Abstract
Aim/Introduction
Studies on a novel point‐of‐care device for nerve conduction study called DPNCheck have been limited to Westerners. We aimed to clarify Japanese normal limits of nerve action potential amplitude (Amp) and conduction velocity by DPNCheck (investigation I), and the validity of DPNCheck to identify diabetic symmetric sensorimotor polyneuropathy (DSPN; investigation II).
Materials and Methods
For investigation I, 463 non‐neuropathic Japanese participants underwent DPNCheck examinations. Regression formulas calculating the normal limits of Amp and conduction velocity (Japanese regression formulas [JRF]) were determined by quantile regression and then compared with regression formulas of individuals from the USA (USRF). For investigation II, in 92 Japanese diabetes patients, ‘probable DSPN’ was diagnosed and nerve conduction abnormalities (NCA1: one or more abnormalities, and NCA2: two abnormalities in Amp and conduction velocity) were determined. Validity of NCAs to identify ‘probable DSPN’ was evaluated by determining sensitivity, specificity, reproducibility (kappa‐coefficient) and the area under the curve of receiver operating characteristic curves.
Results
For investigation I, JRF was different from USRF, and normal limits by JRF were higher than that of USRF. The prevalence of Amp abnormality calculated by JRF was significantly higher than that of USRF. For investigation II, the sensitivity, specificity and reproducibility of NCA1 and NCA2 judged from JRF were 85%, 86% and 0.57, and 43%, 100% and 0.56, respectively. These values of JRF were higher than those of USRF. The area under the curve of JRF (0.89) was larger than USRF (0.82).
Conclusions
A significant difference in the normal limits of nerve conduction parameters by DPNCheck between Japanese and USA individuals was suggested. Validity to identify DSPN of NCAs might improve by changing the judgment criteria from USRF to JRF.
Keywords: Clinical, Complication I – nerve, Diagnosis and pathophysiology
Introduction
Diabetic symmetric sensorimotor polyneuropathy (DSPN) is the most common disorder of heterogeneous diabetic neuropathies1. Early and accurate diagnosis of DSPN is necessary to prevent its progression through appropriate management. Although the presence of DSPN is primarily confirmed by a nerve conduction study1, clinics where nerve conduction studies can be practiced have been limited to only specialized clinics because of the need for skilled laboratory technicians and expensive equipment. A novel point‐of‐care device (POCD) for nerve conduction study called NC‐stat®/DPNCheck™ (Neurometrix Inc, Waltham, MA, USA) has recently been developed. Using DPNCheck, the nerve action potential amplitude (Amp) and conduction velocity (CV) of the sural nerve can be semi‐automatically measured quickly and reliably at the bedside without the need of a skilled physician or laboratory technician. Two reports have evaluated the accuracy of the results by DPNCheck compared with the conventional nerve conduction method2, 3. Although acceptable accuracy in clinical use was indicated in both reports, overestimation of CV2 and underestimation of Amp3 were reported compared with conventional methods. Therefore, the results measured by DPNCheck should not be evaluated by the normal limit values obtained by conventional method. For this reason, the DPNCheck result sheet shows measurement values of Amp and CV, with the normal limits adjusted by age and height estimated from the normative database4 by the attached software. The database is also published on the Neurometrix website4. Normal limit values shown by DPNCheck are based on data from a USA population, mainly Westerners. There might be significant differences because of the differences in physique between the Japanese and USA population. Therefore, it is necessary to determine the normal limit values of Amp and CV in Japanese individuals, and compare them with the value of the USA individuals.
First, we aimed to clarify the normal limit values of Amp and CV in Japanese people, and compare them with the USA values (investigation I). Second, we aimed to compare the validity of diagnosing DSPN when using Japanese normal limit values compared with using the USA value (investigation II).
Methods
Ethics statement
These protocol and consent procedures were carried out in accordance with the World Medical Association's Helsinki Declaration and were approved by the Ethics Board of the Wakayama Medical University (Approval number 92, 468). All participants provided written informed consent.
Investigation I: determinations of normal limit values in Japanese individuals
Research design and participants
The present study was designed to determine the Japanese normal limit values of Amp and CV measured by DPNCheck. The participants were restricted to those among the residential examinees of the regional health examination considered to clinically have no peripheral neuropathy. Eventually, the Japanese normal limit values were compared with the values of the USA participants described in the DPNCheck detabase4.
