Abstract
Monogenic autoinflammatory disorders are an increasingly heterogeneous group of conditions characterised by innate immune dysregulation. Improved genetic sequencing in recent years has led not only to the discovery of a plethora of conditions considered to be ‘autoinflammatory’, but also the broadening of the clinical and immunological phenotypic spectra seen in these disorders. This review outlines the classification strategies that have been employed for monogenic autoinflammatory disorders to date, including the primary innate immune pathway or the dominant cytokine implicated in disease pathogenesis, and highlights some of the advantages of these models. Furthermore, the use of the term ‘autoinflammatory’ is discussed in relation to disorders that cross the innate and adaptive immune divide. The utilisation of next-generation sequencing (NGS) in this population is examined, as are potential in vivo and in vitro methods of modelling to determine pathogenicity of novel genetic findings. Finally, areas where our understanding can be improved are highlighted, such as phenotypic variability and genotype–phenotype correlations, with the aim of identifying areas of future research.
Keywords: Autoinflammatory, Interferonopathy, inflammasome, interferon, Periodic Fever Syndrome
Introduction
The phrase ‘autoinflammatory disease’ was proposed as an alternative to ‘autoimmune disease’ by McDermott et al. [1] in the paper identifying the genetic cause of Tumour Necrosis Factor (TNF) Receptor Associated Periodic Syndrome (TRAPS). This was considered a suitably representative term at the time, as individuals with inherited periodic fever syndromes had innate immune dysregulation but lacked high titres of autoantibodies and self-reactive T cells [1]. Familial Mediterranean Fever (FMF) was the only genetically defined periodic fever syndrome prior to this publication and the clinical and biochemical features appeared to be well defined [2,3].
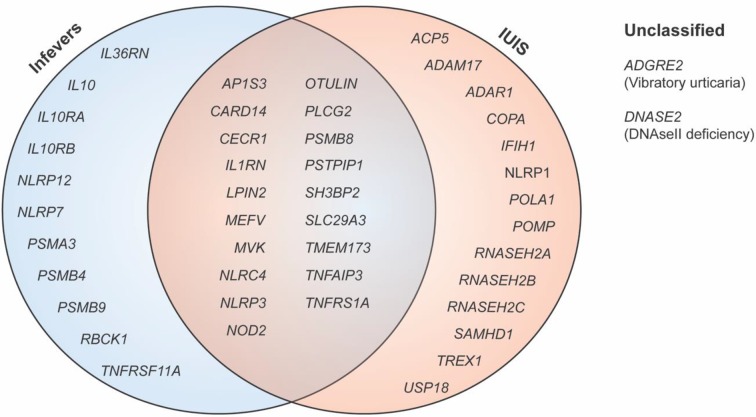
Since this time, over 30 conditions have been added to the list of monogenic autoinflammatory disorders. The significant broadening of clinical features, pathway perturbations and genes involved bring into question the utility of the original definition of these disorders, and whether an alternative is required that better encapsulates the spectrum of immune dysregulation seen. Highlighting the complexity of this task, the list of conditions considered ‘autoinflammatory’ by the International Union of Immunological Societies (IUIS) is incongruent with the Infevers database, a registry of mutations associated with autoinflammatory disorders maintained by the International Society for Systemic Autoinflammatory Diseases (ISSAID) (Figure 1) [4,5].
Figure 1. Genes involved in monogenic autoinflammatory disorders.
Genes involved in monogenic autoinflammatory disorders according to ISSAID as listed in the Infevers database, compared with the IUIS.
Classification
The term inflammasomopathy was introduced in the first review of autoinflammatory disorders categorising conditions based on the pathway implicated in disease pathogenesis [6], including disorders affecting inflammasomes, the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) pathway, the complement system, protein folding, ‘cytokine signalling’ and those resulting in or from macrophage activation. Since this time, a number of reviews have adopted a briefer version, with both inflammasome or interleukin (IL)-1β mediated disorders and NF-κB pathway-associated disorders universally included, but the other categories far less frequently [7]. The term ‘interferonopathies’ was first used to describe a group of monogenic disorders characterised by increased type I interferon (IFN) signalling in 2011 [8] but these disorders were only grouped with autoinflammatory disorders by the IUIS in 2017 [4]. No rationale for this change was provided, preventing a unified strategy of classification to be adopted those researching and managing these conditions.
The pathway model
The pathway model has the advantage of highlighting possible targets for treatment downstream of an abnormal protein, as with Janus kinase (JAK) inhibitors for individuals with stimulator of IFN genes (STING)-associated vasculopathy with onset in infancy (SAVI), as well as possible candidate genes for autoinflammatory disorders, such as that encoding a member of the linear ubiquitin assembly complex (LUBAC), SHARPIN. There are, however, limitations to this classification. A clear example is the case of mutations in TNFRSF1A causing TRAPS. While TNF receptor 1 (TNFR1) is a key receptor in the NF-κB pathway, the disease is not necessarily caused by increased signalling through this pathway alone [1,9–13]. A number of pathogenic mechanisms have been explored, such as defective shedding of TNFR1 [1], retention of TNFR1 in cytoplasmic aggregates with reduced surface expression [11], and abnormal apoptosis and signalling [12].
This classification also neglects the complex interaction between signalling pathways that exist. NF-κB translocation to the nucleus is important for the expression of pro-IL-1β and NOD-like receptor (NLR) pyrin domain containing 3 (NLRP3), and the consequence of NF-κB dysfunction on inflammasome activation cannot be discounted. Key players in the regulation of NF-κB are also implicated in the regulation of NLRP3, as seen with A20 and the possible role of inflammasome activation in the inflammatory manifestations of haploinsufficiency of A20 (HA20) [14]. Furthermore, although not yet shown in human cells, transforming growth factor (TGF)-β activated kinase-1 (TAK1) has been shown to regulate NLRP3, with spontaneous NLRP3 activation documented in TAK1-deficient murine macrophages [15]. The NF-κB and IFN pathways are also intimately linked, with a number of sensors leading to activation of both pathways. In the case of SAVI, literature to date suggests that the IFN pathway is dysregulated in this syndrome [16,17], but whether these two pathways are actually uncoupled in the case of an overactive STING in vivo is unclear. Breeding mutant STING mice to Irf3−/− mice did not rescue the inflammatory phenotype, raising questions, at least in the murine model, of the role of IFN regulatory transcription factor (IRF) 3 in the inflammation associated with SAVI [18]. Furthermore, the role of NF-κB as a member of the IFN-β enhanceosome [19], a multicomponent complex that optimises transcriptional activation of IFN-β, suggests that the pathways are closely connected.
The cytokine model
An alternative classification strategy is based on the primary cytokine dysregulated, either increased or decreased, in individuals with autoinflammatory disorders [20]. This is of potential therapeutic benefit as the primary cytokine driving disease can be therapeutically targeted. An example of this is the treatment of individuals with cryopyrin-associated periodic syndrome (CAPS). In the original manuscript linking Neonatal Onset Multisystem Inflammatory Disease (NOMID) to mutations in NLRP3, cell lysates from unstimulated monocytes of a case had high pro-IL-1β expression as determined by Western blot, and increased IL-1β mRNA in unstimulated peripheral blood mononuclear cells (PBMCs) when compared with healthy controls [21]. The empiric treatment of two cases with recombinant IL-1 receptor antagonist and the rapid resolution of symptoms within hours, and inflammatory markers within days, highlighted the role of IL-1β in the disease pathogenesis [22]. Having said this, the detection of IL-1β in serum of cases is difficult, with both cases and healthy controls having levels below the detection limit of currently available assays. Most publications looking at the IL-1β levels and response to treatment in individuals with CAPS culture PBMCs and measure cytokine release over a 24-h period. The spontaneous secretion of IL-1β by CAPS PBMCs decreases with the initiation of IL-1β-targeted therapy [23]. From this it is clear that even without elevated serum levels, a therapeutic response to IL-1β neutralising therapy suggests that this cytokine is important [23,24]. The response of individuals with colchicine-resistant FMF [25], mevalonate kinase deficiency (MKD) [26], and TRAPS [27] to the neutralising anti-IL-1β antibody canakinumab, suggests that IL-1β is a key cytokine in all of these disorders. Supporting this is evidence of increased expression of IL1B and IL1R1 as determined by microarray in individuals with TRAPS [28]. The gene expression profile of TRAPS moved towards the healthy control profile with canakinumab treatment [27]. More recently, the randomised, double-blind, placebo-controlled study of canakinumab in the above groups demonstrated efficacy in controlling and preventing disease flares [29]. An interesting addition to the literature was a retrospective analysis by Savic et al., of individuals with undifferentiated systemic autoinflammatory disorders who were treated with anakinra [30]. A total of 11 cases were identified over a 3-year period, and nine responded completely to treatment with anakinra within 4–6 weeks of commencement. Although individuals had undergone Sanger sequencing for NLRP3, MEFV, TNFRSF1A and NOD2 with no pathogenic mutations detected, the marked response to treatment suggests that genes in the IL-1β pathway could be further interrogated for variants that may be causing disease. Conversely, subjects could undergo a broader approach with whole exome sequencing (WES) or whole genome sequencing (WGS) and novel genes involved in the IL-1β pathway may be revealed.
Evaluation of the major cytokine/s involved in monogenic autoinflammatory disorders may point to distinctions between conditions within the same pathway. The gain of function mutations in inflammasome forming proteins that lead to disease can be presumed to cause an increase in IL-1β processing and release. Mutations in NLRC4 that result in an autoinflammatory phenotype are associated with markedly increased serum free IL-18 levels in cases when compared with healthy controls and individuals with CAPS [31,32,33].
There are also conditions that may involve pathways distinct from those used to categorise autoinflammatory disorders in the literature. Through the study of autosomal recessive generalised pustular psoriasis (GPP) in a number of multiplex families, Marrakchi et al. [34] identified homozygous missense mutations in IL36RN, which encodes the IL-36 receptor antagonist (IL-36Ra), causing deficiency in IL-36Ra (DITRA). IL-36 is a member of the IL-1 family of cytokines and, like IL-1β, acts via its receptor IL-36R and, in concert with IL-1 receptor accessory protein (IL1RAcP), signals to NF-κB through myeloid differentiation primary response 88 (MyD88). The binding of IL-36Ra to IL-36R prevents the association of IL1RAcP and downstream signalling. While there have been four case reports of the successful treatment of DITRA with anakinra therapy [35–38], therapeutic benefit has also resulted from TNF inhibition [39–41], IL-17 inhibition with secukinumab [42] and IL-12/IL-23 inhibition with ustekinumab [43–45]. This suggests that these agents may be targeting cytokines that are downstream of IL-36 [38]. The possibility of developing a therapeutic agent that is specific for IL-36 has been explored. Mbow et al. characterised a mouse anti-human antibody (MAB92) with high affinity to the IL-36 receptor that blocks signalling through this pathway [46]. Although highly specific for human IL-36R, the authors created MAB04, which cross-reacts with murine IL-36R for in vivo studies. Importantly, MAB04 inhibited imiquimod- and IL-36-induced skin inflammation in mice.
Deficiency in regulatory cytokines have also been described, and the clinical course of these individuals has been tumultuous. Homozygous mutations in IL10, IL10RA or IL10RB, leading to deficiencies in IL-10, IL-10Rα or IL-10Rβ respectively, have been reported to cause monogenic early onset inflammatory bowel disease (EOIBD) [47,48]. IL-10 has regulatory effects on the inflammatory response which are mediated through signal transducer and activator of transcription (STAT) 3, with IL-10-deficient mice developing chronic enterocolitis [49–51]. PBMCs from cases with loss of function of these proteins had higher proinflammatory cytokine responses to lipopolysaccharide (LPS) stimulation, including IL-6, TNF and IL-1β, when compared with healthy controls [48]. Although multiple agents have been trialled in these cases including corticosteroids, azathioprine, methotrexate, cyclosporine A and anti-TNF therapy, only mild clinical benefit has resulted [52]. A number of cases have undergone allogenic haemopoetic stem cell transplantation (HSCT) with marked improvement in their inflammatory bowel disease [52,53]. While recombinant human IL-10 replacement (rhuIL-10) in individuals with IL-10 deficiency would seem to be a therapeutic option, there have been issues with the response to and side effects from rhuIL-10 in trials of individuals with Crohn’s disease [54]. Furthermore, this option would not be effective in individuals with mutations in IL10RA or IL10RB. At this point in time, HSCT is the only curative option.
A number of other conditions are presumed to result from dysregulation of a particular pathway because of their cytokine profile, but little is known about the steps that lead to this alteration. Proteasome-associated autoinflammatory syndrome (PRAAS) is an autosomal recessive autoinflammatory disorder that encompasses conditions previously considered distinct entities: Nakajo-Nishimura syndrome (NKJO), joint contractures, muscular atrophy, microcytic anaemia, and panniculitis-induced lipodystrophy syndrome (JMPS), as well as chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome (CANDLE). Three publications identified mutations in PSMB8, the β5i catalytic component of the immunoproteasome, as the cause of disease [55–57]. Individuals with homozygous loss of function mutations in PSMB8 experienced spontaneous febrile episodes with features of muscle weakness, lipodystrophy as well as neutrophilic and lymphocytic infiltrative skin nodules and evidence of cerebral calcification [55–57]. Homozygous mutations were associated with poor proteasome assembly as well as reduced chymotrypsin-like activity and accumulation of ubiquitinated proteins in either Epstein–Barr virus (EBV)-transformed B cells or immortalised lymphoblastoid cell lines from cases [55–57]. In these early papers, increased serum IL-6 was noted in all cases, but the role of IFN was only identified later [58]. Liu et al. noted an almost 80-fold increase in IFN-γ-inducible protein 10 (IP-10) in cases compared with healthy controls and individuals with CAPS, prompting whole blood microarray analysis to determine the gene signature of these cases. The IFN pathway was the most differentially regulated pathway in individuals with PRAAS, further supported by stronger STAT1 phosphorylation in response to IFN-γ stimulation of monocytes when compared with healthy controls. These authors also highlighted cases with the clinical phenotype of PRAAS without PSMB8 mutations, later explored by Goldbach-Mansky et al. [59]. Digenic mutations involving PSMA3 or PSMB4 and PSMB8 or PSMB9, encoding constitutive proteasome subunits α7 and β7 or inducible subunits β5i and β1i respectively, were found in cases with the clinical diagnosis of PRAAS. One individual harboured a compound heterozygous mutation in PSMB4, and another a heterozygous mutation in POMP, encoding proteasome maturation protein. Similar to earlier reports, the mutant subunits were not efficiently assembled into the proteasome, resulting in reduced proteolytic activity. When compared with cases with homozygous PSMB8 mutations, the chymotrypic proteolytic activity was less impaired, but deficiencies were noted in tryptic and caspase proteolytic activity. Similar to homozygous PSMB8 mutations, there was inefficient clearing of ubiquitinated proteins and the presence of a type I IFN gene signature. Both siRNA models and proteasome inhibitors were used to recapitulate the IFN signature in PBMCs and fibroblasts. However, the mechanism/s by which proteasomal dysfunction leads to this response remains elusive. Classifying PRAAS by its IFN gene signature guides potential treatment considerations and also opens avenues for researchers to determine the role of the immunoproteasome in the IFN pathway.
Clarifying the dominant cytokine or pathway implicated in disease may lead to the development of targeted therapeutic strategies for autoinflammatory conditions, as reviewed in recent publications [60,61]. Recent work has looked at individuals with interferonopathies treated with JAK1/2 inhibitor baricitinib. Initial work was performed by Goldbach-Mansky et al. to determine a dosing regimen for paediatric cases with interferonopathies [62]. This work was then extended with treatment of a number of individuals with interferonopathies including PRAAS and SAVI and longitudinal assessment of response and adverse reactions [63]. Most patients were able to reduce their prednisolone requirements and five out of ten PRAAS cases achieved remission. Adverse events included upper respiratory infections, gastroenteritis and BK viremia. Given that only 18 patients were recruited over a span of 6 years, more work is needed to establish the clinical efficacy and adverse reaction profile in this selected population. The availability of this drug on compassionate grounds through NCT01724580 suggests that more information will become available in the future with ongoing recruitment and follow-up of these cases.
