Abstract
Background
Total hip arthroplasty (THA) requires components that meet a variety of patient factors and needs. This investigation evaluated survivorship of the PINNACLE Acetabular Cup System in primary total hip arthroplasty at 5-10 years.
Methods
In total, 1592 hips (1473 subjects) were enrolled in a multi-center, non-comparative, open-label study of the PINNACLE system: N = 896 metal-on-polyethylene (MOP), N = 667 metal-on-metal (MOM), N = 27 ceramic-on-polyethylene, and N = 2 unknown articulation. Harris Hip Score, Short Form 36, Western Ontario and McMaster Universities Osteoarthritis Index, and radiographs were collected through 10 years. Kaplan-Meier device survivorship was estimated.
Results
There were 41 revisions (23 MOP, 17 MOM, 1 ceramic-on-polyethylene) through 10 years: 56.5% of MOP revisions were for instability and 41.2% of MOM revisions were for adverse local tissue reaction. Kaplan-Meier device survivorship (N with further follow-up) was 97.0% (N = 720) at 5 years and 94.7% (N = 77) at 10 years.
Conclusions
Medium- to long-term survivorship estimates were similar or better than other studies and registries for the PINNACLE system.
Level of Evidence
III.
Keywords: PINNACLE acetabular cup, Total hip arthroplasty, Survivorship
Introduction
Modern techniques in total hip arthroplasty (THA) provide reliable improvement in hip function and reduction in pain. Technical developments, such as cross-linked polyethylene (PE) and alternate bearing surfaces, including ceramic-on-ceramic and metal-on metal (MOM), have extended the application of THA to younger, more active patients. In order to accommodate these changes, surgeons require hip components that provide flexibility in choosing the most suitable hip system for the patient, while maximizing the durability and long-term stability of the implants. The PINNACLE Acetabular Cup System (DePuy Synthes Joint Reconstruction, Warsaw, IN) provides a 2-piece modular acetabular implant (cup and liner) that allows the surgeon the flexibility to choose different levels of fixation of the cup, such as press fit or the use of multiple screws. The design also provides the freedom to choose from several liner options, and the modularity of the system facilitates changing the liner without removing the metal cup, which may reduce the need to revise an otherwise well-positioned, ingrown cup in revision situations.
The primary objective of this investigation was to evaluate the survivorship of the PINNACLE Acetabular Cup System in primary THA at 5 years, and up to 10 years post-operatively if sufficient data were available. Kaplan-Meier (KM) methodology was utilized for estimating device survivorship at 5-10 years post-operatively to adjust for patients who were lost to follow-up; unrevised patients were censored at the time of their last follow-up.
Material and methods
Subjects and participating centers
Between July 2000 and June 2007, 1592 primary THAs in 1473 patients were enrolled in a multi-center, non-comparative, open-label study of the PINNACLE Acetabular Cup System. The study is registered on www.clinicaltrials.gov (NCT #00306930). Surgeries were performed by 17 surgeons at 17 centers throughout the USA; 1 additional site participated in patient follow-up. Enrollment was intended to be prospective, but at least 1 site enrolled some patients after their surgery had taken place. All subjects provided informed patient consent or authorization for release of medical records for participation in a hip study; some provided consent/authorization after the time of their surgery. Thirty-one patients signed an authorization for release of medical records for participation in a stem study, and their data were included in this study of the PINNACLE Acetabular Cup System. Institutional Review Board (IRB) approval was not required in the original study protocol because this study was considered to be a registry type of data collection for products which were cleared for market in the USA. Thirteen sites eventually obtained IRB approval, either from their own institution or Western IRB. Five sites did not obtain IRB approval. Regarding data collection, the sponsor is aware of various record-keeping irregularities at one or more centers that were not in accordance with the initial or subsequently revised study protocols. These record-keeping irregularities did not, in the sponsor's estimation, affect the integrity of the data. Some investigators, including those who did not obtain IRB approval, ended their study participation early at various times between 2005 and 2012, prior to the end of the study. The last patient follow-up for this study occurred in January 2013.
Patients were selected for inclusion into the study in accordance with the normal criteria for total hip replacement and in compliance with the labeling for the device. Additional inclusion criteria consisted of sufficient bone stock to support and seat the prosthesis and signed informed patient consent/authorization. Patients were excluded if they had prior renal transplant, history of active joint sepsis, recent high systemic dose of corticosteroids, carcinoma in the last 5 years, neurological disease (eg, Parkinson's disease), psycho-social disorders that would limit rehabilitation, and use of structural bone graft. Patient follow-up data were collected at 6 weeks, 6 months, and then annually, with the intention of collecting data through a minimum of 5 years and a maximum of 10 years post-operatively.
