Abstract
Objectives
The ability to determine the severity of renal fibrosis, which is involved in most chronic kidney diseases, may be beneficial for monitoring disease progression and management. The aim of this study was to assess a new method involving gold nanoparticles conjugated to an anti-collagen-I antibody (Co-I-AuNPs) as a CT imaging contrast for evaluation of renal fibrosis in situ.
Materials and Methods
Co-I-AuNPs were prepared using gold chloride reduction with sodium citrate, coated with polyethylene glycol (PEG), and their size determined by electron microscopy and nanoparticle tracking analysis. Anti-collagen-I antibody was then conjugated to PEG-SH/COOH on the AuNPs surface. The success of antibody conjugation was tested in vitro using collagen coated plate and mouse stenotic kidney sections, and in vivo using micro-CT and MDCT imaging.
Results
Bare AuNPs were 18.7±0.6 nm and PEG-coated AuNPs 45.3±0.1 nm in size. In vitro, Co-I-AuNPs specifically bound to both a collagen-coated plate and mouse fibrotic kidneys. Furthermore, the stenotic mouse kidney showed increased Co-I-AuNPs retention compared to the contralateral kidney (59.3±5.1 vs 45.1±1.7 HU, p=0.05), which correlated with its collagen deposition. Micro-CT also detected gold signals in situ in the Co-I-AuNPs injected kidney, which co-localized with histological trichrome staining.
Conclusion
Co-I-AuNPs are able to visualize kidney fibrosis in vitro and in situ, and may be useful for nondestructive quantification of tissue fibrosis.
Keywords: Gold nanoparticles, Computer tomography, Kidney, Fibrosis
Introduction
Renal fibrosis is a common pathway of tissue remodeling involved in most chronic kidney diseases(1), regardless of their etiology. We have previously shown that fibrosis plays a pivotal role in ischemic renal injury induced by renal artery stenosis (RAS) in mice and pigs(2, 3). The ability to determine the degrees of renal fibrosis may be beneficial for monitoring disease progression and management. Currently, kidney biopsy is the reference standard for fibrosis evaluation, but being an invasive procedure, its application is limited. Thus, developing non-invasive imaging modalities for kidney fibrosis evaluation is greatly needed.
Among the imaging techniques currently being utilized for evaluating in renal fibrosis, MR imaging(4) with or without gadolinium enhancement shows considerable potential. Yet, computed tomography (CT) provides better temporal and spatial resolution, as well as quantitative power for contrast agents with high atomic numbers. However, identification of renal fibrosis with non-contrast CT remains challenging. Furthermore, gadolinium or iodine administration for MR and CT contrast may not be feasible for all subjects, and introduce a risk of contrast-induced nephropathy(5). Notably, CT offers prominent advantages in terms of speed of image acquisition, relative availability, spatial resolution, and tissue penetration.
Conventional CT contrast agent formulations have charged relatively little over the last 60 years. Thus, there is a need to develop new metal-based agents for X-ray imaging. Gold nanoparticles (AuNP) have been an attractive candidate due to their superior X-ray attenuation properties(6). Polyethylene glycol (PEG) coating prevents bare AuNP aggregation and makes them stable as a contrast agent for CT imaging(7). AuNPs are safe, small, and readily excreted by the kidneys with little retention in the liver and spleen. Antibody-conjugated AuNPs have been used by electron microscopy and as a targeted contrast agent for detecting tumors by CT imaging(8).
Collagen-I is one of the major components of pathological fibrosis(9), and selectively directing AuNP at Collagen-I may be useful for identification of injured kidneys. In this study, we describe the production and evaluation of AuNPs conjugated with an anti-collagen-I antibody (Co-I-AuNPs), and hypothesized that these can be used as CT imaging contrast to detect renal fibrosis.
Methods
All animal protocols were approved by the Mayo Clinic Institute Animal Care and Use Committee. A total of 44 mice were used for this study. RAS was induced in mice by surgical placement of a 0.15 mm diameter arterial cuff, whereas sham surgeries without placement of a cuff were performed in the control group, as we previously described(10). AuNPs were coated with PEG, conjugated with anti-collagen I antibody, and their size determined with an electron microscope and nanoparticle tracking analysis (NTA). The success of antibody conjugation was tested in vitro using a collagen-coated plate and sections of stenotic mouse kidneys (2 weeks after RAS). Subsequently, Co-I-AuNPs were intra-arterially injected in mice with unilateral RAS. Four hours later the mice were scanned with multidetector computed tomography (MDCT) to evaluate renal fibrosis in vivo, and then ex vivo using micro-CT and trichrome staining.
