Abstract
For several decades, cholesterol has been thought to cause ASCVD. Limiting dietary cholesterol intake has been recommended to reduce the risk of the disease. However, several recent epidemiological studies do not support a relationship between dietary cholesterol and/or blood cholesterol and ASCVD. Consequently, the role of cholesterol in atherogenesis is now uncertain. Much evidence indicates that TGF-β, an anti-inflammatory cytokine, protects against ASCVD and that suppression of canonical TGF-β signaling (Smad2-dependent) is involved in atherogenesis. We had hypothesized that cholesterol causes ASCVD by suppressing canonical TGF-β signaling in vascular endothelium. To test this hypothesis, we determine the effects of cholesterol, 7-dehydrocholesterol (7-DHC; the biosynthetic precursor of cholesterol), and other sterols on canonical TGF-β signaling. We use Mv1Lu cells (a model cell system for studying TGF-β activity) stably expressing the Smad2-dependent luciferase reporter gene. We demonstrate that 7-DHC (but not cholesterol or other sterols) effectively suppresses the TGF-β-stimulated luciferase activity. We also demonstrate that 7-DHC suppresses TGF-β-stimulated luciferase activity by promoting lipid raft/caveolae formation and subsequently recruiting cell-surface TGF-β receptors from non-lipid raft microdomains to lipid rafts/caveolae where TGF-β receptors become inactive in transducing canonical signaling and undergo rapid degradation upon TGF-β binding. We determine this by cell-surface 125I-TGF-β-cross-linking and sucrose density gradient ultracentrifugation. We further demonstrate that methyl-β-cyclodextrin (MβCD), a sterol-chelating agent, reverses 7-DHC-induced suppression of TGF-β-stimulated luciferase activity by extrusion of 7-DHC from resident lipid rafts/caveolae. These results suggest that 7-DHC, but not cholesterol, promotes lipid raft/caveolae formation, leading to suppression of canonical TGF-β signaling and atherogenesis. J. Cell. Biochem. 118: 1387–1400, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: 7-DHC, CHOLESTEROL, LIPID RAFTS/CAVEOLAE, NON-LIPID RAFT MICRODOMAINS, CANONICAL TGF-β SIGNALING
Transforming growth factor (TGF-β) is a pleiotropic cytokine involved in many biological processes: cell growth, cell differentiation, apoptosis, angiogenesis, wound healing, and immune regulation [Moses et al., 2016]. TGF-β is produced by macrophages, T cells, vascular cells, and other cell types. It is one of the very few potent growth factors/cytokines active at subpicomolar concentrations. It is also capable of autoinduction in target cells [Huang and Huang, 2005]. Thus, TGF-β activity is regulated at multiple levels of transcription, post-translation (activation of the latent form of TGF-β), and plasma-membrane receptor complex formation [Huang and Huang, 2005; Moses et al., 2016]. Among these, TGF-β receptor complex formation in the plasma membrane of target cells plays a very important role in regulating TGF-β-induced canonical signaling (Smad2-dependent) and cellular responses [Di Guglielmo et al., 2003; Huang and Huang, 2005; Chen et al., 2006, 2007, 2008]. Canonical TGF-β signaling is mediated by type I and type II TGF-β receptor (TβR-I and TβR-II) oligomeric heterocomplexes (ratio of TβR-II>TβR-I) localized in non-lipid raft microdomains following TGF-β binding to these receptors [Huang and Huang, 2005; Chen et al., 2006, 2007]. This occurs at coated-pit stages during clathrin-dependent endocytosis of the TGF-β-bound TβR-I-TβR-II oligomeric heterocomplexes [Chen et al., 2009]. Accordingly, TβR-I-TβR-II oligomeric heterocomplexes (ratio of TβR-II<TβR-I) localized in lipid rafts/caveolae undergo caveolin-dependent endocytosis and rapid degradation upon TGF-β binding [Di Guglielmo et al., 2003; Huang and Huang, 2005; Chen et al., 2006, 2007, 2008]. Localization of the TGF-β receptors in non-lipid raft microdomains occurs mainly in target cells. More canonical TGF-β signaling is then induced in these microdomains [Di Guglielmo et al., 2003; Huang and Huang, 2005; Chen et al., 2006, 2007]. TGF-β signaling is suppressed if TGF-β receptors are mainly localized in lipid rafts/caveolae [Di Guglielmo et al., 2003; Huang and Huang, 2005; Chen et al., 2006, 2007]. Many lines of evidence indicate that the TGF-β-induced cellular responses mediated by this canonical signaling can be enhanced or suppressed by altering the expression ratio of TβR-I and TβR-II and/or changing the cell-surface environment and/or changing plasma membrane components in ways known to exist in various pathophysiological conditions, including ASCVD [Huang and Huang, 2005].
Much evidence indicates that TGF-β is a protective cytokine against ASCVD [Grainger, 2004; Huang and Huang, 2005; Chen et al., 2007] and other diseases such as Alzheimer’s disease and cancer [De Servi et al., 2002; Pickup et al., 2013]. We recently found that hypercholesterolemia and high-level dietary trans fats cause ASCVD, at least in part, by suppressing canonical TGF-β signaling and responsiveness in aortic endothelium in ApoE-knockout and wild-type mice fed a trans fat diet [Chen et al., 2007, 2011]. The suppressed canonical TGF-β signaling in the aortic endothelium is modulated by both hypercholesterolemia and dietary trans fats, increasing the localization of TβR-I and TβR-II in lipid rafts/ caveolae in the aortic endothelium of these mice but not in control mice [Chen et al., 2007, 2011]. This is consistent with the suppressed canonical TGF-β signaling and responsiveness seen in vascular cells derived from atherosclerotic plaques in human patients [McCaffrey et al., 1995]. We also found that dynasore, an endocytosis inhibitor, attenuates ASCVD in ApoE-knockout mice without altering the high plasma cholesterol levels in the animals [Chen et al., 2009]. Dynasore enhances canonical TGF-β signaling by sustained TGF-β-induced canonical signaling at coated-pit stages during clathrin-dependent endocytosis of cell-surface TGF-β receptors stimulated by TGF-β [Chen et al.,2009]. These results indicate that suppression of canonical TGF-β signaling is an important step in ASCVD development and that ASCVD can be attenuated by enhancing canonical TGF-β signaling which counteracts suppressed TGF-β signaling in ASCVD.
Cholesterol is an important structural component of lipid rafts/caveolae [Simons and Ehehalt, 2002]. These are small (10–200 nm) and heterogeneous. Lipid rafts/caveolae are stable rigid sterol and sphingolipid raft domains. Cholesterol is thought to be the primary dynamic component that holds the sphingolipids together in the raft. We hypothesized that hypercholesterolemia and high-level dietary trans fats increase formation of lipid rafts/caveolae in aortic endothelium by directly incorporating and facilitating cholesterol integration into plasma membranes of vascular cells, respectively [Chen et al., 2007, 2011]. This leads to recruitment of TβR-I-TβR-II heterocomplexes from non-lipid raft microdomains to lipid rafts/ caveolae, resulting in suppression of canonical TGF-β signaling, thus causing atherogenesis. This hypothesis is supported by the demonstration that in vitro cholesterol treatment recruits TβR-I and TβR-II from non-lipid raft microdomains to lipid rafts/caveolae, leading to attenuation of TGF-β signaling and responses in Mv1Lu cells (a model cell system for studying TGF-β signaling and responses) and other cell types [Huang and Huang, 2005; Chen et al., 2007, 2008]. However, we found that the role of cholesterol in recruiting TGF-β receptors to lipid rafts/caveolae (thus suppressing TGF-β signaling) was unclear, until we fortuitously found that the cholesterol compound we used in our experiments [Chen et al., 2007, 2008] was impure. This impurity is 7-dehydrocholesterol (7-DHC), an immediate biosynthetic precursor of cholesterol, which is a 5,7-conjugated diene sterol. Pure cholesterol is inactive in recruiting TGF-β receptors to lipid rafts/caveolae and in suppressing canonical TGF-β signaling in target cells [Huang et al., 2015]. Here, we demonstrate that 7-DHC (but not cholesterol and other related sterols) effectively suppresses canonical TGF-β signaling in a concentration-dependent manner by recruiting TGF-β receptors from non-lipid raft microdomains to lipid rafts/caveolae in target cells. Since, suppression of canonical TGF-β signaling is an important step in atherogenesis [McCaffrey et al., 1995; Chen et al., 2007, 2008, 2009, 2011], these results suggest that 7-DHC, but not cholesterol, is likely involved in the development of ASCVD.
MATERIALS AND METHODS
MATERIALS
Na [125I] (17 Ci/mg) was obtained from ICN Biochemicals (Irvine, CA). DMEM, high molecular mass protein standards (myosin, 205 kDa; β-galactosidase, 116 kDa; phosphorylase, 97 kDa; bovine serum albumin, 66 kDa), chloramine-T, disuccinimidyl suberate (DSS), methyl β-cyclodextrin (MβCD), cholesterol (≥99%, Lot#080M5304V), and other sterols/steroids were obtained from Sigma (St Louis, MO). 7-Dehydrocholesterol (7-DHC) (Lot#19172) was purchased from Pfaltz & Bauer (Waterbury, CT). Sterols/steroids were dissolved in 100% ethanol at 25 mg/ml with a final concentration of 0.1%. TGF-β (TGF-β1) was purchased from Austral Biologicals (San Ramon, CA). Rabbit polyclonal antibodies to caveolin-1 (N-20), TβR-I (ALK-5), and TR-II were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
TGF-β-STIMULATED LUCIFERASE ACTIVITY ASSAY IN Mv1Lu CELLS
TGF-β-stimulated luciferase activity assay was performed according to published procedures [Chen et al., 2007, 2009; Huang et al., 2016a,b]. Mv1Lu cells stably expressing a luciferase reporter gene containing Smad2-dependent canonical-signaling-responsive elements (MLE cells-Clone 32) were grown to near-confluence on 12-well dishes, then treated with 100 pM TGF-β or vehicle only in the presence of several concentrations of 7-DHC and/or cholesterol, vitamin D3, vitamin D2, ergosterol or other sterols as indicated (in the medium containing 0.1% fetal calf serum). After 5 hr at 37°C, treated cells were lysed in 100 μl of lysis buffer (Promega). TGF-β-stimulated luciferase activity of cell lysates (~20 mg protein) was then assayed using the luciferase kit from Promega. Cells treated without exogenous TGF-β exhibited a low level of luciferase activity which was stimulated by endogenous TGF-β derived from 0.1% fetal calf serum in the assay medium.
