Abstract
Background
Three-dimensional echocardiography provides a volumetric measurement of global and regional left ventricular (LV) function. It avoids the subjectivity of 2D echocardiography in the assessment of regional wall motion abnormalities (RWMA).
Purpose
Evaluate the feasibility and practicality of 3D echocardiography in the evaluation of ischemic patients with abnormal regional LV contractility.
Methods
The study included 40 patients with ischemic heart disease and RWMA as well as 30 control subjects. They underwent routine clinical examination and conventional 2D echocardiographic assessment. Segments were categorized as; normal, hypokinetic; akinetic or dyskinetic. Three-dimensional echocardiographic images were acquired and later on analyzed offline. Global LV function was semi-automatically calculated by the machine using volumetric measurements. Regional LV function was calculated manually for the 17 LV segments by detecting the end-diastolic (EDD) and end-systolic (ESD) points on the specific segment volume curve and the regional ejection fraction (EF) was calculated by the following formula {(EDDx-ESDx)/EDDx}, where x represents the specific segment. Regional EF was compared between patients and control subjects.
Results
The mean age was 55.0 ± 8.0 and 32.6 ± 8.5 years (P < 0.001) in patients and control groups, respectively. No statistically significant difference in EF between 2D and 3D images (47.3 ± 10.5 vs 48.0 ± 8.0, p = 0.6). There was a good correlation between the 2D-RWMA and 3D-regional EF, and this correlation was consistent in the whole 17 segments.
Conclusion
Three-dimensional echocardiography is an easy, non-invasive and objective tool to detect regional wall motion abnormalities in ischemic patients. It shows comparable results with conventional 2D images with the advantage of quantitative assessment of regional myocardial function.
Keywords: RT3D echocardiography, Wall motion, Abnormalities, Left ventricular regional function
1. Introduction
Three-dimensional echocardiography offers important advantages over conventional two-dimensional echocardiography (2DE) for the determination of global and regional LV function. Assessment of LV volumes and ejection fraction can be accomplished independently of geometrical assumptions which are inherently needed for 2D echocardiography.1, 2, 3, 4
A number of studies have demonstrated that three-dimensional echocardiography (3DE) is more accurate, more reproducible, and more reliable than 2D echocardiography for the quantitation of LV volumes and ejection fraction in LVs with distorted geometry.5, 6, 7, 8 The recent introduction of real-time 3D data acquisition as well as semiautomatic border detection algorithms, have allowed fast, easy, and a more observer-independent quantitation of LV function, requiring only minimal interaction by the investigator.9
The aim of this study is to compare between conventional 2DE and 3DE in the detection of RWMA and evaluation of LV regional and global function.
2. Subjects and methods
2.1. Subjects
This study is an Observational prospective study that enrolled 40 patients with coronary artery disease and 30 healthy control subjects.
2.1.1. Inclusion criteria
Patients presenting with coronary artery disease and their 2D echocardiography shows segmental wall motion abnormalities.
2.1.2. Exclusion criteria
-
1.
Patients with unsatisfactory echocardiographic images.
-
2.
Patients with acute pulmonary edema or cardiogenic shock.
-
3.
Patients with atrial fibrillation.
-
4.
Patients with significant valvular heart disease.
-
5.
Patients with hypertrophic cardiomyopathy (HCM).
-
6.
Patients with congenital heart disease.
-
7.
Patients refusing to be enrolled in the study.
2.2. Methods
On presentation, all included subjects were subjected to the following:
-
•
A consent was taken from recruited subjects.
-
•
Risk factors assessment: prior history of diabetes mellitus (DM), hypertension (HTN), smoking, dyslipidemia or positive family history of premature coronary artery disease.
-
•
Clinical examination: Weight, height, body mass index (BMI).
-
•Two-dimensional echocardiography: Based on the recent American and European guidelines of echocardiography,10 a 2D, M-Mode and Doppler measures were used to obtain the following:
-
1.Chambers dimensions: (LV end diastolic and end systolic dimensions and left atrium).
-
2.Left ventricular ejection fraction (LVEF).
-
3.RWMA assessment based on the 16–Segments model of the LV. Through this model, the culprit coronary artery could be identified.11
-
4.Mitral inflow Doppler pattern: mitral E and A velocities and E/A ratio.