We selected 463 participants who were clinically diagnosed as having no peripheral neuropathy from 626 community medical screening program examinees. The absence of clinical peripheral neuropathy was confirmed by the following exclusion criteria: (i) positive history for clinical cerebral infarction sequelae, renal failure, diabetes, hypothyroidism or alcoholism; (ii) positive symptoms of numbness, pricking sensation or pain in the legs/feet/toes; and (iii) bilaterally abnormal Achilles tendon reflex.
Demographics data (sex, age, height, weight and body mass index) of the Japanese participants were obtained by physical examinations. Demographic data of the USA participants described in the database of the DPNCheck were provided with permission.
Evaluation of neurological functions
Subjective symptoms of peripheral neuropathy were determined by asking whether there were any positive symptoms (numbness, pricking sensation, pain) in the legs/feet/toes by interview. Achilles tendon reflexes on both sides were examined in the knee standing position.
Amp and CV of the bilateral sural nerve were measured by DPNCheck according to the test manual5 by clinical laboratory technicians trained in advance. The average values of the left and right were used for statistical analyses. Seven participants refused the examination of both sides because of a feeling of discomfort while being tested. In these cases, measured values on the tested side were represented as averages.
In order to verify the signs of symmetric decrease of distal sensation at lower limbs, quantitative vibration threshold (VT) at 125 Hz was assessed in both big toe tips using a vibratory sensation meter (AU‐02B™; RION Inc., Tokyo, Japan). The method of VT measurement was described previously6.
Statistical analysis
In the present study, we used the same statistical methods as the DPNCheck normative database4. The cut‐off value of the normal limit of Amp/CV and VT were defined as the 5th‐ and 95th percentile values, respectively. To determine the cut‐off values, the multivariate quantile regression analysis (quantile regression analysis) was used. In this method, the normal limit cut‐off value is expressed as a linear function with demographic variables as follows: normal limit = K + C 1 V 1 + C 2 V 2 + … + C n V n. In this regression formula, K is a constant, V i is the i th demographic variable and C i is the coefficient related to the i th demographic variable. First, demographic variables on which nerve functions (Amp, CV and VT) depend significantly (P < 0.05) were selected from sex, age, height, weight and BMI by forward stepwise analysis. Then, the first quantile regression analysis using selected variables was carried out to determine the variables on which normal limit values of nerve functions significantly depend. Finally, the determined variables were adopted to the second quantile regression analysis, and the constant (K) and the coefficient (C) of the regression formula showing the normal limit value for each of the examinations (Amp, CV and VT) were calculated. The precision of the resulting normal limits was assessed by calculating both point estimates and 95% confidence intervals for representative demographics using the bootstrap method with 100,000 random samples.
The obtained regression formulas showing the cut‐off value for the normal limit of nerve function tests in Japanese participants (Japanese regression formulas [JRF]) were compared with the regression formulas of USA participants (USRF) described in the DPNCheck database4.
Statistical analyses were carried out with JMP (SAS Institute, Cary, NC, USA).
Investigation II: validity of nerve conduction abnormality detection using DPNCheck for diagnosing DSPN
Research design and participants
Nerve conduction abnormality (NCA) was defined based on Amp and CV measurements by DPNCheck. In Japanese diabetes patients, the validity of NCA judged by JRF to detect clinical DSPN was examined. Likewise, the validity of NCA determined by USRF to detect clinical DSPN was also examined. After that, we determined whether the JRF or the USRF were superior in reproducibility, sensitivity and specificity.
A total of 92 outpatients with diabetes who received medical interviews, physical examinations and nerve function tests (Amp, CV and VT) were used as participants. The patients who had a positive history for clinical cerebral infarction sequela, renal failure, hypothyroidism or alcoholism were excluded. Of the 92 diabetes patients, 62 were chosen from the examinees receiving diabetes therapy from the regional medical examination for investigation I, and 30 were selected from outpatients of Wakayama Medical University Hospital, Wakayama, Japan.