CELL-SURFACE 125I-TGF-β-CROSS-LINKING
125I-TGF-β was prepared as described previously [Chen et al., 2006, 2007, 2008; Huang et al., 2016a,b]. Cell-surface 125I-TGF-β-cross-linking was performed at 0°C using the cross-linking agent DSS according published procedures [Chen et al., 2008; Huang et al., 2016a,b]. Briefly, Mv1Lu cells grown to near-confluence on 6-well culture dishes were treated with 7-DHC (12.5, 25, and 50 μg/ml), cholesterol (50 μg/ml), vitamin D2 (50 μg/ml), and vehicle only (control) at 37°C. After 1 h, treated cells were incubated with 100 pM 125I-TGF-β in HEPES buffer, pH 7.4 (binding buffer), containing0.2% bovine serum albumin at 0°C for 2.5 h. 125I-TGF-β-cross-linked cells were then washed with cold binding buffer in the absence of bovine serum albumin and then incubated with 30 μM DSS at 0°C for 15 min. 125I-TGF-β-cross-linked cell lysates were analyzed by 7.5% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and autoradiography. 125I-TGF-β-cross-linked TβR-I (TβR-I ) and 125I-TGF-β-cross-linked TβR-II (TβR-II ) on the SDS–PAGE gel (dried) were quantified using a PhosphoImager.
PLASMA MEMBRANE MICRODOMAIN LOCALIZATION OF TβR-I AND TβR-II IN Mv1Lu CELLS
Mv1Lu cellswere grown on 100 mm dishes (5 × 106 cells per dish). Cells were then incubated with 7-DHC (10 or 50 μg/ml), cholesterol (10 or 50 μg/ml), or vehicle only (control) at 37°C for 4 h. After washing with ice cold phosphate-buffered saline, cells were scraped into 0.85 ml of 500 mM sodium carbonate, pH 11.0. Homogenization was carried out with 10 strokes of a tightfitting Dounce homogenizer followed by three 20-sec bursts of an ultrasonic disintegrator to disrupt cell membranes, as described previously [Chen et al., 2007; Huang et al., 2016a,b]. The homogenates were adjusted to 45% sucrose by addition of 0.85 ml of 90% sucrose in 25 μM 2-(N-morpholino) ethanesulfonic acid, pH 6.5, 0.15 M NaCl (MBS), and placed at the bottom of an ultracentrifuge tube. A discontinuous sucrose gradient was generated by overlaying 1.7 ml of 35% sucrose and 1.7 ml of 5% sucrose in MBS on the top of the 45% sucrose solution. It was then centrifuged at 200,000g for 16–20 h in an SW55 TI rotor. Ten 0.5-ml fractions were collected from the top of the tube, and a portion of each fraction was analyzed by SDS–PAGE followed by Western blot analysis using antibodies to TβR-I, TβR-II, caveolin-1, and EEA-1. The relative amounts of TβR-I, TβR-II, and caveolin-1 on the blot were quantified by densitometry. Fractions 4 and 5, and fractions 7, 8, and 9 contained caveolin-1 (lipid raft/caveolae marker) and EEA-1 (non-lipid raft microdomain marker), respectively.
STATISTICAL ANALYSIS
Statistical analysis was performed using unpaired and two-tailed Student’s t-tests. The P values less than 0.05 were considered to be statistically significant.
RESULTS
7-DHC SUPPRESSES TGF-β-STIMULATED LUCIFERASE ACTIVITY IN Mv1Lu CELLS STABLY EXPRESSING A LUCIFERASE REPORTER GENE
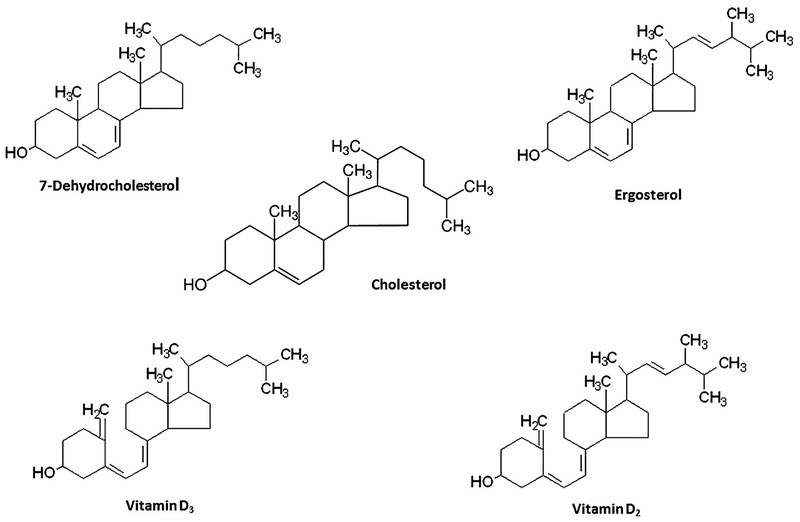
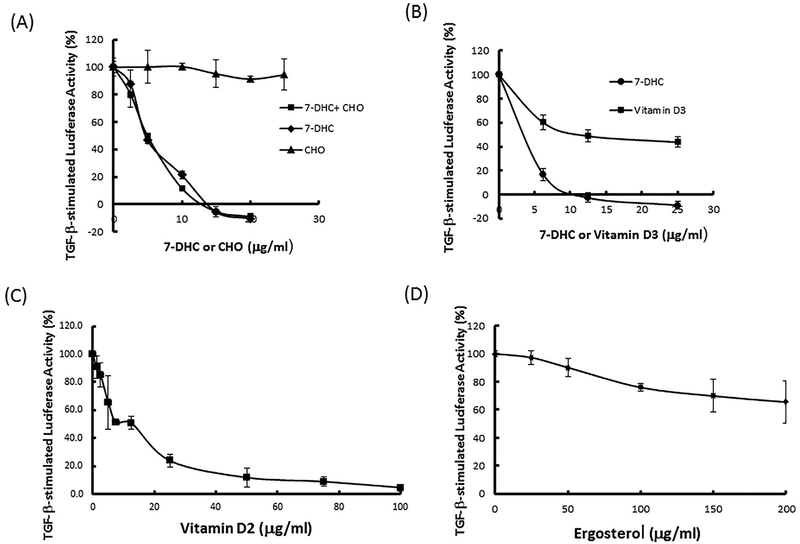
To determine the effects of 7-DHC and ergosterol, which are 5,7-conjugated diene sterols, and their uv-activated derivatives (vitamins D3 and D2) as well as cholesterol (Fig. 1) and its related sterols (Table I) on TGF-β-stimulated canonical signaling, Mv1Lu cells stably expressing a luciferase reporter gene containing Smad2-dependent canonical-signaling-responsive elements [Chen et al., 2007, 2009; Huang et al., 2015, 2016a,b] were used in our experiments. These Mv1Lu cells were treated with 100 pM TGF-β in the presence of several concentrations of these compounds. After 5 h at 37°C, cells were lysed and luciferase activity was measured. As shown in Figure 2A, 7-DHC attenuated TGF-β-stimulated luciferase activity in a concentration-dependent manner with an IC50 of ~ 4 μg/ml). It is important to note that 7-DHC completely inhibited or suppressed the TGF-β-stimulated luciferase activity and that, at higher concentrations (at >10 μg/ml), 7-DHC even inhibited luciferase activity at levels below those seen in cells treated without exogenous TGF-β. This suggests that 7-DHC at higher concentrations inhibits luciferase activity stimulated by both exogenous and endogenous TGF-β derived from 0.1% fetal calf serum in the assay medium. Cholesterol (Fig. 2A) and cholesterol-related compounds (Table I), which lack the 5,7-conjugated diene structure, did not attenuate TGF-β-stimulated luciferase activity. Interestingly, cholesterol (50 μg/ml) did not affect the 7-DHC suppression of TGF-β-stimulated luciferase activity in cells co-treated with 7-DHC and cholesterol (50 μg/ml) (Fig. 2A). These results suggest that 7-DHC, but not cholesterol, suppresses TGF-β-stimulated luciferase activity and/or canonical signaling. The results also indicate that cholesterol does not influence 7-DHC-induced suppression of TGF-β-stimulated luciferase activity in cells treated with 7-DHC in the presence of cholesterol. Vitamin D3 (cholecalciferol), vitamin D2 (ergocalciferol), and ergosterol attenuated TGF-β-stimulated luciferase activity in a concentration-dependent manner with IC50s of ≥ 20,~15, and >50 μg/ml, respectively (Fig. 2B, C, and D). Among these compounds, 7-DHC was the most potent inhibitor whereas ergosterol, a plant sterol, appeared to be the weakest one. The IC50 of vitamin D3 (≥ 20 μg/ml) required to suppress TGF-β-stimulated luciferase activity is much greater than its normal plasma concentrations (35–40 ng/ml or 90–100 nM) [Holick, 2004]. These results suggest that 7-DHC and ergosterol, both of which contain 5,7-conjugated dienes, and their uv-activated derivatives (vitamins D3 and D2) attenuate TGF-β-stimulated luciferase activity or TGF-β-stimulated Smad2-dependent canonical signaling.
Fig. 1.
Chemical structures of cholesterol and, 5,7-conjugated diene sterols (7-dehydrocholesterol and ergosterol) and its uv-activated derivatives (vitamins D3 and D2).
TABLE I.
Sterols Which Are Unable to Suppress TGF-β-Stimulated Luciferase Activity in Mv1Lu Cells Stably Expressing a Smad2-Dependent Luciferase Reporter Genea
| Sterol |
|---|
| Desmosterol |
| 7α-hydroxycholesterol |
| 7β-hydroxycholesterol |
| 25-hydroxycholesterol |
| 27-hydroxycholesterol |
| 7-ketocholesterol |
| 5-Cholesten-3-one |
| 5-Choesten-7-one |
| 5-Cholesten-3β-ol-7-one |
| Lanosterol |
| Lathosterol |
| Zymosterol |
Mv1Lu cells stably expressing a luciferase reporter gene [Chen et al., 2007, 2009] were incubated with 100 pM TGF-β in the presence of several concentrations of sterols (0, 25, and 50 μg/ml) and 7-DHC (25 μg/ml) as a positive control. After 5 h at 37°C, luciferase activity of cell lysates of treated cells was measured. TGF-β-stimulated luciferase activity in cells treated with TGF-β alone was taken as 100%. None of these sterols on the list significantly inhibited the TGF-β-stimulated luciferase activity.
Fig. 2.
Effects of cholesterol (A), 7-DHC (A), vitamin D3 (B), vitamin D2 (C), and ergosterol (D) on TGF-β-stimulated luciferase activity in Mv1Lu cells. Cells were treated with 100 pM TGF-β in the presence of several concentrations (as indicated) of cholesterol (CHO) (A), 7-DHC ± CHO (50 μg/ml) (A), vitamin D3 and 7-DHC (B), vitamin D2 (C) or ergosterol (D) at 37°C for 5 h. TGF-β-stimulated luciferase activity was taken as 100% in cells treated with TGF-β alone. The experiments were performed in triplicate. The data are mean ± s.d.
Fig. 7.
