Abstract
Purpose
To test if a RapidPlan DVH estimation model and its training plans can be improved interactively through a closed‐loop evolution process.
Methods and materials
Eighty‐one manual plans (P0) that were used to configure an initial rectal RapidPlan model (M0) were reoptimized using M0 (closed‐loop), yielding 81 P1 plans. The 75 improved P1 (P1+) and the remaining 6 P0 were used to configure model M1. The 81 training plans were reoptimized again using M1, producing 23 P2 plans that were superior to both their P0 and P1 forms (P2+). Hence, the knowledge base of model M2 composed of 6 P0, 52 P1+, and 23 P2+. Models were tested dosimetrically on 30 VMAT validation cases (Pv) that were not used for training, yielding Pv(M0), Pv(M1), and Pv(M2) respectively. The 30 Pv were also optimized by M2_new as trained by the library of M2 and 30 Pv(M0).
Results
Based on comparable target dose coverage, the first closed‐loop reoptimization significantly (P < 0.01) reduced the 81 training plans’ mean dose to femoral head, urinary bladder, and small bowel by 2.65 Gy/15.63%, 2.06 Gy/8.11%, and 1.47 Gy/6.31% respectively, which were further reduced significantly (P < 0.01) in the second closed‐loop reoptimization by 0.04 Gy/0.28%, 0.18 Gy/0.77%, 0.22 Gy/1.01% respectively. However, open‐loop VMAT validations displayed more complex and intertwined plan quality changes: mean dose to urinary bladder and small bowel decreased monotonically using M1 (by 0.34 Gy/1.47%, 0.25 Gy/1.13%) and M2 (by 0.36 Gy/1.56%, 0.30 Gy/1.36%) than using M0. However, mean dose to femoral head increased by 0.81 Gy/6.64% (M1) and 0.91 Gy/7.46% (M2) than using M0. The overfitting problem was relieved by applying model M2_new.
Conclusions
The RapidPlan model and its constituent plans can improve each other interactively through a closed‐loop evolution process. Incorporating new patients into the original training library can improve the RapidPlan model and the upcoming plans interactively.
Keywords: knowledge‐based planning, model improvement, RapidPlan, rectal cancer
1. INTRODUCTION
Knowledge‐based radiotherapy treatment planning is deemed to reduce the inter‐planner varieties of plan quality1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 and expedite the planning process.14, 15, 16, 17The RapidPlan module in Eclipse treatment planning system of version 13.5 or later (Varian Medical Systems, Palo Alto, CA) has commercialized the knowledge‐based solution18, 19 and displayed good compatibility across patient orientations, treatment techniques, and systems.20, 21
Well‐trained RapidPlan models have outperformed conventional trial and error‐based manual planning by reducing excess organs‐at‐risk (OAR) dose with greater consistency.17, 20, 22, 23, 24, 25, 26, 27, 28, 29, 30 Should the model performance be highly dependent on the library volume31 and average quality of the training plans,17, 32 incorporating the model‐improved constituent training plans into the model (closed‐loop)25 may potentially evolve the model as a cycle of interactive improvement. There has been attempts to iteratively improve KDE (kernel density estimation)‐based DVH prediction model. However, compared with RapidPlan, the KDE algorithm did not consider division between in‐field and out‐of‐field regions, and the generated point objectives were tested on limited sample size based on Pinnacle (Philips Radiation Oncology Systems, Fitchburg, WI),33 whose optimization algorithm, progressive optimization algorithm (POA) is different from Eclipse's Photon Optimizer (PO). This study aims to evaluate the performance of the closed‐loop model evolution on rectal cancer patients in the environment of Eclipse RapidPlan V13.6 knowledge‐based treatment planning system.
2. MATERIALS AND METHODS
As a summary, Fig. 1 displays a schematic workflow explaining the evolution process and naming abbreviations.
Figure 1.
