Abstract
Objective
We aimed to investigate the association of the frequency of three single-nucleotide polymorphisms [glucose transporter isoform 1 (GLUT1) reference single-nucleotide polymorphism (rs) 710218, hypoxia-inducible factor 1 alpha (HIF1α) rs11549465, and T-box transcription factor protein 21 (TBX21) rs17250932], which have been proved to be related with various benign and malignant diseases, with the development of laryngeal cancer and its size and grade.
Methods
In this study, we included 35 patients with laryngeal cancer and 35 volunteers at least 30 years old who had smoked for at least 20 years. DNA was obtained from the blood samples of the participants using an isolation kit. Then, polymorphisms for both the groups were determined using real-time polymerase chain reaction.
Results
No significant differences were detected regarding the genotype and allele frequencies in the three polymorphisms assessed between the two groups. In the patient group, on examining the association of polymorphisms with tumor size and grade, no significant relation was observed in three polymorphisms regarding the related parameters.
Conclusion
GLUT1, HIF1α, and TBX21 polymorphisms have no impact on the development of laryngeal cancer.
Keywords: Cancer, larynx, polymorphism, GLUT1, HIF1α, TBX21
Introduction
Laryngeal cancers constitute about 30% of all cancer cases in the head and neck region. The reported male to female ratio is about 4.6 to 1 (1). Its prevalence increases in the fifth decade and peaks in the seventh to eighth decades (2). A majority (95%) of these cancers are squamous cell carcinomas (3, 4).
The etiology involves environmental and genetic factors. While tobacco and alcohol consumption are the major environmental risk factors, human papillomavirus infection, a diet poor in vegetables and fruits, exposure to coal dust, extensive consumption of pickled meat and fish products are also held responsible (5, 6). Reported genetic factors include the presence of genes responsible for the production of some proteins and enzymes, and polymorphisms (7, 9). Genetic polymorphisms are thought to have a role also in the development of malignancies in organs like the esophagus, the breasts, and the pancreas, and in the development of systemic diseases like diabetes mellitus, systemic lupus erythematosus, and preeclampsia (9–14).
In our review of the literature we identified a limited number of studies that examine the influence of genes or gene polymorphism on parameters like the development and the grade of laryngeal cancer, and tumor size (7, 8). Therefore in this study, we explored the correlation between laryngeal cancer development, tumor grade and size, and the frequency of polymorphisms in Glucose Transporter Isoform 1 (GLUT1) (rs710218) which is responsible for glucose metabolism and the intracellular transport of glucose, in Hypoxia Inducible Factor 1 Alpha (HIF1α) (rs11549465) which regulates cellular response to hypoxia, and in T-box Transcription Factor Protein 21 (TBX21) (rs17250932) which takes part in regulating immune response, in patients who were diagnosed with laryngeal squamous cell carcinoma and in healthy control group patients.
Methods
Study Group
Our study included 35 patients that were diagnosed with laryngeal cancer and 35 healthy volunteers. Mean age was 64.62±10.59 years (range: 43–88) in the study group and 60.11+13.97 years in the control group (range: 31–88). There were no statistically significant differences between the ages and the number of smoking years of the subjects (p=0.132, p=0.062). All participants in the two groups are male. We did not find any reports in the literature on the correlation between gender and the polymorphisms that we have explored in our study. Participants were selected from the patients who were admitted to the ENT Department of Süleyman Demirel University (SDU) Hospital from January 2012 to July 2016.
Inclusion criteria for the study group were:
- Diagnosis of histologically confirmed squamous cell carcinoma of the larynx,
- Smoking history of at least 20 packs/year,
- ≥30 years of age,
- Absence of any other malignancy or systemic disease.
Inclusion criteria for the control group were:
- Smoking history of at least 20 packs/year,
- ≥30 years of age,
- Absence of any malignancy or systemic disease.
Two mL of blood sample was collected during clinical examination from each of the participants in both groups and the samples were placed in tubes containing ethylene diamine triacetic acid. All blood samples were preserved at −200C until deoxyribonucleic acid (DNA) isolation.
Approval was obtained for this prospective study from the Ethics Committee for Clinical Research of SDU (issue date 16 March 2016 and number 46). All volunteers read and signed the informed consent form.
