Abstract
Aims/Introduction
The present multicenter, cross‐sectional survey was initiated to evaluate self‐monitoring of blood glucose (SMBG)‐associated mental distress among patients with diabetes.
Materials and Methods
The survey was carried out in patients with type 1 diabetes and type 2 diabetes using SMBG recruited from 42 medical institutions. Profiles of Mood States 2 and diabetes therapy‐related quality of life questionnaires were used to evaluate mood status and health‐related quality of life. Two original questionnaires were also developed to evaluate SMBG ‘importance,’ ‘painfulness’ and ‘confidence’ among patients, and to evaluate physician attitudes to SMBG use.
Results
Questionnaires from 517 type 1 diabetes and 1,648 type 2 diabetes patients showed that 46.0% of type 1 diabetes and 37.5% of type 2 diabetes patients reported ‘painfulness,’ and that these patients reporting ‘painfulness’ showed significantly higher Profiles of Mood States 2 scores, lower diabetes therapy‐related quality of life scores and higher glycated hemoglobin compared with those not reporting ‘painfulness,’ whereas the number of their daily SMBG tests were comparable. Patients reporting ‘painfulness’ also reported that SMBG use was significantly less important. Whether or not patients recognized the importance of SMBG use was well correlated with the frequency of physicians checking patient diaries.
Conclusions
Type 1 diabetes and type 2 diabetes patients reporting ‘painfulness’ in SMBG use had more mental distress, lower health‐related quality of life and higher glycated hemoglobin regardless of their number of daily SMBG tests. The importance of SMBG use was recognized less by patients experiencing pain, and the importance of SMBG use was recognized more in medical institutions in which physicians regularly checked SMBG diaries to provide meaningful feedback to patients in clinical settings.
Keywords: Education, Health‐related quality of life, Self‐monitoring of blood glucose
Introduction
Diabetes is one of the most serious global health‐related problems, and leads to life‐threatening complications when not properly managed1. Patients with diabetes should therefore receive integrated diabetes self‐management education as well as diabetes self‐management support, such that they can maintain appropriate glycemic control2. Self‐monitoring of blood glucose (SMBG) is widely recognized as a major component of integrated diabetes self‐management education. SMBG is established as a useful tool for guiding healthy diet and exercise, choosing antidiabetic drugs, and preventing hypoglycemia3. In particular, SMBG contributes to appropriate glycemic control among patients with type 1 diabetes and type 2 diabetes receiving insulin injections4, 5. However, SMBG burdens the patient with mental distress and lowers health‐related quality of life (QOL) in some cases6, 7, 8. Finger pricking‐associated pain is one of the most obvious burdens of SMBG use, although few studies have investigated this issue9. Despite recent improvement in lancing devices that reduce the pain of finger pricking, it remains a major burden of overall diabetes management. To clarify this and search for effective remedies, we carried out a multicenter, cross‐sectional survey among Japanese patients with diabetes and their physicians‐in‐charge.
Methods
Study design and participants
The present study was a multicenter, cross‐sectional survey carried out in 42 Japanese medical institutions between October 2016 and January 2017 (clinical trial registration number: UMIN000026761). The survey was carried out in accordance with the Declaration of Helsinki, and the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare of Japan. The protocol was approved by the Ethical Committees of the Japan Association for Diabetes Education and Care, as well as those of each participating institution. All personal information was anonymized before sending to the data managing center at Kansai Electric Power Medical Research Institute from each participating institution. Eligible patients included: (i) those with type 1 diabetes or type 2 diabetes aged ≥20 years and <90 years; (ii) those using SMBG for ≥3 months; (iii) those capable of answering the questionnaires used in the present study; and (iv) those receiving insulin and/or glucagon‐like peptide‐1 receptor agonist, as SMBG is allowed for those using injectables under the Japanese national health insurance coverage. Patients were excluded if they were susceptible to dementia and/or psychological and/or psychiatric disorders, or if they were considered to be ineligible for this study by physicians‐in‐charge.
Data collection and analysis
Clinical data, such as anthropometric measures, duration of diabetes, duration of insulin and/or glucagon‐like peptide‐1 receptor agonist use, glycated hemoglobin (HbA1c), frequency and timing of SMBG measurements, and therapeutic options for diabetes were collected with the questionnaire for the doctors. To evaluate mood status and health‐related QOL, study participants were asked to complete the Profiles of Mood States questionnaire, 2nd edition, Adult‐Short Japanese version (POMS2), which is an instrument for assessing the mood states of individuals aged ≥13 years; and the Diabetes Therapy‐Related QOL (DTR‐QOL) questionnaire, which is an instrument for assessing the influence of diabetes treatment on patient QOL regardless of treatment method10, 11. POMS2 consists of seven domains: Anger‐Hostility (AH), Confusion‐Bewilderment (CB), Depression‐Dejection (DD), Fatigue‐Inertia (FI), Tension‐Anxiety (TA), Vigor‐Activity (VA) and Friendliness (F). Total Mood Disturbance (TMD) was calculated according to the equation as follows: TMD = AH + CB + DD + FI + TA – VA. The T‐score of each domain and TMD was calculated from a T‐score conversion table10. A T‐score of 50 points represents the average T‐score for Japanese individuals. The DTR‐QOL consists of the following four categories: D1, ‘Burden on social activities and daily activities’; D2, ‘Anxiety and dissatisfaction with treatment’; D3, ‘Hypoglycemia’; and D4, ‘Satisfaction with treatment.’ Each score of the domain and the total score was converted to a scale of 0–100 as described elsewhere11. To evaluate attitudes of physicians and patients on SMBG and SMBG use, the participants also were asked to complete an original SMBG questionnaire developed in this study which asks four questions using a 5‐point Likert scale with responses ranging from ‘very unlikely’ (1) to ‘very likely’ (5): Q1 ‘How important is SMBG to you?’; Q2 ‘How painful is SMBG to you?’; Q3 ‘How confident are you to enter SMBG results correctly in your SMBG diary?’; and Q4 ‘Would you like to share your SMBG results with your physician?’. Physicians‐in‐charge were asked to complete another SMBG questionnaire developed in this study with four questions using a 5‐point Likert scale with responses ranging from ‘very unlikely’ (1) to ‘very likely’ (5): QD1 ‘How much do you credit SMBG meter readings with accuracy?’; QD2 ‘How much do you think SMBG readings reflect patients’ self‐management behavior?’; QD3 ‘How accurately do you think patients report SMBG readings?’; and QD4 ‘Do you check patients’ SMBG diary regularly?’. Our questionnaires allow linking each individuals only to medical institution and not to physicians; thus, scores of QD1 to QD4 are expressed as the average scores of physicians in each medical institution for analysis.