Study components
All surgeries utilized the PINNACLE Acetabular Cup System, which provides a variety of cup designs and size options. The PINNACLE cups are cementless titanium alloy cups, available with no screw holes or with various numbers and configurations of screw holes or spikes for adjunct fixation. The 100 Series (no screw holes), 300 Series (3 spikes), and Sector (3 screw holes) options are available in sizes 48-66 mm. The multi-hole (8-12 screw holes) option is available in sizes 48-72 mm, and the Bantam (multi-hole), which is intended for smaller patients or acetabular dimensions, is available in sizes 38-46 mm. All cups feature the POROCOAT Porous Coating on the back of the cup, with the option of hydroxyapatite coating over the POROCOAT on some cup designs. The inside of the PINNACLE Acetabular Cup consists of a central dome region and the Variable Interface Prosthesis taper. The taper design facilitates insertion, retention, and removal of the modular components.
For this study, the PINNACLE Acetabular Cup was used in conjunction with one of the 3 femoral head and acetabular liner combinations: metal-on-polyethylene (MOP), MOM, and ceramic-on-polyethylene (COP). Among the 1592 study hips, there were 56% (896/1592) MOP, 42% (667/1592) MOM, 2% (27/1592) COP, and 0.1% (2/1592) having a PE liner but of undetermined femoral head (MOP or COP). The choice of femoral stem, the type of articulation (MOP, MOM, or COP), and the size of all THA components were determined by surgeon preference and the individual requirements of the patient. This was a post-market study, and all THA components had been cleared by the US Food and Drug Administration prior to initiation of the study. All components were distributed by DePuy Orthopaedics, Inc. (Warsaw, IN).
Clinical evaluation
The Harris Hip Score (HHS) [1] evaluation was used to evaluate clinical outcomes. Subjects completed the Short Form 36 (SF-36) self-assessment to evaluate the physical and mental components of their function [2], and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire [3] to evaluate pain, stiffness, and physical function.
Subjects were followed for safety outcomes throughout the study, and evaluations of adverse events were provided by the investigators. Complications are presented according to the MedDRA international terminology system [4]. MedDRA, the Medical Dictionary for Regulatory Activities terminology, is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA trademark is owned by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) on behalf of ICH.
Survivorship
A revision was defined as the removal of any THA component for any reason, and THA survivorship was defined as the absence of a revision. KM methodology [5] was utilized to estimate THA survivorship, where the time variable for a subject was the time to revision if the THA had been revised, or the time to last follow-up or death if the THA had not been revised. A KM analysis for the survivorship of the PINNACLE Acetabular Cup was also conducted, where a revision of the PINNACLE cup was defined as its removal for any reason; survival of the cup was defined as the absence of its removal. Follow-up by phone was allowed in order to encourage office visit follow-up and to obtain limited information on device survivorship.
Radiographic evaluation
Radiographs taken at clinic visits were non-weight bearing and consisted of anteroposterior pelvis, anteroposterior femur, and lateral femur. An independent radiographic reviewer reviewed the earliest post-operative radiographs for acetabular cup inclination. The radiographs at the longest follow-up interval from the index surgery that were available for each subject were evaluated for radiolucent lines, reactive sclerotic lines, osteolytic lesions, as well as subsidence and migration of components. If radiographic findings were noted, radiographs from the subject's earlier visits were reviewed to determine when the finding was first exhibited.
Analysis datasets
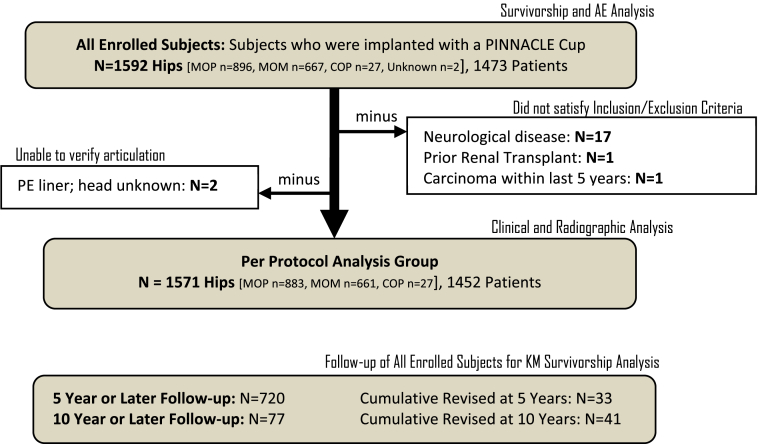
KM estimates of device survivorship were conducted on the dataset of all enrolled subjects, whereas HHS, SF-36, WOMAC, and radiographic summaries were conducted on the Per Protocol analysis set of subjects who satisfied all inclusion and exclusion criteria for the study (Fig. 1).
Figure 1.
Dataset flow diagram.