Polymorphism Determination Method
Deoxyribonucleic acid isolation was performed from the blood samples [High Pure Polymerase Chain Reaction (PCR) Template Preparation Kit; Roche, İstanbul, Turkey] (15). Extracted DNA samples were preserved at −20 C°. Spectrophotometric methods were used to assess DNA concentrations. Concentration and purity of the DNA samples were quantified by measuring absorbance at 260 nm and 280 nm.
Then, polymorphisms were determined for both groups using Real Time-PCR and allele groups were analyzed.
Statistical Analysis
Statistical analyses were conducted using three single nucleotide polymorphism (SNP) variants assessed in the samples taken from the study and control groups. Sizes and grades of tumors were also statistically compared. All statistical analyses were carried out using Statistical Packages for the Social Sciences (SPSS) Windows 15.0 (SPSS Inc.; Chicago, IL, USA). Data were assessed using Student’s t test and Chi-square test and p<0.05 was accepted as significant.
Results
In our study which GLUT1 rs710218 polymorphism (Adenine (A), Thymine (T)) was assessed, AA variant of this polymorphism was present in 22 out of the 35 patients, while AT (10 patients) or homozygous T (3 patients) variants were present in 13 patients (Figure 1). Out of the 35 individuals in the control group, 25 had homozygous A variant, eight had AT and two had homozygous TT variant (Figure 2). In analyzing the differences between the study and the control groups, no statistically significant differences were observed between the AA variant and non-AA variant (AT and TT) rates (p=0.611). No significant differences were found between the two groups for the frequencies of A and T alleles.
Figure 1.
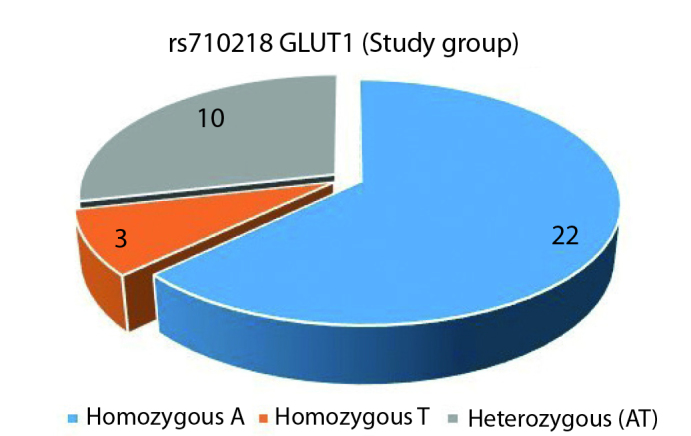
GLUT1 rs710218 study group genotype distribution (A: Adenine, T: Thymine)
Figure 2.
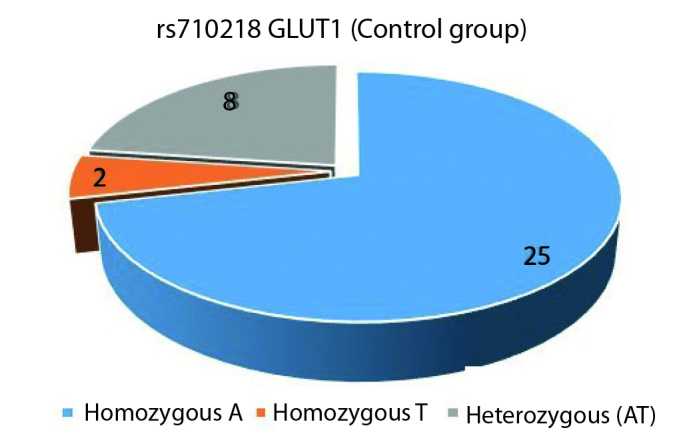
GLUT1 rs710218 control group genotype distribution (A: Adenine, T: Thymine)
Homozygous TT variant of HIF1α rs11549465 polymorphism was not observed in either of the groups (Figures 3, 4). Comparison of homozygous cytosine (C) and CT variants in both groups showed 28 CC variants and CT was found in seven individuals (p=1.0).
Figure 3.