Statistical analysis
Data are expressed as the median (interquartile range) unless otherwise stated. To compare the scores obtained from the questionnaires between two groups (i.e., type 1 diabetes vs type 2 diabetes; group A vs group B; group C vs group D; and group E vs group F), data were analyzed with the Mann–Whitney U‐test. To assess the correlation between the scores obtained from Q1 to Q4 and from QD1 to QD4, Spearman's rank correlation coefficient was used. All statistical analyses were carried out using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P < 0.05.
Results
Valid responses were obtained from 2,253 patients. Of them, 52 patients with other diabetes types and 36 with unknown diabetes types were excluded from the current analysis. The remaining 517 type 1 diabetes and 1,648 type 2 diabetes patients were subjected to further analysis (Table 1). Figure 1 shows the results of POMS2 and DTR‐QOL questionnaires. All POMS2 scores in type 1 diabetes and type 2 diabetes did not differ substantially from those of general Japanese individuals11. There were no significant differences between type 1 diabetes and type 2 diabetes except in AH (P = 0.024) and TA (P = 0.003). All DTR‐QOL scores were comparable with previous studies carried out in Japanese type 1 diabetes and type 2 diabetes patients receiving insulin injections12, 13. Type 1 diabetes showed lower scores in D1, ‘Burden on social activities and daily activities’ (P < 0.001); D2, ‘Anxiety and dissatisfaction with treatment’ (P < 0.001); D3, ‘Hypoglycemia’ (P < 0.001); D4, ‘Satisfaction with treatment’ (P < 0.001); and total score (P < 0.001).
Table 1.
Characteristics of study participants
| Type 1 diabetes | Type 2 diabetes | |
|---|---|---|
| n (male/female) | 517 (234/282) | 1,648 (982/659) |
| Age (years) | 50 (40–65) | 66 (57–72) |
| BMI (kg/m2) | 22.4 (20.5–24.9) | 24.8 (22.3–27.8) |
| Duration of diabetes (years) | 13.5 (6.0–22.0) | 15.0 (10.0–23.0) |
| Duration of insulin use (years) | 12.0 (5.0–20.0) | 6.0 (3.0–11.0) |
| HbA1c (%) | 7.7 (7.0–8.4) | 7.4 (6.8–8.0) |
| Frequency of SMBG instructed by doctors (times/day) | 3 (3–4) | 2 (2–3) |
| Frequency of SMBG performed by patients (times/day) | 3 (2–4) | 2 (1–3) |
| Type of injection(s) | ||
| Basal and bolus combination, n (%) | 436 (84.3) | 484 (29.4) |
| Basal only, n (%) | 11 (2.1) | 418 (25.4) |
| Bolus only, n (%) | 23 (4.4) | 56 (3.4) |
| Mix, n (%) | 19 (3.7) | 306 (18.6) |
| Continuous subcutaneous insulin infusion, n (%) | 11 (2.1) | 0 (0.0) |
| GLP‐1 receptor agonist, basal and bolus combination, n (%) | 0 (0.0) | 32 (1.9) |
| GLP‐1 receptor agonist and basal combination, n (%) | 0 (0.0) | 147 (8.9) |
| GLP‐1 receptor agonist and bolus combination, n (%) | 0 (0.0) | 3 (0.2) |
| GLP‐1 receptor agonist and mix combination, n (%) | 0 (0.0) | 23 (1.4) |
| GLP‐1 receptor agonist, n (%) | 0 (0.0) | 157 (9.5) |
| Others | 17 (3.3) | 22 (1.3) |
Data are shown as the median (interquartile range). Others include patients whose type of injections were unknown. BMI, body mass index; GLP‐1, glucagon‐like peptide‐1; Hba1c, glycated hemoglobin; SMBG, self‐monitoring of blood glucose.
Figure 1.