As noted in Figure 1, follow-up at 5 years or later for KM survivorship analysis was available for 720 subjects; follow-up at 10 years or later was available for 77 subjects. KM analysis of THA survivorship at 5 years was based upon 33 subjects who had been revised prior to 5 years. A total of 41 revisions were reported during the course of the study, all of which occurred prior to 10 years post-operatively.
Sample size and statistical methods
The purpose of this study was to estimate the survivorship of the PINNACLE Acetabular Cup System in primary THA at 5 years and up to 10 years post-operatively, or as long as there were sufficient subjects available for analysis. There were no hypotheses regarding THA survivorship or other clinical study endpoints. It was prospectively planned to estimate THA survivorship using KM methodology for all MOP, MOM, and COP configurations combined, as well as separately for each respective configuration. However, the number of subjects enrolled in the COP group was deemed inadequate for meaningful analysis, so analyses are provided for all enrolled subjects combined and for MOP and MOM subgroups.
At the time of study start-up, sample sizes were based on feasibility and the sponsor's desire for a large dataset rather than on a statistical rationale. After enrollment had ceased, later revisions of the protocol provided a sample size rationale in the context of the anticipated 95% confidence margin of error for THA survivorship at 5 years post-operatively. Specifically, the 95% confidence interval (CI) margin of error for THA survivorship was anticipated to be <1.5% at 5 years post-operatively based on a KM survivorship estimate of ≥95% and an attrition rate of 10% per year.
In addition to KM estimates of THA survivorship, it was prospectively planned to summarize 5-year HHS, WOMAC, and SF-36 results for MOP and MOM configurations, as well as to tabulate radiographic findings and adverse events. Mean outcomes for HHS, SF-36 (physical), SF-36 (mental), and WOMAC, respectively, were compared across MOP and MOM cohorts at 5 years with an analysis of covariance (ANCOVA) model.
Results
Because of the low number of subjects enrolled in the COP group, data summaries are provided for all enrolled subjects combined and for MOP and MOM subgroups.
Baseline demographics are shown in Table 1. Relative to the MOM group, MOP subjects were older on average, with more women, and had a greater percentage of OA cases.
Table 1.
Demographics.
| Variable | All subjects (N = 1592) | MOP (N = 896) | MOM (N = 667) |
|---|---|---|---|
| Age [y, mean (range)] | 62.1 (18-100) | 67.4 (19-91) | 55.1 (20-100) |
| BMI [kg/mm2, mean (range)] | 29.7 (15.7-65.4) | 29.3 (15.7-65.4) | 30.3 (18.1-64.3) |
| Gender (male/female) | 47.8/52.2 | 37.5%/62.5% | 61.9%/38.1% |
| Diagnosis | OA: 86.7% | OA: 89.6% | OA: 83.2% |
| AVN: 7.4% | AVN: 4.6% | AVN: 10.9% | |
| PTA: 1.6% | PTA: 1.3% | PTA: 1.8% | |
| RA: 1.6% | RA: 2.2% | RA: 0.6% | |
| Other: 2.7% | Other: 2.2% | Other: 3.4% |
AVN, avascular necrosis; BMI, body mass index; OA, osteoarthritis; PTA, post-traumatic arthritis; RA, rheumatoid arthritis.
Survivorship outcomes
There were 41 revisions (23 MOP, 17 MOM, 1 COP) reported during the course of the study, all of which occurred prior to 10 years post-operatively. Among MOP revisions, 13 of 23 (56.5%) revisions were for instability (dislocation), and among MOM revisions, 7 of 17 (41.2%) revisions were for adverse local tissue reaction. Eighteen revisions (18/41 or 43.9%) involved a revision of the cup (11 MOP, 6 MOM, 1 COP). After the study closed, but before submission of this article, the sponsor learned about additional revisions through sources outside data collection methods in this study. These additional revisions were not included in the statistical analyses within this article because doing so without also including further follow-up on all unrevised hips from a similar search of sources outside data collection methods in this study would have introduced bias. There were no revisions for osteolysis, PE wear, or liner dissociation. A summary of the number of revisions and the reasons for them is presented in Table 2, where the number in parentheses is the number of revisions that involved revision of the PINNACLE cup.
Table 2.
Reasons for revision.
| Revision reason | MOP (N = 896) | MOM (N = 667) | COP (N = 27) |
|---|---|---|---|
| Dislocation | 13 (6) | 3 (0) | |
| Deep infectiona | 4 (4) | 4a (2) | 1 (1) |
| ALTRb | 7b (3) | ||
| Fracture of femur | 2 (0) | 1 (0) | |
| Stem loosening | 2 (0) | ||
| Head failure | 1 (1) | ||
| Cup loosening | 1 (1) | ||
| Hematoma | 1 (0) | ||
| Pain: not ALTR | 1 (0) |
ALTR, adverse local tissue reaction.
Table entries are N revised (N that involved revision of the PINNACLE cup).
Two revisions for “deep infection” (MOM; cup not revised) had an additional reason for revision: “implant failure: stem.”