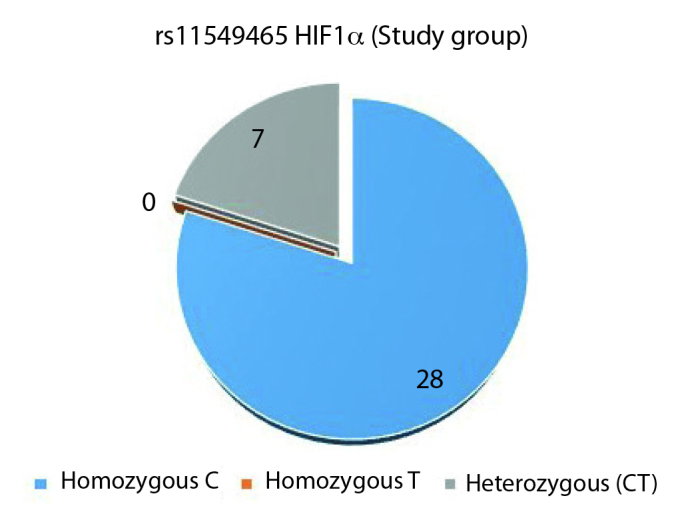
HIF1α rs11549465 study group genotype distribution (C: Cytosine, T: Thymine)
Figure 4.
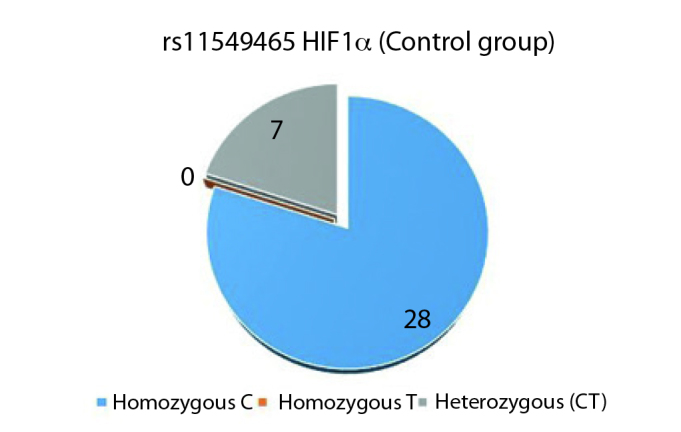
HIF1α rs11549465 control group genotype distribution (C: Cytosine, T: Thymine)
TBX21 rs17250932 polymorphism was assessed by comparing the rate of the TT variant, which was dominant in both groups, with the rates of non-TT variants (CT and CC). In the study group, homozygous T variant was seen in 20, CT variant in 15 patients, while homozygous C variant was seen in none (Figure 5). In the control group, homozygous T variant was seen 20 individuals; and out of the remaining 15, CT variant was seen in 14 and CC variant was seen in one (Figure 6). Statistically significant differences were not found between the two groups for TT and non-TT variants (p=1). The two groups were also found comparable for C and T alleles.
Figure 5.
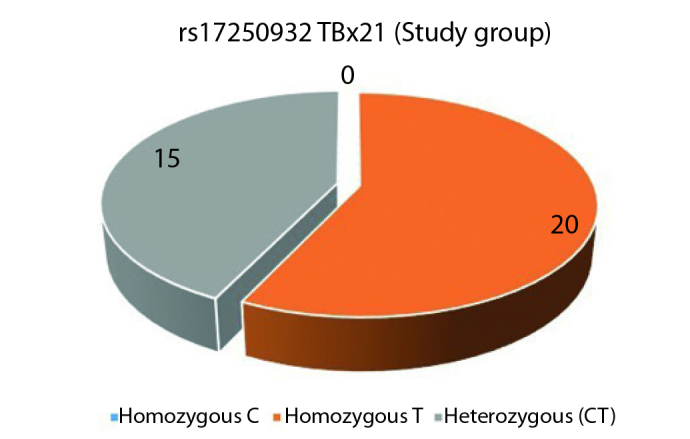
TBX21 rs17250932 study group genotype distribution (C: Cytosine, T: Thymine)
Figure 6.
