement in the HOMA index18. Similarly, data from the USA and Australia suggest that CPAP might only have a beneficial effect on glucose tolerance in individuals with severe OSA14, or on insulin sensitivity after prolonged utilization30.
In a hospital‐based trial recruiting 391 non‐EDS patients with OSA defined by ODI, auto‐adjusting CPAP for 6 months did not affect the HOMA index compared with standard care36. This trial might possibly be affected by very low CPAP adherence (2.9 h/night). Thus, the authors of this trial recommended careful interpretation of these results.
The studies carried out to date on the effect of CPAP on insulin resistance are severely limited due to the use of different diagnostic criteria and experimental designs. Furthermore, many of the clinical trials are not randomized or controlled, and it is thus difficult to draw conclusions from these studies. Therefore, analysis of the global information generated by a meta‐analysis allows for better interpretation.
An initial meta‐analysis of 12 studies (mostly observational and carried out with non‐diabetic OSA patients) showed that CPAP treatment was associated with a reduction in insulin resistance or the HOMA index in non‐diabetic OSA patients37, but did not affect fasting glucose levels or BMI. Another meta‐analysis, which included only observational studies, confirmed the potential benefits of CPAP treatment (8–24 weeks) on insulin resistance in patients with good CPAP adherence (≥4 h/night), but not in those with poor adherence38. This was confirmed by the most recent meta‐analysis, which included only RCTs39; from a total of five studies featuring 244 non‐diabetic OSA patients (83% men), the pooled estimate of the difference in mean (95% CI) HOMA index for therapeutic CPAP compared with sham CPAP or control groups was 20.4 (20.1–20.8; P = 0.02).
From both laboratory experiments and epidemiological data, although OSA is associated with the development of diabetes, CPAP, a primary treatment for OSA, might not significantly improve glucose metabolism and insulin resistance. This disparity might for several reasons. First, good adherence to CPAP therapy is a key factor to achieving the beneficial effect of CPAP therapy on glucose metabolism and insulin resistance, but it might be difficult to achieve such a good adherence for all OSA patients. Second, as obesity, lifestyle and dietary behaviors also contribute to glucose metabolism and insulin resistance (Figure 1), between‐measure variance in blood levels of glucose and insulin could become relatively large, and the effect of CPAP therapy might be less likely to be detectable in a small population. To confirm the impact of OSA on diabetes, further investigations are required.
Impact of OSA on glucose metabolism and insulin resistance in Asian populations
Here, we have reviewed publications regarding the association between OSA and diabetes. Meta‐analysis showed that diabetes is more prevalent among OSA patients, and systematic review showed that more than half of diabetes patients suffer from OSA. A common concern regarding these analyses is the effect of ethnicity‐dependent differences in physical constitution, lifestyle and possibly metabolism. As most of the data reviewed in the present review originated from studies in Western countries, we also focused on some studies carried out in Japan or other Asian nations. One East Asian study was included in the meta‐analysis1, but there are several others that we have not mentioned. For example, a Japanese cross‐sectional study of 536 men showed that mild OSA, but not moderate or severe OSA, was associated with a higher prevalence of impaired glucose metabolism, with ORs (95% CI) for impaired glucose metabolism of 2.9 (1.80–4.60), 1.2 (0.72–2.10) and 1.58 (0.80–3.00), respectively40.
The Circulatory Risk in Communities Study investigated the association between nocturnal intermittent hypoxia as a surrogate marker of OSA and risk of type 2 diabetes in community residents aged 40–69 years (n = 4,398). After a median (interquartile range) follow‐up period of 3.0 years (2.9–4.0 years), 210 participants developed diabetes, and the multivariable‐adjusted HR (95% CI) for developing type 2 diabetes was 1.26 (0.91–1.76) among those with mild nocturnal intermittent hypoxia, and 1.69 (1.04–2.76) among those with moderate‐to‐severe nocturnal intermittent hypoxia (P = 0.03 for trend)41.