Includes one report of ALTR for which no subsequent revision was documented. In this table, the PINNACLE cup is assumed to be not revised.
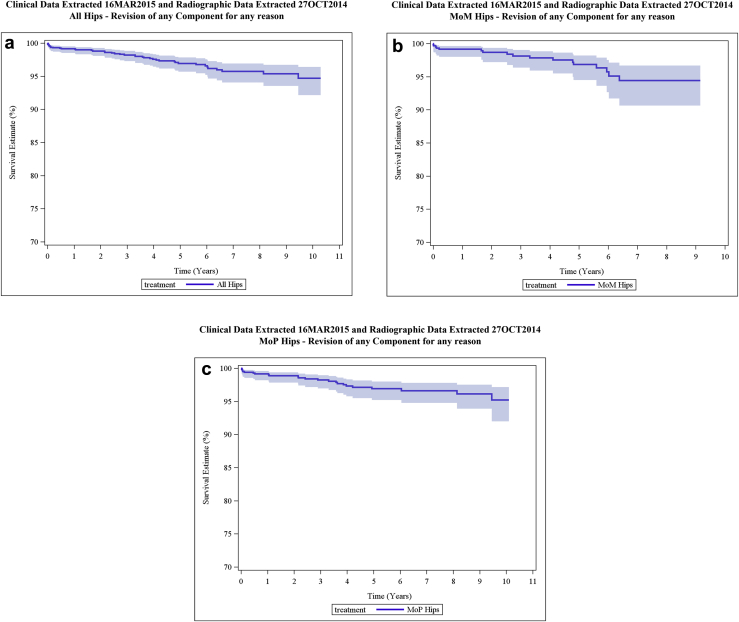
KM THA survivorship estimates for all articulations combined and for MOP and MOM articulations separately are provided in Table 3. A KM THA survivorship plot with 95% CI is provided in Figure 2 for all enrolled subjects combined (N = 1592). KM survivorship for COP hips was not calculated at 5 years or at any subsequent follow-up interval because the number of subjects available for follow-up was insufficient for the KM calculation (N <40). MOP survivorship was calculated at intervals from 5 to 10 years post-operatively (N = 62 with further follow-up at 10 years), but survivorship of MOM hips was calculated only through 9 years because the number of MOM subjects available for follow-up was insufficient for KM survivorship evaluation at 10 years (N <40). At 9 years post-operatively, data from 48 MOM subjects with further follow-up were available for KM survivorship evaluation.
Table 3.
Kaplan-Meier THA survivorship estimates: revision of any component.
| Cohort | 5 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
7 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
8 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
9 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
10 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
|---|---|---|---|---|---|
| All enrolled (N = 1592a) | 97.0% (95.7-97.8) | 95.7% (94.1-97.0) | 95.7% (94.1-97.0) | 95.4% (93.5-96.7) | 94.7% (92.1-96.4) |
| N = 720 (33 revised) | N = 376 (39 revised) | N = 286 (39 revised) | N = 197 (40 revised) | N = 77 (41 revised) | |
| MOP (N = 896) | 96.9% (95.2-98.0) | 96.6% (94.8-97.8) | 96.6% (94.8-97.8) | 96.1% (93.9-97.5) | 95.2% (92.0-97.2) |
| N = 441 (20 revised) | N = 254 (21 revised) | N = 200 (21 revised) | N = 149 (22 revised) | N = 62 (23 revised) | |
| MOM (N = 667) | 96.8% (94.5-98.2) | 94.4% (90.7-96.7) | 94.4% (90.7-96.7) | 94.4% (90.7-96.7) | N/A |
| N = 261 (13 revised) | N = 117 (17 revised) | N = 85 (17 revised) | N = 48 (17 revised) | N <40 (17 revised) | |
| COP (N = 27) | 1 revised KM survivorship not conducted (N <40) |
||||
N/A, not applicable.
Femoral head information was not provided for 2 subjects who received a polyethylene liner. These subjects were not revised at last follow-up (4 and 5 years, respectively).
Figure 2.
KM survivorship: all enrolled subjects (N = 1592), MOP hips (N = 896), and MOM hips (N = 667).
Table 4 presents a KM analysis for the survivorship of the PINNACLE Acetabular Cup. An interim analysis of mid-term data from this study was presented in a poster at the American Academy of Orthopaedic Surgeons meeting in 2007. That poster represented 99.9% cup survivorship at 5 years post-operatively. Subsequent data collection and analyses have revealed cup revisions that occurred before that poster was presented, but were not included in the KM estimate at that time. A revision of the PINNACLE cup was defined as a revision procedure in which the cup was removed. The time variable for a subject was the time to revision of the PINNACLE cup if the cup had been revised, or the time to last follow-up or death if the THA had not been revised.
Table 4.
