Abstract
Background
Acute kidney injury (AKI) is a serious complication after major surgery, which may lead to increased morbidity and mortality. The aim of this study was to identify cost and determinants of AKI after total joint arthroplasty.
Methods
A retrospective case-controlled study was conducted with 1719 primary elective total hip or knee replacements performed from January 2004 through September 2015 at an urban teaching hospital. Patients who developed AKI were matched in a 1:3 ratio with those in a control group who did not develop AKI based on age, sex, race, operated joint, and comorbidities including hypertension and diabetes. Increased postoperative serum creatinine was considered indicative of AKI.
Results
Fifty-four patients (3.1%) had AKI that was significantly associated with increased length of hospital stay (8.07 days) compared with that of the control group (4.50 days, P < .0001) and incurred significantly higher hospital charges ($224,533) than those of the control group ($142,753, P < .0001). We identified high body mass index, undergoing bilateral surgery in one session, high estimated blood loss, and longer duration of surgery as significant risk factors for AKI in univariate analysis. Elevated preoperative creatinine, large postoperative drop in hemoglobin, and high American Society of Anesthesiologists physical status scores were significant independent predictors of AKI in multivariate analysis.
Conclusions
Health-care providers and patients should work together to manage risk factors and to lower the risk of morbidity and mortality, longer in-hospital stay, and high associated costs of AKI.
Keywords: Total joint arthroplasty, Complications, Acute kidney injury, Outcomes improvement
Introduction
Total joint arthroplasty (TJA) surgery can improve the quality of life for many patients suffering from disabling arthritis of the hip and knee. However, complications after TJA can have a profound impact on patients and health-care systems. Periprosthetic joint infection [1], [2], [3], aseptic loosening, implant failure, wound dehiscence, deep vein thrombosis [4], [5], [6], dislocation of the prosthesis [7], [8], and impaired renal function [9], [10], [11], [12] are among the high-impact complications. Renal impairment after TJA is associated with an increased rate of in-hospital stay and long-term mortality [13], [14]. A significant number of patients who undergo TJA have comorbid conditions such as hypertension (HTN) and diabetes, and the medications used to manage these conditions may impair renal function [6], [15], [16], [17], [18]. Risk factors and costs associated with acute kidney injury (AKI) after TJA have not been investigated before using the latest Kidney Disease: Improving Global Outcomes (KDIGO) criteria recommended by the International Society of Nephrology. The aim of this study is to use the latest AKI criteria to identify modifiable risk factors for renal impairment after TJA. Before an elective primary TJA, health-care providers and patients may work together to manage these factors and to lower the risk and associated costs of AKI thereby reducing morbidity and mortality while enhancing quality of life.
Material and methods
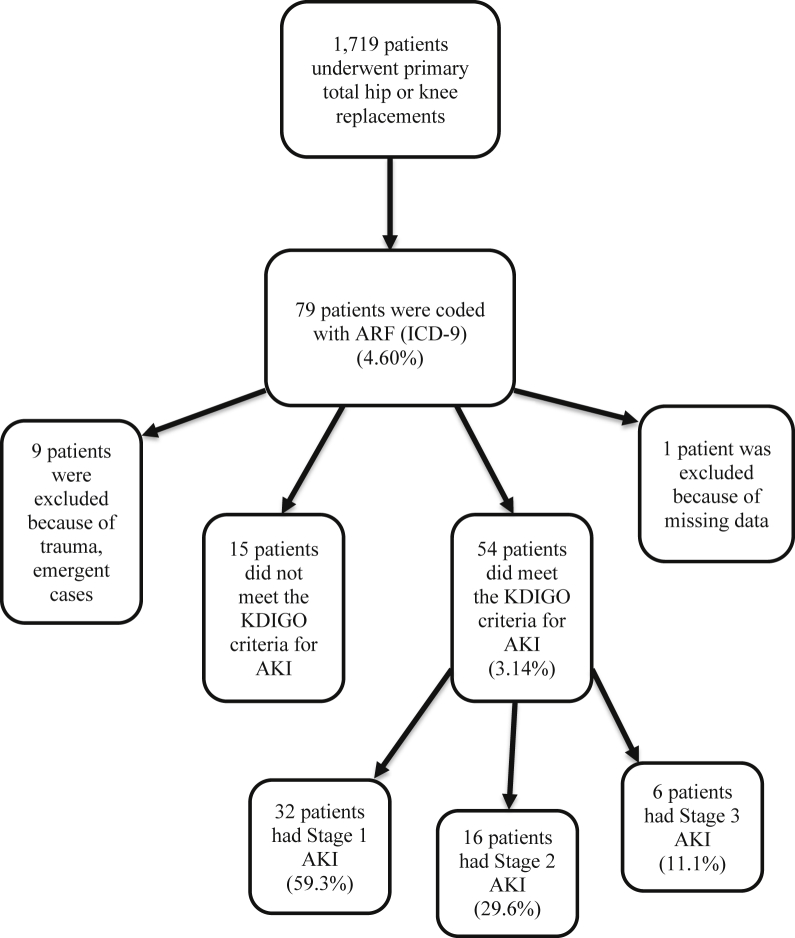