Diagnosis of clinical DSPN
DSPN was diagnosed according to the latest international consensus on diabetic neuropathies, as reported by the Toronto diabetic neuropathy expert group (Toronto Consensus)1. In the consensus, three characteristic clinical symptoms/signs for DSPN were proposed as follows: (i) positive neuropathic sensory symptoms (e.g., ‘asleep numbness,’ prickling or stabbing, burning or aching pain in the toes, feet or legs); (ii) sign of symmetric decrease of distal sensation; and (iii) unequivocally decreased or absent ankle reflexes. Then, DSPN was categorized into four stepwise criteria according to the presence of above symptoms/signs: ‘possible DSPN’: one symptom/sign; ‘probable DSPN’: two or more symptoms/signs; ‘Confirmed DSPN’: one or more symptoms/signs with nerve conduction abnormality (abnormal small fiber neuropathy: small fiber neuropathy can be substituted); and ‘subclinical DSPN’: nerve conduction abnormality or small fiber neuropathy without any symptoms/signs. The consensus has recommended that ‘probable DSPN’ definitions be used for clinical practice.
In the present study, we diagnosed DSPN based on the modified criterion of ‘probable DSPN.’ Modifications were made to increase accuracy and objectivity. Specifically, the bilateral abnormal VT judged by the regression formula for the normal limit of investigation I was adopted as a sign of symmetric decrease of distal sensation in the lower extremities.
Comparison of the validity of diagnosing ‘Probable DSPN’ between when using Japanese normal limit values compared with using USA values
The validity of NCA for diagnosing ‘Probable DSPN’ was evaluated by determining the sensitivity, specificity and reproducibility to predict the presence or absence of ‘Probable DSPN.’ We used two NCA criteria (one or more abnormal value of Amp and CV: NCA1; two abnormal values of both: NCA2), and those NCAs were judged from two regression formulas (JRF and USRF). Sensitivity, specificity and reproducibility of each of the NCAs were compared to assess the superiority in diagnosis of DSPN when compared with each other. Furthermore, the values of sensitivity, 1‐specificity of NCA1 and NCA2 judged by JRF and USRF were plotted in receiver operating characteristic curves, and the areas under the curves (AUC) were calculated.
Reproducibility was assessed by the Cohen's kappa coefficient. Statistical analyses were carried out by using the statistical software Excel statistics (Ekuseru‐Toukei 2010; Social Survey Research Information Co., Ltd., Tokyo, Japan).
Results
Investigation I: determinations of normal limit values in Japanese individuals
Characteristics of the participants
Demographics data (sex, age, height, and weight and body mass index) of the Japanese and USA participants are shown in Table 1. Japanese participants were significantly older, of a shorter height and less obese compared with the USA participants.
Table 1.
Comparison of demographics and nerve function data (nerve action potential amplitude, conduction velocity and vibration threshold) between Japanese and USA participants
| Japanese participants | USA participants | P‐value | |
|---|---|---|---|
| Mean ± SD (n) | Mean ± SD (n) | ||
| Demographics data | |||
| Sex (male/female) | 186/277 | 257/270 | 0.0067 |
| Age (years) | 60.8 ± 9.8 (463) | 48.3 ± 18.5 (527) | <0.0001 |
| Height (cm) | 160.3 ± 8.5 (463) | 167 ± 11.5 (527) | <0.0001 |
| Weight (kg) | 57.7 ± 10.6 (463) | 73.1 ± 16.8 (527) | <0.0001 |
| Body mass index (m/kg2) | 22.3 ± 3.1 (463) | 26.0 ± 4.09 (527) | <0.0001 |
| Nerve function data | |||
| Amp (μV) | 15.2 ± 7.04 (463) | 16.9 ± 8.62 (527) | <0.0001 |
| CV (m/s) | 54.5 ± 4.23 (463) | 53.0 ± 5.17 (523) | <0.0001 |
| VT (dB) | 17.3 ± 7.8 (463) | NE | |
Data of USA participants were provided from DPNCheck normative database (reference 4) with the manufacturer's permission. The unpaired t‐test and χ2‐test were used for statistical analysis. Amp, nerve action potential amplitude; CV, conduction velocity; NE, not examined; SD, standard deviation; VT, vibration threshold.
Demographic variables on which the normal limit values of Amp, CV, VT depend
The results of the first quantile regression analyses after forward stepwise analyses are shown in Table 2. The demographic variable on which normal limit values of Amp depend significantly was age. Demographic variables on which normal limit values of CV depend significantly were age and height. Similarly, the demographic variable on which normal limit values of VT depend significantly was age. There was no difference in the variables showing significant dependency between the data of the present study and the USA database.
Table 2.