Kaplan-Meier survivorship of the cup: revision of PINNACLE Acetabular Cup.
| Cohort | 5 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
7 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
8 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
9 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
10 Years KM survivorship (95% CI) N with later follow-up (cumulative revised) |
|---|---|---|---|---|---|
| All enrolled (N = 1592a) | 98.8% (97.8-99.3) | 97.9% (96.5-98.8) | 97.9% (96.5-98.8) | 97.5% (95.8-98.6) | 96.8% (94.2-98.3) |
| N = 720 (12 cups revised) | N = 376 (16 cups revised) | N = 286 (16 cups revised) | N = 197 (17 cups revised) | N = 77 (18 cups revised) | |
| MOP (N = 896) | 98.4% (97.0-99.2) | 98.4% (97.0-99.2) | 98.4% (97.0-99.2) | 97.9% (95.9-99.0) | 97.0% (93.6-98.6) |
| N = 441 (9 cups revised) | N = 254 (9 cups revised) | N = 200 (9 cups revised) | N = 149 (10 cups revised) | N = 62 (11 cups revised) | |
| MOM (N = 667) | 99.2% (97.4-99.7) | 97.2% (93.5-98.8) | 97.2% (93.5-98.8) | 97.2% (93.5-98.8) | N/A |
| N = 261 (3 cups revised) | N = 117 (6 cups revised) | N = 85 (6 cups revised) | N = 48 (6 cups revised) | N <40 (6 cups revised) | |
| COP (N = 27) | 1 cup revised KM survivorship not conducted (N <40) |
||||
N/A, not applicable.
Femoral head information was not provided for 2 subjects who received a polyethylene liner. These subjects were not revised at last follow-up (4 and 5 years, respectively).
Clinical outcomes
Summaries (mean and standard deviation) for HHS (total), SF-36 (physical and mental component), and WOMAC scores at 5 years post-operatively (4.5-5.5 years to accommodate a ±6-month follow-up window) are provided in Table 5 for subjects in the per protocol analysis group. Because the number of subjects with follow-up for each evaluation varied, the number of subjects with follow-up for each respective outcome is also provided in the table.
Table 5.
Harris Hip Score, SF-36, and WOMAC outcomes: 5-year follow-up.
| Per protocol analysis group | HHS | SF-36 physical component | SF-36 mental component | WOMAC |
|---|---|---|---|---|
| All articulations combined | N = 498 | N = 501 | N = 501 | N = 479 |
| 94.8 (9.0) | 45.2 (10.8) | 55.9 (7.4) | 12.8 (14.9) | |
| MOP | N = 286 | N = 287 | N = 287 | N = 278 |
| 94.1 (9.0) | 43.1 (11.0) | 55.8 (7.1) | 14.0 (14.8) | |
| MOM | N = 204 | N = 206 | N = 206 | N = 193 |
| 95.7 (9.2) | 48.0 (10.0) | 55.9 (7.8) | 11.2 (14.8) |
SD, standard deviation.
Data are represented as N (with 5-year follow-up) or mean (SD).
Mean outcomes for HHS, SF-36 (physical), SF-36 (mental), and WOMAC, respectively, were compared across MOP and MOM at 5 years with an ANCOVA model where age, gender, weight, and presence/absence of inflammatory joint disease were included as covariates. These ANCOVA models had MOP versus MOM P-values of .686, .001, .044, and .379 for HHS, SF-36 (physical), SF-36 (mental), and WOMAC, respectively. The 5 year SF-36 adjusted means were slightly higher for MOM versus MOP, consistent with the raw mean differences shown in Table 5, where a higher score for MOM indicates a better outcome.
Radiographic outcomes
Radiographic assessment of cup inclination was conducted, evaluating the first post-operative radiograph available for the 718 subjects (454 MOP, 257 MOM, 7 COP) for whom post-operative radiographs were available (633/718 = 88.2% in the 6-month interval or earlier). Mean (standard deviation) acetabular cup inclination was 45.4 (6.9) degrees overall: 45.3 (6.8) degrees for MOP and 45.7 (6.6) degrees for MOM; the MOP versus MOM difference was not statistically significant with a t-test P-value of .363.
Radiographs obtained at, or later than, 1 year post-operatively were evaluated for a total of 687 subjects (408 MOP, 272 MOM, 7 COP) for whom a set of radiographic images was available. The latest available set of radiographs was prior to the 5-year follow-up interval for 25.8% (177/687) of this analysis group, while the latest set of radiographs was in the ≥5-year follow-up interval for the other 74.2% (510/687) of this analysis group. Among the available radiographs obtained for subjects at or later than 1 year post-operatively, there were no measurable radiolucent lines, reactive sclerotic lines, or osteolytic lesions observed. One MOM subject had an osteolytic lesion associated with the femoral stem at 2 years that could not be precisely measured, and 1 MOM subject had thin radiolucencies associated with the femoral stem at 3 years that could not be measured. Two subjects exhibited femoral stem subsidence of 0.5 cm (1 MOM subject at 2-year follow-up, and 1 MOP subject at 7-year follow-up). No complications related to these findings were reported, and none of these subjects were revised.