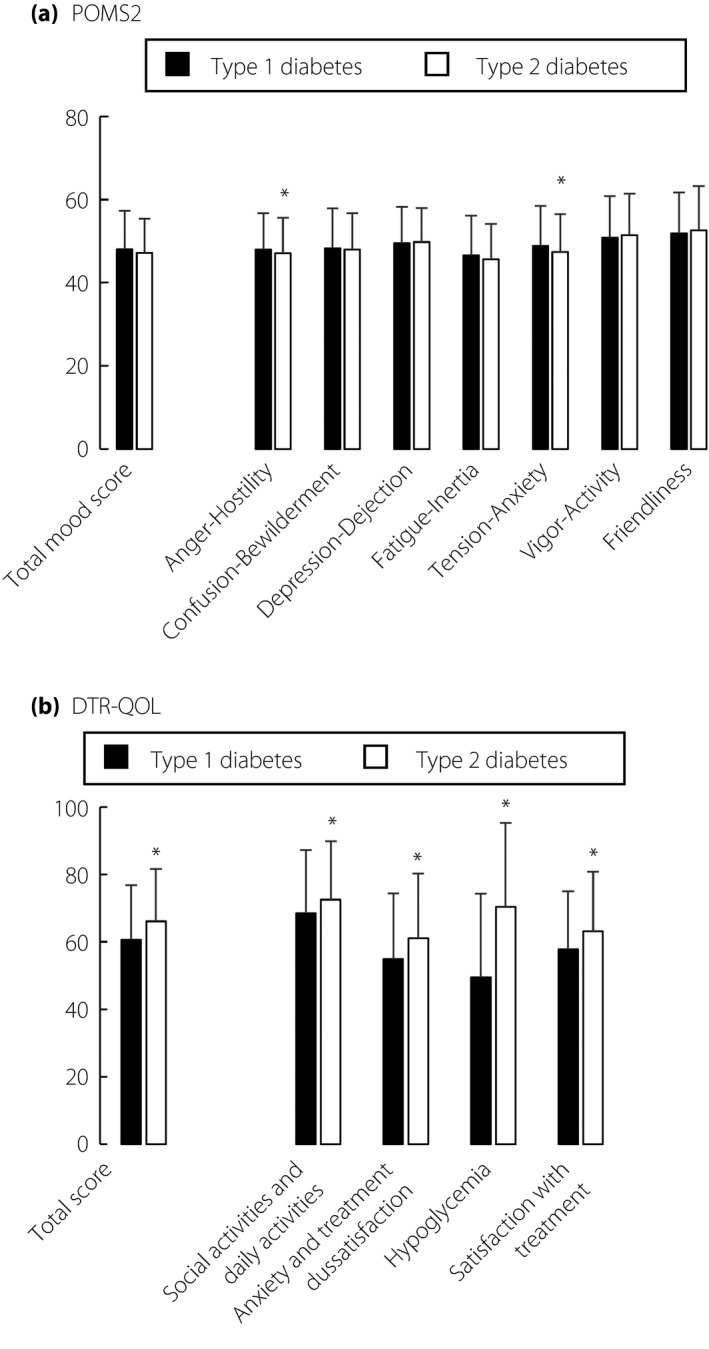
Results of (a) Profiles of Mood States questionnaire, 2nd edition, Adult‐Short Japanese version scores and (b) Diabetes Therapy‐Related Quality of Life scores in type 1 diabetes (black bar) and type 2 diabetes (white bar) patients are shown. Values are mean ± standard error of the mean. *P < 0.05 (vs type 1 diabetes).
Figure 2 shows the results of the SMBG questionnaire developed by the current study. In Q2 ‘How painful is SMBG to you?’, 39.8% of type 1 diabetes and 46.4% of type 2 diabetes patients responded ‘very unlikely’ or ‘unlikely,’ and 46.0% of type 1 diabetes and 37.5% of type 2 diabetes patients responded ‘likely’ or ‘very likely.’ To analyze possible associations of experience of pain with health‐related QOL, the results of POMS2 and DTR‐QOL were compared in two groups: group A, those responding ‘very unlikely’ and ‘unlikely,’ and group B, those responding ‘likely’ and ‘very likely’ in Q2 ‘How painful is SMBG to you?’. Group A showed a significantly lower POMS2 total mood disturbance score in type 1 diabetes and type 2 diabetes patients (Figure 3a). Group A also showed a higher DTR‐QOL total score in type 1 diabetes and type 2 diabetes patients. HbA1c was significantly lower in group A than in group B in both type 1 diabetes and type 2 diabetes patients (Figure 3c), suggesting that mood status and health‐related QOL affect glycemic control. Interestingly, the frequency of SMBG testing reported by patients did not differ between the two groups both in type 1 diabetes and type 2 diabetes (Figure 3d). Group A showed significantly higher scores in Q1 ‘How important is SMBG to you?’. compared with group B in both type 1 diabetes and type 2 diabetes patients (Figure 3e). These results suggest that SMBG‐associated pain might influence patient appreciation of the importance of SMBG use. In Q1 ‘How important is SMBG to you?’, 93.0% of type 1 diabetes and 84.8% of type 2 diabetes patients responded ‘likely’ or ‘very likely’ (group C), and 3.7% of type 1 diabetes and 6.7% of type 2 diabetes patients responded ‘very unlikely’ or ‘unlikely’ (group D). Group C showed lower scores in Q2 ‘How painful is SMBG to you?’ compared with group D in both type 1 diabetes and type 2 diabetes (Figure 3f), although the results of the POMS2 total score (group C 47.8 ± 9.1 and group D 49.7 ± 9.4, P = 0.3287 in type 1 diabetes patients, group C 47.1 ± 8.2 and group D 47.7 ± 8.4, P = 0.5010 in type 2 diabetes) and DTR‐QOL total score (group C 60.7 ± 16.2 and group D 61.0 ± 17.4, P = 0.7381 in type 1 diabetes patients, group C 67.7 ± 14.7 and group D 70.5 ± 17.3, P = 0.0551 in type 2 diabetes patients) were comparable in the two groups. In addition, scores in Q2 ‘How painful is SMBG to you?’ were weakly, but significantly, associated with scores in Q1 ‘How important is SMBG to you?’ (type 1 diabetes r = −0.165, P < 0.01; and type 2 diabetes r = −230, P < 0.01).
Figure 2.