-
5.Tissue Doppler of the lateral mitral annulus E′, A′, S′, E/E′, E′/A′ ratio.
-
6.Regional wall motion score (WMS): assessed for the LV 16-segments as in Table 1. Regional wall motion score index (WMSI) is obtained by diving the regional wall motion score by the number of segments.
-
1.
Table 1.
Assessment of LV wall motion score index.12
| Visual/subjective normal, hypokinetic, akinetic, dyskinetic |
|---|
| Semiquantiative WMS or WMSI 1. = normal 2. = hypokinetic, i.e. reduced endocardial excursion and wall thickening 3. = akinetic, absent endocardial excursion and thickening 4. = dyskinetic, systolic bulging with no thickening WMSI = Total score of segments/Total number of segments. WMS, wall motion score; WMSI, wall motion score index |
2.2.1. Three-dimensional echocardiography
Real-time three-dimensional echocardiography (RT3D) was performed using Philips machine (Epiq-7) equipped with an xMATRIX transducer and data were recorded on CDs and analyzed offline using QLAB, version 10.0.
2.2.1.1. Image acquisition
After having a good image quality and a stable ECG recording, RT3DE images were acquired within a single breath-hold, 4 wedge-shaped sub-volumes were acquired from an apical view to create full-volume data sets. Care was taken to include the entire LV within the 3D scan volume by decreasing the depth and sector width as much as possible to improve the temporal and spatial resolution of the images.13
2.2.1.2. Quantitative analysis by QLAB
Using QLAB version 10.0, 3DQ Advanced software (Philips), a semi-automated border detection biplane LV analysis was performed. For the quantification of LV volumes and function, the longitudinal axes were aligned in the first frame of the loop which corresponds to LV end-diastole, in both the apical four-chamber and two-chamber views. Care was taken in the proper definition of both apical views and orthogonal views to avoid foreshortening. Then, tracing was performed by marking five points: septal, lateral, anterior and inferior on the mitral annulus and the fifth point on the LV apex in either view.13, 14, 15
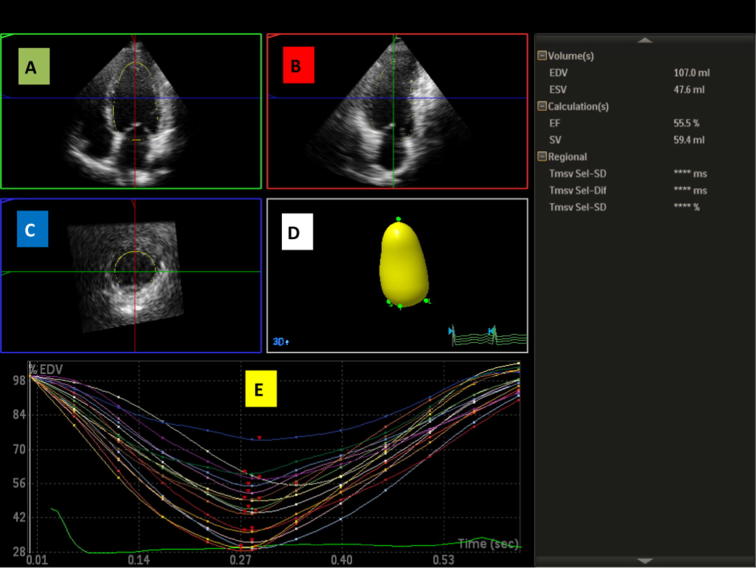
A semi-automated blood–endocardial interface detection algorithm then automatically identified the endocardial border and calculated the LV end-diastolic volume (EDV). Unsatisfactory delineation of the endocardial border was manually adjusted. The end-systole was selected by visually identifying the frame with the smallest LV cavity size just before mitral valve opening where tracing was repeated in the same manner as for the end-diastole to obtain the LV end-systolic volume (ESV). The software automatically detects the endocardial borders in three dimensions, throughout the cardiac cycle and calculates LV volumes and EF13, 14, 15 (Fig. 1). The software automatically draws volume curves for each myocardial segment.
Fig. 1.
Segmental analysis and volume curves of the LV using RT3DE. (A) The apical 4C view and the septal and lateral mitral annulus points are marked. (B) The apical 2C view and the anterior and inferior mitral annulus points are marked. (C) The short axis view. (D) The automatically created LV volume model. (E) The regional time-volume curves. (The image is from one of our patients.)