Complications
Complications reported to the sponsor were coded using MedDRA version 15, which consists of standard categories of System Organ Class and Preferred Term. Table 6 provides a listing of all complications that were reported as “local” (operative site), and the total number of hips, for each configuration, that experienced each respective complication. Neither seriousness nor severity of adverse events was captured during this clinical investigation. The number and type of complications reported are typical for THA, and are generally similar between MOP and MOM. Joint dislocation appears to be more prevalent in the MOP than the MOM group.
Table 6.
Complications by MedDRA preferred term.
| MedDRA system organ class | MedDRA preferred term | MOP | MOM | COP |
|---|---|---|---|---|
| General disorders and administration site conditions | Device dislocation | 4 | 3 | 0 |
| Device failure | 0 | 5 | 0 | |
| Device-device incompatibility | 1 | 0 | 0 | |
| Medical device site reaction | 0 | 6 | 0 | |
| Pain | 1 | 3 | 0 | |
| Infections and infestations | Infection | 4 | 5 | 1 |
| Skin infection | 2 | 0 | 0 | |
| Injury, poisoning, and procedural complications | Fall | 2 | 1 | 0 |
| Femur fracture | 4 | 6 | 0 | |
| Hip fracture | 1 | 0 | 0 | |
| Iliotibial band syndrome | 0 | 1 | 0 | |
| Incision site complication | 1 | 0 | 0 | |
| Joint dislocation | 23 | 6 | 1 | |
| Laceration | 1 | 0 | 0 | |
| Muscle strain | 1 | 0 | 0 | |
| Pelvic fracture | 0 | 1 | 0 | |
| Skeletal injury | 0 | 2 | 0 | |
| Soft tissue injury | 0 | 1 | 0 | |
| Stress fracture | 1 | 0 | 0 | |
| Musculoskeletal and connective tissue disorders | Arthralgia | 8 | 4 | 0 |
| Arthritis | 1 | 0 | 0 | |
| Bursitis | 19 | 11 | 0 | |
| Groin pain | 2 | 3 | 0 | |
| Joint crepitation | 0 | 3 | 0 | |
| Muscular weakness | 0 | 1 | 0 | |
| Musculoskeletal pain | 1 | 0 | 0 | |
| Osteolysis | 0 | 2 | 0 | |
| Pain in extremity | 2 | 1 | 0 | |
| Soft tissue necrosis | 0 | 1 | 0 | |
| Tendonitis | 2 | 2 | 0 | |
| Skin and subcutaneous tissue disorders | Decubitus ulcer | 0 | 1 | 0 |
| Surgical and medical procedures | Joint dislocation reduction | 1 | 0 | 0 |
| Vascular disorders | Hematoma | 6 | 1 | 0 |
Note: The following complications which were reported as local (operative site) have been omitted from this tabulation because they do not appear to have been related to the operative site: post lumbar puncture syndrome, wrist fracture, back pain, spinal column stenosis, prostate cancer, dementia Alzheimer's type, sciatica, hip arthroplasty, osteoarthritis, and spinal laminectomy.
Conclusions
The primary purpose of this study was to evaluate the survivorship of the PINNACLE Acetabular Cup System in primary THA at 5 years, and up to 10 years post-operatively if there were sufficient data. Although follow-up was less than anticipated, data from a large cohort were available at 5 years (all enrolled, N = 720; MOP, N = 441; MOM, N = 261). KM survivorship methodology accounts for subjects who are lost to follow-up by reducing the survivorship estimate at the time of each respective revision in proportion to the number of subjects who have further follow-up beyond that time point; the outcome of patients who are lost to follow-up is assumed to be similar to that of patients who continued to be followed. KM survivorship (with 95% CI) estimates at 5 years for all enrolled, MOP, and MOM cohorts were 97.0% (95.7-97.8), 96.9% (95.2-98.0), and 96.8% (94.5-98.2), respectively. These survivorship estimates at 5 years are in line with the National Institute for Health and Care Excellence guidance [6] which was in place at the time PINNACLE MOM was on the market, as well as the Orthopaedic Data Evaluation Panel's [7] interpretation of this guidance for a 5A rating; they are also in line with the current National Institute for Health and Care Excellence guidance and Orthopaedic Data Evaluation Panel's interpretation for a 5A rating [8], [9]. In addition, the study data suggest that the survivorship performance of the MOP articulation is similar to that of the MOM articulation at 5 years.
