Abstract
Rare diseases (RD) affect approximately 30 million Americans, half of whom are children. This study is the first to comprehensively evaluate their medical device needs via a survey of physicians. The study sought to identify and document the presumed unmet diagnostic and therapeutic device needs for RD management; clarify the magnitude of the potential unmet need; and generate meaningful data to inform medical device stakeholders. A cross-sectional nonprobability survey was conducted. The study population was drawn from the membership files of four groups: FDA Medical Devices Advisory Committee, Pediatric Advisory Committee, Pediatric Device Consortia, and National Institutes of Health (NIH) Rare Diseases Clinical Research Network. Only physician respondents with experience or knowledge regarding RD were eligible. Among eligible respondents, 90% confirmed the need for innovative devices to care for people with RD. Over 850 device needs were identified for 436 RD, with 74% of needs related to children. Pediatric physicians (OR = 2.11, 95% CI 1.01–4.39, P = 0.046) and physicians with more RD experience reflected greater dissatisfaction with existing devices (OR = 4.49, 95% CI 2.25–8.96, P < 0.0001). Creation of entirely new devices is the top recommendation for mitigating needs. This study demonstrates a major public health need for innovative medical devices to care for children and adults with RD. FDA and NIH support and seek opportunities to accelerate device development for these vulnerable patients.
Introduction
The impact of rare diseases (RD) is likely far greater than the term implies. The lives of nearly 30 million Americans, half of whom are children, are directly affected by nearly 7000 RD [1]. Statistics for the number of people seeking care with diseases of unknown or unclear etiology (i.e., undiagnosed RD patients) remain elusive. The evolution of genomics along with the creation of more targeted therapeutics herald an era of medicine with not only greater numbers of RD but increased longevity for those with RD. When this potential is considered with known data, the probability that every healthcare professional in the U.S. cares for a patient with a RD, knowingly or unknowingly, becomes a relevant consideration for healthcare resource allocation and policy development.
The aggregate influence of RD conveys a compelling but limited perspective. The Orphan Drug Act (ODA) of 1983 defines a RD as one affecting fewer than 200,000 people in the U.S. [2], yet many RD affect only tens to hundreds of people. The relatively small and heterogeneous nature of these populations has led to circumscribed education and understanding by clinicians and a relative dearth of resources committed toward research and development for specific RD. Subsequently, people seeking care for rare diseases or conditions are often misdiagnosed and experience inappropriate or insufficient medical management. On average, patients receiving an accurate diagnosis have sought care for at least 7 years and delayed diagnosis can be fatal for pediatric patients [3]. Innovative diagnostic and therapeutic options may improve and save the lives of these patients.
A number of developments in recent decades have fueled medical product development for people with RD. Patient-driven organizations have bolstered awareness and been successful in advocacy. The ODA, via incentives designed for the drug and biologics markets, has engendered a substantial increase in the development of respective medical products. Innovation with respect to medical devices to care for this population remains far less robust.
The U.S. Food and Drug Administration (FDA) and the National Center for Advancing Translational Sciences (NCATS)/Office of Rare Diseases Research (ORDR) at the National Institutes of Health (NIH) seek to better understand the medical device needs of patients with RD in order to inform strategies for device development. From Nov. 4, 2015 through Feb. 29, 2016, we conducted a national needs assessment survey of clinicians (physicians and other health care providers) as part of the Rare Disease Needs Assessment Project (the project). This effort has evolved from the 2010 Institutes of Medicine (IOM) report as well as the FDA's 2011 Section 740 Rare and Neglected Diseases Report to Congress [4,5]. The IOM report examined the opportunities for and obstacles to the development of drugs and medical devices to treat rare diseases. It assessed and proposed strategies to accelerate rare disease research and orphan product development. The IOM report noted that “for rare diseases, efforts to accelerate research and product development clearly focus on drugs and biological products. Devices and the need for devices are much less frequently mentioned in journal articles or stakeholder conversations. When devices for rare conditions are discussed, it is generally tied with pediatric populations.” Both the IOM report and the FDA's report recommended that an assessment be conducted regarding unmet medical device needs for patients with rare diseases. The FDA report also recommended an assessment of “the barriers to, and meaningful incentives for, the development of medical devices for rare diseases.” The project is the first comprehensive assessment for this population and was developed to meet the following goals: (1) identify and document the presumed unmet diagnostic and therapeutic device needs for people with RD; (2) clarify the magnitude of the potential unmet needs; and (3) generate meaningful data to inform medical device stakeholders. In recognition of the prominent influence of RD on the lives of children, the project included a subfocus on pediatrics. Here, we summarize the key findings of the project, focusing on the survey responses of the eligible physician (inclusive of surgeons) cohort. A comprehensive report is available online.2
Methods
Project Development and Stakeholder Collaboration.