In a study carried out in Toon, Japan, the adjusted ORs (95% CI) for impaired fasting glucose, glucose intolerance and diabetes for participants with ODI ≥15.0 events/h were 1.27 (0.72–2.23), 1.69 (1.03–2.76) and 1.28 (0.59–2.79), respectively, compared with participants with ODI <5 events/h. The HOMA index and Matsuda index, both of which reflect insulin tolerance, were significantly associated with the severity of sleep‐related intermittent hypoxemia, as assessed using ODI (P = 0.03 and P = 0.007, respectively)42.
The association between OSA and diabetes has also been investigated in South Korea, where it was found that habitual snorers showed no increase in baseline fasting glucose or insulin levels, but significant increases in both at 1 and 2 h in the OGTT43. Habitual snoring was associated with higher HbA1c, even in non‐obese adults44. More precisely, the study found that the ORs (95% CI) of non‐obese patients with moderate‐to‐severe OSA (AHI ≥ 5.0) were 3.15 (1.44–6.90) and 2.24 (1.43–3.50) for impaired glucose tolerance and impaired insulin tolerance, and diabetes, respectively45. These data suggest that the association between OSA and diabetes is also present in residents of East Asia, including Japan and South Korea.
The population attributable fraction (PAF), defined by formula (i) assigning RRi with a relative risk for group i compared with the reference group and pi with the proportion of cases in group i to overall cases, is an indicator estimating the contributing effect of exposure on outcome for an overall population46.
| (1) |
To evaluate the contributing effect of OSA on type 2 diabetes in the Japanese population, we calculated the PAF of 3% ODI levels in relation to incident type 2 diabetes (Figure 2)41. In total, the PAF of OSA for diabetes development is 11.5% (3% ODI ≥5 events/h), suggesting that if OSA were successfully treated in the population, 11.5% of diabetes cases could be prevented.
Figure 2.
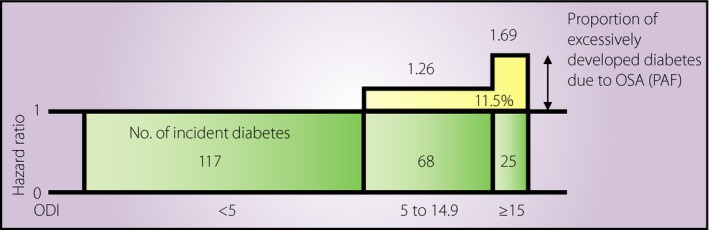
Population attributable fraction of obstructive sleep apnea in relation to incident diabetes. Population attributable fraction (PAF) was calculated using data from the Circulatory Risk in Communities Study41. ODI, oxygen desaturation index.
Conclusion
In conclusion, both disorders together are a risk for future cardiovascular disease, such as stroke, in which thrombosis is a crucial factor in pathogenesis. In Japan, it has been reported that OSA is also associated with atrial fibrillation, which potentiates a cerebral or cardiac infarction47. All clinicians, including those in Asian countries, should thus be aware of the clinical relevance of OSA and diabetes, and of appropriate approaches for diagnosis and therapy. Although CPAP therapy for OSA patients might not prevent diabetes or improve glucose metabolism, to prevent diabetes and subsequent cardiovascular disease, we need to consider improving sleep quality and quantity through OSA treatment in addition to promoting healthy behaviors, such as exercise, diet and smoking cessation.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
Authors declared no funding source.