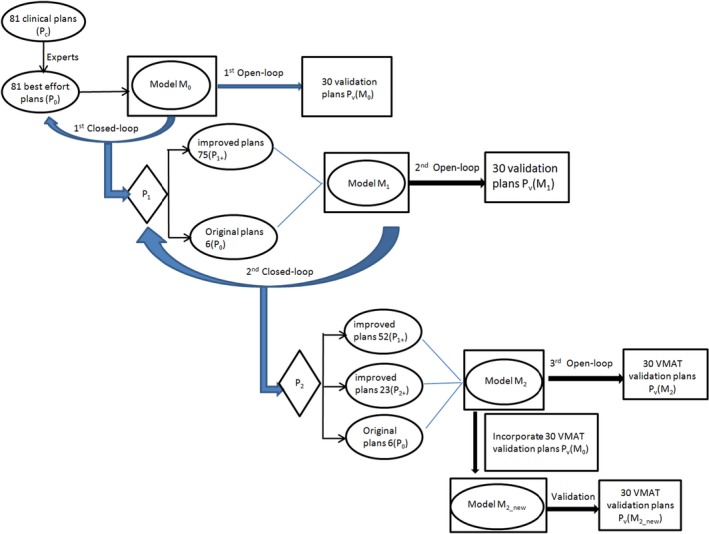
A schematic workflow and naming abbreviations of this work.
2.A. Initial model configuration
The planning and modeling details can be found in our previous publications.17, 20, 34 In summary, 81 clinical VMAT plans (Pc) for preoperative rectal cancer patients were refined manually by experts (best‐effort manual plans, P0) to guarantee the initial plan quality and push a stricter evaluation criteria on the closed‐loop method. Plans were optimized to deliver 50.6 Gy and 41.8 Gy to 95% PTVboost and 95% PTV respectively in 22 fractions.35 The extracted structure sets, prescriptions, and field geometries of P0 were regressed as the initial DVH estimation model (M0) and statistically verified using Varian Model Analytics tool.36 Model‐generated optimization objectives and priorities were assisted by additional manual constraints to make the model comply with our clinical protocols. The validations on 100+ patients have demonstrated that M0‐generated personalized objectives improved plan quality and consistency significantly compared to the clinical plans.17, 20
2.B. Model evolution
As shown in Fig. 1, the 81 constituent P0 of M0 were reoptimized using M0 (closed‐loop), yielding training sets of first iteration (P1). To simplify the scoring of plan quality and avoid observer‐dependent evaluation preferences especially when the DVH lines have crossovers, three explicit endpoints: the mean dose to the femoral head, urinary bladder, and small bowel (D mean_FH, D mean_UB, and D mean_SB) were compared33. Plans with reduced D mean_FH, D mean_UB, and D mean_SB were defined as improved plans (P1+). The first closed‐loop reoptimization using M0 produced 75 P1+, which composed M1 in addition to 6 P0 where the original plans were considered better. Second closed‐loop reoptimization using M1 derived 23 P2+ of better quality than both their P0 and P1 forms. The new model of each iteration was configured with best plans from all previous optimizations, hence M2 included 6 P0, 52 P1+, and 23 P2+. To be cost‐effective, iterations were terminated when no or clinically negligible improvement could be achieved anymore.
2.C. Model assessment
To monitor the impact of modifying the knowledge base, open‐loop validation was performed on other 30 clinical VMAT plans (Pv) that were not included in any model. All plans were renormalized to the target prescriptions before comparing the OAR exposure. Specifically, open‐loop validation on 30 Pv were reoptimized using M0, M1, and M2, producing Pv(M0), Pv(M1), and Pv(M2) respectively. The following metrics were evaluated: (a) homogeneity index (), where indicates the dose to x% of the volume; (b) conformity index (), where and indicate the volumes receiving at least 100% of the prescribed dose and the target volumes respectively; (c) the small bowel volumes receiving at least 35, 40, and 45 Gy (V 35Gy_SB, V 40Gy_SB, V 45Gy_SB); (d) the femoral head and urinary bladder volumes receiving at least 40 and 45 Gy (V 40Gy_FH, V 40Gy_UB, V 45Gy_FH, and V 45Gy_UB); (e) The maximum and mean dose to the small bowel, femoral head, and urinary bladder (D max_SB, D max_FH, D max_UB; D mean_SB, D mean_FH, and D mean_UB). (f) Using an in‐house MATLAB code, mean DVHs of 30 Pv and their reoptimized forms were calculated based on exported DVHs in tabular format, and were plotted for comparison using SigmaPlot software (v. 10.0, Systat, San Jose, CA).
To address the over‐fitting problem, the reoptimized 30 VMAT validation cases using M0 (Pv(M0)) were added to the training library of M2, yielding model M2_new. The performance of M2_new was tested on the 30 validation cases thereafter.