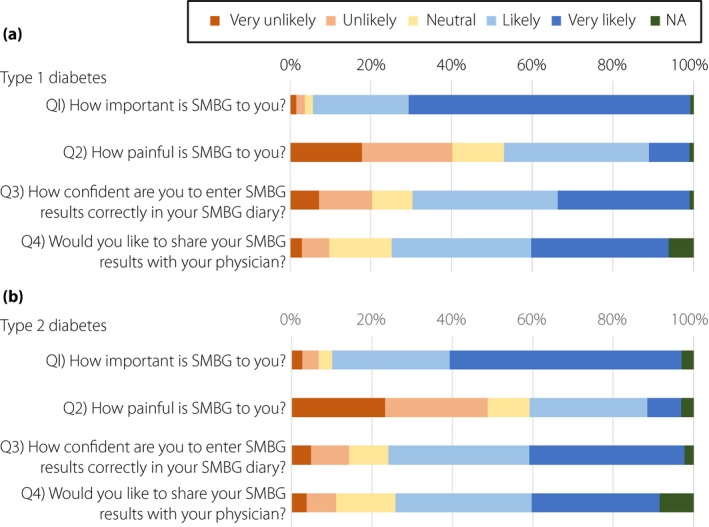
Results of the original self‐monitoring of blood glucose (SMBG) questionnaire from patients with (a) type 1 diabetes (n = 517) and (b) type 2 diabetes (n = 1,648). Each questionnaire was answered by using a 5‐point Likert scale from ‘1: very unlikely’ to ‘5: very likely’, or not answered (NA).
Figure 3.
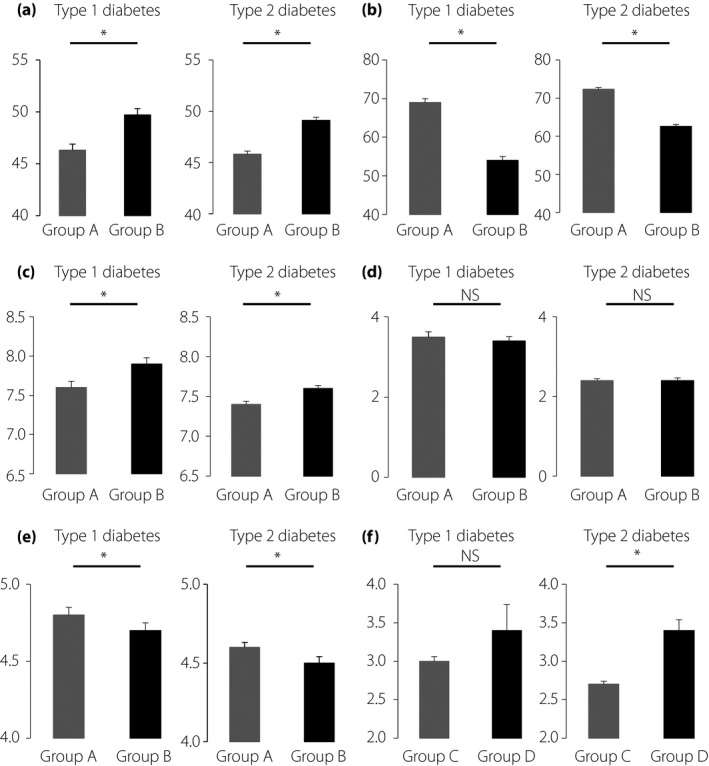
Comparisons of various parameters between patients answering, ‘very unlikely’ or ‘unlikely’ (group A) and those answering ‘likely’ or ‘very likely’ (group B) in Q2 ‘How painful is SMBG to you?’. Parameters include (a) total mood disturbance scores in Profiles of Mood States questionnaire, 2nd edition, Adult‐Short Japanese version, (b) total scores in Diabetes Therapy‐Related Quality of Life, (c) glycated hemoglobin, (d) frequency of self‐monitoring of blood glucose (SMBG) testing and (e) scores in Q1 ‘How important is SMBG to you?’. (f) Comparisons of scores in Q1 ‘How important is SMBG to you’ between patients answering ‘likely’ or ‘very likely’ (group C) and those answering ‘very unlikely’ or ‘unlikely’ (group D) in Q2 ‘How important is SMBG to you?’. Data are shown as mean ± standard error of the mean. *P < 0.05. NS, not significant.
We also investigated possible interactions between attitudes of patients and physicians‐in‐charge to SMBG use, as it is likely that structured education on SMBG and regular checks of SMBG results by physicians‐in‐charge might increase patients’ understanding of the importance of carrying out SMBG testing, thereby reducing the burden of SMBG‐associated pain. In Q4 ‘Would you like to share your SMBG results with your physician?’, 9.5% of type 1 diabetes and 10.0% of type 2 diabetes patients responded ‘very unlikely’ or ‘unlikely,’ and 68.5% of type 1 diabetes and 65.5% of type 2 diabetes patients responded ‘likely’ or ‘very likely.’ As shown in Figure 4, POMS2 total mood disturbance score and DTR‐QOL total score did not differ between group E, those responding ‘very unlikely’ and ‘unlikely,’ and group F, those a responding ‘likely’ and ‘very likely’ in Q4 ‘Would you like to share your SMBG results with your physician?’. Importantly, scores in Q4 ‘Would you like to share your SMBG results with your physician?’ were associated with the scores in Q1 ‘How important is SMBG to you?’ (type 1 diabetes r = 0.328, P < 0.01; and type 2 diabetes r = 0.368, P < 0.01), Q3 ‘How confident are you to enter SMBG results correctly in your SMBG diary?’ (type 1 diabetes r = 0.387, P < 0.01; and type 2 diabetes r = 0.439, P < 0.01) and age (type 1 diabetes r = 0.253, P < 0.01; and type 2 diabetes r = 0.368, P < 0.01), but not with HbA1c and duration of diabetes, as well as POMS2 total score or DTR‐QOL total score. We then turned to physicians’ attitudes on SMBG and SMBG use. Valid responses were obtained from 137 physicians (91 diabetes specialists certified by the Japan Diabetes Society and 46 non‐certified physicians) in the 42 medical institutions. Figure 5 shows results of the SMBG questionnaire for physicians developed by the current study. Notably, in QD4, ‘Do you check patients’ SMBG diary regularly?’, 97.9% of the doctors answered that they were ‘likely’ or ‘very likely’ to check patients’ SMBG diary regularly. To understand possible impacts of physician behavior on patient attitude to SMBG and SMBG use, associations of QD1‐QD4 with results of the SMBG questionnaire for patients, as well as POMS2 and DTR‐QOL scores, were investigated. Significant associations were found with QD4 scores and Q1 ‘How important is SMBG to you?’ (r = 0.311, P < 0.05) and those of Q4 ‘Would you like to share your SMBG results with your physician?’ (r = 0.344, P < 0.05; Figure 6).