The project was a multistakeholder collaborative effort. Strategic and technical leadership was provided by the Needs Assessment Working Group (NAWG), composed of representatives from the FDA's Office of Orphan Products Development, Center for Devices and Radiological Health (CDRH), and the Office of Planning, and the NIH's NCATS/ORDR. Multiple stakeholder groups representing patients, advocacy organizations, researchers, clinicians, and industry were engaged by the NAWG via public meetings and solicited communications to provide input on project goals, methodology, and strategic outcomes. The NAWG contracted with ICF, a global consulting and technology services firm, for survey development, design, implementation, data processing and report drafting.
Project Terminology.
For the project, the definition of a rare disease is a disease or condition with a prevalence of fewer than 200,000 people in the U.S., similar to the definition in the ODA. This differs from the population criteria of up to 8000 individuals annually used for the humanitarian device exemption (HDE) program, which was developed to provide an alternative pathway to receive marketing approval for devices serving people with RD. Utilizing the prevalence of 200,000 provided the opportunity to gather data on a wider range of RD.
For the project, an unmet medical need exists when there are no approved devices for the treatment or diagnosis of a disease or condition or when a novel device could provide a clinically meaningful advantage over existing approved devices. Regulated medical devices represent a broad spectrum of diverse technologies, utilized for both diagnostic and therapeutic indications. These technologies range from simple medical instruments such as sphygmomanometers to commonplace hematology analyzers to complex robotic surgical suites to cutting-edge lab-on-a-chip microfluidics platforms.
Survey Design and Administration.
The online survey was designed to elicit information on a number of aspects regarding unmet diagnostic and therapeutic device needs fundamental to the care of patients with RD. Personalized survey URLs allowed physicians to intermittently engage the survey at their convenience. Fifty open- and closed-ended questions were utilized. Key topic areas included: (1) satisfaction with devices for managing RD; (2) unmet device needs for RD; and (3) impediments to device development and innovation. Professional demographic information was also queried.
The sample size (N) for each survey question varied on the basis of both the primary skip pattern and the ability of respondents to choose to skip the question. See Fig. 1 illustrating the survey organization and primary skip pattern.
Fig. 1.
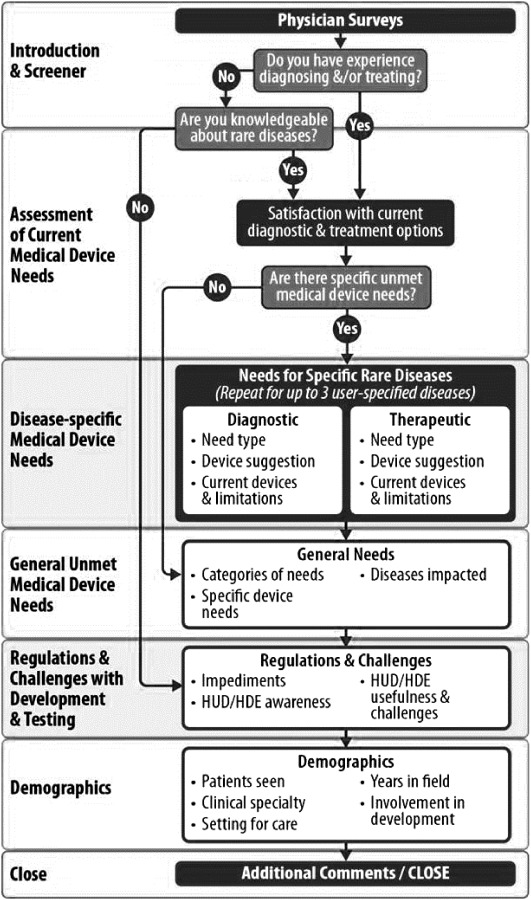
Survey organization and primary skip pattern
Prior to fielding, cognitive and usability testing of the survey was conducted. Cognitive evaluation ensured clarity of instructions, questions, and sequence via nine physicians selected by the NAWG based on their experience with rare disease management. Usability evaluation was subsequently completed with FDA physician employees following programming for mobile and desktop computers.
The FDA and ICF independently sought and received Institutional Review Board (IRB) approval prior to fielding. Communication strategy was designed to maintain confidentiality of all participants. The FDA and NIH had no knowledge of which invitees responded to the survey. Only de-identified data were sent to the NAWG.
Survey Sample.
A national cross-sectional nonprobability sample of 1154 physicians in the U.S. received the survey. The sample was drawn from the 2015 membership files of four groups (663 members of the FDA CDRH Medical Devices Advisory Committee [6], 26 members of the FDA Pediatric Advisory Committee [7], 58 members of the Pediatric Device Consortia [8], and 407 members of the NCATS Rare Diseases Clinical Research Network [9]) that advise or work with the FDA or NIH on public health issues associated with rare diseases and medical device development.