Statistical data obtained by of the first and second multivariate quantile regression analyses (quantile regression analyses)
| Results of the first quantile regression analyses after forward stepwise analyses verifying dependency of Amp, CV, VT on demographic variables | ||||||
|---|---|---|---|---|---|---|
| Demographic variables | ||||||
| Sex (female) | Age | Height | Weight | Body mass index | ||
| Nerve function tests | Constant | Regression coefficient P‐value [95% CI] | Regression coefficient P‐value [95% CI] | Regression coefficient P‐value [95% CI] | Regression coefficient P‐value [95% CI] | Regression coefficient P‐value [95% CI] |
| Amp | 15.87 | – |
−0.095 <0.001 [−0.156, −0.038]* |
– | – |
−0.158 0.097 [−0.344, 0.028] |
| CV | 85.90 |
1.146 0.389 [−1.459, 3.751] |
−0.112 0.020 [−0.206, −0.017]* |
−0.224 0.007 [−0.390, −0.059]* |
0.064 0.244 [−0.044, 0.173] |
– |
| VT | −35.25 | – |
0.487 <0.001 [0.350, 0.625]* |
0.208 0.059 [0.050, 0.365] |
– | – |
| Results of the second quantile regression analysis and the regression formulas representing the cut‐off value of normal limit of nerve function tests | ||||||
|---|---|---|---|---|---|---|
| Age | Height | |||||
| Nerve function tests | Constant |
Regression coefficient P‐value [95% CI] |
Regression coefficient P‐value [95% CI] |
Regression formulas representing the normal limit | ||
| Amp | 12.62 |
−0.103 <0.001 [−0.162, −0.044]* |
Amp = 12.62 – 0.103 × age (years) | |||
| CV | 94.88 |
−0.148 0.016 [−0.269, −0.027]* |
−0.231 0.001 [−0.369, −0.092]* |
CV = 94.88 – 0.148 × age (years) – 0.231 × height (cm) | ||
| VT | 4.615 |
0.385 <0.001[0.224, 0.545]* |
VT = 4.615 + 0.385 × age (years) | |||
*Statistically significant values. –, Excluded by forward stepwise analyses; Amp, amplitude of action potential of sural nerve; CI, confidence interval; CV, conduction velocity of sural nerve; VT, vibratory perception threshold.
Determination of normal limit of Amp/CV and VT
The constants and coefficients of the regression formulas representing the cut‐off value of the normal limit of Amp, CV and VT, which were calculated by the second (final) quantile regression method, are shown in Table 2. The regression formulas themselves are also shown in Table 2. As results, regression formulas representing the cut‐off value of the normal limit of each nerve function test were as follows: Amp = 12.62 – 0.103 × age (years), CV = 94.88 – 0.148 × age (years) – 0.231 × height (cm), VT = 4.615 + 0.385 × age (years). In contrast, the regression formulas Amp and CV of USA participants described in the DPNCheck database4 were as follows: Amp = 11.2 – 0.099 × age (years), CV = 88.5 – 0.13 × age (years) – 0.20 × height (cm). There were differences between the regression formulas of Japanese participants (JRF) and that of US participants (USRF).
Figure 1a shows the relationship between Amp and age. Solid and broken lines show the age‐dependent normal limit calculated by JRF and USRF, respectively. The regression line of JRF is clearly higher than that of USRF. Figure 1b shows the relationship between CV and age. Solid and dotted lines show the age dependent normal limit at the height of 155 cm calculated by JRF and USRF, respectively. In the same way, the double line and broken lines show the age‐dependent normal limit at the height of 165 cm calculated by JFR and USRF, respectively. The regression lines at the height of 155 cm are clearly higher than that of 165 cm. Especially at a younger age, the regression lines by JRF are somewhat higher than those by USRF.
Figure 1.
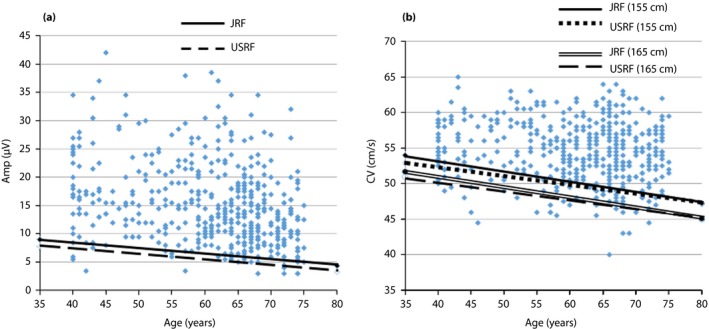
The relationships between (a) nerve action potential amplitude (Amp) and age, and (b) conduction velocity (CV) and age are shown. Details are described in the main text. JRF, Japanese regression formula; USRF, USA regression formula.