Similarly, elucidating elevated serum free IL-18 levels in an individual with autoinflammation with infantile enterocolitis, an NLRC4-associated autoinflammatory disease, lead to the successful therapeutic trial of a recombinant IL-18 binding protein (rhIL-18) [64]. Following from this, there is a Phase 3 randomised, double-blind, placebo-controlled trial of rhIL-18 in NLRC4-associated autoinflammatory diseases (NCT03113760) as well as an open-label extension (NCT03512314) underway. This not only highlights the importance of identifying driver or dominant cytokines for possible therapeutic manipulation, but also the potential differences between inflammasome effector and regulatory mechanisms as well as possible epigenetic factors, that results in one cytokine dominating over another.
Autoinflammation, autoimmunity and immune deficiency
The boundaries of what is classified as an autoinflammatory disorder are also being blurred. The strict definition of innate immune dysregulation without self-reactive T cells or high titres of autoantibodies is increasingly in question, especially when one considers interferonopathies such as Aicardi–Goutieres syndrome (AGS). AGS was originally described in the 1980s as a disorder of the central nervous system (CNS) associated with lymphocytosis on cerebrospinal fluid analysis and bilateral basal ganglia calcifications [65]. The genetic causes of AGS are numerous, and all involve the processing of nucleic acid, either self or foreign, in the cytoplasm. Loss of function mutations in genes encoding deoxyribonuclease three prime repair exonuclease 1 (TREX1) [66], deoxynucleoside triphosphate triphosphohydrolase SAM and HD domain containing protein 1 (SAMHD1) [67], ribonuclease components ribonuclease H2 (RNASEH2) A (RNASEH2A), RNASEH2B or RNASEH2C [68], or adenosine deaminase acting on RNA 1 (ADAR1) [69] have been identified in individuals with AGS. The link between AGS and autoimmunity was initially made when Aicardi and Goutières speculated that the phenotype of two individuals with infantile systemic lupus erythematosus overlapped AGS considerably. They hypothesised that the two may be either the same condition or linked by an increase in IFN-α [70]. The phenotypic link has subsequently been highlighted by a number of groups [71,72], although the number of subjects in a large cohort of 374 mutations confirmed that AGS with clinically diagnosed lupus was low [73]. An abnormal serum autoantibody profile was seen in a minority of individuals with AGS in one cohort study [74], however another detected persistent antinuclear antibodies (ANA) or autoantibodies against extractable nuclear antigens (ENA), dsDNA and cardiolipin in the majority of their cases with mutation confirmed AGS [72]. Subsequent work using multiplex autoantibody microarrays identified unique autoantibodies in cases with AGS [75]. Whether this condition, and indeed other interferonopathies, should be considered autoimmune or autoinflammatory is a matter of debate. AGS highlights that this distinction is not always clear.
Indeed, the spectrum of immune dysregulation and overlap between autoimmunity, autoinflammation and immune deficiencies has been seen in a number of recently described conditions. Homozygous loss of function mutations in HOIL1 or HOIP have been described in cases with evidence of systemic inflammation, susceptibility to pyogenic infections, and amylopectinosis [76,77]. The original description by Boisson et al. highlighted the importance of haem-oxidised IRP2 ubiquitin ligase 1 (HOIL1) in maintaining the stability of the LUBAC, involved in the ubiquitination of components in the NF-κB pathway, as well as promoting the association of inhibitor of NF-κB kinase subunit γ (IKKγ) with TNF or IL-1 receptor signalling complexes [76]. The authors noted cell-type specific defects associated with HOIL1 deficiency. Fibroblasts and EBV-immortalised B cells from subjects displayed impaired canonical NF-κB pathway activation in response to TNF or IL-1β as well as partial impairment of the response to toll-like receptor (TLR) stimuli. The inflammatory phenotype was determined to originate from monocytes, with monocytes displaying hyper-responsiveness to IL-1β in terms of inflammatory cytokines produced compared with healthy control monocytes. The clinical and cellular phenotypes in the HOIL1-deficient cases overlap with those seen in an individual with homozygous loss of function mutations in HOIP [77]. The publication of a series of ten cases from eight families with polyglucosan storage myopathy harbouring either homozygous or compound heterozygous mutations in HOIL1 [78] suggests that mutations in this gene, and potentially other components of LUBAC, may also present with a more limited clinical phenotype, and that much remains to be learnt about genotype–phenotype correlations in these disorders.
Classifying these as autoinflammatory diseases inherently fails to acknowledge the associated immunodeficiency, and vice versa. A similar problem concerns the conditions caused by mutations in PLCG2 encoding phospholipase c γ-2 (PLCγ2), PLCγ2-associated antibody deficiency and immune dysregulation (PLAID) and autoinflammation and PLAID (APLAID) [79,80]. PLCγ2 was linked to autoimmune and autoinflammatory diseases initially through an N-ethyl-N-nitrosourea (ENU) mutagenesis screen [81]. A heterozygous point mutation in PLCG2 in mice led to spontaneous inflammation, arthritis and dermatitis with evidence of immune complex driven glomerulonephritis. Subsequently, by sequencing three families with dominantly inherited cold-induced urticaria, antibody deficiency and autoimmunity, in-frame deletions in PLCG2 were identified and shown to segregate with disease [79]. These deletions affected the autoinhibitory C-terminal Src-homology 2 domain and resulted in constitutive phospholipase activity. Interestingly, and somewhat contradictory to the increased activity of PLCγ2, B cells and natural killer (NK) cells demonstrated reduced calcium flux and reduced phosphorylation of mitogen-activated protein kinase (MAPK) in response to stimulation with either IgM cross-linking or cross-linking of activating receptors respectively. This was determined to be temperature specific, however, with increasing MAPK pathway phosphorylation and cytosolic calcium in response to decreasing temperatures. This description was quickly followed by one of a family with a dominantly inherited autoinflammatory condition who had a missense mutation in PLCG2. Unlike the previous report, the individuals had no evidence of autoimmunity, but did have hypogammaglobulinaemia and markedly reduced class switched memory B cells in addition to inflammatory manifestations in the form of skin inflammation and granulomata, enterocolitis, bronchiolitis and uveitis [80]. Of the two cases described, neither had cold-induced symptoms. Increased baseline PLCγ2 activity was noted in an overexpression COS-7 cell model. Chae et al. [82] progressed the understanding of the inflammatory manifestations of APLAID by showing that the increased activity of PLCγ2 and subsequent increase in inositol and release of Ca2+ from ER stores, previously established by Kurosaki and Tsukada [83], resulted in increased NLRP3 activation and IL-1β release when assessing PBMCs from cases compared with healthy controls. It would be interesting to determine whether the same increase in NLRP3-driven IL-1β is seen when PBMCs from PLAID subjects are examined, as their inflammatory phenotype was not as profound, and was temperature dependent. Furthermore, the partial response to IL-1β-targeted therapy [80] suggests that there may be more than NLRP3 driving the inflammatory disease.
Clearly, more information is needed to tease out the different immunological consequences of mutations in PLCG2. This is, of course, not unique to this autoinflammatory disease. With the description of rare disorders and involvement of novel genes and mutations, one can expect the phenotypic spectrum to evolve as more cases are reported. In the case of deficiency of adenosine deaminase 2 (DADA2), homozygous loss of function mutations in CECR1 were found in individuals with polyarteritis nodosa [84] as well as early onset stroke, vasculopathy and febrile episodes [85]. Although immunodeficiency and autoimmunity were not a major feature, IgM deficiency was noted in a number of cases [85]. Treatment of ten individuals with TNF targeting therapy by Levy-Lahad et al. led to significant clinical improvement, highlighting the role of this cytokine in disease pathogenesis [84]. The response to TNF directed therapy has since been reproduced [86,87]. In a subsequent study of 48 cases with polyarteritis nodosa associated with livedo reticularis and/or strokes, Gattorno et al. performed Sanger sequencing of CECR1 and determined that 15 cases harboured homozygous or compound heterozygous mutations [88]. Since the time of the original description, there has been an expansion of the clinical phenotype of cases with DADA2, from cytopaenias and pure red cell aplasia [89], to lymphoproliferative disease [90–92], and combined immune deficiency, as well as common variable immune deficiency (CVID) [93]. Indeed, a cohort study of 181 cases with antibody deficiency diagnosed 11 individuals with mutation and enzyme activity confirmed DADA2 [94]. An interesting finding in this group was that anti-TNF therapy resulted in an improvement in IgM levels in one case, and there was an inverse correlation between c-reactive protein (CRP) and IgG in another. Further complicating the potential mechanisms of this disease, individuals with DADA2 have also been reported to have an IFN gene signature [95,96]. Researchers investigated individuals with features overlapping with AGS-5 (caused by mutations in SAMHD1). In each report, cases had enhanced IFN stimulated gene expression. These cases were treated with a range of immunosuppressive agents but had not been trialled on anti-TNF therapy. Given the reports of profound response in individuals with DADA2 to this therapy, it would be interesting to determine whether the IFN gene signature is abrogated with the use of anti-TNF therapy.
Furthermore, a number of conditions classified as disorders of predominantly antibody deficiency by the IUIS have marked autoinflammatory features. The syndrome of sideroblastic anemia with B-cell immunodeficiency, periodic fevers and developmental delay (SIFD) was first described by Wiseman et al. in 2013 [97], with 11 out of the 12 cases described experiencing periodic fevers. It was subsequently determined to be caused by homozygous or compound heterozygous mutations in TRNT1 [98], encoding the CCA-adding enzyme tRNA nucleotidyltransferase [99]. Aksentijevich et al. investigated the inflammatory phenotype of these cases, noting markedly elevated acute phase reactants and inflammatory cytokines in cases with active disease [100]. The authors documented reduced expression of mature cytosolic tRNA, as well as increased reactive oxygen species when corrected for live cells in fibroblasts after 72 h compared with healthy controls. Using an siRNA knockdown THP-1 cell model, TRNT1-knockdown cells demonstrated increased IL-1β production at baseline and in response to LPS which was reversed with the small molecule NLRP3 inhibitor MCC950, suggesting an NLRP3-dependent inflammatory phenotype.
Autoinflammatory disease classification summary
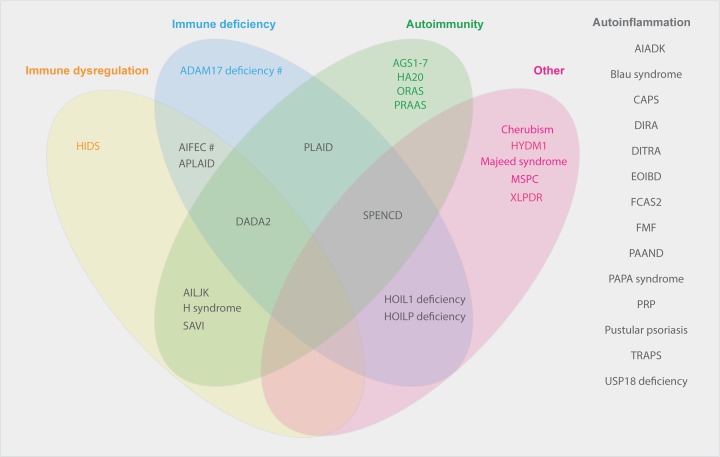
From the above discussion, it is apparent that there are significant barriers to a simple definition or classification criteria for what are considered monogenic autoinflammatory disorders. As research progresses, the inflammatory component of disorders previously considered to be primarily of immune deficiency or autoimmunity will become more apparent. Provided in Figure 2 is a summary of conditions listed as autoinflammatory disorders in the latest IUIS Expert Committee for Primary Immunodeficiency (2017) as well as the Infevers database, documenting the spectrum of immunological manifestations recognised to date.
Figure 2. Phenotypic spectrum of monogenic autoinflammatory disorders.
Abbreviations: AIADK, autoinflammation with arthritis and dyskeratosis; AID, autoinflammatory disorder; AIFEC, autoinflammation with infantile enterocolitis; AILJK, autoimmune interstitial lung, joint and kidney disease; DIRA, deficiency of IL-1 receptor antagonist; FCAS2, familial cold autoinflammatory syndrome 2; HIDS, hyper IgD syndrome; HYDM1, hydatidiform molar pregnancy; MSPC, multiple self-healing palmoplantar carcinoma; ORAS, otulin-related autoinflammatory syndrome; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; PAPA, pyogenic arthritis, pyoderma gangrenosum and acne; PRP, pityriasis rubra pilaris, SPENCD, spondyloenchondrodysplasia; XLPDR x-linked pigmentary disorder, reticulate, with systemic manifestations. #Susceptibility.
Genetic sequencing of autoinflammatory disorders
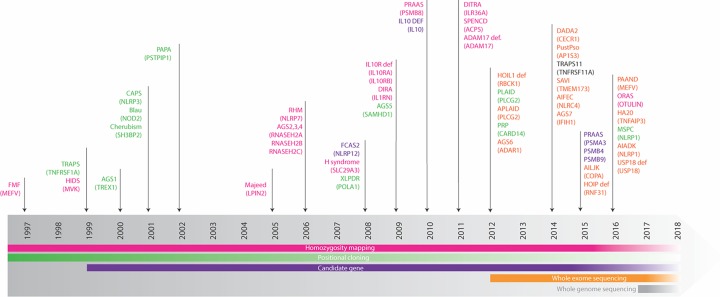
The phenotypic heterogeneity of what is considered a monogenic autoinflammatory disorder, as well as the explosion in the number of newly described conditions, coincides with advances in genetic sequencing techniques. The gene mutated in FMF was determined to be MEFV using positional cloning methods in 1997, and since this time many more disease-causing genes have been recognised (Figure 3). Next-generation sequencing (NGS) technology has been used in the description of these conditions since 2012, starting with the identification of RBCK1 as the gene implicated in HOIL1 deficiency [76].
Figure 3. Timeline of monogenic autoinflammatory disorder discovery and genetic sequencing technique used.
Abbreviations: AIADK, autoinflammation with arthritis and dyskeratosis; AIFEC, autoinflammation with infantile enterocolitis; AILJK, autoimmune interstitial lung, joint and kidney disease; DIRA, deficiency of IL-1 receptor antagonist; Dysreg, dysregulation (including lymphoproliferation); FCAS2, familial cold autoinflammatory syndrome 2; HIDS, hyper IgD syndrome; HYDM1, hydatidiform molar pregnancy; MSPC, multiple self-healing palmoplantar carcinoma; ORAS, otulin-related autoinflammatory syndrome; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; PAPA pyogenic arthritis, pyoderma gangrenosum and acne; RAAS, proteasome associated autoinflammatory syndrome; PRP, pityriasis rubra pilaris; SPENCD, spondyloenchondrodysplasia; XLPDR, x-linked pigmentary disorder, reticulate, with systemic manifestations. Adapted and updated from [101] with permission granted by Springer Nature, licence number: 4371831027106.
NGS
The now widely adopted NGS, also known as massive parallel or deep sequencing, is a broad term encompassing a number of different technologies that share the ability to generate and analyse millions of sequences per run. There are a large number of platforms on which NGS can be performed, and the specifics of the sequencing method varies depending on the instrument used [102]. In general, the sequencing process involves the preparation of a library of short DNA fragments through either enzymatic or sonication techniques. These short strands of DNA are then ligated to generic adapters in vitro. PCR amplification follows, performed using either emulsion PCR in oil–water emulsion micelles, or bridge PCR on a solid surface coated with complementary primers. Subsequent sequencing of the amplicon is performed by either pyrosequencing, sequencing by ligation or sequencing by synthesis. The large number of short reads generated from this process must then be aligned against a reference sequence. A plethora of software have been developed not only to align the reads, but to also determine where deviations from a reference sequence exist. Furthermore, considering that WES or WGS of an individual identifies 20000 or 4000000 variants respectively, an appropriate filtering strategy must be employed to determine which of these variants are potentially pathogenic [103,104].