Figure 4.
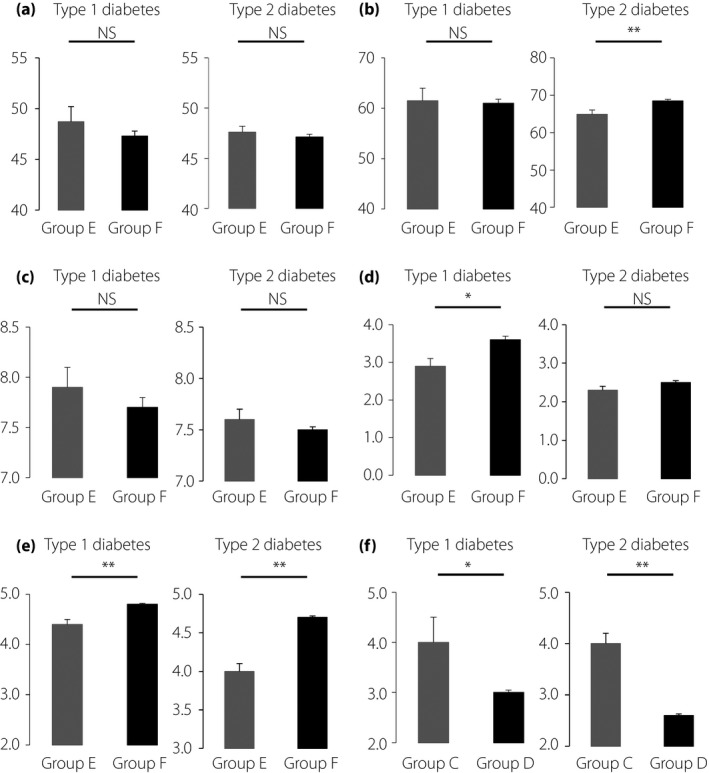
Comparisons of various parameters between patients answering, ‘very unlikely’ or ‘unlikely’ (group E) and those answering ‘likely’ or ‘very likely’ (group F) in Q4 ‘Would you like to share your SMBG results with your physician?’. Parameters include (a) total mood disturbance scores in Profiles of Mood States questionnaire, 2nd edition, Adult‐Short Japanese version, (b) total scores in Diabetes Therapy‐Related Quality of Life, (c) glycated hemoglobin, (d) frequency of SMBG, self‐monitoring of blood glucose (SMBG) testing and (e) scores in Q1 ‘How important is SMBG to you?’. (f) Comparisons of scores in Q1 ‘How important is SMBG to you’ between patients answering ‘likely’ or ‘very likely’ (group C) and those answering ‘very unlikely’ or ‘unlikely’ (group D) in Q4 ‘Would you like to share your SMBG results with your physician?’. Data are shown as mean ± standard error of the mean. *P < 0.05, **P < 0.01. NS, not significant.
Figure 5.
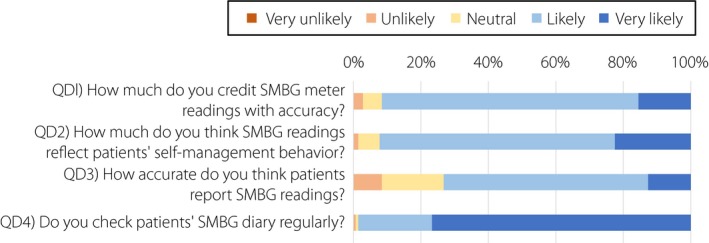
Results of the original self‐monitoring of blood glucose (SMBG) questionnaire from 137 physicians‐in‐charge in the 42 participating medical institutions. Each questionnaire was answered by using a 5‐point Likert scale from ‘1: very unlikely’ to ‘5: very likely.’
Figure 6.