The PINNACLE Acetabular Cup System also yielded THA survivorship estimates that were acceptable in the 9- to 10-year post-operative time frame. At 9 years, the number of hips with further follow-up was 197 overall: 149 for MOP and 48 for MOM. MOP bearings exhibited a slightly higher survivorship than MOM at 9 years, with KM survivorship (with 95% CI) estimates of 96.1% (93.9-97.5) versus 94.4% (90.7-96.7). The MOP THA survivorship estimate in this study at 10 years post-operatively was 95.2%. This survivorship estimate is similar to the KM survivorship estimate at 10 years for a large cohort of MOP primary cementless THAs in the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man (UK NJR) [10]. In the 2016 annual report of the UK NJR, there were 42,469 cementless Corail/PINNACLE MOP procedures (at baseline), for which the 10-year KM survivorship estimate was 96.8% (95% CI 96.3-97.3). The survivorship estimate for the MOP cohort in this study was also similar to the KM survivorship estimate at 10 years for a large cohort of primary cementless THAs, excluding large diameter MOM (≥36 mm), in the Australian Orthopaedic Association National Joint Replacement Registry (AOA NJRR) [11]. In the 2015 annual report of the AOA NJRR, there were 26,938 cementless Corail/PINNACLE procedures (at baseline) excluding large diameter MOM procedures, for which the KM survivorship estimate at 10 years was 94.6% (95% CI 93.5-95.5). In contrast to MOP, the THA survivorship estimate of 94.4% at 9 years post-operatively for the MOM cohort in this study is much higher than the KM survivorship estimate at 10 years for a large cohort of MOM primary THAs in the UK NJR (a 9-year estimate is not provided in the UK NJR). In the 2016 annual report of the UK NJR, there were 11,906 cementless Corail/PINNACLE MOM procedures (at baseline), for which the 10-year KM survivorship estimate was 85.4% (95% CI 84.3-86.5). The Corail/PINNACLE MOM cohort (≥36 mm) in the 2015 annual report of the AOA NJRR had 966 procedures at baseline, with a KM survivorship estimate at 10 years of 86.6% (95% CI 82.4-89.8).
In order to understand how the results of this study compare to what is seen in recent literature, a review of THA survivorship outcomes for PINNACLE MOP and MOM was conducted of clinical studies published from 2013 to 2016. KM survivorship estimates (enrolled sample size in parenthesis) for THA with PINNACLE MOP ranged from 97.9% (100) to 99.2% (150) at 10 years [12], [13]. KM survivorship estimates (enrolled sample size in parenthesis) for THA with PINNACLE MOM ranged from 92.8% (557) to 99.4% (169) at 5 years; 88.9% (578) to 97.1% (169) at 8 years; and 82% (378) to 93.3% (169) at 10 years [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. The long-term survivorship estimates at 9-10 years for PINNACLE MOP in this study were consistent with what is reported in recent literature, whereas the long-term survivorship estimates at 9 years for PINNACLE MOM in this study were somewhat higher than what is reported in recent literature for MOM at 9-10 years.
The results of this study support the integrity and wear characteristics of the PE in the MOP cohort and also the effectiveness of the modular MOP and MOM constructs. Although KM survivorship at 9-10 years was slightly higher for the MOP cohort, it should be noted that the MOM cohort had potentially higher demands than the MOP cohort, with a much higher percentage of men and an average age that was 12 years younger. In the instances of revision, the multi-liner capabilities of this cup were shown to be of significant utility, as the cup was retained in 23 of 41 revisions. The clinical and radiographic data gathered in this study support the use of the PINNACLE Acetabular Cup System as a versatile, robust system for use in primary THA, with advantages in a revision THA situation. There were no significant clinical or radiographic differences between MOP and MOM cohorts at 5-year follow-up other than slightly better SF-36 scores for MOM subjects which may be more reflective of the large sample sizes rather than a clinically meaningful difference.
The major weakness of this study was the subject loss to follow-up rate. At 5 years post-op, there were only 720/1592 (45%) of enrolled subjects who had further follow-up for KM survivorship purposes. Nevertheless, this number constitutes a fairly large cohort (720 total enrolled, 441 MOP and 261 MOM), and was sufficient to provide a valuable estimate of survivorship at the 5-year point, which was the primary goal of this study. The smaller cohort sizes through 9 years (for MOP and MOM) and through 10 years (for MOP and all enrolled), were also sufficient to provide useful estimates of survivorship in the 9- to 10-year post-operative time frame. All these estimates reflect the satisfactory survivorship of the PINNACLE Acetabular Cup System through 10 years post-operatively.
Acknowledgments
The authors thank the patients who participated in this clinical study, and acknowledge the integral contributions of all investigators and clinical research coordinators. We thank Toni Kingsley for her editorial contribution in manuscript development.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2017.07.001.