NGS has been employed in the diagnostic evaluation of individuals with autoinflammatory disorders. Ceccherini et al. compared the performance of three NGS platforms in a pilot study interrogating 10 genes (MEFV, MVK, TNFRSF1A, NLRP3, NLRP12, NOD2, PSTPIP1, IL1RN, LPIN2 and PSMB8) from 50 cases with genetically confirmed autoinflammatory disorders [105]. The expected mutations were correctly called in most cases, although there was a failure to detect p.Val377Ile MVK in a number of cases due to low coverage. Additional variants were also noted, a number of which were false positives and detected on only one of the three platforms used. Importantly, true positive incidental variants did not alter the clinical diagnosis or management of the individual. Taking a different approach, Nakayama et al. [106] prospectively recruited individuals with a clinical diagnosis of an autoinflammatory disorder prior to any genetic testing. Using an MiSeq platform developed in house, they sequenced 9 genes (IL1RN, MEFV, MVK, NLRP12, NLRP3, NOD2, PSMB8, PSTPIP1 and TNFRSF1A) in 108 cases. A total of 27 missense mutations were identified and confirmed with Sanger sequencing. Unfortunately, the authors did not outline any genotype–phenotype correlation, nor did they include positive controls to ensure that all pathogenic mutations were detected. A further addition to the literature was by Omoyinmi et al. [107] with their development of a vasculitis and inflammation panel targeting up to 166 genes. Initially, 16 samples with known pathogenic mutations were analysed and the best performing pipeline carried over to the assessment of individuals with unknown diagnosis. Pathogenic mutations were detected in 12% of cases, and likely pathogenic variants in 22%. Furthermore, the depth of coverage was sufficient to be able to detect a 3% somatic mosaicism in NLRP3.
Somatic mosaicism
Somatic mosaicism in NLRP3 causing disease was first described in 2005 in an individual with a p.Tyr507Cys variant occurring at a frequency of 16.7% detected using Sanger sequencing [108]. Somatic mosaicism in NLRP3 has since been reported by multiple groups with a mutation frequency as low as 2.7% noted [109–116]. Importantly, a recent study highlighted that NGS was able to detect somatic NLRP3 mutations in eight individuals symptomatic of CAPS who had previously tested negative for mutations in NLRP3 sequencing using Sanger techniques [116]. Retrospective review of the Sanger chromatogram identified small peaks in only three of the eight cases, each with an allele frequency of greater than 10%, suggesting that Sanger sequencing is not a sensitive technique for detecting low frequency somatic mosaicism.
Autoinflammatory genetics summary
While the use of NGS panels for the diagnosis of autoinflammatory disorders in the clinical setting is increasing, the key limitation from a research perspective is the inability to discover new disease-causing genes. In using WES or WGS, novel variants in genes known to cause disease, and also variants in novel genes, may be uncovered. The rationale for the use of WES is based on the finding that the majority of pathogenic variants causing Mendelian diseases that have been identified to date are located in protein-coding regions [117–119]. While WGS has the benefit of capturing introns and intergenic regions, and detecting copy number variants [104], a large volume of data must be interrogated, and the bioinformatics analysis is complex. Both strategies raise the possibility of detecting an incidental finding that has implications for the health of the individual and their family. Furthermore, neither method negates the requirement for the validation of pathogenicity of a novel variant.
Modelling monogenic autoinflammatory disorders
Modelling genetic findings experimentally is of great importance in determining the clinical significance of a novel variant. In recent years, many groups have taken advantage of clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 gene editing techniques. CRISPR/Cas9 gene editing utilises features of an adaptive immune response seen in bacteria and archaea.
CRISPR approaches for functional validation
CRISPR/Cas9 techniques have improved the ability to create models of diseases caused by point mutations. Previously, creating point mutations in mice would require homologous recombination in embryonic stem cells, a lengthy and expensive process to generate a homozygous mouse strain [120]. In vivo editing with CRISPR/Cas9 has allowed for genome editing of fertilised mouse eggs [121,122]. Briefly, plasmids with DNA encoding the editing tools, including the guide RNA and Cas9, are injected into the cytoplasm of a one-cell embryo, generating a target-specific double-strand break (DSB). The subsequent repair of this break is mediated by non-homologous end joining (NHEJ) or homology directed repair (HDR). The former often results in frameshift mutations and loss of function. HDR, on the other hand, can result in substitutions, insertions or deletions if the one-cell embryo is co-injected with a single-strand oligonucleotide (ssOligo) that acts as a template. Soon after this technique was first published, the efficiency of gene disruption by frameshift mutations through NHEJ using this method was reported to be approximately 80–90% whereas introducing a point mutation through HDR is approximately 50–80% [122,123].
Introduction of point mutations in human cells using CRISPR/Cas9 techniques has also been described. Early reports of CRISPR/Cas9 editing in HEK293T cells demonstrated NHEJ efficiency of up to 33% [124–127], but HDR efficiency of only 3–8% [126]. Improved efficacy was noted through cell synchronisation techniques that control the timing of delivery of single guide RNA (sgRNA) and ssOligo to HEK293T cells, with HDR in up to 38% of cells [128]. Various strategies have also been employed in an attempt to improve efficiency in cells that are difficult to nucleofect, a process by which components of the editing system are delivered to the nucleus of the target cell, including the use of a ssDNA template provided by recombinant adeno-associated virus (rAAV) [129,130]. The process, however, remains less efficient than NHEJ and its widespread application in research is still limited.
A significant recent addition to the literature has been the description ‘base editors’, able to create point mutations in human cell lines without generating a DSB. In the initial descriptions, a catalytically dead Cas9 was fused to a cytidine deaminase enzyme, with the unit guided to a locus of interest with an sgRNA [131,132]. This complex allowed for a targeted C•G to T•A substitution in human and murine cell lines in up to 40% of total sequencing reads, with a maximum base editing yield possible of 50%. Subsequent base editors have included the adaptation of tRNA adenosine deaminase to edit DNA, allowing for A•T to G•C conversion [133]. The significance of this development in the potential for disease modelling and future gene editing was highlighted by the correction of the c.845G>A HFE mutation implicated in hereditary haemochromatosis in an immortalised-lymphoblastoid cell line [133]. Despite the difficulty with which these cells are transfected, an efficiency rate of 28% was noted, with no off-target effects. Similar to the process of CRISPR/Cas9 with HDR, this technique is not yet used widely. However, significant advances in a short period of time suggests that either may become a routine method for modelling diseases caused by point mutations.
Autoinflammatory disease models using mice
In vivo murine models have provided great insight into disease pathology given genetic homologies between humans and mice and the ability to create transgenic, knockout and knockin mice. For example, the initial murine model of CAPS published in 2009 by Hoffman et al. recapitulated the IL-1β-mediated inflammation [134]. Furthermore, a number of teams have used murine models to explore the skeletal consequences of CAPS [135,136]. More recently, the generation of Nlrp3 mutant mice on Il1b/Il18, casp-1/casp-11 or Tnf-deficient backgrounds raised the possible role of TNF in CAPS disease pathology [137]. There are, however, shortcomings here. The recent attempt to model LPS-responsive and beige-like anchor protein (LRBA) deficiency using Lrba−/− mice, which in humans cause a range of manifestations including autoimmunity, hypogammaglobulinaemia, organomegaly and chronic diarrhoea [138], failed to recapitulate the clinical or immunological phenotype [139,140]. Furthermore, differences between murine and human pyrin led to many years of work predicated on pyrin as anti-inflammatory, rather than an inflammasome forming protein. Modelling of disorders that act through the pyrin pathway, such as Pyogenic Arthritis, Pyoderma gangrenosum and Acne (PAPA) syndrome caused by mutations in PSTPIP1 may thus be similarly problematic. Additionally, humanised mouse models, using immunodeficient mice engrafted with human haematopoietic cells, are useful in the study of haematopoiesis, but have been limited in the investigation of the innate immune system due to quantificative and functional deficiencies of a number of cells including monocytes and macrophages [141].
Autoinflammatory subject derived induced pluripotent stem cells
An alternative method of modelling autoinflammatory disorders is the use of subject-derived induced pluripotent stem cells (iPSCs). This method of reprogramming somatic cells to pluripotency [142] allows for indefinite propagation as well as differentiation to a variety of human cell types that would previously have been unobtainable [143–145]. Saito et al. utilised this method in the investigation of autoinflammatory disorders involving NLRP3 [146] and NLRC4 [147]. Two individuals with somatic mutations in NLRP3 had both wild-type (WT) and mutant NLRP3 iPSC lines generated [146]. The WT iPSC lines served as a comparator, with the authors able to determine that only macrophages differentiated from mutant NLRP3 iPSC lines showed abnormal IL-1β secretion. A subsequent publication generated iPSC lines from an individual suspected of CAPS but without pathogenic mutations in NLRP3 [147]. Heterogeneous responses to LPS stimulation in the iPSC clones prompted WES, with clones having a robust response to LPS possessing a mutation in NLRC4. Subsequent deletion of NLRC4 using CRISPR/Cas9 techniques in the mutant clones abrogated the enhanced response to stimulation, indicating that the mutation was likely pathogenic. As the frequency of the mutation was later determined to be approximately 63%, WES would most likely have identified this as a candidate variant of interest. Furthermore, despite its promise, the generation of a cell line from case samples demands expertise, as well as specific ethical considerations. It also requires access to case samples which may be difficult in the case of critically ill individuals who succumb to disease prior to genetic evaluation.
Having said this, the use of these cells has the potential to overcome a significant limitation in the modelling of autoinflammatory disorders to date. Using either the genetic manipulation of healthy iPSCs or subject-derived iPSCs, it is possible to explore the effect of a genetic mutation on a variety of cell types. For example, in our recent paper exploring a novel NLRC4 variant, THP-1 monocyte like cells were used to model and the variant was determined to be pathogenic [31]. iPSCs would have permitted exploration of the effect of this variant on NK cells and T cells, allowing for addressing questions that remain unanswered including the pathogenesis of macrophage activation syndrome in this population. Likewise, the use of iPSCs differentiated to keratinocytes would allow interrogation of mechanisms of the dermatological manifestations in specific autoinflammatory disorders. As summarised in Table 1, most monogenic autoinflammatory disorders described to date have not had immunological assessment of multiple cell types.
Table 1. Monogenic autoinflammatory disorder summary table.
| Condition | Gene/s | Protein | MOI | GOF/LOF | Pathway | Cytokine group | System involved | Human cell model | Potential murine model | Reference/s |
|---|---|---|---|---|---|---|---|---|---|---|
| ADAM17 deficiency | ADAM17 | ADAM17 | AR | LOF | Unknown | Unknown | Skin GIT | PBMC: ↓TNF-α response to LPS, PMA + anti-CD3/anti-CD28 antibodies | Nil | [148] |
| AGS1 | TREX1 | TREX1 | AR or AD | LOF | IFN | T1IFN | CNS | Human neural stem cell-derived astrocytes, primary astrocytes, brain-derived endothelial cells: shRNA knockdown → IFN gene signature + ↑ proinflammatory cytokines | Trex1–/– mice | [66,149–151] |
| AGS2 | RNASEH2B | RNASEH2B | AR | LOF | IFN | T1IFN | CNS | Rnaseh2b knockout first [KOF] mice | [68,74] | |
| AGS3 | RNASEH2C | RNASEH2C | AR | LOF | IFN | T1IFN | CNS | Rnaseh2c−/− mice | [68,74,152,153,154] | |
| AGS4 | RNASEH2A | RNASEH2A | AR | LOF | IFN | T1IFN | CNS | Human neural stem cell-derived astrocytes, primary astrocytes, brain-derived endothelial cells: shRNA knockdown minimal change in ISG/IFN cytokine profile | Rnaseh2aG37S/G37S | [68,74,149] |
| AGS5 | SAMHD1 | SAMHD1 | AR | LOF | IFN | T1IFN | CNS | HeLa cells: transfection of mutant SAMHD1 showed abnormal localisationHuman neural stem cell-derived astrocytes primary astrocytes, brain-derived endothelial cells: shRNA knockdown → IFN gene signature + ↑ proinflammatory cytokines | Samhd1−/− mice | [67,73,149,155–157] |
| AGS6 | ADAR1 | ADAR1 | AR | LOF | IFN | T1IFN | CNS |
HEK293Tcells: IFN reporter assay Lymphoblastoid cell line: ↓ ADAR1 expression of mutant c/w WTHuman neural stem cell-derived astrocytes, primary astrocytes, brain-derived endothelial cells: shRNA knockdown minimal change in ISG/IFN cytokine profile |
Adarf/− SCL-Cre-ERT * mice | [149,158] |
| AGS7 | IFIH1 | MDA5 | AD | GOF | IFN | T1IFN | CNS | HEK293T: IFN reporter assay | Ifih1gs/+ mice | [159–161] |
| AIADK | NLRP1 | NLRP1 | AD | GOF | Inflam | IL-18 ?IL-1β |
Multiple | Nlrp1aQ593P mice* | [162,153] | |
| AIFEC | NLRC4 | NLRC4 | AD | GOF | Inflam | IL-18 | Multiple |
Monocytes: ↑IL-1β in response to PrgI Monocyte-derived macrophages: ↑cell death, ↑IL-1β, IL-18 with LPS priming + flagellin HEK293T cells: ASC speck analysis and inflammasome reconstitution iPSCs: ↑IL-1β, IL18 to LPS |
mu-NLRC4 transgenic mice | [147,32,163–165] |
| AILJK | COPA | COPA | AD | Dominant negative | ?NF-κB ?IFN |
Multiple incl lungs, kidney |
CD4 T cells: skewing to Th17 response BLCL: ↓ autophagy, ↑ transcription IL-1 β, IL-6, IL-23 HEK293T cells: siRNA knockdown → ↑ER stress |
Nil | [166,167] | |
| APLAID | PLCG2 | PLCγ2 | AD | GOF | Unknown ?Inflam ?NF-κB |
Unknown ?IL-1β |
Multiple |
PBMC: ↑response to NLRP3 activation B cells: ↑ERK phosphorylation |
Multiple* | [80,82] |
| Blau syndrome | NOD2 | NOD2 | AD | GOF | NF-κB | Multiple TNFα |
Multiple |
HEK293T cells: NF-κB luciferase assay. ↑ activity with transfection of mutants PBMC: single patient w novel variant ↓ NF-κB response |
Nod22939ic mice | [168–170] |
| CAPS |
NLRP3 |
NLRP3 | AD | GOF | Inflam | IL-1β | Multiple |
PBMC: constitutively high IL-1β secretion, as well as IL-6 + TNF THP1 cells: ↑ IL-1β and IL-18 when transduced with mutant c/w WT CD4 T cells: α-CD3 + α-CD46 stimulation →↑ IL-1β in patient cells c/w WT |
Nlrp3A350VneoR/+
Nlrp3L351PneoR/+ mice Nlrp3R258W mice |
[21,154,171–173] |
| Cherubism | SH3BP2 | SH3BP2 | AD | ? GOF ? dominant negative |
? NF-κB ? NFATc1 |
TNF-α | Bone | Sh3bp2 P416R/+ mice | [174,175] | |
| DADA2 | CECR1 | ADA2 | AR | LOF | Unknown | ?T1IFN ?TNFα |
Multiple incl vascular |