The raw response rate of 44% was calculated utilizing 502 survey responses (428 complete and 74 partially complete). A survey was considered partially complete if the respondent completed the opening screening questions and at least one additional survey item regarding device needs. Twenty-three respondents who noted no direct experience with RD patients or knowledge of RD were excluded from the final study population, yielding an adjusted response rate of 42%.
Statistical Analysis.
Pearson's Chi-square test was used to evaluate the differences between physician groups in response to questions regarding need for new or improved devices and satisfaction with current diagnostic and therapeutic devices. If the test statistic suggested a difference in the distribution of responses, we used pairwise comparisons to identify the responses where the physician groups differed. P-values of ≤0.05 were considered significant.
Logistic regression was used to evaluate the multivariate relationships between dissatisfaction with devices and physicians' characteristics and perceptions (e.g., impediment factors), using stepwise selection. Variables were entered into the model if they had a significance level of 0.15 and were kept in the model if they had an entry level of 0.05. We calculated odds ratios and 95% confidence intervals for the significant effects.
Factor analysis was used to evaluate the correlation in the nine posed impediment options.
The response categories were ordinal: 0 = not an impediment (or no response), 1 = small extent, 2 = moderate extent, and 3 = large extent. The factor analysis was based on polychoric correlations, which assumes the ordinal variables are discrete representations of underlying continuous latent variables. The factor loadings are based on Varimax rotation.
Results
The study population was comprised only of physician respondents with direct experience or knowledge regarding rare diseases. Their professional characteristics are detailed in Table 1.
Table 1.
Characteristics of the Physician Study Populationa
| Experience with rare disease patients | Physicians (no. and %) | |
|---|---|---|
| Direct experience with rare disease patients | 468/479 | 98 |
| No direct experience with rare disease patients but knowledge of rare diseases | 11/479 | 2 |
| Professional characteristics | Physicians with direct experience or knowledge regarding rare diseases (no. and %) | |
| Years of clinical practice | ||
| Never practiced | 0/377 | 0 |
| 1 year to less than 5 years | 10/377 | 3 |
| 5 years to less than 10 years | 30/377 | 8 |
| 10 years or more | 337/377 | 89 |
| Setting for careb | ||
| Academic medical center | 341/377 | 90 |
| Nonacademic hospital | 16/377 | 4 |
| Patient's home | 3/377 | 1 |
| Group practice | 30/377 | 8 |
| Single practitioner | 6/377 | 2 |
| Other | 6/377 | 2 |
| Experience with device development and/or device clinical trials | ||
| No experience with device development or trials | 103/378 | 27 |
| Experience with device development | 57/378 | 15 |
| Experience with device trials | 45/378 | 12 |
| Experience with device development and trials | 173/378 | 46 |
| Rare disease patients seen within previous 2 years | ||
| None | 17/375 | 5 |
| 1–20 | 97/375 | 26 |
| 21–50 | 63/375 | 17 |
| 51–99 | 63/375 | 17 |
| 100–499 | 100/375 | 27 |
| 500 or more | 35/375 | 9 |
| Proportion of rare disease patients seen within previous 2 years < 21 years of age | ||
| None | 69/358 | 19 |
| Fewer than half | 119/358 | 33 |
| About half | 18/358 | 5 |
| More than half | 80/358 | 22 |
| All | 72/358 | 20 |
| Clinical specialtyb | ||
| Pediatrics | 84/377 | 18 |
| Neurology | 50/377 | 13 |
| Pulmonology | 38/377 | 10 |
| Cardiology | 29/377 | 8 |
| Ophthalmology | 22/377 | 6 |
| Medical genetics | 25/377 | 7 |
| Internal medicine | 25/377 | 7 |
| Orthopedic surgery | 18/377 | 5 |
| Gastroenterology | 20/377 | 5 |
| Surgery | 19/377 | 5 |
| Oncology | 17/377 | 5 |
| Radiology | 16/377 | 4 |
| Pathology | 13/377 | 3 |
| Nephrology | 16/377 | 4 |
| Otolaryngology | 10/377 | 3 |
| Allergy and immunology | 11/377 | 3 |
| Neurological surgery | 11/377 | 3 |
| Thoracic surgery | 11/377 | 3 |
| Endocrinology | 9/377 | 2 |
| Obstetrics and gynecology | 6/377 | 2 |
| Plastic surgery | 5/377 | 1 |
| Psychiatry | 4/377 | 1 |
| Rheumatology | 5/377 | 1 |
| Urology | 3/377 | 1 |
| Physical medicine and rehabilitation | 4/377 | 1 |
| Geriatrics | 2/377 | 1 |
| Anesthesiology | 2/377 | 1 |
| Dermatology | 2/377 | 1 |
| Diagnostic radiology | 1/377 | 0.3 |
| Nuclear medicine | 2/377 | 1 |
| Colon and rectal surgery | 1/377 | 0.3 |
| Physiatry | 1/377 | 0.3 |
| Preventive medicine | 1/377 | 0.3 |
| Emergency medicine | 0/377 | 0 |
The final study population excluded 23 physician respondents who had no experience or knowledge of rare diseases. Physician professional characteristics are based on 378 eligible physicians who completed the survey through the demographic section; a denominator less than 378 indicates item missing data.