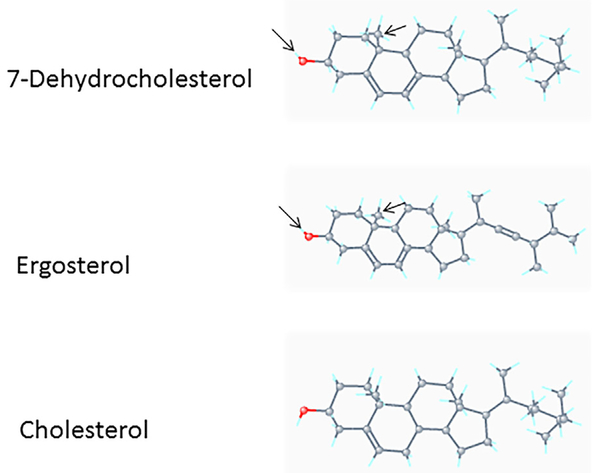
Comparison of 3D conformer structures of 7-DHC, ergosterol, and cholesterol. The 3D conformer structures of 7-DHC, ergosterol, and cholesterol were derived from PubChem (https://pubchem.ncbi.nlm.nih.gov/compound/). The backbones of these sterols consist of rigid, fused four-ring systems. The configuration of the hydrocarbon tail (side chain) in 7-DHC is identical to that in cholesterol but distinct from that in ergosterol because of the presence of a double bond in ergosterol. The configurations of the 3b-ol (marked by red) and 10-methyl groups in 7-DHC appear to be very similar to those in ergosterol but different from those in cholesterol. Both 7-DHC and ergosterol are known to promote lipid raft/caveolae domain formation more strongly than cholesterol [Xu et al., 2001]. This suggests that the specific configuration of the 3b-ol group in 7-DHC plays an important role in its interaction with specific lipids in lipid rafts/caveolae [Ikonen, 2008]. The presence of a double bond on the side chain of ergosterol diminishes its affinity for specific lipids in lipid rafts/ caveolae in animals.
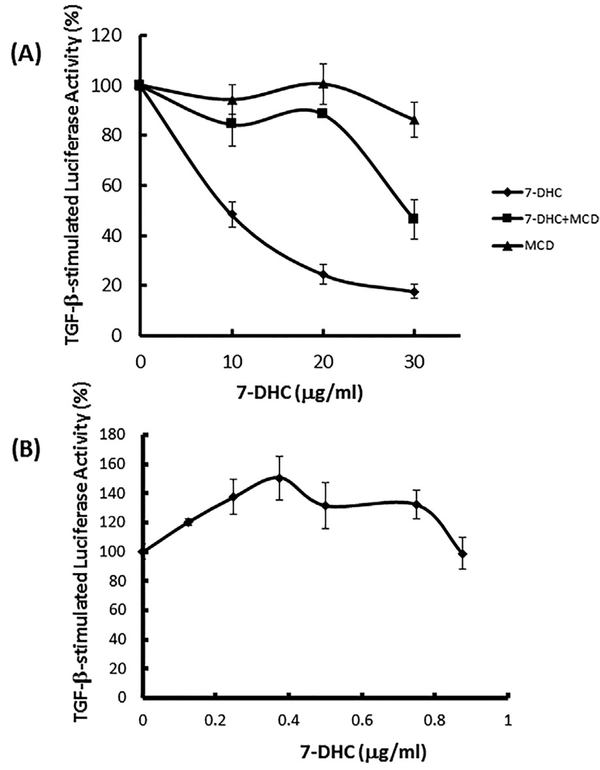
We hypothesize that 7-DHC suppresses TGF-β-stimulated canonical signaling by directly incorporating it into the plasma membrane and promoting formation of lipid rafts/caveolae [Xu et al., 2001; Keller et al., 2004]. This results in recruitment of cell-surface TβR-I-TβR-II oligomeric heterocomplexes from non-lipid raft microdomains to newly formed (or stabilized) lipid rafts/ caveolae. This is where canonical TGF-β signaling is suppressed. To test this hypothesis, Mv1Lu cells stably expressing a luciferase reporter gene were pre-treated with several concentrations (0, 10, 20, and 30 μg/ml) of 7-DHC for 1 h (allowing integration of 7-DHC into the plasma membrane, forming lipid rafts/caveolae) and then treated 100 pM TGF-β with or without several concentrations (0, 5, 10, and 15 μg/ml) of methyl β-cyclodextrin (MβCD). After further incubation for 5 h, luciferase activity of the lysates of treated cells was measured. MβCD, a sterol-chelating agent [Chen et al., 2007], is capable of extracting sterols (including 7-DHC) from the plasma membrane in target cells [Chen et al., 2007]. As shown in Figure 3A, 7-DHC suppressed TGF-β-stimulated luciferase activity in a concentration-dependent manner. The 7-DHC-suppressed TGF-β-stimulated luciferase activity was reversed by MβCD. This result supports the hypothesis.
Fig. 3.
Reversal of 7-DHC-suppressed TGF-β-stimulated luciferase activity by MβCD (MCD) (A) and enhancement of TGF-β-stimulated luciferase activity by low concentrations of 7-DHC (B) in Mv1Lu cells. (A) Cells were pre-treated with several concentrations (0, 10, 20, and 30 μg/ml) of 7-DHC at 37°C for 1 h (allowing integration of 7-DHC into the plasma membrane to form lipid rafts/caveolae), then treated with 100 pM TGF-β in the presence (7-DHC þ MCD) and absence (7-DHC alone) of several concentrations (0, 5, 10, and 15 μg/ml) of MCD at 37°C for an additional 5 h. In a control experiment (MCD alone), cells were treated with vehicle only at 37°C for 1 h and then treated with 100 pM TGF-β in the presence of several concentrations (0, 5, 10, and 15 μg/ml) of MCD at 37°C for an additional 5 h. TGF-β-stimulated luciferase activity of these treated cells was determined. The TGF-β-stimulated luciferase activity was taken as 100% in cells treated with TGF-β alone. The experiments were performed in triplicate. The data are mean ± s.d. (B) Cells were treated with 100 pM TGF-b in the presence of several concentrations (0, 0.125, 0.25, 0.5, 0.75, and 0.875 μg/ml) of 7-DHC at 37°C. After 5 h, TGF-β-stimulated luciferase activity of lysates of treated cells was determined. The TGF-β-stimulated luciferase activity was taken as 100% in cells treated with TGF-β alone. The experiments were performed in triplicate. The data are mean ± s.d.
Vitamins D2 and D3 have recently been shown to exert TGF-β enhancer activity at low concentrations [Huang et al., 2015]. They promote TGF-β-stimulated canonical signaling and cellular responses in Mv1Lu cells in a concentration-dependent manner with an optimal concentration of ~400 ng/ml (by ~1.5-fold as compared with that seen in cells treated with TGF-β only). Both vitamins D2 and D3 appear to promote canonical TGF-β signaling by extrusion of cholesterol from resident lipid rafts/caveolae [Huang et al., 2015], resulting in destabilization of lipid rafts/ caveolae, facilitating translocation of TβR-I and TβR-II there to non-lipid raft microdomains where canonical TGF-β signaling occurs. Thus, we determined the effect of 7-DHC at low concentrations on TGF-β-stimulated luciferase activity. Mv1Lu cells stably expressing the luciferase reporter gene were treated with 100 pM TGF-β in the presence of several concentrations (0, 0.125, 0.25, 0.375, 0.5, 0.75, and 0.875 μg/ml] of 7-DHC. After 5 h at 37°C, luciferase activity in the cell lysates was determined. As shown in Figure 3B, 7-DHC at 0.4 μg/ml promoted TGF-β-stimulated luciferase activity by ~1.5- fold. This suggests that, like vitamins D2 and D3 [Huang et al., 2015], 7-DHC, at certain low concentrations (~0.4 μg/ml), enhances TGF-β-stimulated luciferase activity by extrusion of cholesterol from lipid rafts/caveolae, facilitating translocation of TβR-I and TβR-II from lipid rafts/caveolae to non-lipid raft microdomains where canonical TGF-β signaling occurs [Huang et al., 2015].
7-DHC RECRUITS TβR-I AND TβR-II FROM NON-LIPID RAFT MICRODOMAINS TO LIPID RAFTS/CAVEOLAE
To further test the hypothesis that 7-DHC suppresses TGF-β signaling by directly incorporating it into the plasma membrane and promoting formation of lipid rafts/caveolae, we determined the microdomain localization of cell-surface TβR-I-TβR-II oligomeric heterocomplexes in Mv1Lu cells treated with and/or without 7-DHC using cell-surface 125I-TGF-β-cross-linking [Huang and Huang, 2005; Chen et al., 2006, 2007, 2008, 2011]. This has been used to determine the microdomain localization of TβR-I-TβR-II oligomeric heterocomplexes in cultured cells and tissues (e.g., vascular endothelium) [Chen et al., 2007, 2011]. When the ratio of 125I-TGF-β binding (cross-linked) to TβR-II and TβR-I (TβR-II*/TβR-I*) is decreased after treatment of cells or tissues with any test compound, this suggests that the treatment induces recruitment of TβR-I-TβR-II oligomeric heterocomplexes from non-lipid raft microdomains to lipid rafts/caveolae [Huang and Huang, 2005; Chen et al., 2006, 2007, 2008]. The smaller the ratio of TβR-II*/TβRI* is, the greater the localization of TβR-I-TβR-II oligomeric heterocomplexes in lipid rafts/caveolae [Huang and Huang, 2005]. When the ratio of TβR-II*/TβR-I* is larger than one (i.e., TGF-β binding to TβR-II is dominant), hetero-oligomeric TGF-β receptor complexes are mainly localized in non-lipid raft microdomains. After TGF-β binding, they are internalized by clathrin-mediated endocytosis [Huang and Huang, 2005]. This results in TGF-β-stimulated canonical signaling at coated-pit stages [Chen et al., 2009], ending in endosome localization and subsequent lysosomal degradation. When the ratio of TβR-II*/TβR-I* is smaller than one (i.e., TGF-β binding to TβR-I is dominant), hetero-oligomeric TGF-β receptor complexes are mainly localized in lipid rafts/caveolae. Then, following TGF-β binding, they are internalized by caveolar/ lipid-raft-mediated endocytosis. This results in rapid degradation and suppressed TGF-β signaling [Huang and Huang, 2005; Chen et al., 2007].