PBMC: ↑ B cell death when cultured without stimulation Monocytes: differentiate into M1>M2 |
Nil | [84,85,92,93] |
| DIRA | IL1RN | IL-1Ra | AR | LOF | IL-1β | Multiple | Multiple bone | Mononuclear cells: stimulation with IL-1β → ↑ IL-1α, MIP1α, TNFα, IL-8, IL-6 c/w WT | Il1ra−/− mice* | [176,177,178,179,180,181,40–42] |
| DITRA | IL36RN | IL-36Ra | AR | LOF | Other | IL-36 | Skin | PBMC: IL-36A stimulation → ↑ IL-1α, IL-6, IL-8, TNFα c/w WT | IL1F6 transgenic, IL1F5−/− mice | [182] |
| EOIBD | IL10, IL10RA, IL10RB | IL10, IL10RA, IL10RB | AR | LOF | Other | IL-10 | GIT | PBMC: Failure of IL-10 to ↓ LPS induced TNF α in patients with receptor mutations; More rapid TNF α response to LPS; Failure to phosphorylate STAT3 in response to IL10 |
IL10Trunc/Trunc mice*
IL-10−/− Cx3cr1gfp/+ mice |
[47,48,51,183] |
| FCAS2 | NLRP12 | NLRP12 | AD | LOF | NF-κB ? Inflam |
TNFα, IL-6, IL-1β | Skin Multiple |
HEK293T cells: NF-κB luciferase assay PBMC: ↑ spontaneous TNF-α, IL-6, IL-1β c/w WT |
NLRP12−/− mice | [184–186] |
| FMF | MEFV | Pyrin | AR>>AD | GOF | Inflam | IL-1β | Multiple |
PBMC: no spontaneous IL-1β secretion when cultured. ↑ IL-1β in response to LPS (inconsistent). Anti-CD3/CD28 stimulation → ↑ IL-17 and IL-22 Neutrophils: possible release of IL-1β through NETS |
MefvM680I/M680I mice MefvM694V/M694V mice MefvV726A/V726A mice |
[178,179,187,188] |
| H syndrome | SLC29A3 | SLC29A3 | AR | LOF | Unknown | Unknown | Multiple | ENT3–/– mice | [189,190] | |
| HA20 | TNFAIP3 | A20 | AR | LOF | NF-κB Inflam |
Multiple |
HEK293T cells: NF-κB luciferase assay PBMC, fibroblasts: ↑ nuclear translocation p65 at rest + with TNF stimulation PBMC: LPS → ↑ inflammatory cytokines; Polarisation to Th9, Th17 CD4 T cell linage. LPS → NLRP3 inflam activation |
A20−/− mice* | [14] | |
| HIDS | MVK | MVK | AR | LOF | Inflam | IL-1β | Multiple |
PBMC: ↑ IL-1β, IL-6 and TNFα at rest and with stimulation EBV-LCL: accumulation unprenylated Rab proteins (temperature dependent) |
Mvk−/+ mice | [191–195] |
| HOIL1 deficiency | RBCK1 | HOIL1 | AR | LOF | NF-κB | Multiple | Multiple |
Fibroblasts, B cells: ↓NF-κB activation. JNK phosphorylation normal. Impaired response to IL-1β > TNF CD3+, CD19+, CD56+ cells: No response to TNF or IL-1β stimulation Monocytes: IL-1β stimulation → ↑IL-6 and MIP-1α c/w healthy control |
Rbck1−/− mice* (overtly normal) | [76] |
| HOIP deficiency | RNF31 | HOIP | AR | LOF | NF-κB | Multiple | Multiple |
Fibroblasts: ↓IKK phosphorylation, IL-6 production in response to TNF or IL-1β stimulation B cells: ↓ CD80 up-regulation with CD40L + IL-21 or IL-4 Monocytes: IL-1β stimulation → ↑IL-6 and IL-1β c/w healthy control |
Hoip−/− mice (embryonically lethal). Various crosses | [77] |
| HYDM1 | NLRP7 | NLRP7 | AR | Unknown | ? Inflam ? NF-κB |
Unknown | Placenta |
HEK293T cells: transient transfection → abnormal methylation PBMC: ↓IL-1β and TNF in response to LPS. Conflicting data on secretion as well as effect on pro-IL-1β expression |
Nil (no murine orthologue) | [180,181,196–199] |
| Majeed syndrome | LPIN2 | Lipin 2 | AR | LOF | Inflam | IL-1β | Bone Skin Multiple |
HEK293T cells: PAP activity assay. Hepa1-6 cells: PPARα luciferase assay. |
Lpin2−/− mice | [200–203] |
| MSPC | NLRP1 | NLRP1 | AD | GOF | Inflam | IL-1β | Skin |
HEK293T cells: ASC speck assay, reconstitution of inflam ↑pro-IL-1β cleavage Primary keratinocytes: spontaneous inflam activation PMA differentiated THP1 cells: Doxycycline induced NLRP1 expression constructs. Mutants ↑IL-1β and cell death. ASC dependent |
Nlrp1aQ593P mice* | [153,204] |
| ORAS | OTULIN | Otulin | AR | LOF | NF-κB | TNF | Multiple |
HEK293T cells: transfection of mutant ↑NF-κB pathway c/w WT; NF-κB luciferase assay showed ↓inhibitory effect of mutant Otulin c/w WT T cells: Normal proliferation and NF-κB response to TCR stimulation B cells: Normal proliferation and NF-κB response to BCR stimulation Fibroblasts: Expression undetectable. ↑p-IKBa, p-IKKa/b, p-P38 and P-JNK with TNF stimulation. ↓ ability to deubiquitinate linear chains PBMC: ↓ ability to deubiquitinate linear chains |
CreERT2-OtulinLacZ/flox mice | [205,206] |
| PAAND | MEFV | Pyrin | AD | GOF | Inflam | ?Multiple | Skin Multiple |
Monocyte: ↑ASC speck formation, caspase-1 activity, c/w healthy control PBMC: ↑ IL-18 and IL-1Ra with LPS stimulation c/w healthy control HEK293T cells: ASC speck assay. 14-3-3 binding in overexpression model THP1 cells: Retroviral reconstitution and lentviral reconstitution of MEFV KO cells. Mutants ↑cell death, ↑IL-1β, IL-18 |
Nil | [207,208] |
| PAPA syndrome | PSTPIP1 | PSTPIP1 | AD | Unknown | Inflam | ?IL-1β | Skin, Joints Multiple |
HeLa cells: Transient cotransfection. Mutant PSTPIP1 hyperphosphorylated and ↑binding to pyrin Cos-7L cells: inflammasome reconstitution assay. Mutant PSTPIP1 ↑IL-1β processing THP1 cells: Immunoprecipitation to show interaction between PSTPIP1 and pyrin Macrophages: ↓invasion and podosome formation T cells: ↓numbers, ↓proliferation response to mitogen. Normal migration PBMC: ↓ IL-1Ra, ↑IL-1β, IL-6, TNFα and GMCF in response to multiple stimuli. siRNA knockdown of NLRP3 ↓IL-1β in response to LPS |
Rosa-PSTPIP1 A230TSTOP del/+ mice | [209–213] |
| PLAID | PLCG2 | PLCγ2 | AD | GOF | Unknown | Unknown | Multiple |
COS7, A20 cells: transfection model. Mutants- ↑phospholipase activity at subphysiological temperatures LAD2 mast cells: transfection of mutant → spontaneous degranulation at 20°C B cells and NK cells: ↓ERK phosphorylation in response to stimulation T cells: normal response to CD3 cross-linking |
Multiple* | [79] |
| PRAAS | PSMB8, PSMB9, PSMB4, PSMA3, POMP | PSMB8, PSMB9, PSMB4, PSMA3, POMP | AR | LOF | ?NF-κB ?IFN |
T1IFN | Multiple |
HeLa cells: Transfection studies show poor formation of proteasome with mutant c/w WT Primary fibroblasts: ↑ precursor complexes in patients. siRNA knockdown in control cells → IFN induction and proteasome dysfunction Lymphoblastoid cell line: ↑ precursor complexes, ↓ proteasome formation EBV transformed B cells: generally, ↓ chymotryptic like activity Primary keratinocytes: Ubiquitin aggregation |
Lmp7−/− mice | [59,214] |
| PRP | CARD14 | CARD14 | AD | GOF | NF-κB | Skin |
HEK293T cells: NF-κB luciferase assay Immortalised primary keratinocytes: Expression + NF-κB activity |
Nil | [215,216] | |
| Pustular psoriasis | AP1S3 | AP1S3 | AR | LOF | ?NF-κB | IL36 IL-1 |
Skin | Primary keratinocytes and dermal fibroblasts: abnormal autophagy, accumulation of p62. Abnormal TLR2/6 signalling | Nil | [217,218] |
| SAVI | TMEM173 | STING | AD | GOF | IFN | T1IFN | Multiple incl lungs, vessels |
HEK293T cells: IFNB1 reporter assay. Immunoblot analysis of STING pathway CD4, CD8 T cells, CD19 B cells: constitutive STAT1 phosphorylation PBMC and dermal fibroblasts: ↑ IFNB1 transcription at rest. No change with cGAMP exposure. Transcription of TNF and IL-6 ↑ at baseline and with cGAMP treatment |
StingN153S/+ mice | [16–18] |
| SPENCD | ACP5 | ACP5 | AR | LOF | IFN | T1IFN | Multiple |
Primary human macrophages: colocalisation studiesPlasmacytoid dendritic cells: co-localisation studies. TLR9 stimulation in shRNA ACP5 knockdown studies → ↑ transcription ISGs HEK293T cells: cotransfection TRAP and osteopontin followed by immunoprecipitation THP1 cells: shRNA ACP5 knockdown studies → ↑ phosphorylation of osteopontin |
Acp5−/− mice | [219–222] |
| TRAPS | TNFRS1A | TNFR1 | AD | Unknown | NF-κB | ?IL-1β | Multiple |
PBMC: ↑surface expression TNFR1 + ↓shedding (conflicting data) Monocytes: ↑surface expression TNFR1 and ↓shedding; Abnormal autophagy →↑IL-1β + NF-κB activation Dermal fibroblasts: Mutant TNFR1 ↓ receptor shedding Neutrophils: Mutant TNFR1 abnormal retention in cytoplasm HEK293T cells: minor differences in receptor shedding when TNFR1 WT or mutant overexpressed; Cytoplasmic retention and reduced surface expression of mutant |
Tnfrsf1aT50M/+ mice (13) Tnfrsf1aC33Y/+ mice (232) Tnfrsf1ap55deltNS mice (10) |
[1,9–11,13,223–232] |
| USP18 deficiency | USP18 | USP18 | AR | LOF | IFN | T1IFN | CNS Liver |
Primary dermal fibroblasts: ↑ transcription ISG after IFN stimulation. Persistent STAT2 phosphorylation. No sig difference in IL-6 response to IL-1β or poly(I:C) .↑ISGylation | Usp18−/− mice* | [233] |
| XLPDR | POLA1 | POLA1 | XLR | LOF | IFN > NF-κB |
T1IFN | Multiple |
Primary dermal fibroblasts: ↑IFN + NF-κB response to stimulation with poly(da:dt) or TNF; ↑IRF and NF-κB pathway activation; ↓RNA:DNA levels; Lentiviral transduction of WT rescued phenotype Fibroblast and HeLa cell line: siRNA POLA1 knockdown → ↑ IFN + NF-κB response to stimulation with poly(da:dt) or TNF |
Nil | [234] |
Abbreviations: AD, autosomal dominant; AIADK, autoinflammation with arthritis and dyskeratosis; AID, autoinflammatory disorder; AIFEC, autoinflammation with infantile enterocolitis; AILJK, autoimmune interstitial lung, joint and kidney disease; AR, autosomal recessive; BLCL, EBV-transformed B-lymphoblastoid cell lines; c/w, compared with; CD, cluster of differentiation; COPA, coatomer subunit α; DIRA, deficiency of IL-1 receptor antagonist; ER, endoplasmic reticulum; FCAS2, familial cold autoinflammatory syndrome 2; GIT, gastrointestinal tract; GOF, gain of function; HIDS, hyper IgD syndrome, HYDM1, hydatidiform molar pregnancy; Inflam, inflammasome; ISG, IFN-stimulated gene; LOF, loss of function; MOI, mode of inheritance; MSPC, multiple self-healing palmoplantar carcinoma; NFATc1, nuclear factor of activated T cell, cytoplasmic 1; ORAS, otulin-related autoinflammatory syndrome; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; POLA, DNA polymerase α catalytic subunit; PRP, pityriasis rubra pilaris; SMS, Singleton–Merten syndrome; SPENCD, spondyloenchondrodysplasia; T1IFN, type 1 IFN; XLPDR, x-linked pigmentary disorder, reticulate, with systemic manifestations.
Murine model prior to the description of monogenic condition.
Future directions and questions
Questions remain in the field about the phenotype–genotype correlation as well as phenotypic variability of individuals with a specific genetic variant. This includes disorders of ‘variable penetrance’. There are alternative explanations for differing phenotypes among cases with the same genetic variant. One consideration is the presence of another genetic variant that affects disease presentation. A true digenic disorder requires the inheritance of a distinct heterozygous mutation in two genes that, when inherited separately, do not cause a phenotype [235,236]. The broader term of epistasis refers to possible interactions between genes [237]. Determining genetic epistasis is complex and requires an appropriate pedigree, which includes more than one gene mutated in a single pedigree, a range of genetic permutations and at least one member with WT alleles in both genes [238,239].
Another possible explanation for the phenotypic heterogeneity among cases is epigenetic differences. Epigenetic processes such as DNA methylation, histone modifications, chromatin remodelling and non-coding RNAs can alter the activity of a gene without changing the DNA sequence. For example, a transcriptionally active gene has minimal DNA methylation and an open chromatic structure. Monozygotic and dizygotic twin studies of concordance have been used for some time to determine the contribution of a particular genotype to phenotype [240]. More recently, this has been combined with methods of quantifying epigenetic changes. In a study of monozygotic twins disconcordant for the clinical diagnosis of CVID, a DNA methylation array performed on CD19+ B cells revealed that both switched and unswitched memory B cells of the twin with CVID had higher DNA methylation in genes relevant to B-cell function [241]. This finding highlights that epigenetic factors could account for phenotypic variations. There have been two publications assessing DNA methylation in individuals with monogenic autoinflammatory disorders, but in each case the diseased population was compared with a healthy control [242,243]. Vento-Tormo et al. [243] assessed the DNA methylation status of genes encoding various components of the inflammasome in monocytes of cases with CAPS and compared this with healthy controls. They noted that genes such as IL1B, IL1RN and ASC were demethylated more efficiently in CAPS monocytes when compared with healthy controls, a feature that normalised when individuals were treated with anti-IL1 therapy. Determining the epigenetic factors contributing to the phenotypic variability of a particular genotype is not simple. With a small number of cases, one possible approach is to compare the methylation status of genes potentially involved in the phenotype observed. An alternative approach is genome-wide DNA methylation profiling [244]. However, drawing conclusions from only a few individuals with different genetic backgrounds may not be feasible.
Exploring factors that can account for this phenotypic variability may provide insight into the pathways involved in disease. Furthermore, the comprehensive genetic evaluation and investigation of various immune and non-immune cells of individuals with these conditions will likely enlighten the field to the intimate link between the innate and adaptive immune system as well as the role of ‘innate immune’ proteins in non-immune cells.
Abbreviations
- AGS
Aicardi–Goutieres syndrome
- APLAID
autoinflammation and PLCγ2-associated antibody deficiency and immune dysregulation
- CAPS
cryopyrin-associated periodic syndrome
- CARD
caspase activation and recruitment domain
- CRISPR
clustered regularly interspaced short palindromic repeat
- CVID
common variable immune deficiency
- DADA2
deficiency of adenosine deaminase 2
- DITRA
deficiency in IL-36Ra
- DSB
double-strand break
- EBV
Epstein–Barr virus
- FMF
familial mediterranean fever
- HDR
homology directed repair
- HHMI
Howard Hughes Medical Institute
- HOIL1
haem-oxidised IRP2 ubiquitin ligase 1
- HSCT
haemopoetic stem cell transplantation
- IFN
interferon
- IL
interleukin
- IL-36Ra
IL-36 receptor antagonist
- IL1RAcP
IL-1 receptor accessory protein
- iPSC
induced pluripotent stem cell
- IUIS
International Union of Immunological Societies
- JAK
Janus kinase
- LPS
lipopolysaccharide
- LUBAC
linear ubiquitin assembly complex
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cell
- NGS
next-generation sequencing
- NHEJ
non-homologous end joining
- NHMRC
National Heath and Medical Research Council
- NK
natural killer
- NLR
NOD-like receptor
- NOD
nucleotide-binding oligomerization domaim
- PBMC
peripheral blood mononuclear cell
- PLAID
PLCγ2-associated antibody deficiency and immune dysregulation
- PLCγ2
phospholipase c γ-2
- PRAAS
proteasome-associated autoinflammatory syndrome
- rAAV
recombinant adeno-associated virus
- rhuIL-10
recombinant human IL-10
- RNASEH2
ribonuclease H2
- SAMHD1
SAM and HD domain containing protein 1
- SAVI
STING-associated vasculopathy with onset in infancy
- sgRNA
single guide RNA
- ssOligo
single-strand oligonucleotide
- STAT
signal transducer and activator of transcription
- STING
stimulator of IFN gene
- TAK1
transforming growth factor-β activated kinase-1
- TNF
tumour necrosis factor
- TNFR1
TNF receptor 1
- TRAPS
TNF receptor associated periodic syndrome
- WES
whole exome sequencing
- WGS
whole genome sequencing
- WT
wild-type
Funding
This work was supported by the Henry James Williams Scholarship (University of Melbourne) to F.M.; the Australian Genomics Health Alliance Ph.D. top-up award to F.M.; the NHMRC grants [grant numbers 1144282, 1142354, 1099262 (to S.L.M.)]; the Sylvia and Charles Viertel Foundation to S.L.M.; the HHMI-Wellcome International Research Scholarship to S.L.M.; and GlaxoSmithKline to S.L.M.