1. AuNPs synthesis, characterization, and antibody conjugation
AuNPs were prepared following previously published methods(11, 12) with slight modifications. To prepare 50mg AuNPs in solution, 250 mL of nanopure water were boiled in a 500 mL flask with high speed stirring. Then 1 mL of 5% gold chloride solution (99.99% purity), which contains 50mg gold metal (Salt Lake Metals, UT) was added and boiled for 3 more min, then 2.5 mL 5% sodium citrate dihydrate (Sigma) was added, boiled for 30min, and allowed to cool at room temperature. Pegylation of AuNPs was then performed to prevent aggregation and provide sites for antibody conjugation. For pegylation, mPEG-SH and HS-PEG-COOH (Creative PEGWorks, Chapel Hill, NC) were added to reach 100:1 and 50:1 molecular ratio to gold, and incubated for 1h with slow stirring at room temperature. The PEG-coated AuNP solution was transferred to 50 ml Falcon tubes and centrifuged at 10000 g for 1 hour. Supernatants were carefully removed, and the AuNP pallets re-suspended in 20 ml nanopure water, and centrifuged at 10000 g for 1 hour. After 3 centrifugation-resuspension cycles, phosphate-buffered saline (PBS) was added into AuNP pallets to reach a final volume of 2.5 ml. The final gold concentration was 20 mg/ml, based on 50 mg gold initially added for processing.
AuNPs were characterized using UV-Vis spectroscopy (Nanodrop 2000c, Thermo Scientific) for the specific peak absorbance at 525nm wavelength, and their optical density (OD) recorded. To determine their size, 200 μl (20mg/ml) AuNPs were subjected to transmission electron microscopy analysis (JEOL 1400 TEM, Peabody, MA). Furthermore, the size distribution of AuNPs after coating was assessed by NTA (NanoSight NS300, NanoSight, Amesbury, UK). AuNPs samples were diluted with PBS at a range of concentrations between 4×108 and 8×108 particles per mL in a total volume of 1 mL. Each sample was continuously run through a flow-cell top-plate set up to 20.0°C using a syringe pump (25 μL/min). At least three videos of 120 seconds documenting Brownian motion of nanoparticles were recorded and at least 1000 of completed tracks analyzed by the NanoSight software (NTA 2.3.5).
To conjugate anti-collagen-I antibody to PEG-AuNPs, 1ml of PEG-AuNPs (20mg/ml) were mixed with 1ml of freshly made 1-ethyl-3-(3-(dimethylaminopropyl)-carbodiimide (12.5mg EDC, Life Technologies)/N-hydroxysulfosuccinimide (24mg NHS, Sigma-Aldrich) EDC/NHS mix, and incubated for 30 min at room temperature. Then, 10 mL of PBS with 0.05% Tween® 20 (PBST) were added and vortexed thoroughly, and spun down by centrifugation (6,500 g for 30 min). Most of the supernatant was removed, and 1ml of collagen-I antibody (Abcam, rabbit polyclonal, 1 mg/mL in PBS) added. The solution was then sonicated in a water bath sonicator for 10 sec, incubated for 4 hours at room temperature with mixing, 10 mL of PBST added and vortexed, and spun down by centrifugation at (3,500 g for 30 min). The supernatant was removed, 1ml PBS with 1% bovine serum albumin (BSA) added to keep its original concentration (20 mg/ml), and the solution was stored at 4ºC ready to use. Furthermore, fluorescence labeled (APC)-Co-I-AuNPs were also made for an organ distribution study using the same protocol, except that collagen-I antibody was pre-conjugated with APC using an APC conjugation kit (Abcam ab201807), following the provided protocol.