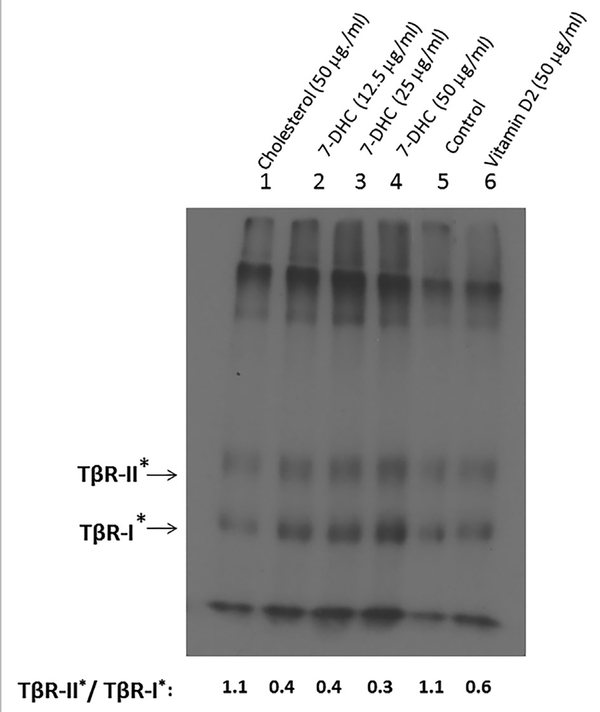
In the experiments, Mv1Lu cells were treated with vehicle only (0.1% ethanol; control), cholesterol (50 μg/ml), 7-DHC (12.5, 25, and 50 μg/ml) and vitamin D2 (50 μg/ml). After 1 h at 37°C, cell-surface 125I-TGF-β-cross-linking was performed at 0°C as described [Huang and Huang, 2005; Chen et al., 2006, 2007, 2008, 2009, 2011]. Lysates of 125I-TGF-β-cross-linked cells were analyzed by 7.5% SDS–PAGE and autoradiography. As shown in Figure 4, cells treated with cholesterol (50 μg/ml], 7-DHC (12.5, 25, and 50 μg/ml), vehicle (ethanol) only (control) and vitamin D2 (50 μg/ml), exhibited TβRII*/TβR-I* ratios of 1.1 0.4, 0.4, 0.3, 1.1, and 0.6, respectively. Both cholesterol-treated cells and control cells had a TβR-II*/TβR-I* ratio of 1.1 (Fig. 4, lanes 1 and lane 5). This indicates that cells treated with cholesterol and vehicle only (control) have predominant non-lipid raft microdomain localization of the TGF-β receptors. Cholesterol does not affect the TGF-β receptor domain localization as compared with that in cells treated with vehicle only. However, 7-DHC and vitamin D2 increased localization of TβR-I-TβR-II oligomeric heterocomplexes in lipid rafts/caveolae (Fig. 4, lanes 2–4 and lane 6 vs. lane 5, respectively) with the potency of 7-DHC (TβR-II*/TβRI* = 0.3 or 0.4) >vitamin D2 (TβR-II*/TβR-I* = 0.6).
Fig. 4.
Recruitment of TβR-I and TβR-II by 7-DHC from non-lipid raft microdomains to lipid rafts/caveolae in Mv1Lu cells, as determined by cell-surface I125-TGF-β-cross-linking. Cells were treated with cholesterol (50 mg/mg), 7-DHC (12.5, 25, and 50 μg/ml), vehicle only (control), or vitamin D2 (50 μg/ml) at 37°C for 1 h. The treated cells were then incubated with I125-TGF-β at 0°C for 2.5 h. After washing with ice-cold binding buffer, cell-surface I125-TGF-β-cross-linking was performed using DSS (as a cross-linking agent) at 0°C for 15 min. I125-TGF-β-cross-linked cells were then analyzed by 7.5% SDS–PAGE and autoradiography, as described [Chen et al., 2007, 2008]. I125-TGF-β-cross-linked TβR-I (TβR-I*) and I125-TGF-β-cross-linked TβR-II (TβR-II*) on the SDS–PAGE (dried gel) were quantified using PhosphoImager. Free I125-TGF-β migrated near the dye front. Representative of three experiments is shown. The relative ratios of TβRII*/TβR-I* in cells treated with cholesterol (50 μg/ml), 7-DHC (12.5, 25, and 50 μg/ml), vehicle only (control) and vitamin D2 (50 μg/ml) were estimated to be 1.1, 0.4, 0.4, 0.3, and 0.6, respectively.ficantly higher or lower than that in cells treated with vehicle only (control) (P < 0.05).
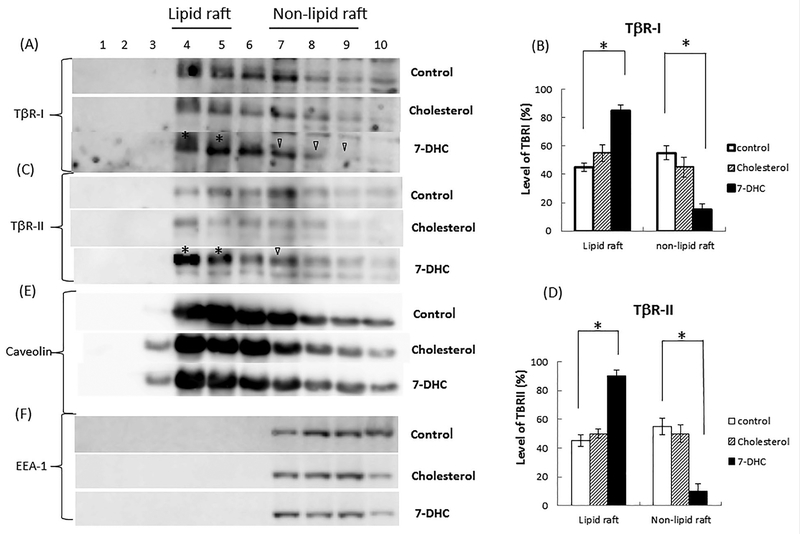
To further define the predominant localization of TβR-I-TβR-II oligomeric heterocomplexes in cells treated with 7-DHC, we analyzed the microdomain localization of TβR-I and TβR-II in Mv1Lu cells treated with and without 7-DHC (10 μg/ml) at 37°C for 4 h using sucrose density gradient ultracentrifugation followed by SDS–PAGE and Western blot analysis/quantitative analysis (Fig. 5A–D for TβR-I and TβR-II, respectively). As shown in Figure 5A and C, 7-DHC treatment induced recruitment of TβR-I and TβR-II from non-lipid raft microdomains to lipid rafts/caveolae (lanes 4 and 5 vs. lanes 7, 8, and 9). 7-DHC increased localization of TβR-I and TβR-II in lipid rafts/caveolae to 85% and 90%, respectively (Fig. 5B and D). In contrast, cholesterol (10 μg/ml at 37°C for 4 h) did not significantly alter the microdomain localization of TβR-I and TβR-II as compared with that in cells treated with vehicle only (control) (Fig. 5B and D, respectively). About ~45–55% of TβR-I and
Fig. 5.
Recruitment of TβR-I and TβR-II by 7-DHC from non-lipid raft microdomains to lipid rafts/caveolae in Mv1Lu cells. Cells were treated with vehicle only (control), cholesterol (10mg/ml), and 7-DHC (10mg/ml) at 37°C for 4 h. The lipid raft/caveolae and non-lipid raft microdomain localization of TβR-I (A and B), TβR-II (C and D), caveolin- 1 (E), and EEA-1 (F) in treated cells was then determined by sucrose gradient ultracentrifugation followed by Western blot analysis using antibodies to TβR-I, TβR-II, caveolin-1, and EEA-1 (early endosome antigen 1). Representative of three experiments is shown. Fractions 4 and 5, which mainly contained caveolin-1, represent the location of lipid rafts/caveolae (lipid raft) (A, C, and E). Fractions 7, 8, and 9, which contained EEA-1, represent the location of non-lipid raft microdomains (non-lipid raft) (A, C, and F). Fractions 7, 8, 9, and 10 contain trace or small amounts of caveolin-1. This is due to the presence of mitochondria in these fractions [Huang et al., 2016a,b]. The * symbol indicates the increased amount of TβR-I or TβR-II in the fraction of cells treated with 7-DHC as compared with that in control cells. The open arrow head indicates the decreased amount of TβR-I or TβR-II in the fraction of 7-DHC-treated cells as compared to that in control cells. The relative amount of TβR-I and TβR-II in the microdomains in treated cells were quantified by densitometry using caveolin-1 as an internal control (B and D). The relative total amount of TβR-I or TβR-II in lipid rafts/cavelolae (fractions 4 and 5) and non-lipid raft microdomains (fractions 7, 8, and 9) in treated cells was taken as 100%. For example, the relative amounts of TβR-I and TβR-II in lipid rafts/caveolae (lipid raft) and non-lipid raft microdomains (non-lipid raft) in cells treated with 7-DHC were estimated to be 85% and 15%, and 90% and 10%, respectively. The experiments were performed in triplicate. The data are mean ± s.d. *Significantly higher or lower than that in cells treated with vehicle only (control) (P <0.05).
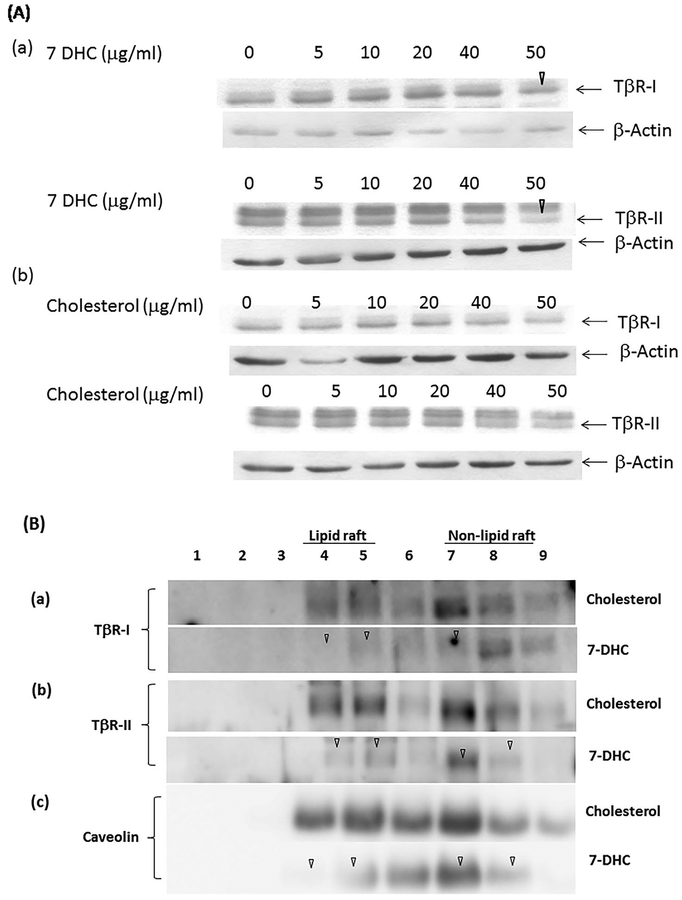
TβR-II are localized in lipid rafts/caveolae in control cells and cells treated with cholesterol (Fig. 5B and D). Treatment of cells with 7-DHC and cholesterol at 10 μg/ml did not affect microdomain localization of caveolin-1 and early endosome antigen 1 (EEA-1; a non-lipid raft marker) as compared with that in cells treated with vehicle only (control cells) (Fig. 5E and F, respectively). To determine the effects of 7-DHC and cholesterol on the stability of TβR-I and TβR-II, Mv1Lu cells were treated with several concentrations (0, 5, 10, 20, 40, and 50 μg/ml) of 7-DHC or cholesterol at 37°C and for 4 h. The cell lysates of these treated cells were subjected to Western blot analysis using antibodies to TβR-I, TβR-II, and b-actin. The relative levels of TβR-I and TβR-II in treated cells were estimated by normalizing the level of b-actin to that in control cells. As shown in Figure 6A(a), 7-DHC, at 5, 10, and 20 μg/ml, did not alter the expression of TβR-I and TβR-II but, at 50 μg/ml, attenuated the expression of TβR-I and TβR-II by ~30% (Fig. 6A(a), marked by an open arrowhead) in the total cell lysates of these cells. Importantly, cholesterol, at all concentration tested, did not significantly affect expression of TβR-I and TβR-II (Fig. 6A(b)).