J Diabetes Investig 2018; 9: 991–997
References
- 1. Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest May2017; 152: 1070–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang SX, Khalyfa A, Wang Y, et al Sleep fragmentation promotes NADPH oxidase 2‐mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes (Lond) 2014; 38: 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu J, Long YS, Gozal D, et al Beta‐cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med 2009; 46: 783–790. [DOI] [PubMed] [Google Scholar]
- 4. Nagayoshi M, Punjabi NM, Selvin E, et al Obstructive sleep apnea and incident type 2 diabetes. Sleep Med 2016; 25: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anothaisintawee T, Reutrakul S, Van Cauter E, et al Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta‐analysis. Sleep Med Rev 2016; 30: 11–24. [DOI] [PubMed] [Google Scholar]
- 6. Resnick HE, Redline S, Shahar E, et al Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care 2003; 26: 702–709. [DOI] [PubMed] [Google Scholar]
- 7. Nakano H, Furukawa T, Nishima S. Relationship between snoring sound intensity and sleepiness in patients with obstructive sleep apnea. J Clin Sleep Med 2008; 4: 551–556. [PMC free article] [PubMed] [Google Scholar]
- 8. Xiong X, Zhong A, Xu H, et al Association between self‐reported habitual snoring and diabetes mellitus: a systemic review and meta‐analysis. J Diabetes Res 2016; 2016: 1958981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al‐Delaimy WK, Manson JE, Willett WC, et al Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol 2002; 155: 387–393. [DOI] [PubMed] [Google Scholar]
- 10. Elmasry A, Janson C, Lindberg E, et al The role of habitual snoring and obesity in the development of diabetes: a 10‐year follow‐up study in a male population. J Intern Med 2000; 248: 13–20. [DOI] [PubMed] [Google Scholar]
- 11. Strand LB, Carnethon M, Biggs ML, et al Sleep disturbances and glucose metabolism in older adults: the cardiovascular health study. Diabetes Care 2015; 38: 2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chin K, Shimizu K, Nakamura T, et al Changes in intra‐abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 1999; 100: 706–712. [DOI] [PubMed] [Google Scholar]
- 13. Henley DE, Buchanan F, Gibson R, et al Plasma apelin levels in obstructive sleep apnea and the effect of continuous positive airway pressure therapy. J Endocrinol 2009; 203: 181–188. [DOI] [PubMed] [Google Scholar]
- 14. Weinstock TG, Wang X, Rueschman M, et al A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep 2012; 35: 617–625B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lam JC, Lam B, Yao TJ, et al A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J 2010; 35: 138–145. [DOI] [PubMed] [Google Scholar]
- 16. Coughlin SR, Mawdsley L, Mugarza JA, et al Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J 2007; 29: 720–727. [DOI] [PubMed] [Google Scholar]
- 17. Sivam S, Phillips CL, Trenell MI, et al Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J 2012; 40: 913–918. [DOI] [PubMed] [Google Scholar]
- 18. Hoyos CM, Killick R, Yee BJ, et al Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham‐controlled study. Thorax 2012; 67: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 19. Shpirer I, Rapoport MJ, Stav D, et al Normal and elevated HbA1C levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath 2012; 16: 461–466. [DOI] [PubMed] [Google Scholar]
- 20. Steiropoulos P, Papanas N, Nena E, et al Markers of glycemic control and insulin resistance in non‐diabetic patients with Obstructive Sleep Apnea Hypopnea Syndrome: does adherence to CPAP treatment improve glycemic control? Sleep Med 2009; 10: 887–891. [DOI] [PubMed] [Google Scholar]
- 21. Nena E, Steiropoulos P, Tzouvelekis A, et al Reduction of serum retinol‐binding protein‐4 levels in nondiabetic obstructive sleep apnea patients under continuous positive airway pressure treatment. Respiration 2010; 80: 517–523. [DOI] [PubMed] [Google Scholar]
- 22. Lindberg E, Berne C, Elmasry A, et al CPAP treatment of a population‐based sample–what are the benefits and the treatment compliance? Sleep Med 2006; 7: 553–560. [DOI] [PubMed] [Google Scholar]
- 23. Trenell MI, Ward JA, Yee BJ, et al Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007; 9: 679–687. [DOI] [PubMed] [Google Scholar]