2.D. Statistical methods
Using SPSS (v21.0, IBM Analytics, Armonk, NY), normality was tested using Shapiro–Wilk method. Normal and abnormal data were analyzed by paired samples t‐test and Wilcoxon signed‐rank test respectively (two‐tailed, significant level 0.05).
3. RESULTS
3.A. Closed‐loop reoptimizations
After replacing the training library with 75 P1+ during the first closed‐loop refinement, the 81 plans used to configure model M1 were of comparable HI and CI (mean difference < 0.03) relative to the library of M0, but of consistently lower mean dose to all OARs. D mean_FH, D mean_UB, and D mean_SB in M1 library were significantly reduced by 2.65 Gy (15.63%), 2.06 Gy (8.11%), and 1.47 Gy (6.31%) respectively (all P < 0.01), relative to M0 library.
After updating the library of model M2 with 23 P2+ that were superior to both P0 and P1 forms after the second closed‐loop reoptimization, the changes of HI and CI were negligible (mean difference < 0.03), yet the D mean_FH, D mean_UB, and D mean_SB of 81 plans were further significantly reduced by 0.04 Gy (0.28%), 0.18 Gy (0.77%), 0.22 Gy (1.01%) on average respectively (all P < 0.01), relative to M1 library.
More details are shown in Tables 1 and 2.
Table 1.
Dosimetric changes of 81 training plans after incorporating improved plans from the closed‐loop reoptimization: targets
| HI | CI | |||
|---|---|---|---|---|
| PTVboost | PTV | PTVboost | PTV | |
| M0 | ||||
| Mean ± SD | 0.06 ± 0.01 | 0.26 ± 0.01 | 1.06 ± 0.07 | 1.02 ± 0.02 |
| 95% CI | 0.06–0.06 | 0.26–0.27 | 1.05–1.08 | 1.02–1.03 |
| M1 | ||||
| Mean ± SD | 0.05 ± 0.01 | 0.26 ± 0.01 | 1.09 ± 0.08 | 1.03 ± 0.02 |
| 95% CI | 0.05–0.05 | 0.26–0.27 | 1.07–1.11 | 1.02–1.03 |
| M2 | ||||
| Mean ± SD | 0.05 ± 0.01 | 0.26 ± 0.01 | 1.09 ± 0.08 | 1.03 ± 0.02 |
| 95% CI | 0.05–0.05 | 0.26–0.27 | 1.07–1.10 | 1.02–1.03 |
HI, homogeneity index; CI, conformity index; PTV, planning target volume; Mx, model after x round of closed‐loop refinement; SD, standard deviation; 95% CI, 95% confidence intervals.
Table 2.
Dosimetric changes of 81 training plans after incorporating improved plans from the closed‐loop reoptimization: organs‐at‐risk
| M0 | M1 | M2 | |
|---|---|---|---|
| Femoral head | |||
| V 40Gy (%) | 0.03 | 0.01 | 0.01 |
| V 45Gy (%) | 0.00 | 0.00 | 0.00 |
| D max (Gy) | 39.76 | 38.71 | 38.88 |
| D mean (Gy) | 16.95 | 14.30 | 14.26 |
| P | <0.01 | <0.01 | |
| Urinary bladder | |||
| V 40Gy (%) | 16.22 | 14.46 | 14.43 |
| V 45Gy (%) | 3.28 | 4.20 | 4.15 |
| D max (Gy) | 49.24 | 49.86 | 49.90 |
| D mean (Gy) | 25.40 | 23.34 | 23.16 |
| P | <0.01 | <0.01 | |
| Small bowel | |||
| V 35Gy (%) | 3.89 | 4.75 | 4.55 |
| V 40Gy (%) | 0.14 | 0.34 | 0.34 |
| V 45Gy (%) | 0.00 | 0.00 | 0.00 |
| D max (Gy) | 39.85 | 41.56 | 41.46 |
| D mean (Gy) | 23.29 | 21.82 | 21.60 |
| P | <0.01 | <0.01 | |
V xGy, volumes receiving at least x Gy; D mean, mean dose; D max, maximum dose; P values are for the comparisons of D mean; Mx, model after x round of closed‐loop refinement.