2.2.1.3. Assessment of regional function
Each regional time-volume curve was analyzed separately. EDV and ESV were detected as the highest and lowest points on the volume curve and regional EF was manually calculated as follows: EDVx − ESVx/EDVx (where x represents the particular myocardial region).
2.2.2. Statistical analysis
The statistical analysis was done using SPSS-17 package. Categorical data were presented as frequency and percentages. Continuous data were presented as a mean and standard deviation. Statistical significance was considered when the P value is < 0.05. Independent sample t-test was used to compare continuous variable measurements taken by 2D and 3D echocardiography in patients and control groups. ANOVA test was used to compare RWMA assessment by 2D and regional EF by 3D echocardiography.
3. Results
The baseline clinical characteristics of the studied subjects are shown in Table 2. The most prevalent risk factors among our patients were smoking (52.5%) followed equally by HTN and DM (47.5%).
Table 2.
Baseline clinical characteristics.
| Variable | Group 1 (mean ± SD) | Group 2 (mean ± SD) | P-value |
|---|---|---|---|
| Age (yrs) | 55.0 ± 8.0 | 32.6 ± 8.5 | <0.001 |
| Male gender, no (%) | 33 (82.5) | 24 (80.0) | 0.8 |
| Weight (kg) | 76.2 ± 9.4 | 68.3 ± 9.5 | 0.001 |
| Height (cm) | 169.2 ± 3.6 | 167.3 ± 28.6 | 0.677 |
| BMI (kg/m2) | 26.6 ± 2.8 | 23 ± 2.8 | <0.001 |
| BSA (m2) | 1.9 ± 0.13 | 1.8 ± 0.26 | 0.010 |
BMI: body mass index.
BSA: body surface area.
The data obtained by 2DE was compared between patients and control groups, as shown in Table 3.
Table 3.
Two-D echocardiographic parameters in patients and control groups.
| Variable | Patients (mean ± SD) | Controls (mean ± SD) | P value |
|---|---|---|---|
| LVEDD (cm) | 5.6 ± 0.6 | 4.6 ± 0.5 | <0.001 |
| LVESD (cm) | 4.4 ± 0.7 | 3.3 ± 0.5 | <0.001 |
| SWT (cm) | 0.92 ± 0.1 | 0.9 ± 0.1 | 0.020 |
| PWT (cm) | 0.95 ± 0.1 | 0.86 ± 0.9 | 0.020 |
| LA (cm) | 3.5 ± 0.5 | 2.8 ± 0.4 | <0.001 |
| AO (cm) | 2.8 ± 0.4 | 2.6 ± 0.3 | 0.032 |
| EF | 47.3 ± 10.5 | 63 ± 5.9 | <0.001 |
| LA VOLUME | 41.4 ± 11.3 | 33.1 ± 9 | 0.002 |
| LA AREA | 18.2 ± 3 | 19.7 ± 27.5 | 0.730 |
| E | 68 ± 21 | 68 ± 19 | 0.987 |
| A | 59.2 ± 16.4 | 49.8 ± 13.6 | 0.014 |
| E/A | 1.3 ± 0.8 | 1.4 ± 0.23 | 0.488 |
| E′ | 8.4 ± 2.9 | 14.9 ± 4.1 | <0.001 |
| A′ | 8.9 ± 2.9 | 10.3 ± 2.7 | 0.039 |
| S′ | 7.0 ± 2.0 | 8.6 ± 2.4 | 0.007 |
| E/E′ | 9.0 ± 4.2 | 4.7 ± 1.5 | <0.001 |
| E′/A′ | 1.0 ± 0.4 | 1.5 ± 0.4 | <0.001 |
| WMSI | 1.53 ± 0.4 | 1.0 ± 0.0 | <0.001 |
LVEDD: left ventricular end diastolic dimension, LVESD: left ventricular end systolic dimension SWT: septal wall thickness, PWT: posterior wall thickness, EF: left ventricular ejection fraction, AO: aortic root dimension, LA: left atrium dimension, WMSI: wall motion score index. Significant p values are marked in bold letters.
Left ventricular volumes and regional EF data taken by RT3DE, are shown in Table 4.