Fig. 6.
Effects of the treatment (at 37°C for 4 h) with several concentrations of 7-DHC and cholesterol on the stability of TβR-I and TβR-II in Mv1Lu cells (A) and with 50 μg/ml of 7-DHC and cholesterol on the stability of TβR-I and TβR-II localized in plasma-membrane microdomains of Mv1Lu cells (B). (A) Mv1Lu cells were treated with several concentrations (0, 5, 10, 20, 40, and 50 μg/ml) of 7-DHC (a) or cholesterol (b) at 37°C for 4 h. The cell lysates of treated cells were subjected to 7.5% SDS–PAGE, followed by Western blot analysis using antibodies to TβR-I, TβR-II, and b-actin as described previously [Huang et al., 2016a,b]. The arrow indicates the location of TβR-I, TβR-II, or b-actin. The data is representative of three independent experiments. The levels of TβR-I, TβR-II, and b-actin were quantified by densitometry. The relative levels of TβR-I and TβR-II in treated cells were estimated by normalizing the level of b-actin to that in control cells treated with vehicle only. 7-DHC at 50 μg/ml appeared to attenuate the expression of TβRI or TβR-II by ~30% (marked by an open arrowhead) as compared to that in control cells treated with vehicle only. (B) Cells were treated with cholesterol (50 μg/ml) or 7-DHC (50 μg/ml) at 37°C for 4 h. The lipid raft/caveolae (lipid raft) and non-lipid raft microdomain (non-lipid raft) localization of TβR-I (a), TβR-II (b), and caveolin-1 (c) in treated cells were then determined by sucrose gradient ultracentrifugation, followed by Western blot analysis using antibodies to TβR-I, TβR-II, and caveolin-1. Treatment of cells with 7-DHC (50 μg/ml) diminished the amounts of TβR-I (a), TβR-II (b), and caveolin-1 (c) in fractions 4 and 5 (lipid raft), and fractions 7 and 8 (non-lipid raft), as marked by an open arrowhead. The fraction was compared to that in cells treated with cholesterol. The data are representative of a total of three independent analyses. The relative amounts of TβR-I and TβR-II in lipid rafts/caveolae (fractions 4 and 5) and non-lipid raft microdomains (fractions 7, 8, and 9) in cells treated with cholesterol or 7-DHC were quantified by densitometry. The total amount of TβR-I, TβR-II, or caveolin-1 in lipid rafts/cavelolae/non-lipid raft microdomains in cells treated with cholesterol was taken as 100%. The relative total amounts of TβR-I, TβR-II and caveolin-1 in lipid rafts/caveolae (lipid raft)/non-lipid raft microdomains (non-lipid raft) in cells treated with 7-DHC were estimated to be ~10%, and ~10% and ~15%, respectively. Cholesterol treatment did not appear to affect the total relative amounts of TβR-I, TβR-II and caveolin-1 in the microdomains.
Cellular TβR-I and TβR-II mainly reside in two major cellular compartments including cytoplasmic vesicles and plasma membrane microomains [Massague and Kelly, 1986; Dore et al., 2001; Asano et al., 2011; Huang et al., 2016a,b]. Approximately, two thirds of the total TGF-β receptors and one third of the TGF-β receptors are localized in cytoplasmic vesicles and plasma membrane micro-domains, respectively [Huang et al., 2016b]. We hypothesize that 7-DHC at 50 μg/ml (at 37°C for 4 h) induces down-regulation of most of the TβR-I and TβR-II, which are localized in plasma membrane microdomains. This leads to a ~30% decrease of TβR-I and TβR-II in the total cell lysates. To test this hypothesis, Mv1Lu cells were treated with 50 μg/ml 7-DHC or cholesterol at 37°C for 4 h. Treated cell homogenates were then subjected to sucrose density gradient ultracentrifugation fractionation, followed by Western blot analysis. As shown in Figure 6B, treatment with 50 μg/ml of 7-DHC at 37°C for 4 h induced turnover of TβR-I and TβR-II, and caveolin-1 localized in lipid rafts and non-lipid raft microdomains by 85–90% (Fig. 6B(a), (b), and (c), respectively). Cholesterol at 50 μg/ml did not significantly alter the stability of TβR-I and TβR-II, and caveolin-1 localized in these plasmamembrane microdomains under the same conditions (37°C for 4 h). This result supports the hypothesis that 7-DHC at 50 μg/ml induces turnover of most TβR-I and TβR-II and caveolin-1 which are localized in the plasma-membrane microdomains. Since, 7-DHC is known to be incorporated into lipid rafts/caveolae in vitro and in vivo [Xu et al., 2001; Keller et al., 2004], the results shown in Figure 5 suggest that 7-DHC at 10 μg/ml promotes lipid raft/caveolae formation and facilitates recruitment of TβR-I and TβR-II from non-lipid raft microdomains to newly formed (or stabilized) lipid rafts/caveolae where TGF-β signaling does not occur. However, TGF-β receptors undergo rapid degradation upon ligand (TGF-β) binding. This leads to suppression of TGF-β-stimulated canonical signaling. However, 7-DHC at a high concentration (50 μg/ml) at 37°C for 4 h induces recruitment of TβR-I, TβR-II, and caveolin-1 from non-lipid raft microdomains to lipid rafts/caveolae and subsequent disruption of lipid rafts/ caveolae, resulting in turnover (~85–90%) of TβR-I, TβR-II, and caveolin-1. 7-DHC has been shown to cause disruption of lipid rafts/caveolae in keratinocytes [Valencia et al., 2006].
DISCUSSION
Here, we demonstrate that 7-DHC, ergosterol and their uv-activated derivatives, which contain the 5,7-conjugated diene structure, (but not cholesterol and other related sterols) suppress or attenuate TGF-β-stimulated luciferase activity in Mv1Lu cells stably expressing a luciferase reporter gene containing TGF-β canonical signaling (Smad-dependent)-responsive elements [Chen et al., 2007, 2008, 2009]. This is consistent with our previous observations that 7-DHC attenuates TGF-β-stimulated canonical signaling in all cell types studied, including Mv1Lu, NRK, CHO-K1, and BAEC cells, as determined by analyzing TGF-β-stimulated expression of phosphorylated Smad2 (P-Smad2) and plasminogen activator inhibitor-1 (PAI-1) [Huang and Huang, 2015]. 7-DHC, ergosterol and their uv-activated derivatives (vitamins D2 and D3) suppress TGF-β-stimulated canonical signaling by recruiting TβR-I and TβR-II from non-lipid raft microdomains to lipid rafts/caveolae as determined by cell-surface 125I-TGF-β-cross-linking and sucrose density gradient ultrafiltration fractionation [Huang et al., 2015]. 7-DHC and ergosterol are both known to promote lipid raft/caveolae domain formation more strongly than cholesterol [Xu et al., 2001]. 7-DHC is distinguishable from cholesterol in its greater ability to be incorporated into lipid rafts in vitro and in vivo [Keller et al., 2004]. The 5,7-conjugated dienes present in 7-DHC and ergosterol are important structural elements that strongly bind sphingolipids together to generate lipid rafts/caveolae. The 5,7-conjugated dienes confer specific configurations of the 3β-ol (hydroxy) and 10-methyl groups (Fig. 7, as marked by an arrow) in 7-DHC and ergosterol molecules as compared to those in the cholesterol molecule. However, the presence of a double bond on the side chain of ergosterol diminishes its ability to promote lipid raft/caveolae formation and suppress TGF-β-stimulated luciferase activity. The 7-DHC-promoted newly formed (or stabilized) lipid rafts/caveolae are characterized by the co-localization of TβR-I and TβR-II with caveolin-1 (a lipid raft/cavelae marker) in the sucrose density gradient ultracentrifugation fractions of Mv1Lu cells treated with 7-DHC. 7-DHC-containing lipid rafts/caveolae exhibit various protein components and stability, dependent on the expression level of 7-DHC [Kovarova et al., 2006; Korade et al., 2009; Gou-F abregas et al., 2016]. We hypothesize that 7-DHC, at certain low concentrations (~0.4 μg/ml), enhances TGF-β canonical signaling by extrusion of cholesterol from resident lipid rafts/caveolae, resulting in destabilization of lipid rafts/caveolae and facilitating translocation of the TGF-β receptors from lipid rafts/caveolae to non-lipid raft microdomains (where canonical signaling occurs) [Huang et al., 2015]. However, at higher concentrations (>1 μg/ml), 7-DHC suppresses TGF-β canonical signaling by promoting formation of lipid rafts/caveolae [Xu et al., 2001], thus recruiting the TGF-β receptors from non-lipid raft microdomains to lipid rafts/ caveolae and, at 50 μg/ml, eventually inducing disruption of lipid rafts/caveolae.
Vitamins D2 and D3 are steroid molecules with 5,7-conjugated dienes and an open B ring. They are capable of promoting formation of lipid rafts/caveolae as demonstrated by 125I-TGF-β cell-surface cross-linking [Huang et al., 2015]. The normal plasma concentrations of vitamin D3 in humans are 35–40 ng/ml [Holick, 2004]. These are plasma bone health-acting concentrations. Here, we show that vitamin D3 suppresses TGF-β canonical signaling with an IC50 of ≥ 20 μg/ml. This is much greater than its physiological plasma concentrations and may not be physiologically relevant. However, like 7-DHC, vitamins D2 and D3, at certain low concentrations (<1 μg/ml), are capable of enhancing canonical TGF-β signaling by ~1.5–2-fold in target cells [Huang et al., 2015]. The TGF-β enhancer activity of vitamins D2 and D3 is likely responsible for their known effects on the prevention of autoimmune disease, cancer, and ASCVD [Holick, 2004]. TGF-β is known to be a protective cytokine against these diseases [Akhurst and Derynck, 2001; Grainger, 2004; Li et al., 2006]. Vitamin D3 apparently exhibits three different potential biological activities of bone vitamin, TGF-β enhancer and TGF-β suppressor (or inhibitor) with optimal concentrations at ~40 ng/ml [Holick, 2004], ~400 ng/ml [Huang et al., 2015], and ~20 μg/ml, respectively.