3.B. Validations
Based on 30 VMAT validation cases, knowledge‐based reoptimizations using various models yielded comparable target coverage (mean difference of HI and CI < 0.01), but the impact on the OARs were more complex and intertwined: relative to the results of using M0, monotonically increased magnitudes of mean dose reduction to two OARs were observed using the refined models M1 and M2, by 0.34 Gy (1.47%) and 0.36 Gy (1.56%) on average for urinary bladder, and by 0.25 Gy (1.13%) and 0.30 Gy (1.36%) on average for small bowel. However, M1 and M2 increased the mean dose to femoral head than M0, by 0.81 Gy (6.64%) and 0.91 Gy (7.46%) on average. More details are shown in Tables 3 and 4.
Table 3.
The open‐loop validation results of various models on 30 additional patients: targets
| HI | CI | |||
|---|---|---|---|---|
| PTVboost | PTV | PTVboost | PTV | |
| Pv(M0) | ||||
| Mean ± SD | 0.05 ± 0.01 | 0.27 ± 0.01 | 1.09 ± 0.05 | 1.05 ± 0.03 |
| 95% CI | 0.05–0.05 | 0.26–0.27 | 1.07–1.11 | 1.03–1.06 |
| Pv(M1) | ||||
| Mean ± SD | 0.05 ± 0.01 | 0.26 ± 0.01 | 1.09 ± 0.06 | 1.04 ± 0.03 |
| 95% CI | 0.05–0.05 | 0.26–0.27 | 1.07–1.11 | 1.03–1.05 |
| Pv(M2) | ||||
| Mean ± SD | 0.05 ± 0.01 | 0.27 ± 0.01 | 1.09 ± 0.06 | 1.04 ± 0.03 |
| 95% CI | 0.05–0.05 | 0.26–0.27 | 1.07–1.11 | 1.03–1.05 |
HI, homogeneity index; CI, conformity index; PTV, planning target volume; Pv(Mx), validation plans reoptimized using model Mx; Mx, model after x round of closed‐loop refinement; SD, standard deviation; 95% CI, 95% confidence intervals.
Table 4.
The open‐loop validation results of various models on 30 additional patients: organs‐at‐risk
| Pv(M0) | Pv(M1) | Pv(M2) | |
|---|---|---|---|
| Femoral head | |||
| V 40Gy (%) | 0.00 | 0.00 | 0.00 |
| V 45Gy (%) | 0.00 | 0.00 | 0.00 |
| D max (Gy) | 37.42 | 38.29 | 38.07 |
| D mean (Gy) | 12.20 | 13.01 | 13.11 |
| P | <0.01 | 0.05 | |
| Urinary bladder | |||
| V 40Gy (%) | 12.94 | 12.87 | 12.85 |
| V 45Gy (%) | 2.84 | 2.83 | 2.78 |
| D max (Gy) | 49.17 | 49.16 | 49.00 |
| D mean (Gy) | 23.08 | 22.74 | 22.72 |
| P | <0.01 | 0.66 | |
| Small bowel | |||
| V 35Gy (%) | 6.26 | 5.92 | 6.14 |
| V 40Gy (%) | 0.83 | 0.82 | 0.83 |
| V 45Gy (%) | 0.15 | 0.02 | 0.03 |
| D max (Gy) | 42.51 | 42.51 | 42.75 |
| D mean (Gy) | 22.10 | 21.85 | 21.80 |
| P | 0.39a | ||
Pv(Mx), validation plans reoptimized using model Mx; Mx, model after x round of closed‐loop refinement; V xGy, volumes receiving at least x Gy; D max, maximum dose; D mean, mean dose; P values are for the comparisons of D mean.
Friedman test.
Figure 2 plots the mean DVHs of 30 VMAT Pv that were reoptimized using models M0, M1, and M2 respectively, as represented by the solid, dashed, and dotted lines. The largely overlapping DVHs of Pv(M1) and Pv(M2) were considered as indicators of terminating further iterations of closed‐loop model refinement. Figure 3 compares the mean DVHs of 30 Pv that were reoptimized using models M0, M2, and M2_new respectively, as represented by the solid, dash, and dash‐dotted lines.
Figure 2.