Physicians could select multiple settings of care and multiple clinical specialties. The percentage will add up to more than 100.
Satisfaction With Current Devices and Need for Innovative Devices.
The physicians' level of satisfaction with existing devices for RD and the number reporting that a need exists for a new or improved device are presented in Table 2. The satisfaction distribution was different between medicine physicians and surgery physicians (P < 0.05), with higher neutrality for surgery physicians.
Table 2.
Satisfaction for current devices, need for new or improved devicesa
| Overall (no. and %) | Pediatric (no. and %) | Nonpediatric (no. and %) | Medicine (no. and %) | Surgery (no. and %) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Satisfaction with diagnostic devices | |||||||||||
| Very satisfied | 45/476 | 9.5 | 22/182 | 12.1 | 23/294 | 7.8 | 26/277 | 9.4 | 15/99 | 15.2 | |
| Somewhat satisfied | 175/476 | 36.8 | 73/182 | 40.1 | 102/294 | 34.7 | 115/277 | 41.5b | 29/99 | 29.3b | |
| Neutral | 88/476 | 18.5 | 28/182 | 15.4 | 60/294 | 20.4 | 40/277 | 14.4b | 27/99 | 27.3b | |
| Somewhat dissatisfied | 120/476 | 25.2 | 43/182 | 23.7 | 77/294 | 26.2 | 67/277 | 24.2 | 18/99 | 18.2 | |
| Very dissatisfied | 48/476 | 10.1 | 16/182 | 8.8 | 32/294 | 10.9 | 29/277 | 10.5 | 10/99 | 10.1 | |
| P value = 0.2509 | P value = 0.0114b | ||||||||||
| Satisfaction with therapeutic devices | |||||||||||
| Very satisfied | 7/471 | 1.5 | 3/181 | 1.7 | 4/290 | 1.4 | 4/277 | 1.4 | 2/99 | 2.0 | |
| Somewhat satisfied | 73/471 | 15.5 | 29/181 | 16.0 | 44/290 | 15.2 | 44/277 | 15.9 | 17/99 | 17.2 | |
| Neutral | 106/471 | 22.5 | 36/181 | 19.9 | 70/290 | 24.1 | 66/277 | 23.8 | 25/99 | 25.3 | |
| Somewhat dissatisfied | 169/471 | 35.9 | 68/181 | 37.6 | 101/290 | 34.8 | 95/277 | 34.3 | 33/99 | 33.3 | |
| Very dissatisfied | 116/471 | 24.6 | 45/181 | 24.9 | 71/290 | 24.5 | 68/277 | 24.6 | 22/99 | 22.2 | |
| P value = 0.8709 | P value = 0.9761 | ||||||||||
| Need for new or improved devices | |||||||||||
| Yes | 426/473 | 90.1 | 170/182 | 93.4 | 256/291 | 88.0 | 248/277 | 89.5 | 86/100 | 86.0 | |
| No | 47/473 | 9.9 | 12/182 | 6.6 | 35/291 | 12.0 | 29/277 | 10.5 | 14/100 | 14.0 | |
| P value = 0.0546 | P value = 0.3411 | ||||||||||
Based on 479 respondents having experience with or knowledge of rare diseases; a denominator less than 479 indicates item missing data. P-values were calculated using the Pearson Chi-square test comparing pediatric versus nonpediatric and medicine and nonmedicine/surgical physicians.
Medicine and surgery significantly different at P < 0.05.
Necessary Device Improvements and Associated Benefits.
Physicians named over 850 RD needs, 19% requiring a diagnostic device, 27% requiring a treatment device, and 54% requiring both. Table 3 presents characteristics about the type of improvement needed and associated potential benefit of innovative devices for RD management.
Table 3.
Characteristics of necessary improvementsa
| Options reported for diagnostic devices (no. and %) | Options reported for treatment devices (no. and %) | |||
| Type of improvement | ||||
| Creation of a new device | 308/438 | 70.3 | 299/448 | 66.7 |
| Modification of an existing device | 87/438 | 19.9 | 86/448 | 19.2 |
| Using an existing device for a different indication | 43/438 | 9.8 | 63/448 | 14.1 |
| Impact of the device options | ||||
| Breakthrough advancement | 204/423 | 48.2 | 251/437 | 57.4 |
| Important incremental improvement | 195/423 | 46.1 | 165/437 | 37.8 |
| Don't know | 24/423 | 5.7 | 21/437 | 4.8 |
| Benefit of diagnostic improvement | ||||
| Quicker | 317/428 | 74.1 | ||
| More specific | 294/428 | 68.7 | ||
| More sensitive | 270/428 | 63.1 | ||
| Less cumbersome | 225/428 | 52.6 | ||
| Less invasive | 191/428 | 44.6 | ||
| Benefit of therapeutic improvement | ||||
| Prolong survival | 275/443 | 62.1 | ||
| Restore/replace organ function | 243/443 | 54.9 | ||
| Provide temporary relief | 155/443 | 35.0 | ||
| Improve quality of life | 377/443 | 85.1 | ||
Base is the number of rare diseases in need of a new or improved device as reported by the sample of experienced or knowledgeable physicians. There were a total of 620 rare diseases/conditions listed in need of improved diagnostic devices and 687 diseases/conditions listed in need of treatment devices, of all listed diseases/conditions. Physicians were given the option to provide details on up to three diseases. Most chose only to provide details for one disease.