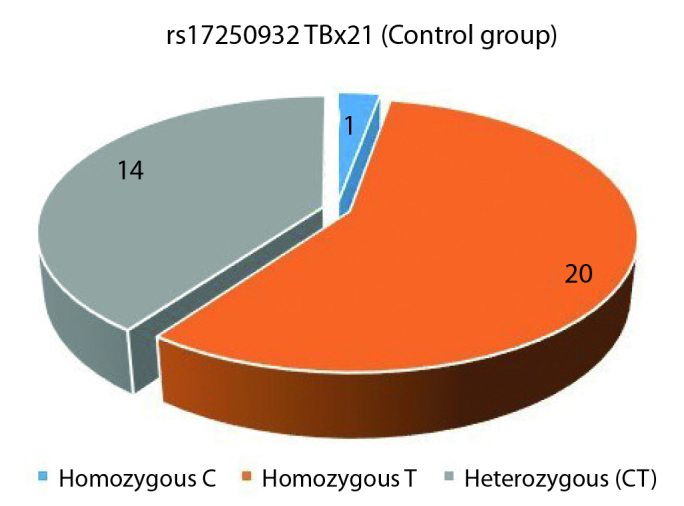
TBX21 rs17250932 control group genotype distribution (C: Cytosine, T: Thymine)
No statistically significant differences were identified between GLUT1 rs710218 polymorphic variants and tumor sizes in the study group (p=0.45). With regards to the correlation between genotype and tumor grade, advanced grade tumor (T3/T4) was seen in 11 of the 21 patients in the first group (AA), and in five of the 11 patients in the non-AA (AA+AT) group. Statistically difference was not found between the group in this respect (p=1).
No statistically significant differences were identified between HIF1α rs11549465 polymorphic variants and tumor size (p=1, p=1).
No statistically significant differences were identified between TBX21 rs17250932 polymorphic variants, and tumor grade and size (p=0.45, p=0.722).
Discussion
There are many factors that can influence cancer development, including environmental and dietary, as well as genetic factors. Many genes are reported to have a role in cancer development including the proteins that are responsible for signal transmission in cell proliferation, mitotic cycle regulators, and the genes which code the proteins that are responsible for mutation expression and repair (16). The presence of two or more genetically identified alternative phenotypes in a population based one various alleles is defined as “genetic polymorphism.” That thousands of candidate polymorphic genes exist within the human genome and that such differences in the genome can affect the individual’s predisposition for cancer, lead many researchers to explore this area (17).
In recent years many studies were published in the literature that address structures like GLUT1 GLUT3, HIF1, TBX, manganese-dependent superoxide dismutase (MnSOD), glutathione peroxidase 1 (GPx1), catalase C-262T polymorphism (CAT-262), and the correlation between their polymorphisms and other types of cancers. Most of these studies were conducted in areas other than ENT and explore genetic polymorphism in the production of a range of enzymes. There are only two studies in the literature that explore the influence of the gene polymorphisms in the development of laryngeal cancer.
Aynali et al. (7), in their 2013 study, examined the polymorphisms of MnSOD, GPx1 and CAT-262 genes. In this study, the smoking laryngeal cancer patients in the study group were compared to the smoking individuals in the control group based on parameters like age, number of smoking years, and prevalence of carcinoma development. The authors reported to have found the homozygous AA genotype of MnSOD Valine16Alanine (Val16Ala) to be significantly more prevalent in the study group than in the control group (93% vs 13%). Similarly, the MnSOD Val16Ala AV genotype was found to be more prevalent than in the control group (87% vs 8%). No significant differences were found between the groups for the polymorphisms examined in the study. This study by Aynali et al. (7) demonstrated that polymorphism of the MnSOD Val16Ala gene could contribute to a predisposition to laryngeal cancer (7). Comparably, we examined one polymorphism in each of the three genes (GLUT1, HIF1α, and TBX21) in our study group of smoking laryngeal cancer patients versus the smoking healthy individuals in the control group. We found no statistically significant differences.