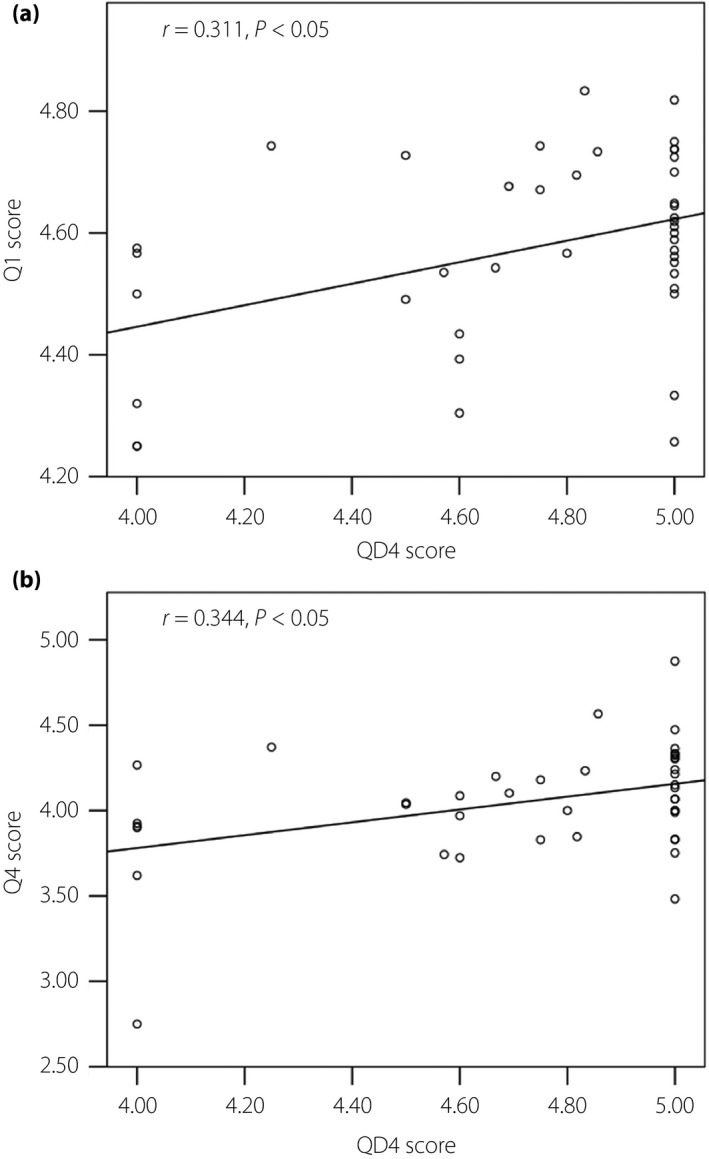
Association of (a) the score of QD4 ‘Do you check patients’ SMBG diary regularly?’ with the score of Q1 ‘How important is SMBG to you?’ (r = 0.311, P < 0.05) and that of (b) Q4 ‘Would you like to share your SMBG results with your physician?’ (r = 0.344, P < 0.05). SMBG, self‐monitoring of blood glucose.
Discussion
The present multicenter, cross‐sectional study shows that type 1 diabetes and type 2 diabetes patients with SMBG‐associated pain have a lower mood state, lower health‐related QOL, higher HbA1c and they appreciate the importance of SMBG use less, regardless of their frequency of daily SMBG testing. The present study also shows that patients who appreciate the importance of SMBG use are more willing to share their SMBG results with their physicians, and that patients realize the importance of conducting SMBG testing and sharing SMBG results with their physicians more when their physicians regularly check patient SMBG results.
The average POMS2 TMD scores of type 1 diabetes and type 2 diabetes patients in the current study did not differ from the average scores of Japanese individuals, suggesting that diabetes and/or SMBG use does not affect patient mood status. However, AH and TA scores were slightly, but significantly, lower in type 1 diabetes patients, possibly due to the more frequent hypoglycemia in type 1 diabetes patients. Although the frequency and severity of hypoglycemia in each patient was not assessed in the current study, type 1 diabetes patients had lower DTR‐QOL scores than type 2 diabetes patients regardless of insulin injection type; and the AH and TA scores were weakly, but significantly, associated with DTR‐QOL scores (Figure S1). In the present study, the scores of total and each DTR‐QOL category were comparable with those of previous studies carried out in Japanese type 1 diabetes and type 2 diabetes patients receiving insulin injections12, 13. Importantly, the treatment satisfaction‐related score was relatively high in the present study population, possibly because most participants consulted regularly with Japan Diabetes Society‐certified diabetes specialists.
The present results suggest that SMBG‐associated pain generates a negative impact on diabetes management overall, which might be furthered by patients’ lack of appreciation of the importance of SMBG use. It has been found that the number of finger pricks required for a SMBG reading is associated with patient fear of insulin injection and SMBG use14. As it has been shown that SMBG use provides substantial clinical benefits when SMBG is implemented by well‐trained healthcare professionals in a structured fashion15 and structured education on SMBG by well‐trained healthcare professionals might well reduce the number of finger pricks required for an SMBG reading, such training clearly would be beneficial. In addition, SMBG‐associated pain might be reduced by patients’ better appreciation of the importance of SMBG use when they receive structured education on SMBG and regular checks of SMBG results from well‐trained healthcare professionals. Indeed, the present study shows that patients who realize the importance of carrying out SMBG testing are more willing to share SMBG results with their physicians, and that patients realize the importance of carrying out SMBG testing and sharing SMBG results with their physicians more when physicians regularly check patients’ SMBG results to provide meaningful feedback. Previous studies have shown that appropriate SMBG use under instructions from healthcare professionals improved glycemic control and well‐being in type 1 diabetes patients16 and also in non‐insulin treated type 2 diabetes patients17, 18, whereas others have failed to show significant benefits19, 20. As the present study shows that physicians’ regular check of patients’ SMBG results affects patients’ attitude to SMBG use, sharing SMBG results between patients and healthcare professionals could play a critical role in making better use of SMBG to improve glycemic control in type 1 diabetes and type 2 diabetes patients. Thus, it would be interesting to test in prospective, randomized, controlled trials whether modalities to facilitate SMBG data sharing between patients and healthcare professionals could improve glycemic control in type 1 diabetes and type 2 diabetes patients.