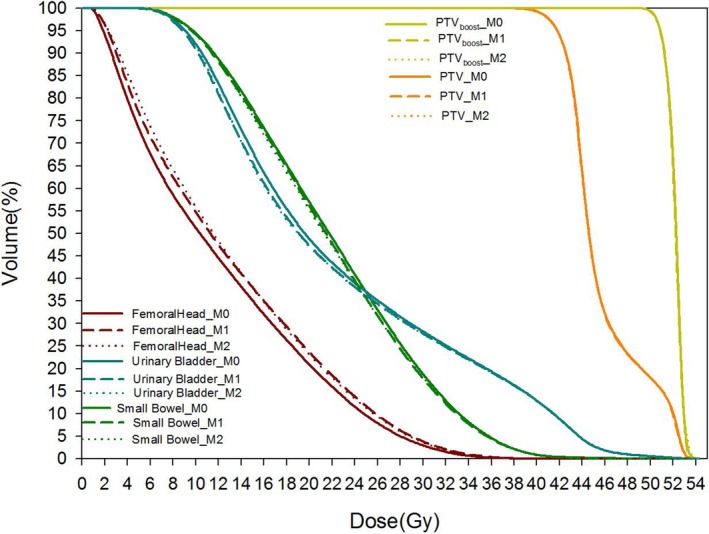
The mean DVH plots of 30 VMAT validation plans using various model M0, M1 and M2.
Figure 3.
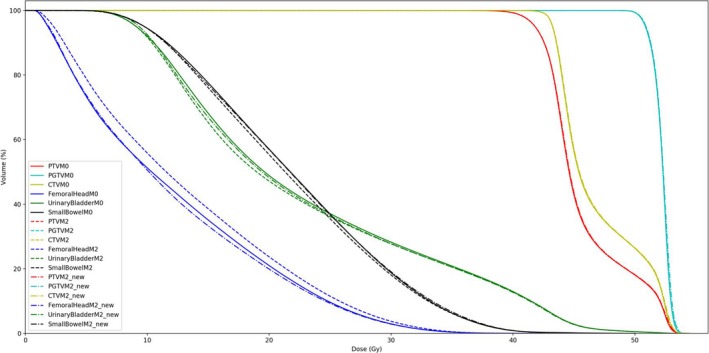
The mean DVH plots of 30 VMAT validation plans optimized using model M0, M2, and M2_new.
4. DISCUSSION
In the first closed‐loop refinement, most (75/81, 92.59%) of the best‐effort manual plans (P0) that were used to train the initial RapidPlan model (M0) can be further improved by closed‐loop knowledge‐based reoptimization using M0‐generated objectives. These results echoed the superiority of knowledge‐based solution over the conventional trial‐and‐error manual planning, in line with previous publications.17, 20, 22, 23, 24, 25, 26, 27 It suggested that knowledge‐ and geometry‐based dosimetric predictions can help avoid selecting suboptimal or conflict optimization constraints as manual limitations.
For 23 out of 81 training sets, excessive dose to all OARs were still deductable in the second closed‐loop reoptimization. However, the magnitudes of improvement were very marginal, which were treated as an indicator of iteration termination. It suggested exhausting potential of model evolution providing the anatomy and selected beam geometry.
To extend the geometric diversity and representativeness of the model, it is clinically desirable to enlarge the training set library by adding new appropriate cases. This work suggests that closed‐loop reoptimization of the new candidate is beneficial before the incorporation, to avoid introducing suboptimal plans into the knowledge base. Our solutions of updating the training library with improved plans may better preserve the anatomical variety, which distinguished our work from Li's work where poor plans were filtered.37 To avoid unacceptable geometric outliers, statistical verification assisted by Model Analytics38 can be helpful, where plans with Z‐scores > 3.5 were reviewed case‐by‐case. By adding the validation plans into the improved model library, the geometric representativeness were further improved.
The largely overlapping target DVHs in Fig. 2 echoed the comparable target numeric in Table 3, providing relatively fair basis for the comparison of the OAR dose in the open‐loop validation. However, incorporating the improved plans into the knowledge pool of M1 and M2 has made marginal and intertwined dosimetric changes in the open‐loop validation than using M0: two OARs were better spared using the models trained with better plans, at cost of excessive dose to one OAR though.
Three possible explanations could be: (a) RapidPlan generates line objectives under the lower bound of the estimation ranges (predicted DVH‐1 standard error), which already makes the optimization fairly challenging. The potential of dosimetric improvement based on the same patient anatomy and beam geometry may have been exhausted during the first round of knowledge‐based planning, making it less sensitive to the even lower estimation and objectives generated by the refined models. (b) A potential limitation of current RapidPlan algorithm may also be ascribed to: achievable DVH of each OAR was modeled and estimated independently, but OARs competed with each other hence optimal results can hardly be achieved simultaneously. Additional feedbacks such as patient‐specific adjustment of objective priorities might be beneficial to balance the complexity. Similar tradeoffs and overfitting problems were reported by Yuan, et al.19: although the training set was good, prediction errors between different OARs were still observed in some validation cases. The model generalization capability might be further improved by considering the OARs collectively, which is worthy of future studies. (c) Table 5 compares the anatomic statistics of the VMAT patients used for closed‐ and open‐loop iterations: although the ranges were largely overlapping, disparities were unavoidable because of the patient diversity. The similar patient anatomy and field geometry may partially explain the prevailed performance of closed‐loop than the open‐loop iterations. That is why it is clinically desirable to enlarge the model library continuously, and our closed‐loop evolution method can better preserve the training set volume.