Table 4.
Three-dimensional echocardiographic data in patients and control groups.
| Variable | Patients (mean ± SD) | Controls (mean ± SD) | P value | |
|---|---|---|---|---|
| EDV | 101.8 ± 28.3 | 74.8 ± 16.8 | <0.001 | |
| ESV | 54 ± 19 | 27.6 ± 6.4 | <0.001 | |
| SV | 48.1 ± 14.5 | 46.6 ± 11.8 | 0.632 | |
| EF | 0.48 ± 0.08 | 0.62 ± 0.06 | <0.001 | |
| Segmental EF | ||||
| Anterior | BASAL | 0.50 ± 0.15 | 0.61 ± 0.06 | <0.001 |
| MID | 0.50 ± 0.18 | 0.64 ± 0.10 | <0.001 | |
| APICAL | 0.48 ± 0.17 | 0.69 ± 0.09 | <0.001 | |
| Anteroseptal | BASAL | 0.47 ± 0.15 | 0.60 ± 0.07 | <0.001 |
| MID | 0.44 ± 0.15 | 0.60 ± 0.09 | <0.001 | |
| Inferoseptal | BASAL | 0.45 ± 0.15 | 0.60 ± 0.07 | <0.001 |
| MID | 0.42 ± 0.17 | 0.63 ± 0.08 | <0.001 | |
| Apical septal | 0.44 ± 0.18 | 0.6 0 ± 0.10 | <0.001 | |
| Inferior | BASAL | 0.42 ± 0.13 | 0.63 ± 0.09 | <0.001 |
| MID | 0.41 ± 0.16 | 0.66 ± 0.07 | <0.001 | |
| APICAL | 0.46 ± 0.15 | 0.63 ± 0.09 | <0.001 | |
| Inferolateral | BASAL | 0.45 ± 0.14 | 0.62 ± 0.08 | <0.001 |
| MID | 0.47 ± 0.15 | 0.64 ± 0.09 | <0.001 | |
| Anterolateral | BASAL | 0.50 ± 0.18 | 0.62 ± 0.08 | <0.001 |
| MID | 0.47 ± 0.14 | 0.63 ± 0.09 | <0.001 | |
| Apical lateral | 0.50 ± 0.18 | 0.65 ± 0.08 | <0.001 | |
| APEX | 0.41 ± 0.14 | 0.58 ± 0.11 | <0.001 | |
EDV; end-diastolic volume, ESV; end-systolic volume, SV; stroke volume, EF; ejection fraction. Significant p values are marked in bold letters.
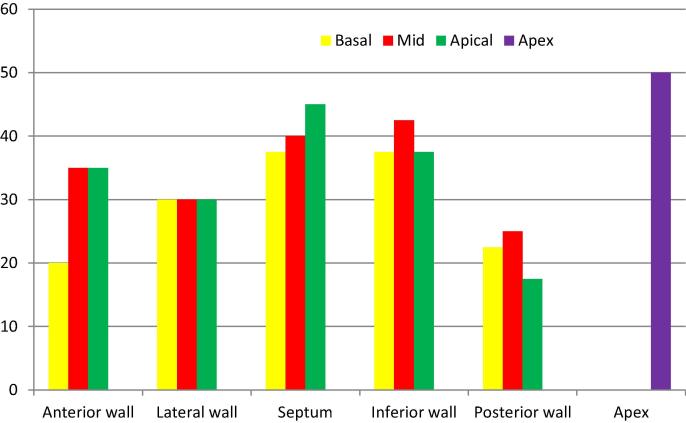
The distribution of abnormal regional wall motion among different myocardial segments is shown in Fig. 2.
Fig. 2.
Percentage of abnormal regional wall motion in different myocardial segments, as detected by 2DE.
Table 5 shows the regional EF as detected by RT3DE as compared to regional wall motion abnormalities detected by 2DE. Data could not be computed for the apical inferior dyskinetic region, as there was only one segment with dyskinesia. As we could see from the table, regional EF of the hypokinetic areas was comparable to that of the akinetic areas. This means that RT3DE could not differentiate between hypokinesia and akinesia.
Table 5.