A retrospective case-controlled study approved by the institutional review board committee of our institution was performed at a large urban teaching hospital. All patients who underwent primary total hip or knee joint arthroplasty from January 2004 through September 2015 were screened for developing acute renal failure using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (code 5845 for acute kidney failure with lesion of tubular necrosis and code 5849 for acute kidney failure, unspecified). Demographic and perioperative data were obtained from the institution's computerized database and electronic medical record. A total of 1719 patient charts were screened, and all episodes of renal impairment were investigated. Patients who underwent arthroplasty for fracture, patients with missing data, and patients who did not meet the KDIGO criteria for AKI were excluded (Fig. 1).
Figure 1.
Flow chart showing the process of selecting the patients.
In the literature, there is no single definition for acute renal impairment. We used the staging guidelines for AKI developed by the KDIGO AKI work group [19]. Recently, this classification has been demonstrated to be a good predictor of AKI in hospitalized patients and cardiac patients [20]. In addition, it was found that the criteria for AKI are independently associated with mortality [20], [21]. Stage 1 AKI is defined as a serum creatinine (SCr) increase of 1.5-1.9 times the baseline (preoperative) value within 1 week or a 0.3-mg/dL increase in SCr within 48 hours. Stage 2 AKI is defined as an increase of 2.0-2.9 times the baseline, and stage 3 AKI is 3.0 times the baseline or increase in SCr to greater than or equal to 4.0 mg/dL [22]. All patients who underwent an elective primary TJA and met the criteria for AKI stages 1-3 were included in this study. We then matched the cases that developed AKI to a control group in a 1:3 ratio. The matching criteria were sex, age (within ±3 years), race, operated joint (hip or knee), and comorbidities including HTN and diabetes. We tried to find the closest possible match for each patient from our database.
The perioperative variables investigated for an association with postoperative AKI are listed in Table 1. Preoperative data collected for analysis as potential risk factors for AKI include body mass index (BMI), smoking status, American Society of Anesthesiologists (ASA) physical status score, SCr, hemoglobin (Hb), nonsteroidal anti-inflammatory drug (NSAID) use, angiotensin-converting enzyme inhibitor use, angiotensin receptor blocker use, and diuretic use. We sought to investigate drugs that target kidney function to assess them as potential risk factors for AKI after TJA. We also chose the preoperative variable of SCr as a proxy of kidney function before TJA because it is inversely proportional to kidney glomerular filtration rate. We categorized patients based on low (1,2) and high (3,4) ASA scores. Intraoperative data collected for analysis as potential risk factors for AKI included type of anesthesia, having either unilateral or bilateral surgery in one session, duration of surgery, and estimated blood loss (EBL). Postoperative variables included the lowest Hb value within 1 week and postoperative NSAID use. We used the postoperative SCr value as an outcome measure to determine AKI status. We calculated the difference between preoperative and postoperative Hb levels to analyze the drop in Hb, postoperatively. We also collected data on length of stay and total hospital charges to evaluate the in-hospital and economic burden associated with AKI after TJA.
Table 1.
Variables examined as potential predictors of AKI after TJA.
| Preoperative variables | Intraoperative variables | Postoperative variables |
|---|---|---|
| 1. BMI | 1. Type of anesthesia | 1. Postoperative Hb difference |
| 2. SCr | 2. Unilateral or bilateral | 2. NSAID use |
| 3. Smoking status | 3. Duration of surgery | 3. LOS |
| 4. ASA score | 4. EBL | 4. Hospital charges |
| 5. NSAID use | ||
| 6. ACE-I use | ||
| 7. ARB use | ||
| 8. Diuretic use |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LOS, length of stay.