The prevalence of Amp abnormal values in the 463 Japanese participants (919 limbs) judged by JRF and USFR were 63 out of 919 (6.6%) and 48 out of 919 (3.8%), respectively. Similarly, the prevalence of CV abnormal values in the 463 Japanese participants (919 limbs) judged by JRF and USFR were 74 out of 919 (8.1%) and 65 out of 919 (7.1%), respectively. Although the prevalence of Amp abnormality calculated in JRF was significantly higher than that in USRF (χ2 = 8.46, P = 0.0036), the prevalence of CV in JRF was not different compared with that of USRF (χ2 = 0.63, P = 0.4272).
Investigation II: validity of NCA detection using DPNCheck for diagnosing ‘Probable DSPN’
Clinical characteristics and averaged nerve function data is shown in Table 3. The diabetes patients were relatively elderly and had good glycemic (average age 65.7 years, average glycated hemoglobin 7.04%). The Amp, CV and VT of diabetes patients were significantly worse than those of normal Japanese individuals in Table 1. We used the unpaired t‐test for statistical analysis (actual data is not shown).
Table 3.
Clinical characteristics, nerve function data and statistical data in 92 Japanese diabetes patients
| Clinical characteristics and averaged nerve function data | Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Sex (male/female) | 59/33 | |||||
| Age (years) | 65.7 ± 7.0 | |||||
| Height (cm) | 161.7 ± 9.15 | |||||
| Weight (kg) | 63.8 ± 13.8 | |||||
| Body mass index (m/kg2) | 24.2 ± 3.97 | |||||
| HbA1c (NGSP) (%) | 7.04 ± 1.13 | |||||
| Amp (μV) | 10.8 ± 5.46 | |||||
| CV (m/s) | 50.8 ± 5.29 | |||||
| VT (dB) | 22.0 ± 8.6 | |||||
| Prevalence of neurological symptoms and signs, ‘probable DSPN’ | ||||||
| Positive neuropathic sensory symptoms | 19/92 (20.7%) | |||||
| Unequivocally decreased or absent ankle reflexes | 32/92 (34.8%) | |||||
| Symmetric decrease of distal sensation (abnormal VT) | 10/92 (10.9%) | |||||
| Probable diabetic symmetrical sensorimotor polyneuropathy (‘probable DSPN’) | 14/92 (15.2%) | |||||
| Prevalence of nerve conduction abnormalities (NCA1, NCA2) judged by JRF or USRF | ||||||
| JRF | USRF | P‐values | ||||
| Bilaterally abnormal Amp | 14/92 (15.2%) | 8/92 (8.70%) | 0.1728 | |||
| Bilaterally abnormal CV | 15/92 (16.3%) | 14/92 (15.2%) | 0.8397 | |||
| NCA1 (one or more abnormal value of Amp and CV) | 23/92 (25.0%) | 18/92 (19.6%) | 0.3757 | |||
| NCA2 (two abnormal values of Amp and CV | 6/92 (6.50%) | 4/92 (4.35%) | 0.5154 | |||
| Sensitivity, specificity and reproducibility of the predictively of diagnosing ‘probable DSPN’ by the each NCAs based on JRF or USRF | ||||||
| RF | USRF | |||||
| Sensitivity | Specificity | Kappa coefficient (P‐values) | Sensitivity | Specificity | Kappa coefficient (P‐values) | |
| NCA1 | 85% | 86% | 0.57 (<0.001) | 71% | 90% | 0.55 (<0.001) |
| NCA2 | 43% | 100% | 0.56 (<0.001) | 29% | 100% | 0.40 (<0.001) |
The χ2‐test and Cohen's kappa coefficient method were used for statistical analysis. Nerve action potential amplitude of the sural nerve (Amp) and conduction velocity of the sural nerve (CV) were measured by a point‐of‐care nerve conduction device – DPNCheck®. VT, quantitative vibration threshold at 125 Hz at the big toe tips using the vibratory sensation meter (AU‐02B®). JRF, regression formulas representing the cut‐off value of normal limit in Japanese participants calculated by the quantile regression method; NGSP, National Glycohemoglobin Standardization Program; SD, standard deviation; USRF, regression formulas representing the cut‐off value of normal limit in USA participants.