Table 4 presents the odds ratios for modeling the need for new or improved devices. The models examine the respondent's current practices for managing RD, perceived impediments to device development, and physician characteristics. Three significant effects are highlighted in the Discussion section.
Table 4.
Odds Ratios for Multivariate Logistic Regression Modelsa
| Characteristic | Odds ratio (95% CI) |
|---|---|
| Need new or improved devicesb | |
| Pediatric physician | |
| Yes | 2.11 (1.01, 4.39) |
| No | 1.00 |
| More than 20 rare disease patients in past 2 years | |
| Yes | 4.49 (2.25, 8.96) |
| No | 1.00 |
| Impediment factor: ROI | 1.50 (1.10, 2.05) |
| Dissatisfied with diagnostic devicesc | |
| Number of named diseases in need of improved medical devices or tests | |
| 2–3 listed | 2.54 (1.24, 5.24) |
| 1 listed | 1.00 |
| Do you use any medical devices or tests to diagnose? | |
| Yes | 1.93 (1.11, 3.34) |
| No | 1.00 |
| Benefit of development: It would make diagnosis more sensitived,e | |
| Selected | 1.68 (1.04, 2.71) |
| Not selected/not diagnostic need | 1.00 |
| Dissatisfied with treatment devicesc | |
| Have any of the following presented a challenge to you in using Humanitarian Use Devices? -Access to humanitarian use devices | |
| Checked | 2.16 (1.18, 3.97) |
| Not selected/Unawaref | 1.00 |
| How would the availability of this device affect the treatment?—restore/replace organ functiond,g | |
| Selected | 2.15 (1.33, 3.48) |
| Not selected/not treatment need | 1.00 |
Model variables were selected using forward selection algorithm. Variables were individually added if their contribution to the model is significant based on the chi-square statistic. Variables were included based on a maximum P-value of 0.05.
Model is based on the 378 physicians who completed the survey through the demographics section.
Model based on 329 respondents who provided at least one disease in need of a new or improved device and completed the demographics section.
Disease referenced is the one first listed by the physician.
Respondent allowed to check multiple benefits. Asked only of respondents who stated need was diagnostic.
Respondent allowed to check multiple barriers. Asked only of respondents who were aware of HUD/HDE.
Respondent allowed to check multiple benefits. Asked only of respondents who stated need was therapeutic.
Table 4 also includes odds ratios for modeling dissatisfaction (somewhat and very dissatisfied) with existing diagnostic and therapeutic devices. The models examine the same characteristics, plus perceived benefits of a new or improved device. For physicians who listed multiple diseases, only the data for the first disease mentioned are included. Three significant effects emerged for the diagnostic device dissatisfaction model. Dissatisfaction was higher among physicians who: (1) named more than one disease (OR = 2.36); (2) currently use a device to diagnose the disease they listed (OR = 1.94); and (3) reported “more sensitive” as a perceived potential benefit (OR = 1.65). Two significant effects emerged in the therapeutic device dissatisfaction model. Dissatisfaction was higher among physicians who: (1) are aware of humanitarian use devices (HUD), but felt access to HUDs have been a challenge (OR = 2.16); and (2) reported “restore/replace organ function” as a perceived benefit to a new or improved device (OR = 2.15).
Impediments to Device Development.
The nine impediments posed in the survey as options for lack of device development for RD are delineated in rank order by overall average score denoting their selected influence on device development (Fig. 2). Differences in rank per physician category are also delineated.
Fig. 2.