We analyzed the relationship between potential risk factors and their distribution among the case and control groups using univariate analysis. The means for continuous variables and the frequency distribution for categorical variables are reported. To compare continuous variables between cases and controls, the Student's t test was used; to compare frequency distributions, χ2 analysis was used. Multivariable binary logistic regression analysis was performed, using the enter method to minimize bias, to identify variables that were significant predictors associated with AKI after adjusting for potential confounders. Results were considered statistically significant when the P value was <.05. We used SPSS 24 software for the statistical analysis.
Results
During the study period, 54 episodes of AKI occurred in patients who underwent elective primary TJA. In this group, there were 19 (35.2%) total hip arthroplasties and 35 (64.8%) total knee arthroplasties. There were 32 (59.3%) women and 22 (40.7%) men in this group, with a mean age of 61 years (range, 26-89 years). Forty-two (77.8%) patients were identified as black, 10 (18.5%) patients were identified as white, and 2 (3.7%) patients were identified as other. Within this group, 51 (94.4%) patients had HTN and 21 (38.9%) patients had diabetes. The incidence of AKI under the KDIGO criteria in this group was 3.14%. Of these patients, 32 patients (59.3%) developed stage 1 AKI, 16 patients (29.6%) developed stage 2 AKI, and 6 patients (11.1%) developed stage 3 AKI.
The control group consisted of 162 patients who did not develop postoperative renal damage. There were 94 (58.8%) women and 68 (42.0%) men in this group, with a mean age of 60 years (range, 41-89 years). One hundred twenty-eight (79.0%) patients were identified as black, 30 (18.5%) were identified as white, and 4 (2.5%) were identified as other. Within this group, 155 (95.7%) had HTN and 74 (45.7%) had diabetes.
Univariate analysis of individual preoperative and intraoperative risk factors, postoperative variables, and the corresponding P values are summarized in Table 2. Patients who developed postoperative AKI had a higher BMI, an elevated preoperative SCr, and a high ASA physical status score. Patients undergoing bilateral surgery and those who had a higher EBL were also at increased risk of developing postoperative AKI. Patients who developed AKI also had an increased drop in Hb postoperatively. Univariate analysis also revealed that smoking status, NSAID use, angiotensin-converting enzyme inhibitor use, angiotensin receptor blocker use, diuretic use, and type of anesthesia were not significant predictors of developing AKI.
Table 2.
Univariate analysis of variables examined as potential predictors of AKI after TJA.
| Variable | Group |
Statistics | P value | |
|---|---|---|---|---|
| Case (n = 54) | Control (n = 162) | |||
| Preoperative | ||||
| BMI | Mean = 38.54 | Mean = 35.20 | T = 2.46 | .016 |
| Preoperative SCr | Mean = 1.282 | Mean = 0.985 | T = 3.25 | .002 |
| Smoker | ||||
| Yes | 20 (37.0%) | 42 (25.9%) | χ2 = 2.44 | .118 |
| No | 34 (63.0%) | 120 (74.1%) | ||
| ASA score | ||||
| Low score (1-2) | 9 (16.7%) | 61 (37.7%) | χ2 = 8.14 | .004 |
| High score (3-4) | 45 (83.3%) | 101 (62.3%) | ||
| NSAID use | ||||
| Yes | 27 (50.0%) | 60 (37.0%) | χ2 = 2.83 | .093 |
| No | 27 (50.0%) | 102 (63.0%) | ||
| ACE-I use | ||||
| Yes | 21 (38.9%) | 73 (45.1%) | χ2 = 0.63 | .428 |
| No | 33 (61.1%) | 89 (54.9%) | ||
| ARB use | ||||
| Yes | 14 (25.9%) | 50 (30.9%) | χ2 = 0.47 | .491 |
| No | 40 (74.1%) | 112 (69.1%) | ||
| Diuretic use | ||||
| Yes | 30 (55.6%) | 78 (48.1%) | χ2 = 0.89 | .346 |
| No | 24 (44.4%) | 84 (51.9%) | ||
| Intraoperative | ||||
| Type of anesthesia | ||||
| General | 43 (79.6%) | 128 (79.0%) | χ2 = 0.01 | .92 |
| Spinal | 11 (20.4%) | 34 (21.0%) | ||
| Bilaterality | ||||
| Unilateral | 41 (75.9%) | 145 (89.5%) | χ2 = 6.25 | .012 |
| Bilateral | 13 (24.1%) | 17 (10.5%) | ||
| Duration of surgery | Mean = 118.24 | Mean = 102.57 | T = 3.15 | .002 |
| EBL | Mean = 498.11 | Mean = 340.13 | T = 2.65 | .009 |
| Postoperative | ||||
| Postoperative Hb difference | Mean = −4.37 | Mean = −2.75 | T = −7.22 | <.0001 |
| LOS | Mean = 8.07 | Mean = 4.50 | T = 8.03 | <.0001 |
| Hospital charges | Mean = $224,533.26 | Mean = $142,752.73 | T = 54.74 | <.0001 |
| Postoperative NSAID use | ||||
| Yes | 16 (29.6%) | 37 (22.8%) | ||
| No | 38 (70.4%) | 125 (77.2%) | χ2 = 1.01 | .315 |
Bold values indicate that there is a statistically significant association between the variable and post-operative AKI development (P < .05).