Comparison between 2DE (RWMA, judged by the operator) and 3DE (segmental EF; mean ± SD).
| Segment | Normal segments EF (m ± SD) |
Hypokinetic segments EF (m ± SD) | Akinetic segments EF (m ± SD) |
Dyskinetic segments EF (m ± SD) | p-Value | Post hoc analysis | |
|---|---|---|---|---|---|---|---|
| Apex | 0.51 ± 0.08 | 0.40 ± 0.12 | 0.28 ± 0.10 | – | <0.001 | 1 and 2 = 0.062 1 and 3 ≤ 0.001 2 and 3 = 0.61 |
|
| Anterior | Basal | 0.60 ± 0.10 | 0.37 ± 0.13 | 0.11 ± 0.10 | – | <0.001 | 1 and 2 ≤ 0.001 1 and 3 ≤ 0.001 2 and 3 = 0.125 |
| Mid | 0.60 ± 0.09 | 0.47 ± 0.14 | 0.25 ± 0.19 | – | <0.001 | 1 and 2 = 0.176 1 and 3 ≤ 0.001 2 and 3 = 0.007 |
|
| Apical | 0.58 ± 0.93 | 0.36 ± 0.12 | 0.24 ± 0.11 | – | <0.001 | 1 and 2 ≤ 0.001 1 and 3 = 0.756 2 and 3 = 1.000 |
|
| Septum | Basal | 0.49 ± 0.11 | 0.38 ± 0.12 | 0.42 ± 0.19 | – | 0.099 | 1 and 2 = 0.126 1 and 3 = 0.756 2 and 3 = 1.000 |
| Mid | 0.48 ± 0.14 | 0.41 ± 0.14 | 0.32 ± 0.13 | – | 0.004 | 1 and 2 = 0.602 1 and 3 = 0.003 2 and 3 = 0.678 |
|
| Apical | 0.51 ± 0.18 | 0.34 ± 0.10 | 0.30 ± 0.15 | – | 0.004 | 1 and 2 = 1.000 1 and 3 = 0.003 2 and 3 = 0.217 |
|
| Inferior | Basal | 0.49 ± 0.06 | 0.35 ± 0.11 | 0.25 ± 0.11 | – | <0.001 | 1 and 2 = 0.001 1 and 3 ≤ 0.001 2 and 3 = 0.083 |
| Mid | 0.49 ± 0.11 | 0.34 ± 0.12 | 0.27 ± 0.18 | <0.001 | 1 and 2 = 0.037 1 and 3 ≤ 0.001 2 and 3 = 0.930 |
||
| Apical | 0.52 ± 0.10 | 0.50 ± 0.20 | 0.28 ± 0.08 | EF = 0.22 | <0.001 | NAa | |
| Posterior | Basal | 0.49 ± 0.11 | 0.40 ± 0.13 | 0.26 ± 0.18 | – | 0.001 | 1 and 2 = 0.615 1 and 3 = 0.001 2 and 3 = 0.211 |
| Mid | 0.50 ± 0.13 | 0.45 ± 0.11 | 0.27 ± 0.13 | – | 0.003 | 1 and 2 = 1.000 1 and 3 = 0.002 2 and 3 = 0.128 |
|
| Lateral | Basal | 0.58 ± 0.13 | 0.37 ± 0.13 | 0.27 ± 0.08 | – | <0.001 | 1 and 2 = 0.015 1 and 3 ≤ 0.001 2 and 3 = 0.557 |
| Mid | 0.51 ± 0.11 | 0.43 ± 0.09 | 0.33 ± 0.18 | – | 0.002 | 1 and 2 = 0.696 1 and 3 = 0.002 2 and 3 = 0.516 |
|
| Apical | 0.55 ± 0.16 | 0.53 ± 0.25 | 0.33 ± 0.13 | – | 0.002 | 1 and 2 = 1.000 1 and 3 = 0.002 2 and 3 = 0.359 |
|
NA; not available.
4. Discussion
Real-Time 3-DE has many advantages over visual 2-DE assessment of RWMA. It is quantitative and it provides rapid image acquisition, requires a lower level of operator skills, and avoids LV foreshortening by correct alignment of imaging planes.16
For each of the LV segments, a time-volume curve is displayed, demonstrating maximum and minimum volumes of that specific segment during the cardiac cycle. Hypo- or non-contractile (akinetic) segments can easily be identified based on the pattern of the curve, while diseased segments have a flattened curve. This approach has been validated using MRI as the standard of Ref. 17.