Prevalence of ‘Probable DSPN’ and NCAs
The prevalence of positive neuropathic sensory symptoms, unequivocally decreased/absent ankle reflexes and symmetric decrease of distal sensation (abnormal VT) were 20.7, 34.8 and 10.9%, respectively. ‘Probable DSPN’ (two or more symptoms/signs) was observed in 14 out of 92 (15.2%). Asymptotic DSPN was four out of 14 (28.5%).
The prevalence of NCA1 (one or more abnormal value of Amp and CV) judged by JRF and USRF were 25.0 and 19.6%, respectively. Similarly, the prevalence of NCA2 (two abnormal values of Amp and CV) determined by JRF and USRF were 6.50 and 4.35%, respectively (Table 3).
Validity for diagnosing ‘Probable DSPN’ by NCAs judged from JRF and USRF
Sensitivity, specificity and Cohen's kappa coefficient (reproducibility) to identify ‘Probable DSPN’ of NCA1 judged from JRF or USRF were 85%, 86% and 0.57, and 71%, 90% and 0.55, respectively. In the same way, those values of NCA2 judged from JRF or USRF were 43%, 100% and 0.56, and 29%, 100% and 0.40, respectively. All values of sensitivity and kappa coefficient of both of NCA1 and NCA2 judged by JRF were higher than those values judged by USRF (Table 3).
The receiver operating characteristic curve showing the validity of NCAs assessed by DPNCheck to diagnose ‘Probable DSPN’ is shown in Figure 2. The solid line plots the sensitivity points ‘1‐specificity’ coordinate points of NCA1 (*) and NCA2 (#) determined by JRF. Similarly, the broken line plots the sensitivity points ‘1‐specificity’ coordinate points of NCA1 (&) and NCA2 ($) determined by USRF. The AUC of JRF (0.89) was larger than AUC of USRF (0.82).
Figure 2.
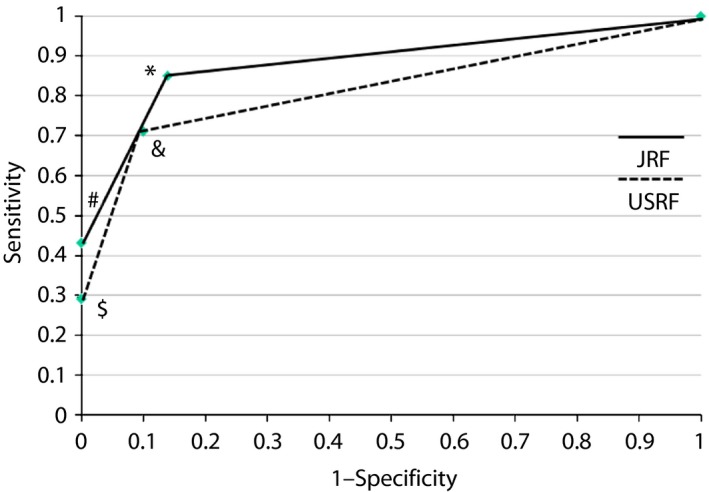
Receiver operating characteristic curve showing the validity of nerve conduction abnormalities (NCAs) assessed by DPNCheck to diagnose ‘probable DSPN.’ The solid line is a line connecting the sensitivity ‘1‐specificity’ coordinate points of NCA1 (*) and NCA2 (#) determined by the Japanese regression formula (JRF). Similarly, the broken line is a line connecting the sensitivity – ‘1‐specificity’ coordinate points of NCA1 (&) and NCA2 ($) determined by the USA regression formula (USRF). The area under the curve of the JRF (0.89) is larger than area under the curve of the USRF (0.82).
Discussion
Investigation I was carried out with Japanese participants who were supposed to have no peripheral neuropathy. The associated demographic factors with the Amp and CV measured by DPNCheck and the normal limits of them were determined. These were compared with the USA values. Two major findings were observed.
First, among demographic factors (age, sex, height, weight, body mass index), age showed a significant association with both AMP and CV, and height showed a significant association with CV. The associated demographic factors with nerve conduction parameters of Japanese participants did not differ from the database4 of USA participants. These findings confirm the deteriorating effect of aging on nerve conduction functions (Amp and CV), which is generally well known. Several studies reported the significant relationship between nerve conduction velocity and height in normal7 and diabetic8 Caucasian participants.