Author contribution
This article has been adapted by F.M. and S.L.M. from the introductory chapter of F.M.’s doctoral thesis; ‘Novel genes and mechanisms in monogenic autoinflammatory disorders’ (2018, University of Melbourne).
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.McDermott M.F., Aksentijevich I., Galon J., McDermott E.M., Ogunkolade B.W., Centola M. et al. (1999) Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–144 10.1016/S0092-8674(00)80721-7 [DOI] [PubMed] [Google Scholar]
- 2.FF Consortium (1997) A candidate gene for familial Mediterranean fever. Nat. Genet. 17, 25–31 10.1038/ng0997-25 [DOI] [PubMed] [Google Scholar]
- 3.TIF Consortium (1997) Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 90, 797–807 10.1016/S0092-8674(00)80539-5 [DOI] [PubMed] [Google Scholar]
- 4.Bousfiha A., Jeddane L., Picard C., Ailal F., Bobby Gaspar H., Al-Herz W. et al. (2018) The 2017 IUIS phenotypic classification for primary immunodeficiencies. J. Clin. Immunol. 38, 129–143 10.1007/s10875-017-0465-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milhavet F., Cuisset L., Hoffman H.M., Slim R., El-Shanti H., Aksentijevich I. et al. (2008) The infevers autoinflammatory mutation online registry: update with new genes and functions. Hum. Mutat. 29, 803–808 10.1002/humu.20720 [DOI] [PubMed] [Google Scholar]
- 6.Masters S.L., Simon A., Aksentijevich I. and Kastner D.L. (2009) Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol. 27, 621–668 10.1146/annurev.immunol.25.022106.141627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manthiram K., Zhou Q., Aksentijevich I. and Kastner D.L. (2017) The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation, Nat. Immunol. 18, 832–842 [DOI] [PubMed] [Google Scholar]
- 8.Crow Y.J. (2011) Type I interferonopathies: a novel set of inborn errors of immunity. Ann. N.Y. Acad. Sci. 1238, 91–98 10.1111/j.1749-6632.2011.06220.x [DOI] [PubMed] [Google Scholar]
- 9.Huggins M.L., Radford P.M., McIntosh R.S., Bainbridge S.E., Dickinson P., Draper-Morgan K.A. et al. (2004) Shedding of mutant tumor necrosis factor receptor superfamily 1A associated with tumor necrosis factor receptor-associated periodic syndrome: differences between cell types. Arthritis Rheum. 50, 2651–2659 10.1002/art.20380 [DOI] [PubMed] [Google Scholar]
- 10.Xanthoulea S., Pasparakis M., Kousteni S., Brakebusch C., Wallach D., Bauer J. et al. (2004) Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J. Exp. Med. 200, 367–376 10.1084/jem.20040435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd I., Radford P.M., Draper-Morgan K.A., McIntosh R., Bainbridge S., Dickinson P. et al. (2004) Mutant forms of tumour necrosis factor receptor I that occur in TNF-receptor-associated periodic syndrome retain signalling functions but show abnormal behaviour. Immunology 113, 65–79 10.1111/j.1365-2567.2004.01942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siebert S., Amos N., Fielding C.A., Wang E.C., Aksentijevich I., Williams B.D. et al. (2005) Reduced tumor necrosis factor signaling in primary human fibroblasts containing a tumor necrosis factor receptor superfamily 1A mutant. Arthritis Rheum. 52, 1287–1292 10.1002/art.20955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon A., Park H., Maddipati R., Lobito A.A., Bulua A.C., Jackson A.J. et al. (2010) Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc. Natl. Acad. Sci. U.S.A. 107, 9801–9806 10.1073/pnas.0914118107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q., Wang H., Schwartz D.M., Stoffels M., Park Y.H., Zhang Y. et al. (2016) Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 48, 67–73 10.1038/ng.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malireddi R.K.S., Gurung P., Mavuluri J., Dasari T.K., Klco J.M., Chi H. et al. (2018) TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J. Exp. Med. 10.1084/jem.20171922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Jesus A.A., Marrero B., Yang D., Ramsey S.E., Montealegre Sanchez G.A. et al. (2014) Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 10.1056/NEJMoa1312625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melki I., Rose Y., Uggenti C., Van Eyck L., Fremond M.L., Kitabayashi N. et al. (2017) Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J. Allergy Clin. Immunol. 140, 543.e5–552.e5 10.1016/j.jaci.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 18.Warner J.D., Irizarry-Caro R.A., Bennion B.G., Ai T.L., Smith A.M., Miner C.A. et al. (2017) STING-associated vasculopathy develops independently of IRF3 in mice. J. Exp. Med. 214, 3279–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M. et al. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 10.1038/ni1087 [DOI] [PubMed] [Google Scholar]
- 20.Moghaddas F. and Masters S.L. (2015) Monogenic autoinflammatory diseases: cytokinopathies. Cytokine 74, 237–246 10.1016/j.cyto.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 21.Aksentijevich I., Nowak M., Mallah M., Chae J.J., Watford W.T., Hofmann S.R. et al. (2002) De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 46, 3340–3348 10.1002/art.10688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins P.N., Lachmann H.J. and McDermott M.F. (2003) Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N. Engl. J. Med. 348, 2583–2584 10.1056/NEJM200306193482523 [DOI] [PubMed] [Google Scholar]
- 23.Goldbach-Mansky R., Dailey N.J., Canna S.W., Gelabert A., Jones J., Rubin B.I. et al. (2006) Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N. Engl. J. Med. 355, 581–592 10.1056/NEJMoa055137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lachmann H.J., Kone-Paut I., Kuemmerle-Deschner J.B., Leslie K.S., Hachulla E., Quartier P. et al. (2009) Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360, 2416–2425 10.1056/NEJMoa0810787 [DOI] [PubMed] [Google Scholar]
- 25.Laskari K., Boura P., Dalekos G.N., Garyfallos A., Karokis D., Pikazis D. et al. (2017) Longterm beneficial effect of canakinumab in colchicine-resistant Familial Mediterranean fever. J. Rheumatol. 44, 102–109 10.3899/jrheum.160518 [DOI] [PubMed] [Google Scholar]
- 26.Arostegui J.I., Anton J., Calvo I., Robles A., Iglesias E., Lopez-Montesinos B. et al. (2017) Open-label, Phase II study to assess the efficacy and safety of canakinumab treatment in active hyperimmunoglobulinemia D with periodic fever syndrome. Arthritis Rheumatol. 69, 1679–1688 10.1002/art.40146 [DOI] [PubMed] [Google Scholar]
- 27.Gattorno M., Obici L., Cattalini M., Tormey V., Abrams K., Davis N. et al. (2017) Canakinumab treatment for patients with active recurrent or chronic TNF receptor-associated periodic syndrome (TRAPS): an open-label, phase II study. Ann. Rheum. Dis. 76, 173–178 10.1136/annrheumdis-2015-209031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borghini S., Ferrera D., Prigione I., Fiore M., Ferraris C., Mirisola V. et al. (2016) Gene expression profile in TNF receptor-associated periodic syndrome reveals constitutively enhanced pathways and new players in the underlying inflammation. Clin. Exp. Rheumatol. 34, S121–S128 [PubMed] [Google Scholar]
- 29.De Benedetti F., Gattorno M., Anton J., Ben-Chetrit E., Frenkel J., Hoffman H.M. et al. (2018) Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med. 378, 1908–1919 10.1056/NEJMoa1706314 [DOI] [PubMed] [Google Scholar]
- 30.Harrison S.R., McGonagle D., Nizam S., Jarrett S., van der Hilst J., McDermott M.F. et al. (2016) Anakinra as a diagnostic challenge and treatment option for systemic autoinflammatory disorders of undefined etiology. JCI Insight 1, e86336 10.1172/jci.insight.86336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moghaddas F., Zeng P., Zhang Y., Schutzle H., Brenner S., Hofmann S.R. et al. (2018) Autoinflammatory mutation in NLRC4 reveals an LRR-LRR oligomerization interface. J. Allergy Clin. Immunol. 10.1016/j.jaci.2018.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romberg N., Al Moussawi K., Nelson-Williams C., Stiegler AL., Loring E., Choi M., Overton J., Meffre E., Khokha MK., Huttner AJ., West B., Podoltsev NA., Boggon TJ., Kazmierczak BI. and Lifton RP. et al. (2014) Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation.. Nat. Genet. 46, 1135–1139 10.1038/ng.3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canna SW., de Jesus AA., Gouni S., Brooks SR., Marrero B., Liu Y., DiMattia MA., Zaal KJ., Sanchez GA., Kim H., Chapelle D., Plass N., Huang Y., Villarino AV., Biancotto A., Fleisher TA., Duncan JA., O’Shea JJ., Benseler S., Grom A., Deng Z., Laxer RM. and Goldbach-Mansky R. (2014) An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–6 10.1038/ng.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrakchi S., Guigue P., Renshaw B.R., Puel A., Pei X.Y., Fraitag S. et al. (2011) Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 365, 620–628 10.1056/NEJMoa1013068 [DOI] [PubMed] [Google Scholar]
- 35.Huffmeier U., Watzold M., Mohr J., Schon M.P. and Mossner R. (2014) Successful therapy with anakinra in a patient with generalized pustular psoriasis carrying IL36RN mutations. Br. J. Dermatol. 170, 202–204 10.1111/bjd.12548 [DOI] [PubMed] [Google Scholar]
- 36.Podlipnik S., Morgado-Carrasco D., Fusta-Novell X., Mensa-Vilaro A., Arostegui J.I., Alsina-Gibert M. et al. (2017) Dynamics of plasma cytokines in a patient with deficiency of interleukin-36 receptor antagonist successfully treated with anakinra. Br. J. Dermatol., 10.1111/bjd.16063 [DOI] [PubMed] [Google Scholar]
- 37.Rossi-Semerano L., Piram M., Chiaverini C., De Ricaud D., Smahi A. and Kone-Paut I. (2013) First clinical description of an infant with interleukin-36-receptor antagonist deficiency successfully treated with anakinra. Pediatrics 132, e1043–7 10.1542/peds.2012-3935 [DOI] [PubMed] [Google Scholar]
- 38.Tauber M., Viguier M., Le Gall C., Smahi A. and Bachelez H. (2014) Is it relevant to use an interleukin-1-inhibiting strategy for the treatment of patients with deficiency of interleukin-36 receptor antagonist? Br. J. Dermatol. 170, 1198–1199 10.1111/bjd.12805 [DOI] [PubMed] [Google Scholar]
- 39.Fialova J., Vojackova N., Vanousova D. and Hercogova J. (2014) Juvenile generalized pustular psoriasis treated with etanercept. Dermatol. Ther. 27, 105–108 10.1111/dth.12065 [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto A., Komine M., Karakawa M., Kishimoto M. and Ohtsuki M. (2017) Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J. Dermatol. 44, 202–204 10.1111/1346-8138.13632 [DOI] [PubMed] [Google Scholar]
- 41.Zangrilli A., Papoutsaki M., Talamonti M. and Chimenti S. (2008) Long-term efficacy of adalimumab in generalized pustular psoriasis. J. Dermatolog. Treat. 19, 185–187 10.1080/09546630701759587 [DOI] [PubMed] [Google Scholar]
- 42.Kostner K., Prelog M., Almanzar G., Fesq H., Haas J.P. and Hugle B. (2018) Successful use of secukinumab in a 4-year-old patient with deficiency of interleukin-36 antagonist. Rheumatology (Oxford) 10.1093/rheumatology/kex510 [DOI] [PubMed] [Google Scholar]
- 43.Arakawa A., Ruzicka T. and Prinz J.C. (2016) Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 152, 825–828 10.1001/jamadermatol.2016.0751 [DOI] [PubMed] [Google Scholar]
- 44.Bonekamp N., Caorsi R., Viglizzo G.M., Graaf M., Minoia F., Grossi A. et al. (2017) High-dose ustekinumab for severe childhood deficiency of interleukin-36 receptor antagonist (DITRA). Ann. Rheum. Dis. 10.1136/annrheumdis-2017-211805 [DOI] [PubMed] [Google Scholar]
- 45.Cherqaoui B. Jr, Rossi-Semerano L., Piram M. and Kone-Paut I. (2017) Standard dose of Ustekinumab for childhood-onset deficiency of interleukin-36 receptor antagonist. Ann. Rheum. Dis. 10.1136/annrheumdis-2017-212793 [DOI] [PubMed] [Google Scholar]
- 46.Ganesan R., Raymond E.L., Mennerich D., Woska J.R. Jr, Caviness G., Grimaldi C. et al. (2017) Generation and functional characterization of anti-human and anti-mouse IL-36R antagonist monoclonal antibodies. MAbs 9, 1143–1154 10.1080/19420862.2017.1353853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glocker E.O., Frede N., Perro M., Sebire N., Elawad M., Shah N. et al. (2010) Infant colitis–it’s in the genes. Lancet 376, 1272 10.1016/S0140-6736(10)61008-2 [DOI] [PubMed] [Google Scholar]
- 48.Glocker E.O., Kotlarz D., Boztug K., Gertz E.M., Schaffer A.A., Noyan F. et al. (2009) Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 10.1056/NEJMoa0907206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams L., Bradley L., Smith A. and Foxwell B. (2004) Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J. Immunol. 172, 567–576 10.4049/jimmunol.172.1.567 [DOI] [PubMed] [Google Scholar]
- 50.Berg D.J., Kuhn R., Rajewsky K., Muller W., Menon S., Davidson N. et al. (1995) Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Invest. 96, 2339–2347 10.1172/JCI118290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhn R., Lohler J., Rennick D., Rajewsky K. and Muller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 10.1016/0092-8674(93)80068-P [DOI] [PubMed] [Google Scholar]
- 52.Engelhardt K.R., Shah N., Faizura-Yeop I., Kocacik Uygun D.F., Frede N., Muise A.M. et al. (2013) Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 131, 825–830 10.1016/j.jaci.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 53.Kotlarz D., Beier R., Murugan D., Diestelhorst J., Jensen O., Boztug K. et al. (2012) Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology 143, 347–355 10.1053/j.gastro.2012.04.045 [DOI] [PubMed] [Google Scholar]
- 54.Buruiana F.E., Sola I. and Alonso-Coello P. (2010) Recombinant human interleukin 10 for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev., CD005109, 10.1002/14651858.CD005109.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitamura A., Maekawa Y., Uehara H., Izumi K., Kawachi I., Nishizawa M. et al. (2011) A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J. Clin. Invest. 121, 4150–4160 10.1172/JCI58414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arima K., Kinoshita A., Mishima H., Kanazawa N., Kaneko T., Mizushima T. et al. (2011) Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc. Natl. Acad. Sci. U.S.A. 108, 14914–14919 10.1073/pnas.1106015108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agarwal A.K., Xing C., DeMartino G.N., Mizrachi D., Hernandez M.D., Sousa A.B. et al. (2010) PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am. J. Hum. Genet. 87, 866–872 10.1016/j.ajhg.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y., Ramot Y., Torrelo A., Paller A.S., Si N., Babay S. et al. (2012) Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 64, 895–907 10.1002/art.33368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brehm A., Liu Y., Sheikh A., Marrero B., Omoyinmi E., Zhou Q. et al. (2015) Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Invest. 125, 4196–4211 10.1172/JCI81260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Awad F., Assrawi E., Louvrier C., Jumeau C., Georgin-Lavialle S., Grateau G. et al. (2018) Inflammasome biology, molecular pathology and therapeutic implications. Pharmacol. Ther. 187, 133–149 10.1016/j.pharmthera.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 61.Lachmann H.J. (2017) Periodic fever syndromes. Best Pract. Res. Clin. Rheumatol. 31, 596–609 10.1016/j.berh.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 62.Kim H., Brooks K.M., Tang C.C., Wakim P., Blake M., Brooks S.R. et al. (2017) Pharmacokinetics, pharmacodynamics, and proposed dosing of the oral JAK1 and JAK2 inhibitor baricitinib in pediatric and young adult CANDLE and SAVI patients. Clin. Pharmacol. Ther., 10.1002/cpt.936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez G.A.M., Reinhardt A., Ramsey S., Wittkowski H., Hashkes P.J., Berkun Y. et al. (2018) JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J. Clin. Invest. 10.1172/JCI98814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novick D. and Dinarello C.A. (2017) IL-18 binding protein reverses the life-threatening hyperinflammation of a baby with the NLRC4 mutation. J. Allergy Clin. Immunol. 140, 316 10.1016/j.jaci.2017.02.037 [DOI] [PubMed] [Google Scholar]