- 24. Vgontzas AN, Zoumakis E, Bixler EO, et al Selective effects of CPAP on sleep apnoea‐associated manifestations. Eur J Clin Invest 2008; 38: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuhadaroğlu C, Utkusavaş A, Oztürk L, et al Effects of nasal CPAP treatment on insulin resistance, lipid profile, and plasma leptin in sleep apnea. Lung 2009; 187: 75–81. [DOI] [PubMed] [Google Scholar]
- 26. Chung S, Yoon IY, Lee CH, et al The effects of nasal continuous positive airway pressure on vascular functions and serum cardiovascular risk factors in obstructive sleep apnea syndrome. Sleep Breath 2011; 15: 71–76. [DOI] [PubMed] [Google Scholar]
- 27. Garcia JM, Sharafkhaneh H, Hirshkowitz M, et al Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir Res 2011; 12: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harsch IA, Schahin SP, Radespiel‐Tröger M, et al Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169: 156–162. [DOI] [PubMed] [Google Scholar]
- 29. de Lima AM, Franco CM, de Castro CM, et al Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respiration 2010; 79: 370–376. [DOI] [PubMed] [Google Scholar]
- 30. Patruno V, Aiolfi S, Costantino G, et al Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest 2007; 131: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 31. Barceló A, Barbé F, de la Peña M, et al Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax 2008; 63: 946–950. [DOI] [PubMed] [Google Scholar]
- 32. Cizza G, Piaggi P, Lucassen EA, et al Obstructive sleep apnea is a predictor of abnormal glucose metabolism in chronically sleep deprived obese adults. PLoS One 2013; 8: e65400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nena E, Steiropoulos P, Papanas N, et al Sleepiness as a marker of glucose deregulation in obstructive sleep apnea. Sleep Breath 2012; 16: 181–186. [DOI] [PubMed] [Google Scholar]
- 34. Drager LF, Bortolotto LA, Maki‐Nunes C, et al The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 2010; 208: 490–495. [DOI] [PubMed] [Google Scholar]
- 35. Bonsignore MR, Esquinas C, Barceló A, et al Metabolic syndrome, insulin resistance and sleepiness in real‐life obstructive sleep apnoea. Eur Respir J 2012; 39: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 36. Craig SE, Kohler M, Nicoll D, et al Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax 2012; 67: 1090–1096. [DOI] [PubMed] [Google Scholar]
- 37. Yang D, Liu Z, Yang H, et al Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: a meta‐analysis. Sleep Breath 2013; 17: 33–38. [DOI] [PubMed] [Google Scholar]
- 38. Yang D, Liu Z, Yang H. The impact of effective continuous positive airway pressure on homeostasis model assessment insulin resistance in non‐diabetic patients with moderate to severe obstructive sleep apnea. Diabetes Metab Res Rev 2012; 28: 499–504. [DOI] [PubMed] [Google Scholar]
- 39. Iftikhar IH, Khan MF, Das A, et al Meta‐analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Ann Am Thorac Soc 2013; 10: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kawada T, Katsumata M, Inagaki H, et al Sleep‐disordered breathing and disorders of glucose metabolism. Diabetes Metab Syndr 2017; 11: 189–191. [DOI] [PubMed] [Google Scholar]
- 41. Muraki I, Tanigawa T, Yamagishi K, et al Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS). Diabetologia 2010; 53: 481–488. [DOI] [PubMed] [Google Scholar]
- 42. Tanno S, Tanigawa T, Saito I, et al Sleep‐related intermittent hypoxemia and glucose intolerance: a community‐based study. Sleep Med 2014; 15: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 43. Shin C, Kim J, Kim J, et al Association of habitual snoring with glucose and insulin metabolism in nonobese Korean adult men. Am J Respir Crit Care Med 2005; 171: 287–291. [DOI] [PubMed] [Google Scholar]
- 44. Joo S, Lee S, Choi HA, et al Habitual snoring is associated with elevated hemoglobin A1c levels in non‐obese middle‐aged adults. J Sleep Res 2006; 15: 437–444. [DOI] [PubMed] [Google Scholar]
- 45. Kim NH, Cho NH, Yun CH, et al Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care 2013; 36: 3909–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flegal KM, Graubard BI, Williamson DF. Methods of calculating deaths attributable to obesity. Am J Epidemiol 2004; 160: 331–338. [DOI] [PubMed] [Google Scholar]
- 47. Tanigawa T, Yamagishi K, Sakurai S, et al Arterial oxygen desaturation during sleep and atrial fibrillation. Heart 2006; 92: 1854–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]