As nutritional metabolites to maintain axonal fibers are delivered from neurons through axonal transport, supply of nutritive metabolites to the terminal of the longest fibers is most challenging. Thus, impairment of metabolic processes essential to maintaining axonal integrity is thought to explain the length‐dependency of distal dominant nerve dysfunction. Mild impairment of metabolic processes is supposed to be caused by not only hyperglycemia, but also ischemia (arteriosclerosis), hypothermia and aging. The taller the stature, the longer the axonal fiber length, the tall persons might be more susceptible to distal axonal dysfunction, such as nerve conduction delay, than short stature persons. In our search scope, the present study is the first to show the relationship between conduction velocity and height in Japanese individuals (East Asian individuals).
Second, the normal limit of Amp calculated by JRFs was higher than that calculated by the USRF, and the prevalence of abnormal Amp values judged by JRF was significantly higher than that by USRF. In contrast, the normal limit of CV calculated by JRF was slightly higher than that calculated by USRF, but there was no significant difference between the prevalence of abnormal CV values judged by JRF and that by USRF. These findings suggest that the normal limit of nerve conduction parameters, especially Amp, of Japanese participants determined by DPNCheck might be different from that of USA participants. The difference of the normal limit of Amp between Japanese and US participants is supposed to be due to the degree of obesity. In obese people, the subcutaneous tissue is thicker than the thin tissue, the distance between the electrode of the nerve conduction device and the nerve beneath it becomes longer, and as a result, the action potential amplitude (Amp) is considered to decay. Although it is a children's study, the sural nerve SNAP in the obesity and insulin‐resistant group has been reported to be significantly lower than the normal control group9.
These findings suggest that there are differences that cannot be ignored in the normal limits of nerve conduction parameters measured by DPNCheck between the USA and Japanese participants. Therefore, it is considered necessary to compare the validity of diagnosing peripheral neuropathy using DPNCheck between the two regression formulas that calculate the normal lower limit of Japanese and USA participants (JRF and USRF).
In investigation II, we evaluated the validity of predicting ‘probable DSPN’ defined by the Toronto consensus1 according to the detection of NCAs by DPNCheck in Japanese diabetes patients. This investigation aimed to clarify whether the validity improves when changing the regression formula that decides NCAs from USRF to JRF. The validity of the predictability of diagnosing ‘probable DSPN’ by NCAs was assessed by the sensitivity, specificity and Cohen's kappa coefficient (reproducibility). Two major findings were observed.
First, all values of sensitivity and Cohen's kappa coefficient (reproducibility) to predict for diagnosing ‘probable DSPN’ of both of NCA1 and NCA2 judged from JRF were higher than those values judged from USRF. These findings might suggest that the validity to predict ‘probable DSPN’ by NCAs judged by JRF was superior to that judged by USRF. In particular, the sensitivity (85%), specificity (86%) and kappa coefficient (0.57) to predict ‘probable DSPN’ by NCA1 judged from JRF were very good. Previous studies have also reported good sensitivity and specificity for DPNCheck to identify diabetic polyneuropathy. Lee et al.2 reported the sensitivity and specificity were 95 and 71%, respectively. Perkins et al.10 reported the sensitivity and specificity were 88 and 82%, respectively.
Second, from the receiver operating characteristic curve analysis, the AUC of JRF (0.89) was larger than the AUC of USRF (0.82). This finding supports the validity of NCA1 judged by JRF. Lee et al.2 also reported results similar to the present study. In their study, the AUC calculated by the same method as the present study was 0.88 Therefore, NCA assessment using DPNCheck is thought to have excellent reliability and acceptable accuracy to identify diabetic polyneuropathy. The usefulness of NCA assessment using DPNCheck has been reported in peripheral neuropathy other than diabetic polyneuropathy, such as chemotherapy‐induced peripheral neuropathy11 and carpal tunnel syndrome12. Thus, this POCD for nerve conduction study might be expected to be widely used in many fields of clinical practice. The present study, showing the racial difference in the normal limits of this POCD, is considered important for accurate clinical diagnosis of peripheral neuropathy.