Dietary and blood cholesterol have been thought to play important roles in the development of ASCVD for decades. Recommendations to reduce the risk of ASCVD in humans include maintaining low cholesterol intake and low plasma cholesterol levels. However, in the last few years, there have been a number of epidemiological studies that do not support a relationship between dietary cholesterol and/or blood cholesterol and ASCVD [Kanter et al., 2012; Berger et al., 2015; Eckel, 2015]. This led to the 2015 USDA guidelines indicating that cholesterol is not a nutrient of concern for overconsumption. Consequently, the role of cholesterol in the development of ASCVD is now uncertain. Much evidence indicates that TGF-β in the blood circulation is a protective cytokine against ASCVD [Mallat et al., 2001; Tashiro et al., 2002; Gojova et al., 2003; Robertson et al., 2003; Grainger, 2004; Huang and Huang, 2005; Chen et al., 2007, 2008, 2011; Frutkin et al., 2009; Reifenberg et al., 2012]. Suppressed canonical TGF-β signaling is an important step in atherogenesis [McCaffrey et al., 1995; Huang and Huang, 2005; Chen et al., 2007, 2008, 2011] and has been identified in atherosclerotic lesions in human patients and ApoE-knockout or wild-type mice [McCaffrey et al., 1995; Chen et al., 2007, 2011]. In this communication, we demonstrate that 7-DHC (but not cholesterol) suppresses TGF-β-stimulated canonical signaling by promoting formation of lipid rafts/caveolae. This facilitates translocation of cell-surface TGF-β receptors from non-lipid rafts/ caveolae to lipid rafts/caveolae (where canonical TGF-β signaling is suppressed). These results provide molecular evidence suggesting that 7-DHC (but not cholesterol) is likely to be important in the development of ASCVD. These results also imply that dietary cholesterol is not involved in atherogenesis because 7-DHC is known to be mainly produced in animal bodies at very low levels.
The 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitors [Tobert, 2003], also known as statins, exert beneficial effects independent of blood cholesterol level reduction on inflammation, endothelial dysfunction, and atherosclerosis [Arnaud et al., 2005; Liao, 2005; Marzilli, 2010]. Statins are the most effective known class of drugs for lowering serum cholesterol levels. However, cholesterol-independent or “pleiotropic” effects of statins are most likely mediated by their ability to limit production of key HMG-CoA reductase downstream molecules other than cholesterol [Arnaud et al., 2005; Liao, 2005; Marzilli, 2010]. For example, statins inhibit the synthesis of important isoprenoids which are biosynthetic precursors of cholesterol [Arnaud et al., 2005; Liao, 2005; Marzilli, 2010]. These isoprenoid intermediates serve as lipid moieties for the post-translational modification of nuclear and cytoplasmic proteins. Protein isoprenylation directs proper subcellular localization and trafficking of intracellular proteins which are involved in diverse cellular functions [Arnaud et al., 2005; Liao, 2005; Marzilli, 2010]. However, the identity of the key molecules involved in the pleiotropic effects of statins on ASCVD is not clear. Here, we demonstrate that 7-DHC (the immediate biosynthetic precursor of cholesterol), but not cholesterol, promotes formation of lipid rafts/ caveolae as evidenced by increased localization of TβR-I and TβR-II in these microdomains (as determined by the co-localization with caveolin-1 in the fractions obtained from sucrose density gradient ultracentrifugation of plasma membranes). We hypothesize that 7-DHC is such a key HMG-CoA reductase downstream molecule involved in mediating inflammation, endothelial dysfunction and suppression of canonical TGF-β signaling in atherogenesis by promoting formation of lipid rafts/caveolae. This hypothesis is supported by the following evidence: (i) 7-DHC is an important structural and functional component in lipid rafts/caveolae. Increased levels of 7-DHC promote formation of lipid rafts/caveolae [Xu et al., 2001; Keller et al., 2004]. (ii) Lipid rafts/caveolae are cell-surface platforms in vascular cells for Toll-like receptor 4 (TLR4) signaling and other signals which are involved in mediating vascular inflammation during atherogenesis [Villacorta et al., 2013; Sorci-Thomas and Thomas, 2016]. Statins may exert their anti-inflammatory pleiotropic effects by lowering levels of 7-DHC, decreasing lipid rafts/caveolae, thus diminishing inflammatory signaling mediated by lipid rafts/caveolae in vascular cells [Chansrichavala et al., 2010]. (iii) Endothelial dysfunction is an early manifestation of ASCVD. It is characterized by the impaired synthesis of endothelium-derived nitric oxide. This results from the localization of nitric oxide synthase (eNOS) in lipid rafts/caveolae where eNOS does not produce nitric oxide [Liao, 2005; Lemaire-Ewing et al., 2012], Statins may improve endothelial function by lowering levels of 7-DHC and decreasing lipid rafts/caveolae in vascular cells, resulting in increasing production of nitric oxide by the eNOS present in non-lipid raft microdomains [Liao, 2005; Lemaire-Ewing et al., 2012]. (iv) Suppression of canonical TGF-β signaling is important in the development of ASCVD [Chen et al., 2007, 2008; Villacorta et al., 2013]. Statins appear to enhance canonical TGF-β signaling, which counteracts suppressed canonical TGF-β signaling in atherogenesis, by lowering 7-DHC levels, decreasing lipid rafts/caveolae and activating the TGF-β receptors localized in non-lipid raft microdomains [Chen et al., 2007, 2008]. And (v) Statin therapy has been shown to reduce the risk of ASCVD independent of its cholesterol-lowering effect [Arnaud et al., 2005; Liao, 2005; Marzilli, 2010]. This is likely due to the inhibition of 7-DHC biosynthesis by statins.
ASCVD accounts for ~80% of all deaths among diabetic patients. Active HMG-CoA reductase produced by prolonged exposure to hyperglycemia is now recognized as an important factor in the pathogenesis of ASCVD in diabetics [Ness et al., 1994]. Increased levels of 7-DHC caused by persistently active HMG-CoA reductase may be involved in the development of ASCVD in diabetics. The plasma concentrations of 7-DHC and cholesterol in normal subjects are 55 (40–66) ng/ml and 2.3 (2.0–2.4) μg/ml, respectively [Axelson et al., 1998]. The level of 7-DHC (but not the level of cholesterol) in human plasma reflects the activity of the HMG-CoA reductase in the liver, suggesting that HMG-CoA reductase inhibitors would be useful agents to lower the 7-DHC level, thus preventing and treating ASCVD [Axelson et al., 1998]. 7-DHC is partially converted to 8-dehydrocholesterol by an active isomerase found mainly in the liver [Batta et al., 1995]. The IC50 (4,000 ng/ml) of 7-DHC required to suppress canonical TGF-β signaling is ~70-fold greater than the plasma concentration of 7-DHC in normal subjects. It is possible that accumulation of 7-DHC in lipid rafts/caveolae (which results in suppressed TGF-β signaling) in vascular endothelium is a rate-limiting step in atherogenesis. It is also possible that accumulation of other 5,7 (8)-conjugated diene-containing sterols (e.g., 8-DHC and 7-dehydrodesmosterol) in lipid rafts/caveolae is also involved in suppressing canonical TGF-β signaling and atherogenesis.
We hypothesize that TGF-β enhancers are therapeutic agents for ASCVD. They counteract the 7-DHC-mediated suppression of TGF-β canonical signaling in aortic endothelium. This hypothesis is supported by our observation that dynasore, a TGF-β enhancer, attenuates ASCVD without altering high plasma cholesterol levels in ApoE-knockout mice [Chen et al., 2009]. To test this hypothesis, we identified several TGF-β enhancers which promote canonical TGF-β signaling at the plasma membrane in target cells [Chen et al., 2009; Huang et al., 2015, 2016a,b]. Based on their known mechanisms, TGF-β enhancers can be classified into 4 types: (i) Type I TGF-β enhancers: cholesterol biosynthesis inhibitors (e.g., statins) [Huang and Huang, 2005; Chen et al., 2009; Huang et al., 2015]. These enhance TGF-β signaling (~threefold) by lowering 7-DHC levels in target cells and tissues. This results in recruitment of TβR-I and TβRII from lipid rafts/caveolae to non-lipid raft microdomains. This is where canonical signaling occurs. (ii) Type II TGF-β enhancers: 7-DHC (or cholesterol)-extruding compounds. They include triterpenoids (e.g., betulinic acid and CDDO-Im) [Suh et al., 2003], polyphenols (e.g., cyanidin) [Fu et al., 2014; Huang et al., 2015], antioxidants (e.g., vitamin E) [Huang et al., 2015; Zingg, 2015], and ethanol [Huang et al., 2016a,b]. These compounds enhance TGF-β signaling (~2–5-fold) by extrusion of 7-DHC from resident lipid rafts/caveolae, resulting in destabilization of lipid rafts/caveolae, thus facilitating translocation of TβR-I and TβR-II from lipid rafts/ caveolae to non-lipid raft mcrodomains. (iii) Type III TGF-β enhancers: endocytosis inhibitors (e.g., dynasore) [Chen et al., 2009]. These agents enhance TGF-β signaling (~fivefold) by sustaining TGF-β receptor signaling at coated-pit stages during clathrin-dependent endocytosis. This occurs in non-lipid raft microdomains. And (iv) Type IV TGF-β enhancers: fusogenic compounds such as DMSO [Huang et al., 2016b], ethanol [Huang et al., 2016a,b], and resveratrol [Huang et al., 2015]. They enhance TGF-β signaling (~2–8-fold) by inducing fusion of cytoplasmic vesicles and plasma membranes, resulting in recruitment of TβR-I or TβR-II from their cytoplasmic vesicle pools to plasma membranes. Type I TGF-β enhancers have been used as therapeutic agents to treat human patients with ASCVD. Since, these four types of TGF-β enhancers utilize different mechanisms to enhance such signaling, combinations of these types of TGF-β enhancers with additive and/ or synergistic effects may lead to strategies to treat and/or prevent ASCVD. Vitamin E is classified as a type II TGF-β enhancer which promotes TGF-β activity, presumably by extrusion of 7-DHC from lipid rafts/caveolae [Zingg, 2015]. The TGF-β enhancer activity of ethanol at low doses appears to be responsible for certain beneficial effects (prevention of ASCVD and other diseases) [Huang et al., 2016a,b]. DMSO has been shown to ameliorate atherosclerosis in animal models [Huang et al., 2016b]. Many agents or compounds which are known to reduce the risk of ASCVD have been found to exhibit TGF-β enhancer activity [Fu et al., 2014; Huang et al., 2015, 2016a,b].