Table 5.
The anatomic statistics of the 81 model cases (closed‐loop) and 30 validation cases (open‐loop)
| 81 model cases | 30 validation cases | |||||
|---|---|---|---|---|---|---|
| Min. | Max. | Mean | Min. | Max. | Mean | |
| Total volume (cm3) | ||||||
| PTVboost | 53.31 | 618.09 | 179.54 | 65.99 | 474.06 | 170.69 |
| PTV | 844.12 | 1675.05 | 1207.99 | 776.29 | 1550.59 | 1090.8 |
| Femoral head | 95.82 | 334.91 | 199.88 | 108.43 | 346.00 | 241.89 |
| Urinary bladder | 55.19 | 744.34 | 283.19 | 63.88 | 693.7 | 250.41 |
| Small bowel | 60.27 | 1152.52 | 457.90 | 120.69 | 1025.27 | 517.89 |
| Overlap with targets (cm3) | ||||||
| Femoral head | 0 | 0.05 | 0 | 0 | 0 | 0 |
| Urinary bladder | 0.29 | 161.44 | 38.18 | 0.11 | 107.48 | 32.71 |
| Small bowel | 0 | 0 | 0 | 0 | 0 | 0 |
| Overlap with targets (%) | ||||||
| Femoral head | 0 | 0.02 | 0 | 0 | 0 | 0 |
| Urinary bladder | 0.21 | 39.01 | 13.70 | 0.17 | 51.06 | 12.40 |
| Small bowel | 0 | 0 | 0 | 0 | 0 | 0 |
Min., minimum; Max., maximum.
The overfitting problem of Fig. 2 can be relieved by Model M2_new. As shown in Fig. 3, the reoptimized Pv using M2_new spared femoral head better at comparable if not lower dose to the other two OARs than the results of M0. This might be ascribed to the improved geometric similarities of M2_new with Pv, which can be applied clinically to further improve knowledge‐based planning. Considering the effects of overfitting reduction, similar methodology of incorporating optimized plans into model training library and then reoptimizing the plan is recommended in future clinical practice.
As a preliminary feasibility study, this work is limited by the single treatment site and single treatment technique. Further work is anticipated in the future to validate the extensibility of the method in a clinical environment. In addition, the extra workload of refining the training library with new patients can be partially conducted by a scripting program, which will be developed in the future.
5. CONCLUSIONS
Based on a rectal RapidPlan model, this study demonstrated that the constituent plans used to develop the DVH estimation model can be interactively improved by the model in a closed‐loop reoptimization. Incorporating new patients into the original training library can improve the RapidPlan model and the upcoming plans interactively.
CONFLICT OF INTEREST
This work was partially supported by Varian Research Collaboration Grant. Dr. Yibao Zhang and Mr. Hao Wu received speaker's honorarium from Varian Medical Systems.
ACKNOWLEDGMENT
This work was jointly supported by Beijing Natural Science Foundation (7172048), National Natural Science Foundation of China (11505012), Capital's Funds for Health Improvement and Research (2018‐4‐1027), the Fund for Fostering Young Scholars of Peking University Health Science Center (BMU2017PY028 and BMU2017PY003), Foundation of Science and Technology Department of Sichuan Province (2018HH0099), and Beijing Natural Science Foundation (1174016 and 1184014). The authors thank all physicists and dosimetrists at Beijing Cancer Hospital for their assistance. This work was partially presented at AAPM 2017 Annual Meeting.
Meijiao Wang and Sha Li contributed equally to this work.