Mera-Menéndez et al. (8) examined the correlation between glottic laryngeal cancer and genetic polymorphism. This study compared the polymorphic variants of C1772T and G1790A of HIF1α in glottic laryngeal cancer patients versus healthy volunteers. Advanced grade tumors (T3/T4) were associated with the TT genotype. The study demonstrated that patients with nodal metastasis carried the TT and GA variants at a significantly higher rate. As a result, the study has identified that the presence of the TT and GA variants were associated with lymph node metastasis, and the presence of the TT variant was associated with larger tumor size (8). In our study, CC homozygous variant was seen at 80% both in the study and the control groups. Homozygous TT variant was not observed in either of the groups. The study and control groups in which the indicated variants were assessed were also compared for polymorphism variants and cancer grades and no significant differences were found.
Correlation of genetic polymorphism has also been extensively studied in non-malignant diseases, as well as malignant diseases, and interestingly, were found to carry strong associations (18, 20). According to the results of our study, there is no definitive evidence that laryngeal cancer is influenced by the polymorphisms of GLUT1, HIF1α, and TBX21. Multiple studies can be planned with large-series for assessing enzymes, proteins and polymorphisms to demonstrate a correlation.
Conclusion
GLUT1 rs710218, HIF1α rs11549465 and TBX21 rs17250932 polymorphisms were concluded to have no influence on laryngeal cancer.
Footnotes
This study was presented at the 39th Turkish National Congress of Otorhinolaryngology Head and Neck Surgery, November 8–12 2017, Antalya, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Süleyman Demirel University, School of Medicine (16.03.2016-46).
Informed Consent: Informed consent was obtained from all individual participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.U., M.T.; Design - C.U., M.T.; Supervision - C.U., M.T., H.Y., E.O.; Resource - C.U.; Materials - C.U.; Data Collection and/or Processing - C.U., M.T.; Analysis and/or Interpretation - C.U., M.T., H.Y., E.O.; Literature Search - C.U., M.T.; Writing - C.U., M.T.; Critical Reviews - M.T., H.Y., E.O.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: This study was funded by The Coordinatorship of Scientific Research Projects Department (BAP), Süleyman Demirel University by the number of 4726-TU1-16.
References
- 1.Sasaki CT, Carlson RD. Malignant neoplasms of the larynx. In: Cummins CW, editor. Otolaryngology - Head and Neck Surgery. St. Louis: Mosby; 1993. pp. 1925–54. [Google Scholar]
- 2.Mollahaliloğlu S, Başara BB, Eryılmaz Z, editors. Health Statistics Yearbook. Turkey: Ministry of Health; 2010. p. 26. [Google Scholar]
- 3.Pilch BZ, Dorfman DM, Brodsky GL, Goodman ML. Pathology of laryngeal malignancies. In: Fried MP, editor. The Larynx, A Multidisciplinary Approach. Second Edition. St. Louis: Mosby; 2015. pp. 461–85. [Google Scholar]
- 4.Rosai J. Larynx and trachea. In: Rosai J, editor. Ackerman’s Surgical Pathology. St. Louis: Mosby; 1996. pp. 314–37. [Google Scholar]
- 5.Shangina O, Brennan P, Szeszenia-Dabrowska N, Mates D, Fabiánová E, Fletcher T, et al. Occupational exposure and laryngeal and hypopharyngeal cancer risk in central and eastern Europe. Am J Epidemiol. 2006;164:367–75. doi: 10.1093/aje/kwj208. [DOI] [PubMed] [Google Scholar]
- 6.Zheng W, Blot WJ, Shu XO, Gao YT, Ji BT, Ziegler RG, et al. Diet and other risk factors for laryngeal cancer in Shangai, China. Am J Epidemiol. 1992;136:178–91. doi: 10.1093/oxfordjournals.aje.a116484. [DOI] [PubMed] [Google Scholar]