The lipid raft/caveolae domain-promoting function of 7-DHC demonstrated herein, will also increase our understanding of the pathogenesis of the Smith-Lemli-Opitz syndrome (SLOS] [Smith et al., 1964] and may lead to development of effective therapy. SLOS is an autosomal recessive disease associated with multiple congenital anomalies and mental retardation [Nowaczyk and Irons, 2012]. It is caused by disabled mutations of the 7-DHC reductase gene (DHCR7), which encodes the enzyme that catalyzes reduction of 7-DHC to cholesterol. Deficiency of 7-DHC reductase results in a low concentration of cholesterol and a high concentration (2.7–470 μg/ml; 10–2,000-fold normal) of 7-DHC in blood and tissues of patients with SLOS [DeBarber et al., 2012; Griffiths et al., 2016]. In SLOS, there is a wide clinical spectrum ranging from early death due to severe organ malformations to minimal facial abnormalities and near-normal mental development. Since, cholesterol supplementation does not improve developmental progress in SLOS [Sikora et al., 2004], the multiple malformations and mental retardation in subjects are mainly caused by excessive levels of 7-DHC in tissues. However, how the accumulation of a single compound (7-DHC) leads to the multiple malformations, mental retardation, and wide phenotypic variability in SLOS has been unknown in the field for decades since the syndrome was discovered. Here, we demonstrate that 7-DHC (at ~4 μg/ml) promotes formation of lipid raft/caveolae, resulting in recruitment of TGF-β receptors and other membrane proteins (e.g., eNOS) from non-lipid raft domains to lipid rafts/caveolae where they become inactive in transducing signaling. Accumulating at high levels in lipid rafts/ caveolae (in SLOS patients), 7-DHC disturbs the structure, and function ofthese microdomains,resultingin alterations ofstabilityand functions of resident proteins (including TGF-β receptors and other membrane proteins) [Valencia et al., 2006]. For example, markedly reduced expression of lipid raft/caveolae-resident proteins (including caveolin-1, Na/K ATPase, folate transporter, and BKCa K+ channel) and reduced fibroblast proliferation activity, and altered functions of the voltage-gated Kv1.3 channel have been found in SLOS fibroblasts and T lymphocytes, respectively [Ren et al., 2011; Balajthy et al., 2016]. Since TGF-β is a mitogen for fibroblasts, reduced proliferation activity in SLOS fibroblasts is likely due to suppression of TGF-β canonical signaling. This results from the predominant localization of TβR-I and TβR-II in lipid rafts/caveolae in these cells [Ren et al., 2011]. We hypothesize that high-level accumulation (10–2,000-fold normal) of 7-DHC, which varies in severity in SLOS patients and SLOS tissues, results inlipidraft/caveolae dysfunction, turnover, and down-regulation of the many resident membrane proteins in lipid rafts/caveolae, including TGF-β receptors [Huang and Huang, 2005; Chen et al., 2007, 2008, 2009, 2011], neurotrophin receptors [Sebastîao et al., 2011; Zhang et al., 2013; Gou-Fàbregas et al., 2016], G protein-coupled receptors (GPCRs) (neurological receptors) [Björk and Svenningsson, 2011], ion channels [Jaffrès et al., 2016], receptor protein kinases (RTKs) (e.g., growth factor receptors) [Basu et al., 2008] and growth hormone receptor [Yang et al., 2004]. Down-regulation of these important membrane proteins in tissues during embryonic development may result in prenatal and postnatal growth retardation, microcephaly, intellectual disability, multiple external malformations (e.g., cleft palate), internal anomalies, and mental retardation characteristic of SLOS. For example, the TGF-β receptor signaling pathway is involved in many cellular processes in both the adult organism and the developing embryo including cell growth, cell differentiation, apoptosis, cellular homeostasis, and other cellular functions. TGF-β receptor signaling plays crucial roles in regulating palate development in both the plate epithelium and mesenchyme [Iwata et al., 2011]. 7-DHC-mediated suppression of TGF-β receptor signaling during embryonic development may result in external malformations, including cleft palate [Iwata et al., 2011] which is the most characteristic facial feature of SLOS.
Therapeutic trials using dietary supplementation of cholesterol have been shown to increase plasma cholesterol levels in some SLOS patients but have thus far failed to alter plasma levels of 7-DHC in the patients. Therapeutic trials in SLOS with simvastatin, which inhibits biosynthesis of both 7-DHC and cholesterol, have been shown to decrease 7-DHC plasma levels, with variable clinical improvement [Jira et al., 2000]. Safety and efficacy of simvastatin therapy in 23 patients with mild to typical SLOS have been recently evaluated in a randomized, double-blind, placebo-controlled trial. Simvastatin appears to be relatively safe in patients with SLOS, improves the serum dehydrocholesterol (7-DHC + 8-DHC)-to-total sterol ratio, and significantly improves irritability symptoms in patients with mild to classic SLOS [Wassif et al., 2016]. 7-DHC is known to undergo free radical peroxidation to produce oxysterols that exert cytotoxicity and attenuate cell proliferation and differentiation. It has been suggested that the accumulation of 7-DHC-derived oxysterols may contribute to the abnormal development of SLOS patients. Dietary supplementation of antioxidants has been recently shown to decrease 7-DHC oxidation in a mouse model of SLOS [Korade et al., 2014]. It was hypothesized that preventing formation of oxysterols derived from 7-DHC is critical for countering the detrimental effects of DHCR7 mutations. In fact, vitamin E, a TGF-β enhancer, has been shown to be capable of altering the structure and function of lipid rafts/caveolae [Zingg, 2015]. This may lead to extrusion of 7-DHC from resident lipid rafts/caveolae, resulting in decreased formation of oxysterols derived from 7-DHC. In addition, these 7-DHC-derived oxysterols, which were decreased by vitamin E in their study [Korade et al., 2014], lack the 5,7-conjugated diene structure and thus should not affect TGF-β signaling as 7-DHC does. Vitamin E is a weak TGF-β enhancer. Recently, over 30 small-molecule compounds, which decrease the production and/or accumulation of 7-DHC in cultured cells, have been identified by screening 727 compounds using 7-DHC reductase (DHCR7)-deficient Neuro2a cells [Korade et al., 2016]. Many of these identified compounds are putative TGF-β enhancers. This suggests that potent TGF-β enhancers, particularly the Type II enhancers, warrant clinical trials for treating SLOS patients.
ACKNOWLEDGMENTS
We thank Lai-Ping Yan and Ying Fei for their technical assistance. This work was supported by NIH grants HL 095261 and AR 052578 to Auxagen. This work was also supported by the President’s Research Fund, Saint Louis University (J.S.H.).
Grant sponsor: NIH; Grant numbers: HL 095261, AR 052578; Grant sponsor: President’s Research Fund.
Footnotes
Conflicts of interest: The authors declare that they have conflict of interest. Jun San Huang and Shuan Shian Huang had an equity position in Auxagen Inc. during the time the research was carried out.
REFERENCES
- Akhurst RJ, Derynck R. 2001. TGF-β signaling in cancer—A double-edged sword. Trends Cell Biol 11:S44–S51. [DOI] [PubMed] [Google Scholar]
- Arnaud C, Veillard NR, Mach F. 2005. Cholesterol-independent effects of statins in inflammation, immunomodulation and atherosclerosis. Curr Drug Targets Cardiovasc Haematol Disord 5:127–134. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Jinnin M, Tamaki K, Sato S. 2011. Altered dynamics of transforming growth factor b (TGF-β) receptors in scleroderma fibroblasts. Ann Rheum Dis 70:384–387. [DOI] [PubMed] [Google Scholar]
- Axelson M, Angelin B, Hillebrant CG, Reihn er E, Einarsson C. 1998. The level of 7-dehydrocholesterol in plasma reflects the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the human liver. Biochim Biophys Acta 1394:153–157. [DOI] [PubMed] [Google Scholar]
- Balajthy A, Somodi S, Petho Z, Peter M, Varga Z, Szabo GP, Paragh G, V ıgh L, Panyi G, Hajdu P. 2016. 7DHC-induced changes of Kv1.3 operation contributes to modified T cell function in Smith-Lemli-Opitz syndrome. Pflugers Arch 468:1403–1418. [DOI] [PubMed] [Google Scholar]
- Basu S, Pathak SK, Chatterjee G, Pathak S, Basu J, Kundu M. 2008. Helicobacter pylori protein HP0175 transactivates epidermal growth factor receptor through TLR4 in gastric epithelial cells. J Biol Chem 283:32369–32376. [DOI] [PubMed] [Google Scholar]
- Batta AK, Tint GS, Shefer S, Abuelo D, Salen G. 1995. Identification of 8-dehydrocholesterol (cholesta-5,8-dien-3 b-ol) in patients with Smith-Lemli-Opitz syndrome. J Lipid Res 36:705–713. [PubMed] [Google Scholar]
- Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ. 2015. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am J Clin Nutr 102:276–294. [DOI] [PubMed] [Google Scholar]
- Bjork K, Svenningsson P. 2011. Modulation of monoamine receptors by adaptor proteins and lipid rafts: Role in some effects of centrally acting drugs and therapeutic agents. Annu Rev Pharmacol Toxicol 51:211–242. [DOI] [PubMed] [Google Scholar]
- Chansrichavala P, Chantharaksri U, Sritara P, Ngaosuwankul N, Chaiyaroj SC. 2010. Atorvastatin affects TLR4 clustering via lipid raft modulation. Int Immunopharmacol 10:892–899. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Huang SS, Huang JS. 2006. Cellular heparan sulfate negatively regulates transforming growth factor-b responsiveness in epithelial cells. J Biol Chem 281:11506–11514. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Liu I-H, Huang SS, Fliesler SJ, Han X, Huang SS, Huang JS. 2007. Cholesterol suppresses cellular TGF-β responsiveness: Implications in atherogenesis. J Cell Sci 120:3509–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-L, Huang SS, Huang JS. 2008. Cholesterol modulates cellular TGF-β responsiveness by altering TGF-β binding to TGF-β receptors. J Cell Physiol 215:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-L, Hou W- H, Liu I-H, Huang SS, Huang JS. 2009. Inhibitors of clarthrin-dependent endocytosis inhibitors enhance TGF-β signaling and responses. J Cell Sci 122:1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-L, Tetri LH, Neuschwander-Tetri B, Huang SS, Huang JS. 2011. A mechanism by which dietary trans fats cause atherosclerosis. J Nutr Biochem 22:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Servi B, La Porta CAM, Bontempelli M, Comolli R. 2002. Decrease of TGF-β1 plasma levels and increase of nitric oxide synthaseactivity in leukocytes as potential biomarkers of Alzheimer’s disease. Exp Gerontol 37:813–821. [DOI] [PubMed] [Google Scholar]
- DeBarber AE, Eroglu Y, Merkens LS, Pappu AS, Steiner RD. 2012. Smith-Lemli-Opitz syndrome. Expert Rev Mol Med 13:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. 2003. Distinct endocytic pathways regulate TGF-β receptor signaling and turnover. Nat Cell Biol 5:410–421. [DOI] [PubMed] [Google Scholar]
- Dore JJ Jr., Yao D, Edens M, Garamszegi N, Sholl EL, Leof EB. 2001. Mechanisms of transforming growth factor-b receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol Biol Cell 12:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH. 2015. Eggs and beyond: Is dietary cholesterol no longer important? Am J Clin Nutr 102:235–236. [DOI] [PubMed] [Google Scholar]
- Frutkin AD, Otsuka G, Stempien-Otero A, Sesti C, Du L, Jaffe M, Dichek HL, Pennington CJ, Edwards DR, Nieves-Cintron M, Minter D, Preusch M, Hu JH, Marie JC, Dichek DA. 2009. TGF-β1 limits plaque growth, stabilizes plaque structure, and prevents aortic dilation in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 29:1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wei Z, Zhou E, Zhang N, Yang Z. 2014. Cyanidin-3-O-b-glucoside inhibits lipopolysaccharide-induced inflammatory response in mouse mastitis model. J Lipid Res 55:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojova A, Brun V, Esposito B, Cottrez F, Gourdy P, Ardouin P, Tedgui A, Mallat Z, Groux H. 2003. Specific abrogation of transforming growth factor-b signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood 102:4052–4058. [DOI] [PubMed] [Google Scholar]
- Gou-F abregas M, Maci a A, Anerillas C, Vaquero M, Jov e M, Jain S, Ribera J, Encinas M. 2016. 7-dehydrocholesterol efficiently supports Ret signaling in a mouse model of Smith-Opitz-Lemli syndrome. Sci Rep 6:28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger DJ. 2004. Transforming growth factor b and atherosclerosis: So far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol 24:399–404. [DOI] [PubMed] [Google Scholar]
- Griffiths WJ, Abdel-Khalik J, Crick PJ, Ogundare M, Shackleton CH, Tuschl K, Kwok MK, Bigger BW, Morris AA, Honda A, Xu L, Porter NA, Bjorkhem I, Clayton PT, Wang Y. 2016. Sterols and oxysterols in plasma from Smith-Lemli-Opitz syndrome patients. J Steroid Biochem Mol Biol pii: S0960–0760(16):30064–30064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. 2004. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–16788S. [DOI] [PubMed] [Google Scholar]
- Huang SS, Huang JS. 2005. TGF-β control of cell proliferation (Review). J Cell Biochem 96:447–462. [DOI] [PubMed] [Google Scholar]
- Huang SS, Huang JS. 2015. Methods for inhibiting TGF-β. United States Patent No.:US 8,946,201 B2 Date of Patent: Feb. 3 2015.