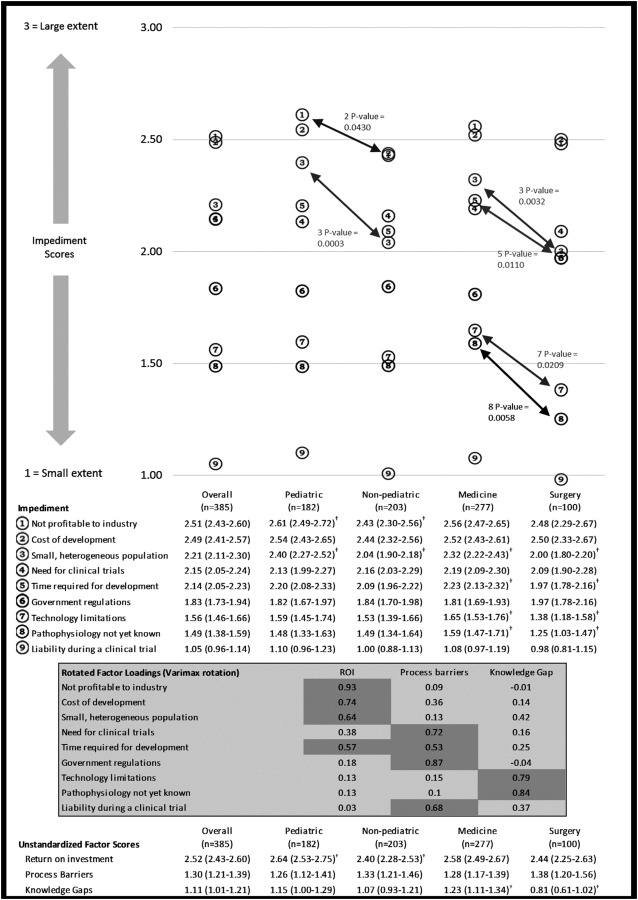
Mean factor scores for impediments and exploratory factor analysis*. *Based on 385 respondents; a denominator less than 385 indicates item missing data; CI denotes confidence interval; P values were calculated using t-test comparing pediatric versus nonpediatric and medicine and nonmedicine; Score is calculated as 0 = not an impediment/no answer, 1 = small extent, 2 = moderate extent, and 3 = large extent. †Significant difference at P < 0.05.
The mean factor scores (unstandardized) are also presented in Fig. 2. Exploratory factor analysis resulted in three factors, which explained 70% of the total variability in the impediment scores:
Return on investment (ROI) explained 46% of the variation. This factor had the largest factor loadings from cost of development and industry profitability. Small, heterogeneous population and time for development also load high on this factor.
Process barriers explained 13% of the variation. Need for clinical trials, government regulations, and liability load high on this factor. Time required for development also loads on this factor. Time required for development is split between ROI and process barrier. This seems appropriate in that development time could be seen as how soon an investment will pay off as well as a barrier to initiating the new product development.
Knowledge gaps explained 12% of the variation. Technology limitations and pathophysiology not yet known load high on this factor, indicating the knowledge for an improved device is not yet at the point of advancement.
Surgery physicians had a higher knowledge gaps score than medicine physicians. There was no significant difference for the process barriers and ROI scores.
Discussion
Children and adults whose lives are influenced by RD need diagnostic and therapeutic devices tailored for their care. It has long been assumed, though not documented, that these device needs are largely unmet. This assumption is now corroborated by our national survey, with the vast majority (90%) of the study physicians noting at least one RD required a new or improved device.
The survey results are bolstered by a high response rate from a knowledgeable, experienced, and medically diverse physician cohort [10,11]. The entire eligible cohort had direct experience managing (98%) or knowledge of (2%) RD. Nearly three-quarters (73%) of those responding have experience with device development and/or device related clinical trials. The vast majority (89%) of those responding has practiced for more than 10 years and more than half (53%) have cared for more than 50 patients with RD within the past 2 years. Nearly all (over 30) medical and surgical specialties are represented with nearly half (47%) of those responding having a predominantly pediatric rare-disease practice (i.e., at least half of their rare-disease patients are ≤21 years old).
The project demonstrates the magnitude, heterogeneity, and ubiquity of the need. Over 850 diagnostic and/or therapeutic device needs were documented spanning 436 unique RD (see comprehensive report). Of the documented needs, 23% were specific to pediatrics and 51% had applicability to both adult and pediatric patients, yielding 74% of device needs pertaining to the pediatric population (see comprehensive report). Our statistical modeling was designed to understand whether physician characteristics, including medical or surgical specialty, pediatric or adult-based practice, or experience with RD, were associated with distinctions in noting device needs. Generally, the multivariate modeling did not reveal meaningful distinctions, reflecting the ubiquity of needs across the lifespan and across specialties. However, pediatric physicians were more than twice as likely (OR = 2.11, 95% CI 1.01–4.39, P = 0.046) and physicians with greater experience caring for patients with RD were nearly 4.5 times as likely (OR = 4.49, 95% CI 2.25–8.96, P < 0.0001) to report that new and improved devices are needed.
The study physicians indicate that device innovation is a key aspect necessary to optimize care for people with RD. In both the diagnostic and therapeutic device needs categories, physicians more often reported that a new device, versus modification or repurposing of a current device, is the optimal solution (70% and 67%, respectively). Speed (74%) and specificity (69%) were most often noted as the reason for needing innovative diagnostic devices.