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LOS, length of stay.
Multivariate analysis examining the aforementioned factors that were statistically significant in the univariate analysis is summarized in Table 3. Preoperative creatinine, postoperative Hb difference, and ASA score were significant predictors of AKI. Increased EBL and bilateral surgery were not shown to increase the risk of postoperative AKI. BMI was also not a significant predictor of postoperative AKI in multivariate analysis.
Table 3.
Multivariate analysis of variables examined as potential predictors of AKI after TJA adjusted for confounding variables.
| Variables | Odds ratio | P value | 95% CI |
|---|---|---|---|
| BMI | 1.051 | .098 | 0.99-1.11 |
| Preoperative SCr | 7.23 | .001 | 2.33-22.39 |
| Postoperative Hb difference | 2.59 | <.0001 | 1.84-3.64 |
| EBL | 1.00 | .110 | 1.00-1.00 |
| Bilaterality | 2.12 | .282 | 0.54-8.36 |
| Duration of surgery | 1.01 | .083 | 1.000-1.03 |
| High ASA score | 3.28 | .03 | 1.13-9.51 |
Bold values indicate that there is a statistically significant association between the variable and post-operative AKI development (P < .05).
Postoperative AKI was significantly associated with increased length of hospital stay (8.07 days) compared with that of the control group (4.50 days, P < .0001) and incurred significantly higher hospital charges ($224,533) compared with those of the control group ($142,753, P < .0001).
Discussion
The incidence of AKI in our study was 3.14%. The incidence of AKI is within the range of recent reports, that is, from 0.5% to 6.2% [14], [23], [24], [25]. In this study, each patient who developed AKI was matched to a control group based on age, race, gender, and comorbidities such as HTN and diabetes. This case-controlled model has not been used in the past to identify risk factors for AKI after TJA.
In the univariate analysis, the risk factors for AKI were high BMI, elevated preoperative SCr, high ASA scores, undergoing bilateral surgery in one session, long duration of surgery, increased EBL, and postoperative Hb drop (Table 2). The multivariable model showed that elevated preoperative SCr, wide postoperative Hb difference, and high ASA score were independent risk factors for postoperative AKI (Table 3). These multivariate results are consistent with prior research findings [13], [14], [24], [26]. Patients may have an increased preoperative SCr due to a prerenal, intrarenal, or postrenal problem. Because almost all patients in this study have HTN as a comorbidity and over one-third are diabetic, it is possible that patients with an elevated preoperative SCr are at increased risk for postoperative AKI due to intrarenal damage caused by these nephrotoxic comorbidities. Another explanation is that patients may have general renal hypoperfusion due to longstanding HTN and congestive heart failure, causing decreased kidney glomerular filtration rate and increased preoperative SCr. We recommend health-care providers to take caution before operating patients who have an elevated preoperative SCr and high ASA score to prevent postoperative AKI.
EBL is not a statistically significant predictor of AKI after adjusting for potential confounders in the multivariate analysis. This is most likely because EBL is neither precisely nor accurately measured in the operating room. However, the postoperative Hb difference is a more reliable biomarker of blood loss because it is a more accurately and precisely obtained laboratory value.