- Huang JS, Huang FW, Johnson FE, Huang SS. 2015. Molecular basis of diets, exercise and lifestyle interventions in atherosclerotic cardiovascular disease In: Lewis BS, Borer JS, Halon DA, editors. In the Proceedings of the 11th International Congress on Coronary Artery Disease. Pianoro (Bologna), Italy: Medimond; pp 49–52. [Google Scholar]
- Huang SS, Chen CL, Huang FW, Johnson FE, Huang JS. 2016a. Ethanol enhances TGF-β activity by recruiting TGF-β receptors from intracellular vesicles/lipid rafts/caveolae to non-lipid raft microdomains. J Cell Biochem 117:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Chen CL, Huang FW, Hou WH, Huang JS. 2016b. DMSO enhances TGF-β activity by recruiting the type II TGF-β receptor from intracellular vesicles to the plasma membrane. J Cell Biochem 117:1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E 2008. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol 9:125–138. [DOI] [PubMed] [Google Scholar]
- Iwata J, Parada C, Chai Y. 2011. The mechanism of TGF-β signaling during palate development. Oral Dis 17:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffr es PA, Gajate C, Bouchet AM, Couthon-Gourv es H, Chantome A, Potier-Cartereau M, Besson P, Bougnoux P, Mollinedo F, Vandier C. 2016. Alkyl ether lipids, ion channels and lipid raft reorganization in cancer therapy. Pharmacol Ther pii: S0163-7258(16):30093–30096. [DOI] [PubMed] [Google Scholar]
- Jira PE, Wevers RA, de Jong J, Rubio-Gozalbo E, Janssen-Zijlstra FS, van Heyst AF, Sengers RC, Smeitink JA. 2000. Simvastatin. A new therapeutic approach for Smith-Lemli-Opitz syndrome. J Lipid Res 41:1339–1346. [PubMed] [Google Scholar]
- Kanter MM, Kris-Etherton PM, Fernandez ML, Vickers KC, Katz DL. 2012. Exploring the factors that affect blood cholesterol and heart disease risk: Is dietary cholesterol as bad for you as history leads us to believe? Adv Nutr 3:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RK, Arnold TP, Fliesler SJ. 2004. Formation of 7-dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith-Lemli-Opitz syndrome. J Lipid Res 45:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Kenworthy AK, Mirnics K. 2009. Molecular consequences of altered neuronal cholesterol biosynthesis. J Neurosci Res 87:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Xu L, Harrison FE, Ahsen R, Hart SE, Folkes OM, Mirnics K, Porter NA. 2014. Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biol Psychiatry 75:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Kim HY, Tallman KA, Liu W, Koczok K, Balogh I, Xu L, Mirnics K, Porter NA. 2016. The effect of small molecules on sterol homeostasis: Measuring 7-dehydrocholesterol in Dhcr7-deficient neuro2a cells and human fibroblasts. J Med Chem 59:1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarova M, Wassif CA, Odom S, Liao K, Porter FD, Rivera J. 2006. Cholesterol deficiency in a mouse model of Smith-Lemli-Opitz syndrome reveals increased mast cell responsiveness. J Exp Med 203:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire-Ewing S, Lagrost L, N eel D. 2012. Lipid rafts: A signalling platform linking lipoprotein metabolism to atherogenesis. Atherosclerosis 221:303–310. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. 2006. Transforming growth factor-b regulation of immune responses. Annu Rev Immunol 24:99–146. [DOI] [PubMed] [Google Scholar]
- Liao JK. 2005. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol 96:24F–33F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. 2001. Inhibition of transforming growth factor-b signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res 89:930–934. [DOI] [PubMed] [Google Scholar]
- Marzilli M 2010. Pleiotropic effects of statins: Evidence for benefits beyond LDL-cholesterol lowering. Am J Cardiovasc Drugs 10(Suppl1):3–9. [DOI] [PubMed] [Google Scholar]
- Massague J, Kelly B. 1986. Internalization of transforming growth factor-band its receptor in BALB/c 3T3 fibroblasts. J Cell Physiol 128:216–222. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, Bush HL, Jr. 1995. Decreased type II/type I TGF-β receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-β1. J Clin Invest 96:2667–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses HL, Roberts AB, Derynck R. 2016. The discovery and early days of TGF-β: A historical perspective. Cold Spring Harb Perspect Biol 8(7):pii: a021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness GC, Zhao Z, Wiggins L. 1994. Insulin and glucagon modulate hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity by affecting immunoreactive protein levels. J Biol Chem 269:29168–29172. [PubMed] [Google Scholar]
- Nowaczyk MJ, Irons MB. 2012. Smith-Lemli-Opitz syndrome: Phenotype, natural history, and epidemiology. Am J Med Gene C Semin Med Genet 160C:250–262. [DOI] [PubMed] [Google Scholar]
- Pickup M, Novitskiy S, Moses HL. 2013. The roles of TGFb in the tumour microenvironment. Nat Rev Cancer 13:788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberg K, Cheng F, Orning C, Crain J, Kupper I, Wiese E, Protschka M, Blessing M, Lackner KJ, Torzewski M. 2012. Overexpression of TGF-ß1 in macrophages reduces and stabilizes atherosclerotic plaques in ApoE-deficient mice. PLoS ONE 7:e40990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Jacob RF, Kaulin Y, Dimuzio P, Xie Y, Mason RP, Tint GS, Steiner RD, Roullet JB, Merkens L, Whitaker-Menezes D, Frank PG, Lisanti MP, Cox RH, Tulenko TN. 2011. Alterations in membrane caveolae and BKCa channel activity in skin fibroblasts in Smith-Lemli-Opitz syndrome. Mol Genet Metab 104:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. 2003. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J Clin Invest 112:1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastîao AM, Assaife-Lopes N, Diogenes MJ, Vaz SH, Ribeiro JA. 2011. Modulation of brain-derived neurotrophic factor (BDNF) actions in the nervous system by adenosine A(2A) receptors and the role of lipid rafts. Biochim Biophys Acta 1808:1340–1349. [DOI] [PubMed] [Google Scholar]
- Sikora DM, Ruggiero M, Petit-Kekel K, Merkens LS, Connor WE, Steiner RD. 2004. Cholesterol supplementation does not improve developmental progress in Smith-Lemli-Opitz syndrome. J Pediatr 144:783–791. [DOI] [PubMed] [Google Scholar]
- Simons K, Ehehalt R. 2002. Cholesterol, lipid rafts, and disease. J Clin Invest 110:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DW, Lemli L, Opitz JM. 1964. A newly recognized syndrome of multiple congenital anomalies. J Pediatr 64:210–217. [DOI] [PubMed] [Google Scholar]
- Sorci-Thomas MG, Thomas MJ. 2016. Microdomains, inflammation, and atherosclerosis. Circ Res 118:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh N, Roberts AB, Birkey Reffey S, Miyazono K, Itoh S, ten Dijke P, Heiss EH, Place AE, Risi, ngsong R, Williams CR, Honda T, Gribble GW, Sporn MB. 2003. Synthetic triterpenoids enhance transforming growth factor b/Smad signaling. Cancer Res 63:1371–1376. [PubMed] [Google Scholar]
- Tashiro H, Shimokawa H, Sadamatu K, Yamamoto K. 2002. Prognostic significance of plasma concentrations of transforming growth factor-b in patients with coronary artery disease. Coron Artery Dis 13:139–143. [DOI] [PubMed] [Google Scholar]
- Tobert JA. 2003. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat Rev Drug Discov 2:517–526. [DOI] [PubMed] [Google Scholar]
- Valencia A, Rajadurai A, Bjorn Carle A, Kochevar IE. 2006. 7-dehydrocholesterol enhances ultraviolet A-induced oxidative stress in keratinocytes: Roles of nadph oxidase, mitochondria and lipid rafts. Free Radic Biol Med 41:1704–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. 2013. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res 98:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassif CA, Kratz L, Sparks SE, Wheeler C, Bianconi S, Gropman A, Calis KA, Kelley RI, Tierney E, Porter FD. 2016. A placebo-controlled trial of simvastatin therapy in Smith-Lemli-Opitz syndrome. Genet Med. DOI: 10.1038/gim.2016.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- Yang N, Huang Y, Jiang J, Frank SJ. 2004. Caveolar and lipid raft localization of the growth hormone receptor and its signaling elements: Impact on growth hormone signaling. J Biol Chem 279:20898–208905. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Khanna R, Nicol GD. 2013. Nerve growth factor/p75 neurotrophin receptor-mediated sensitization of rat sensory neurons depends on membrane cholesterol. Neuroscience 248:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg JM. 2015. Vitamin E: A role in signal transduction. Annu Rev Nutr 35:135–173. [DOI] [PubMed] [Google Scholar]