2. Collagen binding tests of Co-I-AuNPs
The rationale for tests is summarized in Figure 1D. We used an anti-rabbit secondary antibody to visualize Co-I-AuNPs bound to collagen in a plate or the kidney tissue, while mouse collagen-I and anti-mouse secondary antibody served as positive control in kidney tissue. A 24-well plate was coated with soluble collagen (50 μg/ml) and blocked with 1% BSA, then incubated with collagen I antibody (3.5 μl per well), Co-I-AuNPs (100 μl per well), or unconjugated AuNPs (100 μl/well) for 1h. After washing with PBS 3 times using a plate washer (Elx 50 Auto strip washer, Bio-TEK Instruments), it was incubated with FITC secondary antibody (1:5000) for 1h, and washed 3 times with PBS. The plate was then detected in a plate reader at 490nm wavelength. To evaluate the capability of Co-I-AuNPs in detecting RAS-induced kidney fibrosis, frozen sections of both the stenotic and contralateral kidneys were incubated with Co-I-AuNPs or a mouse anti-collagen I antibody (1:100, Abcam) overnight, washed with PBS, and then incubated with anti-rabbit or anti-mouse secondary antibody for 1h at room temperature. Images were taken with a Zeiss fluorescence microscope (20x).
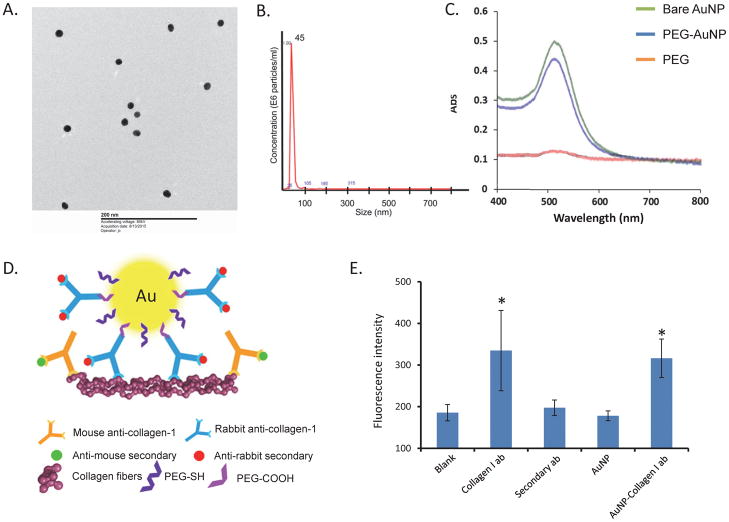
Figure 1.
A. Electron microscope image showing AuNPs of approximately 20nm in size. B. PEG-coated AuNPs measured 45nm in size with nanoparticle tracking analysis. C. UV-Vis spectroscopy showing that peak absorbance of both bare and PEG-coated AuNPs at 525nm. D. A schematic of the experimental approach for staining. An anti-rabbit secondary antibody was used to probe collagen-I-AuNPs collagen in plate or kidney tissue, while mouse collagen I and anti-mouse secondary antibody served as positive controls in kidney tissue. E. Co-I-AuNPs reached signal similar to the same concentration of collagen-I antibody on a collagen coated plate. *p<0.05 vs. Blank
3. AuNPs organ distribution and short-term safety
To investigate the in vivo distribution of Co-I-AuNPs, APC-Co-I-AuNPs (200 μl, 200 mg/kg) were injected through the left carotid artery in mice with unilateral RAS. After 2, 4, or 24 hours (n=5 each), both the stenotic and contralateral kidneys, spleen, liver, heart, and lung were collected, homogenized in 300 μl PBS, and transferred to a 96-well plate to measure APC signals with a fluorescence plate reader. Two hundred μl APC-Co-I-AuNPs were included in the plate and served as injected APC signals. Organ distributions were calculated from organ fluorescence intensity divided by the total fluorescence intensity injected. The kidneys were also homogenized for a hydroxyproline assay to quantify collagen products, and the correlation between kidney APC fluorescence and collagen levels calculated. Blood samples were collected from the left ventricle 24 h after AuNPs injection (n=3) and control mice (n=3) for hematology studies on the Abaxis VetScan HM5 Analyzer, and for chemistry on the Piccolo Xpress Chemistry analyzer.
4. Detection of AuNP in vivo
For a proof of concept, to test the capability of Co-I-AuNPs in detecting fibrosis in vivo, mice with RAS were injected with 200 mg/kg Co-I-AuNPs or unconjugated AuNP (n=3 each), and 2 hours later placed at the center of an annular solid water phantom (Siemens Healthcare, Germany) 20 cm in diameter. They were then scanned by the A-subsystem of a dual-source scanner (SOMATOM CounT, Siemens Healthcare) with a spiral head protocol with 0.35 pitch, 140 kV, 550 mAs, 128×0.6 mm collimation, and 124.5 mGy CTDIvol. All images were reconstructed using the weighted filtered back-projection algorithm, with a medium smooth kernel (D30), the smallest available field-of-view (50mm × 50mm), and the thinnest available slice thickness (0.6 mm)(13). All images were processed on a CT workstation (Syngo.via, Siemens Healthcare).