- 65.Aicardi J. and Goutieres F. (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 15, 49–54 10.1002/ana.410150109 [DOI] [PubMed] [Google Scholar]
- 66.Crow Y.J., Hayward B.E., Parmar R., Robins P., Leitch A., Ali M. et al. (2006) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 10.1038/ng1845 [DOI] [PubMed] [Google Scholar]
- 67.Rice G.I., Bond J., Asipu A., Brunette R.L., Manfield I.W., Carr I.M. et al. (2009) Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 10.1038/ng.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crow Y.J., Leitch A., Hayward B.E., Garner A., Parmar R., Griffith E. et al. (2006) Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat. Genet. 38, 910–916 10.1038/ng1842 [DOI] [PubMed] [Google Scholar]
- 69.Rice G.I., Kasher P.R., Forte G.M., Mannion N.M., Greenwood S.M., Szynkiewicz M. et al. (2012) Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat. Genet. 44, 1243–1248 10.1038/ng.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aicardi J. and Goutieres F. (2000) Systemic lupus erythematosus or Aicardi-Goutieres syndrome? Neuropediatrics 31, 113 10.1055/s-2000-7533 [DOI] [PubMed] [Google Scholar]
- 71.De Laet C., Goyens P., Christophe C., Ferster A., Mascart F. and Dan B. (2005) Phenotypic overlap between infantile systemic lupus erythematosus and Aicardi-Goutieres syndrome. Neuropediatrics 36, 399–402 10.1055/s-2005-873058 [DOI] [PubMed] [Google Scholar]
- 72.Ramantani G., Kohlhase J., Hertzberg C., Innes A.M., Engel K., Hunger S. et al. (2010) Expanding the phenotypic spectrum of lupus erythematosus in Aicardi-Goutieres syndrome. Arthritis Rheum. 62, 1469–1477 10.1002/art.27367 [DOI] [PubMed] [Google Scholar]
- 73.Crow Y.J., Chase D.S., Lowenstein Schmidt J., Szynkiewicz M., Forte G.M., Gornall H.L. et al. (2015) Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am. J. Med. Genet. Part A 167a, 296–312 10.1002/ajmg.a.36887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rice G., Patrick T., Parmar R., Taylor C.F., Aeby A., Aicardi J. et al. (2007) Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am. J. Hum. Genet. 81, 713–725 10.1086/521373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cuadrado E., Vanderver A., Brown K.J., Sandza A., Takanohashi A., Jansen M.H. et al. (2015) Aicardi-Goutieres syndrome harbours abundant systemic and brain-reactive autoantibodies. Ann. Rheum. Dis. 74, 1931–1939 10.1136/annrheumdis-2014-205396 [DOI] [PubMed] [Google Scholar]
- 76.Boisson B., Laplantine E., Prando C., Giliani S., Israelsson E., Xu Z. et al. (2012) Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 13, 1178–1186 10.1038/ni.2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boisson B., Laplantine E., Dobbs K., Cobat A., Tarantino N., Hazen M. et al. (2015) Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J. Exp. Med. 212, 939–951 10.1084/jem.20141130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilsson J., Schoser B., Laforet P., Kalev O., Lindberg C., Romero N.B. et al. (2013) Polyglucosan body myopathy caused by defective ubiquitin ligase RBCK1. Ann. Neurol. 74, 914–919 10.1002/ana.23963 [DOI] [PubMed] [Google Scholar]
- 79.Ombrello M.J., Remmers E.F., Sun G., Freeman A.F., Datta S., Torabi-Parizi P. et al. (2012) Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 366, 330–338 10.1056/NEJMoa1102140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Q., Lee G.S., Brady J., Datta S., Katan M., Sheikh A. et al. (2012) A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 91, 713–720 10.1016/j.ajhg.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu P., Constien R., Dear N., Katan M., Hanke P., Bunney T.D. et al. (2005) Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase C gamma 2 that specifically increases external Ca2+ entry. Immunity 22, 451–465 10.1016/j.immuni.2005.01.018 [DOI] [PubMed] [Google Scholar]
- 82.Chae J.J., Park Y.H., Park C., Hwang I.Y., Hoffmann P., Kehrl J.H. et al. (2015) Connecting two pathways through Ca2+ signaling: NLRP3 inflammasome activation induced by a hypermorphic PLCG2 mutation. Arthritis Rheumatol. 67, 563–567 10.1002/art.38961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurosaki T. and Tsukada S. (2000) BLNK: connecting Syk and Btk to calcium signals. Immunity 12, 1–5 10.1016/S1074-7613(00)80153-3 [DOI] [PubMed] [Google Scholar]
- 84.Navon Elkan P., Pierce S.B., Segel R., Walsh T., Barash J., Padeh S. et al. (2014) Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N. Engl. J. Med. 370, 921–931 10.1056/NEJMoa1307362 [DOI] [PubMed] [Google Scholar]
- 85.Zhou Q., Yang D., Ombrello A.K., Zavialov A.V., Toro C., Zavialov A.V. et al. (2014) Early-onset stroke and vasculopathy associated with mutations in ADA2. N. Engl. J. Med. 370, 911–920 10.1056/NEJMoa1307361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ombrello A., Stone D., Hoffmann P., Jones A., Barham B., Barron K. et al. (2015) The deficiency of adenosine deaminase type 2-results of therapeutic intervention. Pediatr. Rheumatol. 13, O40 10.1186/1546-0096-13-S1-O40 [DOI] [Google Scholar]
- 87.Sahin S., Adrovic A., Barut K., Ugurlu S., Turanli E.T., Ozdogan H. et al. (2018) Clinical, imaging and genotypical features of three deceased and five surviving cases with ADA2 deficiency. Rheumatol. Int. 38, 129–136 10.1007/s00296-017-3740-3 [DOI] [PubMed] [Google Scholar]
- 88.Caorsi R., Penco F., Grossi A., Insalaco A., Omenetti A., Alessio M. et al. (2017) ADA2 deficiency (DADA2) as an unrecognised cause of early onset polyarteritis nodosa and stroke: a multicentre national study. Ann. Rheum. Dis. 76, 1648–1656 10.1136/annrheumdis-2016-210802 [DOI] [PubMed] [Google Scholar]
- 89.Ben-Ami T., Revel-Vilk S., Brooks R., Shaag A., Hershfield M.S., Kelly S.J. et al. (2016) Extending the clinical phenotype of adenosine deaminase 2 deficiency. J. Pediatr. 177, 316–320 10.1016/j.jpeds.2016.06.058 [DOI] [PubMed] [Google Scholar]
- 90.Van Eyck L., Liston A. and Wouters C. (2014) Mutant ADA2 in vasculopathies. N. Engl. J. Med. 371, 480 [DOI] [PubMed] [Google Scholar]
- 91.Van Eyck L. Jr, Hershfield M.S., Pombal D., Kelly S.J., Ganson N.J., Moens L. et al. (2015) Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase 2 deficiency. J. Allergy Clin. Immunol. 135, 2835–287.e5 10.1016/j.jaci.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alsultan A., Basher E., Alqanatish J., Mohammed R. and Alfadhel M. (2017) Deficiency of ADA2 mimicking autoimmune lymphoproliferative syndrome in the absence of livedo reticularis and vasculitis. Pediatr. Blood Cancer, 10.1002/pbc.26912 [DOI] [PubMed] [Google Scholar]
- 93.Schepp J., Bulashevska A., Mannhardt-Laakmann W., Cao H., Yang F., Seidl M. et al. (2016) Deficiency of adenosine deaminase 2 causes antibody deficiency. J. Clin. Immunol. 36, 179–186 10.1007/s10875-016-0245-x [DOI] [PubMed] [Google Scholar]
- 94.Schepp J., Proietti M., Frede N., Buchta M., Hubscher K., Rojas Restrepo J. et al. (2017) Screening of 181 patients with antibody deficiency for deficiency of adenosine deaminase 2 sheds new light on the disease in adulthood. Arthritis Rheumatol. 69, 1689–1700 10.1002/art.40147 [DOI] [PubMed] [Google Scholar]
- 95.Belot A., Wassmer E., Twilt M., Lega J.C., Zeef L.A., Oojageer A. et al. (2014) Mutations in CECR1 associated with a neutrophil signature in peripheral blood. Pediatr. Rheumatol. Online J. 12, 44 10.1186/1546-0096-12-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skrabl-Baumgartner A., Plecko B., Schmidt W.M., Konig N., Hershfield M., Gruber-Sedlmayr U. et al. (2017) Autoimmune phenotype with type I interferon signature in two brothers with ADA2 deficiency carrying a novel CECR1 mutation. Pediatr. Rheumatol. Online J. 15, 67 10.1186/s12969-017-0193-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiseman D.H., May A., Jolles S., Connor P., Powell C., Heeney M.M. et al. (2013) A novel syndrome of congenital sideroblastic anemia, B-cell immunodeficiency, periodic fevers, and developmental delay (SIFD). Blood 122, 112–123 10.1182/blood-2012-08-439083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chakraborty P.K., Schmitz-Abe K., Kennedy E.K., Mamady H., Naas T., Durie D. et al. (2014) Mutations in TRNT1 cause congenital sideroblastic anemia with immunodeficiency, fevers, and developmental delay (SIFD). Blood 124, 2867–2871 10.1182/blood-2014-08-591370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lizano E., Schuster J., Muller M., Kelso J. and Morl M. (2007) A splice variant of the human CCA-adding enzyme with modified activity. J. Mol. Biol. 366, 1258–1265 10.1016/j.jmb.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 100.Giannelou A., Wang H., Zhou Q., Park Y.H., Abu-Asab M.S., Ylaya K. et al. (2018) Aberrant tRNA processing causes an autoinflammatory syndrome responsive to TNF inhibitors. Ann. Rheum. Dis. 77, 612–619 10.1136/annrheumdis-2017-212401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sarrabay G., Barat-Houari M., Annakib S. and Touitou I. (2015) The autoinflammatory diseases: a fashion with blurred boundaries ! Semin. Immunopathol. 37, 359–362 10.1007/s00281-015-0495-3 [DOI] [PubMed] [Google Scholar]
- 102.Goodwin S., McPherson J.D. and McCombie W.R. (2016) Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet. 17, 333–351 10.1038/nrg.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chou J., Ohsumi T.K. and Geha R.S. (2012) Use of whole exome and genome sequencing in the identification of genetic causes of primary immunodeficiencies. Curr. Opin. Allergy Clin. Immunol. 12, 623–628 10.1097/ACI.0b013e3283588ca6 [DOI] [PubMed] [Google Scholar]
- 104.Belkadi A., Bolze A., Itan Y., Cobat A., Vincent Q.B., Antipenko A. et al. (2015) Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants. Proc. Natl. Acad. Sci. U.S.A. 112, 5473–5478 10.1073/pnas.1418631112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rusmini M., Federici S., Caroli F., Grossi A., Baldi M., Obici L. et al. (2016) Next-generation sequencing and its initial applications for molecular diagnosis of systemic auto-inflammatory diseases. Ann. Rheum. Dis. 75, 1550–1557 10.1136/annrheumdis-2015-207701 [DOI] [PubMed] [Google Scholar]
- 106.Nakayama M., Oda H., Nakagawa K., Yasumi T., Kawai T., Izawa K. et al. (2017) Accurate clinical genetic testing for autoinflammatory diseases using the next-generation sequencing platform MiSeq. Biochem. Biophys. Rep. 9, 146–152 10.1016/j.bbrep.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Omoyinmi E., Standing A., Keylock A., Price-Kuehne F., Melo Gomes S., Rowczenio D. et al. (2017) Clinical impact of a targeted next-generation sequencing gene panel for autoinflammation and vasculitis. PLoS ONE 12, e0181874 10.1371/journal.pone.0181874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saito M., Fujisawa A., Nishikomori R., Kambe N., Nakata-Hizume M., Yoshimoto M. et al. (2005) Somatic mosaicism of CIAS1 in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 52, 3579–3585 10.1002/art.21404 [DOI] [PubMed] [Google Scholar]
- 109.Arostegui J.I., Lopez Saldana M.D., Pascal M., Clemente D., Aymerich M., Balaguer F. et al. (2010) A somatic NLRP3 mutation as a cause of a sporadic case of chronic infantile neurologic, cutaneous, articular syndrome/neonatal-onset multisystem inflammatory disease: novel evidence of the role of low-level mosaicism as the pathophysiologic mechanism underlying mendelian inherited diseases. Arthritis Rheum. 62, 1158–1166 10.1002/art.27342 [DOI] [PubMed] [Google Scholar]
- 110.Tanaka N., Izawa K., Saito M.K., Sakuma M., Oshima K., Ohara O. et al. (2011) High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 63, 3625–3632 10.1002/art.30512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jimenez-Trevino S., Gonzalez-Roca E., Ruiz-Ortiz E., Yague J., Ramos E. and Arostegui J.I. (2013) First report of vertical transmission of a somatic NLRP3 mutation in cryopyrin-associated periodic syndromes. Ann. Rheum. Dis. 72, 1109–1110 10.1136/annrheumdis-2012-202913 [DOI] [PubMed] [Google Scholar]
- 112.Nakagawa K., Gonzalez-Roca E., Souto A., Kawai T., Umebayashi H., Campistol J.M. et al. (2015) Somatic NLRP3 mosaicism in Muckle-Wells syndrome. A genetic mechanism shared by different phenotypes of cryopyrin-associated periodic syndromes. Ann. Rheum. Dis. 74, 603–610 10.1136/annrheumdis-2013-204361 [DOI] [PubMed] [Google Scholar]
- 113.Zhou Q., Aksentijevich I., Wood G.M., Walts A.D., Hoffmann P., Remmers E.F. et al. (2015) Brief report: cryopyrin-associated periodic syndrome caused by a myeloid-restricted somatic NLRP3 mutation. Arthritis Rheumatol. 67, 2482–2486 10.1002/art.39190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mensa-Vilaro A., Teresa Bosque M., Magri G., Honda Y., Martinez-Banaclocha H., Casorran-Berges M. et al. (2016) Brief report: late-onset cryopyrin-associated periodic syndrome due to myeloid-restricted somatic NLRP3 mosaicism. Arthritis Rheumatol. 68, 3035–3041 10.1002/art.39770 [DOI] [PubMed] [Google Scholar]
- 115.Lasiglie D., Mensa-Vilaro A., Ferrera D., Caorsi R., Penco F., Santamaria G. et al. (2017) Cryopyrin-associated periodic syndromes in Italian patients: evaluation of the rate of somatic NLRP3 mosaicism and phenotypic characterization. J. Rheumatol. 44, 1667–1673 10.3899/jrheum.170041 [DOI] [PubMed] [Google Scholar]
- 116.Rowczenio D.M., Gomes S.M., Arostegui J.I., Mensa-Vilaro A., Omoyinmi E., Trojer H. et al. (2017) Late-onset cryopyrin-associated periodic syndromes caused by somatic NLRP3 mosaicism-UK single center experience. Front. Immunol. 8, 1410 10.3389/fimmu.2017.01410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Botstein D. and Risch N. (2003) Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat. Genet. 33, 228–237 10.1038/ng1090 [DOI] [PubMed] [Google Scholar]
- 118.Majewski J., Schwartzentruber J., Lalonde E., Montpetit A. and Jabado N. (2011) What can exome sequencing do for you? J. Med. Genet. 48, 580–589 10.1136/jmedgenet-2011-100223 [DOI] [PubMed] [Google Scholar]
- 119.Cooper D.N., Chen J.M., Ball E.V., Howells K., Mort M., Phillips A.D. et al. (2010) Genes, mutations, and human inherited disease at the dawn of the age of personalized genomics. Hum. Mutat. 31, 631–655 10.1002/humu.21260 [DOI] [PubMed] [Google Scholar]
- 120.Hochheiser K., Kueh A.J., Gebhardt T. and Herold M.J. (2018) CRISPR/Cas9: a tool for immunological research. Eur. J. Immunol. 10.1002/eji.201747131 [DOI] [PubMed] [Google Scholar]
- 121.Shen B., Zhang J., Wu H., Wang J., Ma K., Li Z. et al. (2013) Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723 10.1038/cr.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li D., Qiu Z., Shao Y., Chen Y., Guan Y., Liu M. et al. (2013) Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol. 31, 681–683 10.1038/nbt.2661 [DOI] [PubMed] [Google Scholar]
- 123.Yang H., Wang H. and Jaenisch R. (2014) Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9, 1956–1968 10.1038/nprot.2014.134 [DOI] [PubMed] [Google Scholar]