Jafari et al. [14] observed BMI to be an independent risk factor for AKI, and our study did not. This could be because our study was underpowered to detect a difference between cases and controls in the multivariate analysis despite observing a significant difference in BMI between the 2 groups in univariate analysis. Another possibility is that obesity is a confounding variable for AKI risk because obesity may cause more blood loss due to a greater dissection of tissues needed to reach the joint. Our multivariable model has shown blood loss to be an independent risk factor for AKI and not obesity, suggesting that obesity could be an indirect risk for AKI due to increased blood loss in these patients.
We were interested in investigating whether smoking or nephrotoxic drug prescriptions were significant risk factors for AKI in this case-controlled model. Because case and control patients were matched on comorbidities and therefore prescribed similar medications (especially for HTN), factors such as nephrotoxic drug use or smoking may not have been significant predictors for AKI in this study design. Prospective, randomized clinical trials can better and more accurately evaluate a causal relation between medications and AKI.
In this study, we were particularly interested in investigating the economic burden of post-TJA AKI, including length of hospital stay and hospital charges. Postoperative AKI was significantly associated with increased length of hospital stay (8.07 days) compared with that of the control group (4.50 days, P < .0001) and incurred significantly higher hospital charges ($224,533) compared with those of the control group ($142,753, P < .0001). Under the Comprehensive Care for Joint Replacement model, which aims to improve the outcome of patients who undergo total hip and knee replacement, hospitals and physicians are responsible for the quality and cost of care delivered to patients from the time of surgery through 90 days after discharge [27]. Instead of the fee-for-service model that was used in the past, the reimbursement of physicians is now based on outcomes and value [20]. A large component of optimizing outcomes and value in TJA resides in patient selection. Patient-related independent risk factors shown to affect postoperative TJA complications should be weighed carefully before proceeding to surgery. Specifically, health-care providers should consider high ASA score and manage elevated preoperative Cr before surgery and minimize intraoperative blood loss to avoid the high charges and in-hospital stay associated with postoperative AKI.
This study does have some limitations. The retrospective nature of this study did not allow us to validate data entry in the medical records. Another limitation is that we identified our cases by checking the renal failure codes, which means we may have missed some renal failures that might not have been coded at all. This also means it is possible our incidence of AKI was slightly below its true value in this patient population. Future studies may include implementing a multiinstitutional study with a larger sample size and investigating more preoperative and intraoperative potential risk factors such as congestive heart failure, diabetic nephropathy, use of cement, and use of antibiotic cement.
Conclusions
This study used a novel case-controlled design with matching criteria of age, sex, race, and comorbidities such as HTN and diabetes. We determined that elevated preoperative creatinine, large postoperative drop in Hb, and high ASA physical status scores were significant independent predictors of AKI after primary TJA. Health-care providers and patients should work together to manage those at risk for post-TJA AKI (elevated preoperative Cr and elevated ASA) and work on the one controllable finding (blood loss) to lower the risk of morbidity and mortality, longer in-hospital stay, and high associated charges of AKI.
Acknowledgments
The authors would like to thank the Drexel University Medical Student Summer Research Fellowship (MSSRF) program for support of this project as well as the attending physicians and residents of the orthopedic surgery department. We also extend our gratitude to Dr. Edward Gracely of the Dornsife School of Public Health for his guidance in performing the statistical analysis.
Footnotes
No author associated with this article has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2018.05.002.