This study identified a good agreement between RT3DE and conventional 2DE in the assessment of RWMA of most segments. Cainai et al.,18 reported an agreement with the expert visual interpretation of 2DE associated with 91% sensitivity, 80% specificity, and 84% accuracy. The results of previous studies19, 20 evaluating images generated from 3D echocardiography have also demonstrated its ability to identify myocardial ischemia correctly with good agreement to black and white 2DE images.
The results of this study are also consistent with the ones that were found by Corsi et al.17 who studied RT3DE images in 30 patients for detection of LV endocardial surface throughout the cardiac cycle, from which global and regional LV volume (LVV)–time and wall motion (WM)–time curves were obtained. They tested the feasibility of automated detection of RWM abnormalities in each segment; abnormality was detected when regional shortening fraction was below a threshold obtained in normal subjects. The automated detection agreed with the expert interpretation of 2D RWMA in 86% of segments.
Collins21 assessed the feasibility of using RT3D echocardiography to detect RWMA in patients with abnormal LV function. A higher percentage of segments were visualized with 2DE versus RT3D echocardiography (97% vs 83%, respectively, P < 0.001). With the use of the 2-dimensional echocardiographic results as the standard, RT3D echocardiography detected 55 (96%) of 57 regional wall motion abnormalities. An excellent correlation was found between the 2 techniques for assessment of the regional wall motion score index (r = 0.89, P < 0.001). This is consistent with our study that demonstrates the feasibility and potential advantages of RT3D echocardiography for the assessment of regional LV function.
Unlike the previously reported non-real-time three-dimensional methods,22 RT3DE provides a comprehensive evaluation of LV wall motion by completing the entire imaging sequence in a single heartbeat.23, 24
Real-time three-dimensional echocardiography technique is relatively easy to learn and can be mastered quickly by sonographers trained on routine 2DE. The ability to obtain multiple views from a single acquisition makes the technique less demanding. Real-time three-dimensional echocardiography is an evolving technology, which has some limitations in its current form.
5. Conclusion
This study demonstrates that RT3DE is feasible and offers a number of advantages including rapid acquisition and simultaneous visualization of the same segments in different planes. It offers an entirely new approach to the evaluation of patients with coronary artery disease (CAD) and regional wall motion abnormalities.
Evaluation of regional EF generated from RT3DE full volume dataset may correctly identify abnormal regional motion in patients with CAD and it shows non-inferiority to visual assessment by conventional 2DE. Yet, RT3DE could not differentiate between the hypokinetic and the akinetic areas.
Conflicts of interest
The authors declare that none of them had any conflict of interests.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.Lang R.M., Mor-Avi V., Sugeng L., Nieman P.S., Sahn D.J. Three-dimensional echocardiography: the benefits of the additional dimension. J Am Coll Cardiol. 2006;48:2053–2069. doi: 10.1016/j.jacc.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Gopal A.S., King D.L., Keller A.M., Rigling R. Left ventricular volume and endocardial surface area by three-dimensional echocardiography: comparison with two-dimensional echocardiography and nuclear magnetic resonance imaging in normal subjects. J Am Coll Cardiol. 1993;22:258–270. doi: 10.1016/0735-1097(93)90842-o. [DOI] [PubMed] [Google Scholar]
- 3.Siu S.C., Rivera J.M., Guerrero J.L. Three-dimensional echocardiography. In vivo validation for left ventricular volume and function. Circulation. 1993;88:1715–1723. doi: 10.1161/01.cir.88.4.1715. [DOI] [PubMed] [Google Scholar]
- 4.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification. Eur Heart J – Cardiovas Imag. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins C., Bricknell K., Hanekom L. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004;44:878–886. doi: 10.1016/j.jacc.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Gopal A.S., Shen Z., Sapin P.M. Assessment of cardiac function by three-dimensional echocardiography compared with conventional noninvasive methods. Circulation. 1995;92:842–853. doi: 10.1161/01.cir.92.4.842. [DOI] [PubMed] [Google Scholar]