- 124.Pattanayak V., Lin S., Guilinger J.P., Ma E., Doudna J.A. and Liu D.R. (2013) High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 31, 839–843 10.1038/nbt.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E. et al. (2013) RNA-guided human genome engineering via Cas9. Science 339, 823–826 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N. et al. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cho S.W., Kim S., Kim J.M. and Kim J.S. (2013) Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 10.1038/nbt.2507 [DOI] [PubMed] [Google Scholar]
- 128.Lin S., Staahl B.T., Alla R.K. and Doudna J.A. (2014) Enhanced homology-directed human genome engineering by controlled timing. Elife 3, e04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vasileva A. and Jessberger R. (2005) Precise hit: adeno-associated virus in gene targeting. Nat. Rev. Microbiol. 3, 837–847 10.1038/nrmicro1266 [DOI] [PubMed] [Google Scholar]
- 130.Kaulich M. and Dowdy S.F. (2015) Combining CRISPR/Cas9 and rAAV templates for efficient gene editing. Nucleic Acid Ther. 25, 287–296 10.1089/nat.2015.0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nishida K., Arazoe T., Yachie N., Banno S., Kakimoto M., Tabata M. et al. (2016) Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, 10.1126/science.aaf8729 [DOI] [PubMed] [Google Scholar]
- 132.Komor A.C., Kim Y.B., Packer M.S., Zuris J.A. and Liu D.R. (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 10.1038/nature17946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gaudelli N.M., Komor A.C., Rees H.A., Packer M.S., Badran A.H., Bryson D.I. et al. (2017) Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 551, 464–471 10.1038/nature24644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brydges S.D., Mueller J.L., McGeough M.D., Pena C.A., Misaghi A., Gandhi C. et al. (2009) Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity 30, 875–887 10.1016/j.immuni.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bonar S.L., Brydges S.D., Mueller J.L., McGeough M.D., Pena C., Chen D. et al. (2012) Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS ONE 7, e35979 10.1371/journal.pone.0035979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Snouwaert J.N., Nguyen M., Repenning P.W., Dye R., Livingston E.W., Kovarova M. et al. (2016) An NLRP3 mutation causes arthropathy and osteoporosis in humanized mice. Cell Rep. 17, 3077–3088 10.1016/j.celrep.2016.11.052 [DOI] [PubMed] [Google Scholar]
- 137.McGeough M.D., Wree A., Inzaugarat M.E., Haimovich A., Johnson C.D., Pena C.A. et al. (2017) TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J. Clin. Invest. 10.1172/JCI90699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alkhairy O.K., Abolhassani H., Rezaei N., Fang M., Andersen K.K., Chavoshzadeh Z. et al. (2016) Spectrum of phenotypes associated with mutations in LRBA. J. Clin. Immunol. 36, 33–45 10.1007/s10875-015-0224-7 [DOI] [PubMed] [Google Scholar]
- 139.Gamez-Diaz L., Neumann J., Jager F., Proietti M., Felber F., Soulas-Sprauel P. et al. (2017) Immunological phenotype of the murine Lrba knockout. Immunol. Cell Biol. 95, 789–802 10.1038/icb.2017.52 [DOI] [PubMed] [Google Scholar]
- 140.Burnett D.L., Parish I.A., Masle-Farquhar E., Brink R. and Goodnow C.C. (2017) Murine LRBA deficiency causes CTLA-4 deficiency in Tregs without progression to immune dysregulation. Immunol. Cell Biol. 95, 775–788 10.1038/icb.2017.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rongvaux A., Willinger T., Martinek J., Strowig T., Gearty S.V., Teichmann L.L. et al. (2014) Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 32, 364–372 10.1038/nbt.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takahashi K. and Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 143.Avior Y., Sagi I. and Benvenisty N. (2016) Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 17, 170–182 10.1038/nrm.2015.27 [DOI] [PubMed] [Google Scholar]
- 144.Anderson R.H. and Francis K.R. (2018) Modeling rare diseases with induced pluripotent stem cell technology. Mol. Cell Probes 10.1016/j.mcp.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grskovic M., Javaherian A., Strulovici B. and Daley G.Q. (2011) Induced pluripotent stem cells–opportunities for disease modelling and drug discovery. Nat. Rev. Drug Discov. 10, 915–929 [DOI] [PubMed] [Google Scholar]
- 146.Tanaka T., Takahashi K., Yamane M., Tomida S., Nakamura S., Oshima K. et al. (2012) Induced pluripotent stem cells from CINCA syndrome patients as a model for dissecting somatic mosaicism and drug discovery. Blood 120, 1299–1308 10.1182/blood-2012-03-417881 [DOI] [PubMed] [Google Scholar]
- 147.Kawasaki Y., Oda H., Ito J., Niwa A., Tanaka T., Hijikata A. et al. (2017) Identification of a High-Frequency somatic NLRC4 mutation as a cause of autoinflammation by pluripotent cell-based phenotype dissection. Arthritis Rheumatol. 69, 447–459 10.1002/art.39960 [DOI] [PubMed] [Google Scholar]
- 148.Blaydon D.C., Biancheri P., Di W.L., Plagnol V., Cabral R.M., Brooke M.A. et al. (2011) Inflammatory skin and bowel disease linked to ADAM17 deletion. N. Engl. J. Med. 365, 1502–1508 10.1056/NEJMoa1100721 [DOI] [PubMed] [Google Scholar]
- 149.Cuadrado E., Michailidou I., van Bodegraven E.J., Jansen M.H., Sluijs J.A., Geerts D. et al. (2015) Phenotypic variation in Aicardi-Goutieres syndrome explained by cell-specific IFN-stimulated gene response and cytokine release. J. Immunol. 194, 3623–3633 10.4049/jimmunol.1401334 [DOI] [PubMed] [Google Scholar]
- 150.Thomas C.A., Tejwani L., Trujillo C.A., Negraes P.D., Herai R.H., Mesci P. et al. (2017) Modeling of TREX1-dependent autoimmune disease using human stem cells highlights L1 accumulation as a source of neuroinflammation. Cell Stem Cell 21, 319–331.e8 10.1016/j.stem.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stetson D.B., Ko J.S., Heidmann T. and Medzhitov R. (2008) Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 10.1016/j.cell.2008.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hiller B., Achleitner M., Glage S., Naumann R., Behrendt R. and Roers A. (2012) Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J. Exp. Med. 209, 1419–1426 10.1084/jem.20120876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Masters S.L., Gerlic M., Metcalf D., Preston S., Pellegrini M., O’Donnell J.A. et al. (2012) NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37, 1009–1023 10.1016/j.immuni.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haverkamp M.H., van de Vosse E., Goldbach-Mansky R. and Holland S.M. (2014) Impaired cytokine responses in patients with cryopyrin-associated periodic syndrome (CAPS). Clin. Exp. Immunol. 177, 720–731 10.1111/cei.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dale R.C., Gornall H., Singh-Grewal D., Alcausin M., Rice G.I. and Crow Y.J. (2010) Familial Aicardi-Goutieres syndrome due to SAMHD1 mutations is associated with chronic arthropathy and contractures. Am. J. Med. Genet. Part A 152a, 938–942 10.1002/ajmg.a.33359 [DOI] [PubMed] [Google Scholar]
- 156.Berger A., Sommer A.F., Zwarg J., Hamdorf M., Welzel K., Esly N. et al. (2011) SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutieres syndrome are highly susceptible to HIV-1 infection. PLoS Pathog. 7, e1002425 10.1371/journal.ppat.1002425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goncalves A., Karayel E., Rice G.I., Bennett K.L., Crow Y.J., Superti-Furga G. et al. (2012) SAMHD1 is a nucleic-acid binding protein that is mislocalized due to aicardi-goutieres syndrome-associated mutations. Hum. Mutat. 33, 1116–1122 10.1002/humu.22087 [DOI] [PubMed] [Google Scholar]
- 158.Hartner J.C., Walkley C.R., Lu J. and Orkin S.H. (2009) ADAR1 is essential for maintenance of hematopoiesis and suppression of interferon signaling. Nat. Immunol. 10, 109–115 10.1038/ni.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oda H., Nakagawa K., Abe J., Awaya T., Funabiki M., Hijikata A. et al. (2014) Aicardi-Goutieres syndrome is caused by IFIH1 mutations. Am. J. Hum. Genet. 95, 121–125 10.1016/j.ajhg.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rice G.I., del Toro Duany Y., Jenkinson E.M., Forte G.M., Anderson B.H., Ariaudo G. et al. (2014) Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46, 503–509 10.1038/ng.2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Funabiki M., Kato H., Miyachi Y., Toki H., Motegi H., Inoue M. et al. (2014) Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 40, 199–212 10.1016/j.immuni.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 162.Grandemange S., Sanchez E., Louis-Plence P., Tran Mau-Them F., Bessis D., Coubes C. et al. (2017) A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann. Rheum. Dis. 76, 1191–1198 10.1136/annrheumdis-2016-210021 [DOI] [PubMed] [Google Scholar]
- 163.Canna S.W., Girard C., Malle L., de Jesus A., Romberg N., Kelsen J. et al. (2017) Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J. Allergy Clin. Immunol. 139, 1698–1701 10.1016/j.jaci.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kitamura A., Sasaki Y., Abe T., Kano H. and Yasutomo K. (2014) An inherited mutation in NLRC4 causes autoinflammation in human and mice. J. Exp. Med. 211, 2385–2396 10.1084/jem.20141091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Volker-Touw C.M., de Koning H.D., Giltay J.C., de Kovel C.G., van Kempen T.S., Oberndorff K.M. et al. (2017) Erythematous nodes, urticarial rash and arthralgias in a large pedigree with NLRC4-related autoinflammatory disease, expansion of the phenotype. Br. J. Dermatol. 176, 244–248 10.1111/bjd.14757 [DOI] [PubMed] [Google Scholar]
- 166.Watkin L.B., Jessen B., Wiszniewski W., Vece T.J., Jan M., Sha Y. et al. (2015) COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 47, 654–660 10.1038/ng.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Volpi S., Tsui J., Mariani M., Pastorino C., Caorsi R., Sacco O. et al. (2018) Type I interferon pathway activation in COPA syndrome. Clin. Immunol. 187, 33–36 10.1016/j.clim.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 168.Kanazawa N., Okafuji I., Kambe N., Nishikomori R., Nakata-Hizume M., Nagai S. et al. (2005) Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105, 1195–1197 10.1182/blood-2004-07-2972 [DOI] [PubMed] [Google Scholar]
- 169.Ong L.T., Nachbur U., Rowczenio D., Ziegler J.B., Fischer E. and Lin M.W. (2017) A novel nucleotide oligomerisation domain 2 mutation in a family with Blau syndrome: phenotype and function. Innate Immun. 23, 578–583 10.1177/1753425917727063 [DOI] [PubMed] [Google Scholar]
- 170.Maeda S., Hsu L.C., Liu H., Bankston L.A., Iimura M., Kagnoff M.F. et al. (2005) Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science 307, 734–738 10.1126/science.1103685 [DOI] [PubMed] [Google Scholar]
- 171.Janssen R., Verhard E., Lankester A., Ten Cate R. and van Dissel J.T. (2004) Enhanced interleukin-1beta and interleukin-18 release in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 50, 3329–3333 10.1002/art.20494 [DOI] [PubMed] [Google Scholar]
- 172.Feldmann J., Prieur A.M., Quartier P., Berquin P., Certain S., Cortis E. et al. (2002) Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 10.1086/341357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hoffman H.M., Mueller J.L., Broide D.H., Wanderer A.A. and Kolodner R.D. (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305 10.1038/ng756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ueki Y., Lin C.Y., Senoo M., Ebihara T., Agata N., Onji M. et al. (2007) Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell 128, 71–83 10.1016/j.cell.2006.10.047 [DOI] [PubMed] [Google Scholar]
- 175.Mukai T., Ishida S., Ishikawa R., Yoshitaka T., Kittaka M., Gallant R. et al. (2014) SH3BP2 cherubism mutation potentiates TNF-α-induced osteoclastogenesis via NFATc1 and TNF-α-mediated inflammatory bone loss. J. Bone Miner. Res. 29, 2618–2635 10.1002/jbmr.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Horai R., Saijo S., Tanioka H., Nakae S., Sudo K., Okahara A. et al. (2000) Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist–deficient mice. J. Exp. Med. 191, 313–320 10.1084/jem.191.2.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aksentijevich I., Masters S.L., Ferguson P.J., Dancey P., Frenkel J., van Royen-Kerkhoff A. et al. (2009) An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 360, 2426–2437 10.1056/NEJMoa0807865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Skendros P., Chrysanthopoulou A., Rousset F., Kambas K., Arampatzioglou A., Mitsios A. et al. (2017) Regulated in development and DNA damage responses 1 (REDD1) links stress with IL-1beta-mediated familial Mediterranean fever attack through autophagy-driven neutrophil extracellular traps. J. Allergy Clin. Immunol. 140, 1378.e13–1387.e13 10.1016/j.jaci.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 179.Apostolidou E., Skendros P., Kambas K., Mitroulis I., Konstantinidis T., Chrysanthopoulou A. et al. (2016) Neutrophil extracellular traps regulate IL-1beta-mediated inflammation in familial Mediterranean fever. Ann. Rheum. Dis. 75, 269–277 10.1136/annrheumdis-2014-205958 [DOI] [PubMed] [Google Scholar]
- 180.Messaed C., Chebaro W., Di Roberto R.B., Rittore C., Cheung A., Arseneau J. et al. (2011) NLRP7 in the spectrum of reproductive wastage: rare non-synonymous variants confer genetic susceptibility to recurrent reproductive wastage. J. Med. Genet. 48, 540–548 10.1136/jmg.2011.089144 [DOI] [PubMed] [Google Scholar]
- 181.Singer H., Biswas A., Zimmer N., Messaed C., Oldenburg J., Slim R. et al. (2014) NLRP7 inter-domain interactions: the NACHT-associated domain is the physical mediator for oligomeric assembly. Mol. Hum. Reprod. 20, 990–1001 10.1093/molehr/gau060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Onoufriadis A., Simpson M.A., Pink A.E., Di Meglio P., Smith C.H., Pullabhatla V. et al. (2011) Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am. J. Hum. Genet. 89, 432–437 10.1016/j.ajhg.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zigmond E., Bernshtein B., Friedlander G., Walker C.R., Yona S., Kim K.W. et al. (2014) Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40, 720–733 10.1016/j.immuni.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 184.Jéru I., Duquesnoy P., Fernandes-Alnemri T., Cochet E., Yu J.W., Lackmy-Port-Lis M. et al. (2008) Mutations in NALP12 cause hereditary periodic fever syndromes. Proc. Natl. Acad. Sci. U.S.A. 105, 1614–1619 10.1073/pnas.0708616105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jeru I., Hentgen V., Normand S., Duquesnoy P., Cochet E., Delwail A. et al. (2011) Role of interleukin-1beta in NLRP12-associated autoinflammatory disorders and resistance to anti-interleukin-1 therapy. Arthritis Rheum. 63, 2142–2148 10.1002/art.30378 [DOI] [PubMed] [Google Scholar]
- 186.Jeru I., Le Borgne G., Cochet E., Hayrapetyan H., Duquesnoy P., Grateau G. et al. (2011) Identification and functional consequences of a recurrent NLRP12 missense mutation in periodic fever syndromes. Arthritis Rheum. 63, 1459–1464 10.1002/art.30241 [DOI] [PubMed] [Google Scholar]