Improved patient quality of life (86%) is the primary reason for needing innovative therapeutic devices. Nearly 50% of the new or improved diagnostic device needs and 57% of the new or improved treatment device needs are considered breakthrough advancements.
The data also support a subtle and underappreciated aspect of the perspectives of physicians. When generally asked about dissatisfaction regarding devices, the percent reporting dissatisfaction was relatively low: 35% noted dissatisfaction with diagnostic devices or tests and 61% noted dissatisfaction with therapeutic devices. However, when engaged about specific solutions and needs the percentage expressing a need for novel options was dramatically greater. We speculate that the high engagement in identifying diagnostic and treatment needs, despite relatively low dissatisfaction, is a reflection of physicians utilizing available resources, despite existing limitations, to care for their patients, while knowing that a new or improved device would enhance care options. For instance, 52% of respondents report currently using an FDA approved or cleared device in accordance with the indications for use for 239 diseases or conditions (see comprehensive report). Nevertheless, they list these same diseases or conditions as requiring a new therapeutic device to optimally serve their patients. Nearly 50% of the new or improved diagnostic devices and 57% of the new or improved treatment devices are considered a breakthrough advancement by the study physicians.
We also developed models to better understand the perceptions, their magnitude and interrelationships, regarding impediments to the development of devices for RD (Fig. 2). Distinctions between pediatric and adult physicians and medical and surgical physicians were investigated in these models. Overall, “costs of development” and “lack of profitability to industry” are the two largest independent perceived impediments (magnitude score 2.49 and 2.51, respectively). This finding is generally consistent with prior literature [12]. However, our evaluation elucidated two unique aspects. First, these two factors are among the top three rated impediments across all physician categories. Pediatric physicians rated lack of profitability and small heterogeneous populations as having the greatest influence (P = 0.0430 and 0.0003, respectively). Second, prior literature has noted government regulations as a significant impediment to pediatric device development [13]. Our study did not corroborate this finding; in this study population of physicians who are knowledgeable regarding government regulations, “government regulations” factored sixth (among 9) with a relatively low magnitude score (1.83) across all physician categories. In addition, to better understand the interrelationship between the 9 perceived independent impediments, exploratory factor analysis was conducted. This evaluation also demonstrated that issues associated with ROI remain the major perceived contributor to inadequate device development for people with RD.
Our study has several limitations. First, due to certain restrictions on government surveys, the study population includes only physicians associated with government entities. However, these physicians practice in public and private institutions, may work with industry, and, due to the selection process for the government groups with which they are affiliated, have a unique understanding of the regulatory, research, and economic issues associated with medical product development. Second, physicians practicing in academic medical centers are over-represented. However, physicians with expertise and experience regarding RD are relatively rare and more likely to practice at these centers [14]. Third, a significant portion of our survey respondents have medical device development experience, which might have helped them to parse out certain device development impediments. Their background could also be a source of bias toward dissatisfaction and wanting to develop new devices. Fourth, due to government policies associated with confidentiality we were unable to evaluate our nonresponder demographic. Nevertheless, we expect that the knowledge and experience of the nonresponders would be comparable due to the membership criteria for the four groups. Fifth, the study did not prioritize the elicited needs. Finally, the project did not solicit patient or industry input due to limited resources. Despite these limitations, the study achieved its primary goals.
Conclusion
This project, the first to comprehensively assess the unmet medical device needs as noted by physicians to improve care for people with RD, documents a public health issue potentially affecting millions of Americans. Addressing the unmet needs will require concerted efforts by a broad range of stakeholders to develop new and enhanced solutions that will improve medical product development for children and adults living with RD.
Footnotes
Contributor Information
Vasum Peiris, Center for Devices and Radiological Health, , Food and Drug Administration, , 10903 New Hampshire Avenue, , WO Building 66, Suite 5422, , Silver Spring, MD 20993.
Kui Xu, Office of Orphan Products Development, , Food and Drug Administration, , 10903 New Hampshire Avenue, , WO Building 32, Suite 5295, , Silver Spring, MD 20993.
Heather L. Agler, Center for Devices and Radiological Health, , Food and Drug Administration, , 10903 New Hampshire Avenue, , WO Building 66, Suite 5570, , Silver Spring, MD 20993
Eric A. Chen, Office of Orphan Products Development, , Food and Drug Administration, , 10903 New Hampshire Avenue, , WO Building 32, Suite 5295, , Silver Spring, MD 20993 , e-mail: eric.chen@fda.hhs.gov.
Rashmi Gopal-Srivastava, National Center for Advancing , Translational Sciences, , Office of Rare Diseases , Research at National , Institutes of Health, , 6701 Democracy Blvd, , Bethesda, MD 20817.