- 7.Aynali G, Doğan M, Sütcü R, Yüksel Ö, Yarıktaş M, Ünal F, et al. Polymorphic variants of MnSOD Val16Ala, CAT-262 C < T and GPx1 Pro198Leu genotypes and the risk of laryngeal cancer in a smoking population. J Laryngol Otol. 2013;127:997–1000. doi: 10.1017/S0022215113002028. [DOI] [PubMed] [Google Scholar]
- 8.Mera-Menéndez F, Hinojar-Gutiérrez A, Guijarro Rojas M, de Gregorio J, Mera-Menéndez EJ, Sánchez JJ, et al. Polymorphisms in HIF-1alpha affect presence of lymph node metastasis and can influence tumor size in squamous-cell carcinoma of the glottic larynx. Clin Transl Oncol. 2013;15:358–63. doi: 10.1007/s12094-012-0930-z. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Zhen H, Han L, Yan B, Yu J, Zhu S, et al. Association between the genetic variations within TBX21 gene promoter and the clinicopathological characteristics of esophageal squamous cell carcinoma in a high-risk Chinese population. Tumour Biol. 2015;36:3985–93. doi: 10.1007/s13277-015-3042-x. [DOI] [PubMed] [Google Scholar]
- 10.Grabellus F, Sheu SY, Bachmann HS, Lehmann N, Otterbach F, Heusner T, et al. The XbaI G.T Polymorphism of the Glucose Transporter 1 Gene Modulates 18F-FDG Uptake and Tumor Aggressiveness in Breast Cancer. J Nucl Med. 2010;51:1191–7. doi: 10.2967/jnumed.110.075721. [DOI] [PubMed] [Google Scholar]
- 11.Li P, Cao Q, Shao PF, Cai H, Zhou H, Chen J, et al. Genetic polymorphisms in HIF1A are associated with prostate cancer risk in a Chinese population. Asian J Androl. 2012;14:864–9. doi: 10.1038/aja.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontiroli AE, Capra F, Veglia F, Ferrari M, Xiang KS, Bell GI, et al. Genetic contribution of polymorphism of the GLUT1 and GLUT4 genes to the susceptibility to type 2 (non-insulin-dependent) diabetes mellitus in different populations. Acta Diabetol. 1996;33:193–7. doi: 10.1007/BF02048542. [DOI] [PubMed] [Google Scholar]
- 13.You Y, Zhao W, Chen S, Tan W, Dan Y, Hao F, et al. Association of TBX21 gene haplotypes in a Chinese population with systemic lupus erythematosus. Scand J Rheumatol. 2010;39:254–8. doi: 10.3109/03009740903347983. [DOI] [PubMed] [Google Scholar]
- 14.Andraweera PH, Dekker GA, Thompson SD, Dissanayake VH, Jayasekara RW, Roberts CT. Hypoxia-inducible factor-1α gene polymorphisms in early and late onset preeclampsia in Sinhalese women. Placenta. 2014;35:491–5. doi: 10.1016/j.placenta.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 15.High Pure PCR Template Preparation Kit. Rapidly purify genomic DNA for diverse applications. Available from: https://lifescience.roche.com/documents/High-Pure-PCR-Template-Preparation-Kit.pdf.
- 16.Nussbaum RL, McInnes RR, Willard HF, editors. Cancer Genetics and Genomics. 7th edition. Holland: Thompson & Thompson Genetics in Medicine; 2007. [DOI] [Google Scholar]
- 17.Ekmekçi A, Konaç E, Önen Hİ. Gen polimorfizmi ve kansere yatkınlık. MMJ. 2008;21:282–95. [Google Scholar]
- 18.Nagy G, Kovacs-Nagy R, Kereszturi E, Somogyi A, Szekely A, Nemeth N, et al. Association of hypoxia inducible factor-1 alpha gene polymorphism with both type 1 and type 2 diabetes in a Caucasian (Hungarian) sample. BMC Med Genet. 2009;10:79. doi: 10.1186/1471-2350-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wipff J, Dieude P, Avouac J, Tiev K, Hachulla E, Granel B, et al. Association of hypoxia-inducible factor 1A (HIF1A) gene polymorphisms with systemic sclerosis in a French European Caucasian population. Scand J Rheumatol. 2009;38:291–4. doi: 10.1080/03009740802629432. [DOI] [PubMed] [Google Scholar]
- 20.Zhu DY, Jiang LF, Deng XZ, Xiao W, Pei JP, Li BJ, et al. TBX21 polymorphisms are associated with virus persistence in hepatitis C virus infection patients from a high-risk Chinese population. Eur J Clin Microbiol Infect Dis. 2015;34:1309–18. doi: 10.1007/s10096-015-2337-6. [DOI] [PubMed] [Google Scholar]