- 187.Ibrahim J.N., Jounblat R., Delwail A., Abou-Ghoch J., Salem N., Chouery E. et al. (2014) Ex vivo PBMC cytokine profile in familial Mediterranean fever patients: involvement of IL-1beta, IL-1alpha and Th17-associated cytokines and decrease of Th1 and Th2 cytokines. Cytokine 69, 248–254 10.1016/j.cyto.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 188.Van Gorp H., Saavedra P.H., de Vasconcelos N.M., Van Opdenbosch N., Vande Walle L., Matusiak M. et al. (2016) Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc. Natl. Acad. Sci. U.S.A. 113, 14384–14389 10.1073/pnas.1613156113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Molho-Pessach V., Lerer I., Abeliovich D., Agha Z., Abu Libdeh A., Broshtilova V. et al. (2008) The H syndrome is caused by mutations in the nucleoside transporter hENT3. Am. J. Hum. Genet. 83, 529–534 10.1016/j.ajhg.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Melki I., Lambot K., Jonard L., Couloigner V., Quartier P., Neven B. et al. (2013) Mutation in the SLC29A3 gene: a new cause of a monogenic, autoinflammatory condition. Pediatrics 131, e1308–13 10.1542/peds.2012-2255 [DOI] [PubMed] [Google Scholar]
- 191.Drenth J.P., Goertz J., Daha M.R. and van der Meer J.W. (1996) Immunoglobulin D enhances the release of tumor necrosis factor-alpha, and interleukin-1 beta as well as interleukin-1 receptor antagonist from human mononuclear cells. Immunology 88, 355–362 10.1046/j.1365-2567.1996.d01-672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Drenth J.P., van der Meer J.W. and Kushner I. (1996) Unstimulated peripheral blood mononuclear cells from patients with the hyper-IgD syndrome produce cytokines capable of potent induction of C-reactive protein and serum amyloid A in Hep3B cells. J. Immunol. 157, 400–404 [PubMed] [Google Scholar]
- 193.Drenth J.P., van Deuren M., van der Ven-Jongekrijg J., Schalkwijk C.G. and van der Meer J.W. (1995) Cytokine activation during attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Blood 85, 3586–3593 [PubMed] [Google Scholar]
- 194.Stoffels M., Jongekrijg J., Remijn T., Kok N., van der Meer J.W. and Simon A. (2015) TLR2/TLR4-dependent exaggerated cytokine production in hyperimmunoglobulinaemia D and periodic fever syndrome. Rheumatol. (Oxf.) 54, 363–368 10.1093/rheumatology/keu341 [DOI] [PubMed] [Google Scholar]
- 195.Jurczyluk J., Munoz M.A., Skinner O.P., Chai R.C., Ali N., Palendira U. et al. (2016) Mevalonate kinase deficiency leads to decreased prenylation of Rab GTPases. Immunol. Cell Biol. 94, 994–999 10.1038/icb.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Messaed C., Akoury E., Djuric U., Zeng J., Saleh M., Gilbert L. et al. (2011) NLRP7, a nucleotide oligomerization domain-like receptor protein, is required for normal cytokine secretion and co-localizes with Golgi and the microtubule-organizing center. J. Biol. Chem. 286, 43313–43323 10.1074/jbc.M111.306191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hayward B.E., De Vos M., Talati N., Abdollahi M.R., Taylor G.R., Meyer E. et al. (2009) Genetic and epigenetic analysis of recurrent hydatidiform mole. Hum. Mutat. 30, E629–E639 10.1002/humu.20993 [DOI] [PubMed] [Google Scholar]
- 198.Kou Y.C., Shao L., Peng H.H., Rosetta R., del Gaudio D., Wagner A.F. et al. (2008) A recurrent intragenic genomic duplication, other novel mutations in NLRP7 and imprinting defects in recurrent biparental hydatidiform moles. Mol. Hum. Reprod. 14, 33–40 10.1093/molehr/gam079 [DOI] [PubMed] [Google Scholar]
- 199.El-Maarri O., Seoud M., Coullin P., Herbiniaux U., Oldenburg J., Rouleau G. et al. (2003) Maternal alleles acquiring paternal methylation patterns in biparental complete hydatidiform moles. Hum. Mol. Genet. 12, 1405–1413 10.1093/hmg/ddg152 [DOI] [PubMed] [Google Scholar]
- 200.Herlin T., Fiirgaard B., Bjerre M., Kerndrup G., Hasle H., Bing X. et al. (2013) Efficacy of anti-IL-1 treatment in Majeed syndrome. Ann. Rheum. Dis. 72, 410–413 10.1136/annrheumdis-2012-201818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Donkor J., Zhang P., Wong S., O’Loughlin L., Dewald J., Kok B.P.C. et al. (2009) A Conserved Serine residue is required for the phosphatidate phosphatase activity but not the transcriptional coactivator functions of Lipin-1 and Lipin-2. J. Biol. Chem. 284, 29968–29978 10.1074/jbc.M109.023663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ferguson P., Chen S., Tayeh M., Ochoa L., Leal S., Pelet A. et al. (2005) Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome). J. Med. Genet. 42, 551–557 10.1136/jmg.2005.030759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lorden G., Sanjuan-Garcia I., de Pablo N., Meana C., Alvarez-Miguel I., Perez-Garcia M.T. et al. (2017) Lipin-2 regulates NLRP3 inflammasome by affecting P2X7 receptor activation. J. Exp. Med. 214, 511–528 10.1084/jem.20161452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhong F.L., Mamai O., Sborgi L., Boussofara L., Hopkins R., Robinson K. et al. (2016) Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell 167, 187–202.e17 10.1016/j.cell.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 205.Damgaard R.B., Walker J.A., Marco-Casanova P., Morgan N.V., Titheradge H.L., Elliott P.R. et al. (2016) The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell 166, 1215–1230.e20 10.1016/j.cell.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhou Y., Zhang Y., Moorman J.P., Yao Z.Q. and Jia Z.S. (2014) Viral (hepatitis C virus, hepatitis B virus, HIV) persistence and immune homeostasis. Immunology 143, 319–330 10.1111/imm.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Masters S.L., Lagou V., Jeru I., Baker P.J., Van Eyck L., Parry D.A. et al. (2016) Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci. Transl. Med. 8, 332ra45 10.1126/scitranslmed.aaf1471 [DOI] [PubMed] [Google Scholar]
- 208.Moghaddas F., Llamas R., De Nardo D., Martinez-Banaclocha H., Martinez-Garcia J.J., Mesa-Del-Castillo P. et al. (2017) A novel pyrin-associated autoinflammation with neutrophilic dermatosis mutation further defines 14-3-3 binding of pyrin and distinction to Familial Mediterranean Fever. Ann. Rheum. Dis. 10.1136/annrheumdis-2017-211473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cortesio C.L., Wernimont S.A., Kastner D.L., Cooper K.M. and Huttenlocher A. (2010) Impaired podosome formation and invasive migration of macrophages from patients with a PSTPIP1 mutation and PAPA syndrome. Arthritis Rheum. 62, 2556–2558 10.1002/art.27521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Geusau A., Mothes-Luksch N., Nahavandi H., Pickl W.F., Wise C.A., Pourpak Z. et al. (2013) Identification of a homozygous PSTPIP1 mutation in a patient with a PAPA-like syndrome responding to canakinumab treatment. JAMA Dermatol. 149, 209–215 10.1001/2013.jamadermatol.717 [DOI] [PubMed] [Google Scholar]
- 211.Demidowich A.P., Freeman A.F., Kuhns D.B., Aksentijevich I., Gallin J.I., Turner M.L. et al. (2012) Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis Rheum. 64, 2022–2027 10.1002/art.34332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shoham N.G., Centola M., Mansfield E., Hull K.M., Wood G., Wise C.A. et al. (2003) Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl. Acad. Sci. U.S.A. 100, 13501–13506 10.1073/pnas.2135380100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Omenetti A., Carta S., Caorsi R., Finetti M., Marotto D., Lattanzi B. et al. (2016) Disease activity accounts for long-term efficacy of IL-1 blockers in pyogenic sterile arthritis pyoderma gangrenosum and severe acne syndrome. Rheumatol. (Oxf.) 55, 1325–1335 10.1093/rheumatology/kew031 [DOI] [PubMed] [Google Scholar]
- 214.Fehling H.J., Swat W., Laplace C., Kuhn R., Rajewsky K., Muller U. et al. (1994) MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 265, 1234–1237 10.1126/science.8066463 [DOI] [PubMed] [Google Scholar]
- 215.Fuchs-Telem D., Sarig O., van Steensel M.A., Isakov O., Israeli S., Nousbeck J. et al. (2012) Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am. J. Hum. Genet. 91, 163–170 10.1016/j.ajhg.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jordan C.T., Cao L., Roberson E.D., Pierson K.C., Yang C.F., Joyce C.E. et al. (2012) PSORS2 is due to mutations in CARD14. Am. J. Hum. Genet. 90, 784–795 10.1016/j.ajhg.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mahil S.K., Twelves S., Farkas K., Setta-Kaffetzi N., Burden A.D., Gach J.E. et al. (2016) AP1S3 Mutations cause skin autoinflammation by disrupting keratinocyte autophagy and up-regulating IL-36p roduction. J. Invest. Dermatol. 136, 2251–2259 10.1016/j.jid.2016.06.618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Setta-Kaffetzi N., Simpson M.A., Navarini A.A., Patel V.M., Lu H.C., Allen M.H. et al. (2014) AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am. J. Hum. Genet. 94, 790–797 10.1016/j.ajhg.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Briggs T.A., Rice G.I., Adib N., Ades L., Barete S., Baskar K. et al. (2016) Spondyloenchondrodysplasia due to mutations in ACP5: a comprehensive survey. J. Clin. Immunol. 36, 220–234 10.1007/s10875-016-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Briggs T.A., Rice G.I., Daly S., Urquhart J., Gornall H., Bader-Meunier B. et al. (2011) Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat. Genet. 43, 127–131 10.1038/ng.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.An J., Briggs T.A., Dumax-Vorzet A., Alarcon-Riquelme M.E., Belot A., Beresford M. et al. (2017) Tartrate-resistant acid phosphatase deficiency in the predisposition to systemic lupus erythematosus. Arthritis Rheumatol. 69, 131–142 10.1002/art.39810 [DOI] [PubMed] [Google Scholar]
- 222.Bune A.J., Hayman A.R., Evans M.J. and Cox T.M. (2001) Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disordered macrophage inflammatory responses and reduced clearance of the pathogen, Staphylococcus aureus. Immunology 102, 103–113 10.1046/j.1365-2567.2001.01145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lobito A.A., Kimberley F.C., Muppidi J.R., Komarow H., Jackson A.J., Hull K.M. et al. (2006) Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS). Blood 108, 1320–1327 10.1182/blood-2005-11-006783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bachetti T., Chiesa S., Castagnola P., Bani D., Di Zanni E., Omenetti A. et al. (2013) Autophagy contributes to inflammation in patients with TNFR-associated periodic syndrome (TRAPS). Ann. Rheum. Dis. 72, 1044–1052 10.1136/annrheumdis-2012-201952 [DOI] [PubMed] [Google Scholar]
- 225.Nedjai B., Hitman G.A., Church L.D., Minden K., Whiteford M.L., McKee S. et al. (2011) Differential cytokine secretion results from p65 and c-Rel NF-kappaB subunit signaling in peripheral blood mononuclear cells of TNF receptor-associated periodic syndrome patients. Cell. Immunol. 268, 55–59 10.1016/j.cellimm.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 226.Nedjai B., Hitman G.A., Yousaf N., Chernajovsky Y., Stjernberg-Salmela S., Pettersson T. et al. (2008) Abnormal tumor necrosis factor receptor I cell surface expression and NF-kappaB activation in tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 58, 273–283 10.1002/art.23123 [DOI] [PubMed] [Google Scholar]
- 227.Rebelo S.L., Bainbridge S.E., Amel-Kashipaz M.R., Radford P.M., Powell R.J., Todd I. et al. (2006) Modeling of tumor necrosis factor receptor superfamily 1A mutants associated with tumor necrosis factor receptor-associated periodic syndrome indicates misfolding consistent with abnormal function. Arthritis Rheum. 54, 2674–2687 10.1002/art.21964 [DOI] [PubMed] [Google Scholar]
- 228.Yousaf N., Gould D.J., Aganna E., Hammond L., Mirakian R.M., Turner M.D. et al. (2005) Tumor necrosis factor receptor I from patients with tumor necrosis factor receptor-associated periodic syndrome interacts with wild-type tumor necrosis factor receptor I and induces ligand-independent NF-kappaB activation. Arthritis Rheum. 52, 2906–2916 10.1002/art.21268 [DOI] [PubMed] [Google Scholar]
- 229.D’Osualdo A., Ferlito F., Prigione I., Obici L., Meini A., Zulian F. et al. (2006) Neutrophils from patients with TNFRSF1A mutations display resistance to tumor necrosis factor-induced apoptosis: pathogenetic and clinical implications. Arthritis Rheum. 54, 998–1008 10.1002/art.21657 [DOI] [PubMed] [Google Scholar]
- 230.Kamata H., Honda S., Maeda S., Chang L., Hirata H. and Karin M. (2005) Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120, 649–661 10.1016/j.cell.2004.12.041 [DOI] [PubMed] [Google Scholar]
- 231.Miscia S., Marchisio M., Grilli A., Di Valerio V., Centurione L., Sabatino G. et al. (2002) Tumor necrosis factor alpha (TNF-alpha) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 13, 13–18 [PubMed] [Google Scholar]
- 232.Negm O.H., Mannsperger H.A., McDermott E.M., Drewe E., Powell R.J., Todd I. et al. (2014) A pro-inflammatory signalome is constitutively activated by C33Y mutant TNF receptor 1 in TNF receptor-associated periodic syndrome (TRAPS). Eur. J. Immunol. 44, 2096–2110 10.1002/eji.201344328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Meuwissen M.E., Schot R., Buta S., Oudesluijs G., Tinschert S., Speer S.D. et al. (2016) Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 213, 1163–1174 10.1084/jem.20151529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Starokadomskyy P., Gemelli T., Rios J.J., Xing C., Wang R.C., Li H. et al. (2016) DNA polymerase-α regulates type I interferon activation through cytosolic RNA:DNA synthesis. Nat. Immunol. 17, 495–504 10.1038/ni.3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gazzo A., Raimondi D., Daneels D., Moreau Y., Smits G., Van Dooren S. et al. (2017) Understanding mutational effects in digenic diseases. Nucleic Acids Res. 45, e140 10.1093/nar/gkx557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lupski J.R. (2012) Digenic inheritance and Mendelian disease. Nat. Genet. 44, 1291–1292 10.1038/ng.2479 [DOI] [PubMed] [Google Scholar]
- 237.Cordell H.J. (2002) Epistasis: what it means, what it doesn’t mean, and statistical methods to detect it in humans. Hum. Mol. Genet. 11, 2463–2468 10.1093/hmg/11.20.2463 [DOI] [PubMed] [Google Scholar]
- 238.Gazzo A.M., Daneels D., Cilia E., Bonduelle M., Abramowicz M., Van Dooren S. et al. (2016) DIDA: a curated and annotated digenic diseases database. Nucleic Acids Res. 44, D900–D907 10.1093/nar/gkv1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ameratunga R., Woon S.T., Bryant V.L., Steele R., Slade C., Leung E.Y. et al. (2017) Clinical implications of digenic inheritance and epistasis in primary immunodeficiency disorders. Front. Immunol. 8, 10.3389/fimmu.2017.01965, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ben-Zvi I., Brandt B., Berkun Y., Lidar M. and Livneh A. (2012) The relative contribution of environmental and genetic factors to phenotypic variation in familial Mediterranean fever (FMF). Gene 491, 260–263 10.1016/j.gene.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 241.Rodríguez-Cortez V.C., del Pino-Molina L., Rodríguez-Ubreva J., Ciudad L., Gómez-Cabrero D., Company C. et al. (2015) Monozygotic twins discordant for common variable immunodeficiency reveal impaired DNA demethylation during naïve-to-memory B-cell transition. Nat. Commun. 6, 10.1038/ncomms8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kirectepe A.K., Kasapcopur O., Arisoy N., Celikyapi Erdem G., Hatemi G., Ozdogan H. et al. (2011) Analysis of MEFV exon methylation and expression patterns in familial Mediterranean fever. BMC Med. Genet. 12, 105 10.1186/1471-2350-12-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Vento-Tormo R., Álvarez-Errico D., Garcia-Gomez A., Hernández-Rodríguez J., Buján S., Basagaña M. et al. (2017) DNA demethylation of inflammasome-associated genes is enhanced in patients with cryopyrin-associated periodic syndromes. J. Allergy Clin. Immunol. 139, 202.e6–211.e6 10.1016/j.jaci.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 244.Zuo T., Tycko B., Liu T.M., Lin J.J. and Huang T.H. (2009) Methods in DNA methylation profiling. Epigenomics 1, 331–345 10.2217/epi.09.31 [DOI] [PMC free article] [PubMed] [Google Scholar]