Brian M. Lappin, Office of Planning, , Food and Drug Administration, , 10903 New Hampshire Avenue, , WO Building 32, Suite 3352, , Silver Spring, MD 20993
Debra Y. Lewis, Office of Orphan Products Development, , Food and Drug Administration, , 10903 New Hampshire Avenue, , WO Building 32, Suite 5295, , Silver Spring, MD 20993
Gayatri R. Rao, Office of Orphan Products Development, , Food and Drug Administration, , 10903 New Hampshire Avenue, , WO Building 32, Suite 5295, , Silver Spring, MD 20993
Nomenclature
- RD =
Rare diseases
- ODA =
Orphan Drug Act
- FDA =
U.S. Food and Drug Administration
- NCATS/ORDR =
National Center for Advancing Translational Sciences/Office of Rare Diseases Research
- NIH =
National Institutes of Health
- NAWG =
Needs Assessment Working Group
- CDRH =
Center for Devices and Radiological Health
- HDE =
Humanitarian device exemption
- IRB =
Institutional Review Board
- HUD =
Humanitarian use devices
- ROI =
Return on investment
References
- [1].Global Genes, 2015, “ RARE Diseases: Facts and Statistics,” Global Genes, Aliso Viejo, CA, accessed May 22, 2018, http://globalgenes.org/rare-diseases-facts-statistics/
- [2].Genetic and Rare Diseases Information Center, 2018, “ Frequently Asked Questions,” Genetic and Rare Diseases Information Center, Gaithersburg, MD, accessed May 22, 2018, https://rarediseases.info.nih.gov/about-gard/pages/31/frequently-asked-questions
- [3].Rare Disease Impact Report, 2018, “Insights From Patients and the Medical Community,” Global Genes, Aliso Viejo, CA, accessed May 22, 2018, https://globalgenes.org/wp-content/uploads/2013/04/ShireReport-1.pdf
- [4].Institute of Medicine (U.S.), 2010, “Committee on Accelerating Rare Diseases Research and Orphan Product Development,” M. J. Field and T. F. Boat, eds., National Academies Press, Washington, DC, accessed May 22, 2018, http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2010/Rare-Diseases-and-Orphan-Products-Accelerating-Research-and-Development/Rare%20Disease%20Research%202010%20Report%20Brief.pdf
- [5].Department of Health and Human Services, Food and Drug Administration, 2018, “Report to Congress: Improving the Prevention, Diagnosis, and Treatment of Rare and Neglected Diseases,” FDA, White Oak, MD, accessed May 22, 2018, https://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/HowtoapplyforOrphanProductDesignation/ucm283745.htm
- [6].U.S. Food and Drug Administration, 2018, “Medical Device Advisory Committee,” Food and Drug Administration (FDA), Silver Spring, MD, accessed May 22, 2018, https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/default.htm
- [7].U.S. Food and Drug Administration, 2018, “Pediatric Advisory Committee,” Food and Drug Administration (FDA), Silver Spring, MD, accessed May 22, 2018, https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/default.htm
- [8].U.S. Food and Drug Administration, 2018, “Pediatric Device Consortia Grants Program,” Food and Drug Administration (FDA), Silver Spring, MD, accessed May 22, 2018, https://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/PediatricDeviceConsortiaGrantsProgram/
- [9].Rare Diseases Clinical Research Network, 2018, The National Center for Advancing Translational Sciences, Bethesda, MD, accessed May 22, 2018, https://www.rarediseasesnetwork.org/
- [10]. Braithwaite, D. , Emery, J. , de Lusignan, S. , and Sutton, S. , 2003, “ Using the Internet to Conduct Surveys of Health Professionals: A Valid Alternative?,” Fam. Pract., 20(5), pp. 545–551. 10.1093/fampra/cmg509 [DOI] [PubMed] [Google Scholar]
- [11]. Cunningham, C. T. , Quan, H. , Hemmelgarn, B. , Noseworthy, T. , Beck, C. A. , Dixon, E. , Samuel, S. , Ghali, W. A. , Sykes, L. L. , and Jette, N. , 2015, “ Exploring Physician Specialist Response Rates to Web-Based Surveys,” BMC Med. Res. Methodol., 15(1), p. 32. 10.1186/s12874-015-0016-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Buntz, B. , 2016, “ 5 Reasons Why Medical Device Innovation Is so Tough, Medical Product Manufacturing News Medtech Pulse,” The Medical Device and Diagnostic Industry newsletter, Santa Monica, CA, accessed May 22, 2018, http://www.qmed.com/mpmn/medtechpulse/5-reasons-why-medical-device-innovation-so-tough
- [13]. Bergsland, J. , Elle, O. J. , and Fosse, E. , 2014, “ Barriers to Medical Device Innovation,” Med. Devices, 7, pp. 205–209. 10.2147/MDER.S43369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Schieppati, A. , Henter, J. I. , Daina, E. , and Aperia, A. , 2008, “ Why Rare Diseases Are an Important Medical and Social Issue,” Lancet, 371(9629), pp. 2039–2041. 10.1016/S0140-6736(08)60872-7 [DOI] [PubMed] [Google Scholar]