There were two major limitations to this study. First, ‘painful’ in the current questionnaire could mean emotional burden rather than somatic pain, which could underlie higher negative mood disturbance and lower QOL with similar SMBG frequency. However, insofar as ‘painful’ was construed as emotional burden by study participants, improved SMBG data sharing between patients and healthcare professionals would more readily improve patients’ appreciation of the importance of SMBG. Second, we could not correlate physicians’ attitudes on SMBG and SMBG use with each patient, because all personal information of each patient and physician was anonymized before it was sent from each participating institute.
In conclusion, the current questionnaire‐based survey shows that type 1 diabetes and type 2 diabetes patients experiencing SMBG‐associated pain have more mental distress, lower health‐related QOL and higher HbA1c; they also appreciate the importance of SMBG use less, regardless of their number of daily SMBG tests. The current study also shows that patients who realize the importance of SMBG testing are more willing to share their SMBG results with their physicians, and that patients realize the importance of carrying out SMBG testing and sharing SMBG results with their physicians more when physicians regularly check patients’ SMBG results to provide meaningful feedback in clinical settings.
Disclosure
D Yabe received consulting or speaker fees from MSD K.K. and Novo Nordisk Pharma Ltd. D Yabe also received clinically commissioned/joint research grants from MSD K.K., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., Arklay Co. Ltd., Takeda Pharmaceutical Company Limited and Terumo Corporation. Y Hamamoto received consulting or speaker fees from Novo Nordisk Pharma Ltd. T Kurose received consulting or speaker fees from Sanofi K.K. T Kurose also received clinically commissioned/joint research grants from the Japan Vascular Disease Research Foundation. T Osonoi received consulting or speaker fees from Sanwa Kagaku Kenkyuso Co., Ltd., Novo Nordisk Pharma Inc., Astellas Pharma Inc., Tanabe Mitsubishi Co., Ltd. and Takeda Pharmaceutical Company Limited. T Osonoi also received clinically commissioned/joint research grants from Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., Sanwa Kagaku Kenkyuso Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Nippon Eli Lilly Co., Ltd., Taisho Pharmaceutical Co. Ltd., Tanabe Mitsubishi Co., Ltd., Daiichi‐Sankyo Company Limited, Bayer Yakuhin, Ltd and AbbVie Inc. M Minami received consulting or speaker fees from Eli Lilly Japan K.K. D Shimono received consulting or speaker fees from MSD K.K. and AstraZeneca K.K. Y Seino received consulting or speaker fees from Eli Lilly Japan K.K., Sanofi K.K., Novo Nordisk Pharma Inc., Glaxo‐Smith‐Kline, Taisho Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Astellas Pharma Inc., BD, Nippon Boehringer Ingelheim Co., Ltd., Johnson & Johnson and Takeda Pharmaceutical Company Limited. Y Seino also received clinically commissioned/joint research grants from Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly and Company, Taisho Toyama Pharmaceutical Co. Ltd., MSD K.K., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., Arklay Co. Ltd., and Terumo Corporation. N Tanaka, S Ueno, H Kuwata, HK Murotani and T Akashi declare no conflict of interest.
Supporting information
Figure S1 | Hypoglycemia‐related Diabetes Therapy‐Related Quality of Life (DTR‐QOL) scores in type 1 and type 2 diabetes patients in the current study.
Acknowledgments
The authors are grateful to H Abe of Kansai Electric Power Medical Research Institute and M Yamane of Kansai Electric Power Hospital for secretarial assistance. The authors also deeply thank the patients and colleagues who contributed to this study. This study was funded by Terumo Corporation.
Positive SMBG study group investigator
Positive SMBG study group investigators: Masatomo Sekiguchi (Sapporo‐Kosei General Hospital, Sapporo, Japan), Norihiko Hirota (Hakodate Medical Association Hospital, Hakodate, Japan), Hiroki Yokoyama (Jiyugaoka Medical Clinic Internal Medicine, Obihiro, Japan), Osamu Uehara (Kuroishi General Hospital, Kuroishi, Japan), Akira Kanamori (Kanamori Diabetes Clinic, Sagamihara, Japan), Akira Kubota (Mukogaoka Kubota Clinic for Internal Medicine, Kawasaki, Japan), Minori Ishitobi (Kondo Clinic, Kodaira, Japan), Koji Nagayama (Hamamatsu Medical Center, Hamamatsu, Japan), Taiga Shibata (Ogaki Municipal Hospital, Ogaki, Japan), Mayumi Yamamoto (Gifu University Hospital, Gifu, Japan), Yoshio Hiraiwa (Toyama Red Cross Hospital, Toyama, Japan), Kazuhito Fukuda (Fukuda Clinic, Himi, Japan), Takao Taniguchi (Otsu Red Cross Hospital, Otsu, Japan), Akira Kuroe (Hikone Municipal Hospital, Hikone, Japan), Shinji Kagimoto (Kagimoto Diabetes Clinic, Kyoto, Japan), Yu Ihara (Ihara Clinic, Kyoto, Japan), Sachiko Honjo (Tazuke Kofukai Foundation, Medical Research Institute, Kitano Hospital, Osaka, Japan), Haruo Nishimura (Osaka Saiseikai Nakatsu Hospital, Osaka, Japan), Koichiro Yasuda (Osaka Saiseikai Noe Hospital, Osaka, Japan), Seiji Muro (Osaka Red Cross Hospital, Osaka, Japan), Hiroki Ikeda (Seimeikai Ikeda Hospital, Amagasaki, Japan), Yoshio Nakamura (Hyogo Prefectural Amagasaki General Medical Center, Amagasaki, Japan), Seizo Kadowaki (Eikoukai Ono Hospital, Ono, Japan), Seiichi Kawamoto (Kawamoto Clinic, Osaka, Japan), Saburo Kashii (Kashii Clinic, Osaka, Japan), Shinpei Fujimoto (Kochi Medical School Hospital, Nangoku, Japan), Bunzo Matsuura (Ehime University School of Medicine, Toon, Japan), Syuji Nakamura (Heiwadai Hospital, Miyazaki, Japan), Yasushi Yokogawa (Yokogawa Clinic, Fukuoka, Japan), Koji Oida (Fukui Chuoh Clinic, Fukui, Japan), Masahiro Iwamoto (Iwamoto Clinic, Zentsuji, Japan), Yu Kouchi (Orimoto Hospital, Kiyose, Japan), Tetsuji Niiya (Matsuyama Shimin Hospital, Matsuyama, Japan), Makoto Furukawa (Kushiro Red Cross Hospital, Kushiro, Japan) and Jo Satoh (Tohoku Medical and Pharmaceutical University Wakabayashi Hospital, Sendai, Japan).