REFERENCES
- 1. Chanyavanich V, Das SK, Lee WR, et al. Knowledge‐based IMRT treatment planning for prostate cancer. Med Phys. 2011;38:2515–2522. [DOI] [PubMed] [Google Scholar]
- 2. Zhu X, Ge Y, Li T, et al. A planning quality evaluation tool for prostate adaptive IMRT based on machine learning. Med Phys. 2011;38:719–726. [DOI] [PubMed] [Google Scholar]
- 3. Wu B, McNutt T, Zahurak M, et al. Fully automated simultaneous integrated boosted‐intensity modulated radiation therapy treatment planning is feasible for head‐and‐neck cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys. 2012;84:e647–e653. [DOI] [PubMed] [Google Scholar]
- 4. Lian J, Yuan L, Ge Y, et al. Modeling the dosimetry of organ‐at‐risk in head and neck IMRT planning: an intertechnique and interinstitutional study. Med Phys. 2013;40:121704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu B, Pang D, Simari P, et al. Using overlap volume histogram and IMRT plan data to guide and automate VMAT planning: a head‐and neck case study. Med Phys. 2013;40:021714. [DOI] [PubMed] [Google Scholar]
- 6. Yang Y, Ford EC, Wu B, et al. An overlap‐volume‐histogram based method for rectal dose prediction and automated treatment planning in the external beam prostate radiotherapy following hydrogel injection. Med Phys. 2013;40:011709. [DOI] [PubMed] [Google Scholar]
- 7. Good D, Lo J, Lee WR, et al. A knowledge‐based approach to improving and homogenizing intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Radiat Oncol Biol Phys. 2013;87:176–181. [DOI] [PubMed] [Google Scholar]
- 8. Berry SL, Ma R, Boczkowski A, et al. Evaluating inter‐campus plan consistency using a knowledge based planning model. Radiother Oncol. 2016;120:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fogliata A, Nicolini G, Clivio A, et al. A broad scope knowledge based model for optimization of VMAT in esophageal cancer: validation and assessment of plan quality among different treatment centers. Radiat Oncol. 2015;10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tol JP, Dahele M, Delaney AR, et al. Can knowledge‐based DVH predictions be used for automated, individualized quality assurance of radiotherapy treatment plans? Radiat Oncol. 2015;10:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schubert C, Waletzko O, Weiss C, et al. Intercenter validation of a knowledge based model for automated planning of volumetric modulated arc therapy for prostate cancer. The experience of the German RapidPlan Consortium. PLoS ONE. 2017;12:e0178034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J, Hu W, Yang Z, et al. Is it possible for knowledge‐based planning to improve intensity modulated radiation therapy plan quality for planners with different planning experiences in left‐sided breast cancer patients? Radiat Oncol. 2017;12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu B, Kusters M, Kunze‐Busch M, et al. Cross‐institutional knowledge‐based planning (KBP) implementation and its performance comparison to Auto‐Planning Engine (APE). Radiother Oncol. 2017;123:57–62. [DOI] [PubMed] [Google Scholar]
- 14. Chang ATY, Hung AWM, Cheung FWK, et al. Comparison of planning quality and efficiency between conventional and knowledge‐based algorithms in nasopharyngeal cancer patients using intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:981–990. [DOI] [PubMed] [Google Scholar]
- 15. Zarepisheh M, Long T, Li N, et al. A DVH‐guided IMRT optimization algorithm for automatic treatment planning and adaptive radiotherapy replanning. Med Phys. 2014;41:061711. [DOI] [PubMed] [Google Scholar]
- 16. Moore K, Scott Brame R, Low D, Mutic S. Experience based quality control of clinical intensity modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81:545–551. [DOI] [PubMed] [Google Scholar]
- 17. Wu H, Jiang F, Yue H, Li S, Zhang Y. A dosimetric evaluation of knowledge‐based VMAT planning with simultaneous integrated boosting for rectal cancer patients. J Appl Clin Med Phys. 2016;17:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Appenzoller L, Michalski J, Thorstad W, Mutic S, Moore K. Predicting dose‐volume histograms for organs‐at‐risk in IMRT planning. Med Phys. 2012;39:7446–7461. [DOI] [PubMed] [Google Scholar]
- 19. Yuan L, Ge Y, Lee WR, et al. Quantitative analysis of the factors which affect the interpatient organ‐at‐risk dose sparing variation in IMRT plans. Med Phys. 2012;39:6868–6878. [DOI] [PubMed] [Google Scholar]