Appendix A. Supplementary data
References
- 1.Lindeque B., Hartman Z., Noshchenko A., Cruse M. Infection after primary total hip arthroplasty. Orthopedics. 2014;37(4):257. doi: 10.3928/01477447-20140401-08. [DOI] [PubMed] [Google Scholar]
- 2.Kane P., Chen C., Post Z., Radcliff K., Orozco F., Ong A. Seasonality of infection rates after total joint arthroplasty. Orthopedics. 2014;37(2):e182. doi: 10.3928/01477447-20140124-23. [DOI] [PubMed] [Google Scholar]
- 3.Delaunay C., Hamadouche M., Girard J., Duhamel A., So F.G. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12):3863. doi: 10.1007/s11999-013-2935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantilla C.B., Horlocker T.T., Schroeder D.R., Berry D.J., Brown D.L. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Bruce W., Van der Wall H., Peters M., Liaw Y., Morgan L., Storey G. Occurrence of pulmonary thromboembolism immediately after arthroplasty. Nucl Med Commun. 2001;22(11):1237. doi: 10.1097/00006231-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Parvizi J., Mui A., Purtill J.J., Sharkey P.F., Hozack W.J., Rothman R.H. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27. doi: 10.2106/JBJS.E.01443. [DOI] [PubMed] [Google Scholar]
- 7.Heithoff B.E., Callaghan J.J., Goetz D.D., Sullivan P.M., Pedersen D.R., Johnston R.C. Dislocation after total hip arthroplasty: a single surgeon's experience. Orthop Clin North Am. 2001;32(4):587. doi: 10.1016/s0030-5898(05)70229-7. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen C.C., Kjaersgaard-Andersen P., Solgaard S., Kehlet H., Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group Hip dislocations after 2,734 elective unilateral fast-track total hip arthroplasties: incidence, circumstances and predisposing factors. Arch Orthop Trauma Surg. 2014;134(11):1615. doi: 10.1007/s00402-014-2051-3. [DOI] [PubMed] [Google Scholar]
- 9.Aveline C., Leroux A., Vautier P., Cognet F., Le Hetet H., Bonnet F. Risk factors for renal dysfunction after total hip arthroplastyAnn Fr Anesth Reanim. 2009;28(9):728. doi: 10.1016/j.annfar.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 10.Nergelius G., Vinge E., Grubb A., Lidgren L. Renal impairment after hip or knee arthroplasty. Urinary excretion of protein markers studied in 59 patients. Acta Orthop Scand. 1997;68(1):34. doi: 10.3109/17453679709003972. [DOI] [PubMed] [Google Scholar]
- 11.Abelha F.J., Botelho M., Fernandes V., Barros H. Determinants of postoperative acute kidney injury. Crit Care. 2009;13(3):R79. doi: 10.1186/cc7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lainiala O., Reito A., Jamsa P., Eskelinen A. Mild or moderate renal insufficiency does not increase circulating levels of cobalt and chromium in patients with metal-on-metal hip arthroplasty. Bone Joint J. 2017;99-B(9):1147. doi: 10.1302/0301-620X.99B9.BJJ-2016-0773.R2. [DOI] [PubMed] [Google Scholar]
- 13.Jamsa P., Jamsen E., Lyytikainen L.P., Kalliovalkama J., Eskelinen A., Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop. 2017;88(4):370. doi: 10.1080/17453674.2017.1301743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafari S.M., Huang R., Joshi A., Parvizi J., Hozack W.J. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty. 2010;25(6 Suppl):49. doi: 10.1016/j.arth.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Hawker G.A., Guan J., Croxford R. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212. doi: 10.1002/art.22146. [DOI] [PubMed] [Google Scholar]
- 16.Namba R.S., Paxton L., Fithian D.C., Stone M.L. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 Suppl 3):46. doi: 10.1016/j.arth.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Pulido L., Parvizi J., Macgibeny M. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139. doi: 10.1016/j.arth.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Weaver F., Hynes D., Hopkinson W. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693. doi: 10.1016/s0883-5403(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 19.Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1. [Google Scholar]
- 20.Halawi M.J., Greene K., Barsoum W.K. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop (Belle Mead NJ) 2016;45(3):E112. [PubMed] [Google Scholar]
- 21.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 22.Shinjo H., Sato W., Imai E. Comparison of kidney disease: improving global outcomes and acute kidney injury network criteria for assessing patients in intensive care units. Clin Exp Nephrol. 2014;18(5):737. doi: 10.1007/s10157-013-0915-4. [DOI] [PubMed] [Google Scholar]
- 23.Perregaard H., Damholt M.B., Solgaard S., Petersen M.B. Renal function after elective total hip replacement. Acta Orthop. 2016;87(3):235. doi: 10.3109/17453674.2016.1155130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowicka A., Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth. 2016;34:520. doi: 10.1016/j.jclinane.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Warth L.C., Noiseux N.O., Hogue M.H., Klaassen A.L., Liu S.S., Callaghan J.J. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty. 2016;31(1):253. doi: 10.1016/j.arth.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Hassan B., Sahlstrom A., Dessau R. Risk factors for renal dysfunction after total knee joint replacement. Acta Orthop Belg. 2015;81(4):647. [PubMed] [Google Scholar]
- 27.2016. Comprehensive Care for Joint Replacement Model | Center for Medicare & Medicaid Innovation. https://innovation.cms.gov/initiatives/cjr; [accessed 05.11.17] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.