- 7.Qin J.X., Shiota T., McCarthy P.M. Real-time three-dimensional echocardiographic study of left ventricular function after infarct exclusion surgery for ischemic cardiomyopathy. Circulation. 2000;102(suppl 3):100–101. doi: 10.1161/01.cir.102.suppl_3.iii-101. [DOI] [PubMed] [Google Scholar]
- 8.Qin J.X., Jones M., Shiota T. Validation of real-time three-dimensional echocardiography for quantifying left ventricular volumes in the presence of a left ventricular aneurysm: in vitro and in vivo studies. J Am Coll Cardiol. 2000;36:900–907. doi: 10.1016/s0735-1097(00)00793-2. [DOI] [PubMed] [Google Scholar]
- 9.Monaghan M.J. Role of real-time 3D echocardiography in evaluating the left ventricle. Heart. 2006;92(1):131–136. doi: 10.1136/hrt.2004.058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Heller G.V., Cerqueira M.D., Weissman N.J. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol. 2002;9:240–245. doi: 10.1067/mnc.2002.123122. [DOI] [PubMed] [Google Scholar]
- 12.Schiller N.B., Shah P.M., Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Muller S., Bartel T., Katz M.A. Partial cut-off of the left ventricle: determinants and effects on volume parameters assessed by real-time 3-D echocardiography. Ultrasound Med Biol. 2003;29:25–30. doi: 10.1016/s0301-5629(02)00684-1. [DOI] [PubMed] [Google Scholar]
- 15.Amuthan V, Jegadeewaris A. Manual of 3D echocardiography; 2013 [chapter 6].
- 16.Abusaid G.H., Ahmad M. Real time three-dimensional stress echocardiography advantages and limitations. Echocardiography. 2012;29:200–206. doi: 10.1111/j.1540-8175.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 17.Corsi C., Lang R.M., Veronesi F. Volumetric quantification of global and regional left ventricular function from real-time three-dimensional echocardiographic images. Circulation. 2005;112:1161–1170. doi: 10.1161/CIRCULATIONAHA.104.513689. [DOI] [PubMed] [Google Scholar]
- 18.Caiani E.G., Corsi C., Veronesi F. Automated assessment of left ventricular wall motion based on surface detection and color-encoding of real time three-dimensional echocardiographic images. Comput Cardiol. 2006;33:121–124. [Google Scholar]
- 19.Kachenoura N., Delouche A., Dominguez C.R., Nardi O., Frouin F., Diebold B. An automated four-point scale scoring of segmental wall motion in echocardiography using quantified parametric images. Phys Med Biol. 2010;55:5753. doi: 10.1088/0031-9155/55/19/009. [DOI] [PubMed] [Google Scholar]
- 20.Gudmundsson P., Shahgaldi K., Winter R. Parametric quantification of myocardial ischaemia using real-time perfusion adenosine stress echocardiography images, with SPECT as reference method. Clin Physiol Funct Imag. 2010;30:30–42. doi: 10.1111/j.1475-097X.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 21.Collins M., Hsieh A., Ohazama C.J. Assessment of regional wall motion abnormalities with real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 1999;12:7–14. doi: 10.1016/s0894-7317(99)70167-7. [DOI] [PubMed] [Google Scholar]
- 22.Pandian N.G., Roelandt J., Nanda N.C. Dynamic three-dimensional echocardiography. Echocardiography. 1994;11:237–259. doi: 10.1111/j.1540-8175.1994.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 23.Buszman P., Szkróbka I., Tendera Z. Early and late results of percutaneous revascularization in patients with ischemic cardiomyopathy and decreased left ventricular ejection fraction. (Revascularisation in Heart Failure Trial, REHEAT Registry) EuroIntervention. 2005;1:186–192. [PubMed] [Google Scholar]
- 24.Sedlis S.P., Ramanathan K.B., Morrison D.A., Sethi G., Sacks J., Henderson W. Department of Veterans Affairs Cooperative Study# 385, Angina With Extremely Serious Operative Mortality Evaluation (AWESOME) Investigators. Outcome of percutaneous coronary intervention versus coronary bypass grafting for patients with low left ventricular ejection fractions, unstable angina pectoris, and risk factors for adverse outcomes with bypass (the AWESOME Randomized Trial and Registry) Am J Cardiol. 2004;94(1):118–120. doi: 10.1016/j.amjcard.2004.03.041. [DOI] [PubMed] [Google Scholar]