- 20. Wu H, Jiang F, Yue H, Zhang H, Wang K, Zhang Y. Applying a RapidPlan model trained on a technique and orientation to another: a feasibility and dosimetric evaluation. Radiat Oncol. 2016;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cagni E, Botti A, Micera R, et al. Knowledge‐based treatment planning: an inter‐technique and inter‐system feasibility study for prostate cancer. Phys Med. 2017;36:38–45. [DOI] [PubMed] [Google Scholar]
- 22. Fogliata A, Belosi F, Clivio A, et al. On the pre‐clinical validation of a commercial model‐based optimisation engine: application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiother Oncol. 2014;113:385–391. [DOI] [PubMed] [Google Scholar]
- 23. Nwankwo O, Mekdash H, Sihono DSK, et al. Knowledge‐based radiation therapy (KBRT) treatment planning versus planning by experts: validation of a KBRT algorithm for prostate cancer treatment planning. Radiat Oncol. 2015;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tol JP, Delaney AR, Dahele M, et al. Evaluation of a knowledge‐based planning solution for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;91:612–620. [DOI] [PubMed] [Google Scholar]
- 25. Fogliata A, Wang PM, Belosi F, et al. Assessment of a model based optimization engine for volumetric modulated arc therapy for patients with advanced hepatocellular cancer. Radiat Oncol. 2014;9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hussein M, South CP, Barry MA, et al. Clinical validation and benchmarking of knowledge‐based IMRT and VMAT treatment planning in pelvic anatomy. Radiother Oncol. 2016;120:473–479. [In press, 10.1016/j.radonc.2016.06.022]. [DOI] [PubMed] [Google Scholar]
- 27. Fogliata A, Nicolini G, Bourgier C, et al. Performance of a knowledge‐based model for optimization of volumetric modulated arc therapy plans for single and bilateral breast irradiation. PLoS ONE. 2015;10:e0145137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chatterjee A, Serban M, Abdulkarim B, et al. Performance of knowledge‐based radiation therapy planning for the glioblastoma disease site. Int J Radiat Oncol Biol Phys. 2017;99:1021–1028. [In press, 10.1016/j.ijrobp.2017.07.012]. [DOI] [PubMed] [Google Scholar]
- 29. Kubo K, Monzen H, Ishii K, et al. Dosimetric comparison of RapidPlan and manually optimized plans in volumetric modulated arc therapy for prostate cancer. Phys Med. 2017;44:199–204. [In press, 10.1016/j.ejmp.2017.06.026]. [DOI] [PubMed] [Google Scholar]
- 30. Fogliata A, Reggiori G, Stravato A, et al. RapidPlan head and neck model: the objectives and possible clinical benefit. Radiat Oncol. 2017;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boutilier JJ, Craig T, Sharpe MB, et al. Sample size requirements for knowledge‐based treatment planning. Med Phys. 2016;43:1212–1221. [DOI] [PubMed] [Google Scholar]
- 32. Shiraishi S, Moore KL. Knowledge‐based prediction of three‐dimensional dose distributions for external beam radiotherapy. Med Phys. 2016;43:378–387. [DOI] [PubMed] [Google Scholar]
- 33. Fan J, Wang J, Zhang Z, et al. Iterative dataset optimization in automated planning: implementation for breast and rectal cancer radiotherapy. Med Phys. 2017;44:2515–2531. [DOI] [PubMed] [Google Scholar]
- 34. Jiang F, Wu H, Yue H, et al. Photon Optimizer (PO) prevails over Progressive Resolution Optimizer (PRO) for VMAT planning with or without knowledge‐based solution. J Appl Clin Med Phys. 2017;18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Ji J, Cai Y, et al. Preoperative concomitant boost intensity‐modulated radiotherapy with oral capecitabine in locally advanced mid‐low rectal cancer: a phase II trial. Radiother Oncol. 2012;102:4–9. [DOI] [PubMed] [Google Scholar]
- 36. Delaney AR, Tol JP, Dahele M, et al. Effect of dosimetric outliers on the performance of a commercial knowledge‐based planning solution. Int J Radiat Oncol Biol Phys. 2016;94:469–477. [DOI] [PubMed] [Google Scholar]
- 37. Li N, Carmona R, Sirak I, et al. Highly efficient training, refinement, and validation of a knowledge‐based planning quality‐control system for radiation therapy clinical trials. Int J Radiat Oncol Biol Phys. 2016;97:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang M, Li S, Yue H, et al. Modeling for knowledge‐based plan assisted with model analytics. Chin J Med Phys. 2017;34:870–873. [Google Scholar]