J Diabetes Investig 2018; 9: 1203–1211
Clinical Trial Registry University Hospital Medical Information Network UMIN000026761
Contributor Information
Yutaka Seino, Email: seino.yutaka@e2.kepco.co.jp.
the Positive SMBG Study Group Investigators:
Masatomo Sekiguchi, Norihiko Hirota, Hiroki Yokoyama, Osamu Uehara, Akira Kanamori, Akira Kubota, Minori Ishitobi, Koji Nagayama, Taiga Shibata, Mayumi Yamamoto, Yoshio Hiraiwa, Kazuhito Fukuda, Takao Taniguchi, Akira Kuroe, Shinji Kagimoto, Yu Ihara, Sachiko Honjo, Haruo Nishimura, Koichiro Yasuda, Seiji Muro, Hiroki Ikeda, Yoshio Nakamura, Seizo Kadowaki, Seiichi Kawamoto, Saburo Kashii, Shinpei Fujimoto, Bunzo Matsuura, Syuji Nakamura, Yasushi Yokogawa, Koji Oida, Masahiro Iwamoto, Yu Kouchi, Tetsuji Niiya, Makoto Furukawa, and Jo Satoh
References
- 1. IDF . IDF Diabetes Atlas, 7th edn Brussels: IDF, 2015. [Google Scholar]
- 2. Association AD . 4. Lifestyle management. Diabetes Care 2017; 40: S33–S43. [DOI] [PubMed] [Google Scholar]
- 3. Statement AP, Education DS. AADE position statement. Individualization of diabetes self‐management education. Diabetes Educ 2014; 33: 45–49. [DOI] [PubMed] [Google Scholar]
- 4. Miller KM, Beck RW, Bergenstal RM, et al Evidence of a strong association between frequency of self‐monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013; 36: 2009–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Educators AAoD . AADE7 position statement: AADE7 self‐care behaviors. Diabetes Educ 2008; 2008: 445–449. [DOI] [PubMed] [Google Scholar]
- 6. Malanda UL, Welschen LM, Riphagen II, et al Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev 2012; 1: CD005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franciosi M, Pellegrini F, De Berardis G, et al The impact of blood glucose self‐monitoring on metabolic control and quality of life in type 2 diabetic patients: an urgent need for better educational strategies. Diabetes Care 2001; 24: 1870–1877. [DOI] [PubMed] [Google Scholar]
- 8. Russo GT, Scavini M, Acmet E, et al The burden of structured self‐monitoring of blood glucose on diabetes‐specific quality of life and locus of control in patients with noninsulin‐treated type 2 diabetes: the PRISMA study. Diabetes Technol Ther 2016; 18: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol 2008; 2: 919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heuchert JP, McNair DM. Profile of Mood States ‐ Second Edition (POMS‐2). Tonawanda, NY: HMS, 2012. [Google Scholar]
- 11. Ishii H. Development and psychometric validation of the Diabetes Therapy‐Related QOL (DTR‐QOL) questionnaire. J Med Econ 2012; 15: 556–563. [DOI] [PubMed] [Google Scholar]
- 12. Okada M, Okada M, Nishigami J, et al Effect of switching basal insulin regimen to degludec on quality of life in Japanese patients with type 1 and type 2 diabetes mellitus. J Pharm Health Care Sci 2015; 1: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iga R, Uchino H, Kanazawa K, et al Glycemic variability in type 1 diabetes compared with degludec and glargine on the morning injection: an open‐label randomized controlled trial. Diabetes Ther 2017; 8: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al Hayek AA, Robert AA, Babli S, et al Fear of self‐injecting and self‐testing and the related risk factors in adolescents with type 1 diabetes: a cross‐sectional study. Diabetes Ther 2017; 8: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polonsky WH, Fisher L. Self‐monitoring of blood glucose in noninsulin‐using type 2 diabetic patients: right answer, but wrong question: self‐monitoring of blood glucose can be clinically valuable for noninsulin users. Diabetes Care 2013; 36: 179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diabetes C , Complications Trial Research G , Nathan DM, et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 17. Polonsky WH, Fisher L, Schikman CH, et al Structured self‐monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin‐treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care 2011; 34: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ 2007; 335: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Kane MJ, Bunting B, Copeland M, et al Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ 2008; 336: 1174–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farmer A, Wade A, Goyder E, et al Impact of self monitoring of blood glucose in the management of patients with non‐insulin treated diabetes: open parallel group randomised trial. BMJ 2007; 335: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Hypoglycemia‐related Diabetes Therapy‐Related Quality of Life (DTR‐QOL) scores in type 1 and type 2 diabetes patients in the current study.