From all of our investigations, we could find a difference in the normal limit of nerve conduction parameters, especially Amp, between Japanese and USA participants. We could propose a Japanese formula calculating age and/or height adjusted normal limits of the nerve conduction parameters determined by the POCD (DPNCheck). We also would like to emphasize that the validity of identifying diabetic polyneuropathy might improve by changing the judgement criteria of nerve conduction abnormalities from USA to Japanese normal limits, which will lead to excellent reliability. The findings obtained in the present study might be applicable to East Asians individuals other than Japanese.
The advantage of our studies is that we set normal limits by an appropriate method called the quantile regression method13 using a sufficient number of non‐neuropathic individuals confirmed by the Achilles tendon reflex. Another advantage is that we modified the criteria of ‘probable DSPN’ by exchanging the sign of symmetric decrease of distal sensation in the legs to quantitative vibration sensation abnormality in order to improve objectivity.
One limitation of our studies was that the number of diabetes patients was not so large. Therefore, in order to confirm the improved accuracy to identify diabetic polyneuropathy using the POCD for nerve conduction study by using Japanese normal limits instead of USA normal limits, a larger‐scale study is required.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Professor Toshio Shimokawa for excellent advice on statistical analysis. We also thank the staff of the Divisions of Health and Welfare of Katsuragi town for their cooperation. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP15K01723.
J Diabetes Investig 2018; 9: 1173–1181
References
- 1. Tesfaye S, Malik RA, Boulton AJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee JA, Halpern EM, Lovblom LE, et al Reliability and validity of a point‐of‐care sural nerve conduction device for identification of diabetic neuropathy. PLoS One 2014; 9: e86515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perkins BA, Ngo M, Grewel J, et al Validation of a novel point‐of‐care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care 2006; 29: 2023–2027. [DOI] [PubMed] [Google Scholar]
- 4. Neurometrix . NC‐stat® DPNCheck™ Normative Database: Collection, Analysis and Recommended Normal Limits. Available from http://www.dpncheck.com/resources/Resources/nc-stat_dpncheck_normative_data_monograph_for_software_version_2_0_pn2203866_rev_a.pdf Accessed July 19, 2017.
- 5. Neurometrix . NC‐stat® DPNCheck™ User Manual. (2013). Available from http://www.dpncheck.com/resources/Resources/nc-stat_dpncheck_user_manual_pn2203282_rev_g.pdf Accessed July 18, 2017.
- 6. Matsuno S, Sasaki H, Yamasaki H, et al Pro198Leu missense polymorphism of the glutathione peroxidase 1 gene might be a common genetic predisposition of distal symmetric polyneuropathy and macrovascular disease in Japanese type 2 diabetic patients. J Diabetes Investig 2011; 2: 457–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trojaborg WT, Moon A, Andersen BB, et al Sural nerve conduction parameters in normal subjects related to age, gender, temperature, and height: a reappraisal. Muscle Nerve 1992; 15: 666–671. [DOI] [PubMed] [Google Scholar]
- 8. Gadia MT, Natori N, Ramos LB, et al Influence of height on quantitative sensory, nerve‐conduction, and clinical indices of diabetic peripheral neuropathy. Diabetes Care 1987; 10: 613–616. [DOI] [PubMed] [Google Scholar]
- 9. Akın O, Eker İ, Arslan M, et al Association of nerve conduction impairment and insulin resistance in children with obesity. Childs Nerv Syst 2016; 32: 2219–2224. [DOI] [PubMed] [Google Scholar]
- 10. Perkins BA, Ng E, Orszag A, et al Multi‐site testing with a point‐of‐care nerve conduction device can be used in an algorithm to diagnose diabetic sensorimotor polyneuropathy. Diabetes Care 2008; 31: 522–524. [DOI] [PubMed] [Google Scholar]
- 11. Matsuoka A, Mitsuma A, Maeda O, et al Quantitative assessment of chemotherapy‐induced peripheral neurotoxicity using a point‐of‐care nerve conduction device. Cancer Sci 2016; 107: 1453–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shepherd MM. Clinical outcomes of electrodiagnostic testing conducted in primary care. J Am Board Fam Med 2010; 23: 584–590. [DOI] [PubMed] [Google Scholar]
- 13. Peng L, Wuu J, Benatar M. Developing reference data for nerve conduction studies: an application of quantile regression. Muscle Nerve 2009; 40: 763–771. [DOI] [PubMed] [Google Scholar]