Appendix A. Supplementary data
References
- 1.Harris W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of evaluation. J Bone Joint Surg Am. 1969;51:737. [PubMed] [Google Scholar]
- 2.Ware J.E., Brook R.H., Williams K.N. Rand Corp; Santa Monica (CA): 1980. Conceptualisation and measurement of health care in adults in the health insurance study, vol. 1. Model of health and methodology. [Google Scholar]
- 3.Bellamy N., Buchanan W.W., Goldsmith C.H. Validation study of WOMAC: a health status instrument for measuring clinically important relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833. [PubMed] [Google Scholar]
- 4.MedDRA® the medical dictionary for regulatory activities terminology. www.meddra.org; [accessed 22.09.16].
- 5.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;81:66. [Google Scholar]
- 6.NICE Technology Appraisal Guidance 2. Guidance on the selection of prostheses for primary total hip replacement. 2000. https://www.nice.org.uk/guidance/TA2. [Google Scholar]
- 7.Orthopaedic data evaluation panel rating criteria. 2005. http://www.odep.org.uk/. [Google Scholar]
- 8.Developing NICE guidelines: the manual. NICE process [PMG20] 2014. https://www.nice.org.uk/process/pmg20/chapter/introduction-and-overview; [accessed 08.08.17]. [Google Scholar]
- 9.Orthopaedic data evaluation panel. www.odep.org.uk; [accessed 22.09.16].
- 10.National Joint Registry for England, Wales, Northern Ireland and the Isle of Man, 13th Annual Report, 2016. Table 3.9. www.njrreports.org.uk; [accessed 22.09.16].
- 11.Australian Orthopaedic Association National Joint Replacement Registry. Annual Report, Adelaide; AOA 2015. Table HT13. https://aoanjrr.sahmri.com/; [accessed 22.09.16].
- 12.Bedard N.A., Callaghan J.J., Stefl M.D. Fixation and wear with a contemporary acetabular component and cross-linked polyethylene at minimum 10-year follow-up. J Arthroplasty. 2014;29(10):1961. doi: 10.1016/j.arth.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Greiner J.J., Callaghan J.J., Bedard N.A. Fixation and wear with contemporary acetabular components and cross-linked polyethylene at 10-years in patients aged 50 and under. J Arthroplasty. 2015;30(9):1577. doi: 10.1016/j.arth.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Atrey A., Hussain N., Shepherd A., Young S. 557 metal-on-metal Corail-PINNACLE total hip replacements with 36 mm heads. A 5 year follow up: levels of ARMD remain low despite a comprehensive screening program. Hip Int. 2015;25:S16. doi: 10.1016/j.jor.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernasek T.L., Polikandriotis J.A., Levering M.F. Five- to ten-year outcomes for modular metal-on-metal total hip arthroplasty. J Arthroplasty. 2013;28(7):1231. doi: 10.1016/j.arth.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Engh C.A., Jr., Sritulanondha S., Korczak A. No difference in reoperations at 2 years between ceramic-on-metal and metal-on-metal THA: a randomized trial. Clin Orthop Relat Res. 2016;474:447. doi: 10.1007/s11999-015-4424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greiner J.J., Callaghan J.J., Bedard N.A. Metal-on-metal total hip arthroplasty at five to twelve years follow-up: a concise follow-up of a previous report. J Arthroplasty. 2016;31:1773. doi: 10.1016/j.arth.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Lainiala O., Eskelinen A., Elo P. Adverse reaction to metal debris is more common in patients following MoM total hip replacement with a 36 mm femoral head than previously thought: results from a modern MoM follow-up programme. Bone Joint J. 2014;96-B(12):1610. doi: 10.1302/0301-620X.96B12.33742. [DOI] [PubMed] [Google Scholar]
- 19.Langton D.J., Sidaginamale R.P., Avery P. Retrospective cohort study of the performance of the PINNACLE metal on metal (MoM) total hip replacement: a single-centre investigation in combination with the findings of a national retrieval centre. BMJ Open. 2016;6:e007847. doi: 10.1136/bmjopen-2015-007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liudahl A.A., Liu S.S., Goetz D.D., Mahoney C.R., Callaghan J.J. Metal on metal total hip arthroplasty using modular acetabular shells. J Arthroplasty. 2013;28(5):867. doi: 10.1016/j.arth.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Matharu G.S., Theivendran K., Pynsent P.B. Outcomes of a metal-on-metal total hip replacement system. Ann R Coll Surg Engl. 2014;96(7):530. doi: 10.1308/003588414X14055925058030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangaletti R., Barbieri F., Castelli C.C. Hip arthroplasty with metal-on-metal tribology: 10-year follow-up and ionic release trend in 36 mm head implants. Hip Int. 2015;25:S16. [Google Scholar]
- 23.Whitehouse M.R., Aquilina A.L., Patel S., Eastaugh-Waring S.J., Blom A.W. Survivorship, patient reported outcome and satisfaction following resurfacing and total hip arthroplasty. J Arthroplasty. 2013;28(5):842. doi: 10.1016/j.arth.2013.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.