6. Micro-CT imaging
Given the high resolution of micro-CT, spatial distributions of AuNPs within single kidneys were studied in mice with RAS. Mice were injected with 200 mg/kg Co-I-AuNPs or unconjugated AuNPs (n=3 each). After 2, 4, and 24 hours injection, kidneys were harvested and processed for micro-CT scan, as previously described(3). After scanning, renal fibrosis was histologically evaluated by trichrome staining. Micro-CT and histological images from the same kidney were co-registered using anatomic landmarks and compared side-by-side for AuNPs signals and tissue fibrosis.
Statistics
Results were expressed as mean ±SEM. Statistical analysis was performed using JMP version 13.0 (SAS Institute, Cary, NC). Comparison between 2 groups was performed using Student’s t-test.
Results
Gold chloride/sodium citrate ratio in our study created bare AuNPs 18.7±0.6 nm in size, which were detected in electron microscope images (Figure 1A), whereas NTA showed PEG-coated AuNPs to be 45.3±0.1 nm in size (Figure 1B), and Co-I-AuNPs 62.5±6.8 nm. UV-Vis spectroscopy showed that both bare and PEG-coated AuNPs had peak gold absorbance at 525nm (Figure 1C), the typical wavelength for gold(12). Fluorescence plate reader data showed that Co-I-AuNPs produced a signal comparable to that of a similar concentration of collagen-I antibody on a collagen coated plate, indicating successful collagen-I conjugation to the AuNP surface (Figure 1E).
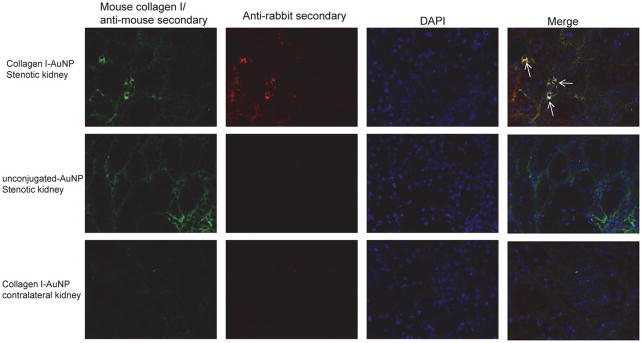
We then tested whether Co-I-AuNPs can be used to detect renal fibrosis in excised kidneys. Immunohistological staining showed that rabbit Co-I-AuNPs in the stenotic kidney (red) co-stained with a mouse anti-collagen-I antibody (green) (Figure 2B), suggesting detection of renal fibrosis. Contrarily, unconjugated AuNPs in the stenotic kidney or Co-I-AuNPs in the contralateral kidney did not produce a red signal.
Figure 2.
Immunohistological staining showing rabbit Co-I-AuNPs detecting fibrosis in the stenotic kidney (red), co-staining with a mouse anti-collagen I antibody (green). The co-localization (yellow) suggested Co-I-AuNPs retention in renal fibrosis areas.
APC signals were higher in the liver, spleen, and stenotic kidneys than in other organs, 2, 4, and 24 hours after APC-collagen-I-AuNPs injection (Figure 3A). Furthermore, AuNPs fluorescence (stenotic/contralateral ratio) modestly but significantly correlated with the amount of renal collagen quantified by hydroxyproline levels in the stenotic kidneys (Figure 3B).
Figure 3.
A. Organ distribution pattern of collagen-I-AuNPs. APC signals were higher in the liver, spleen, and stenotic kidney than in other organs, 2, 4, and 24 hours after injection of APC-collagen-I-AuNPs. B. AuNPs fluorescence (stenotic/contralateral ratio) was significantly correlated with hydroxyproline levels in the stenotic kidneys. C-D. MDCT images showed that the stenotic kidney was smaller and brighter than the contralateral kidney 2hrs after injection of Co-I-AuNPs (C), but not of unconjugated AuNPs (D).
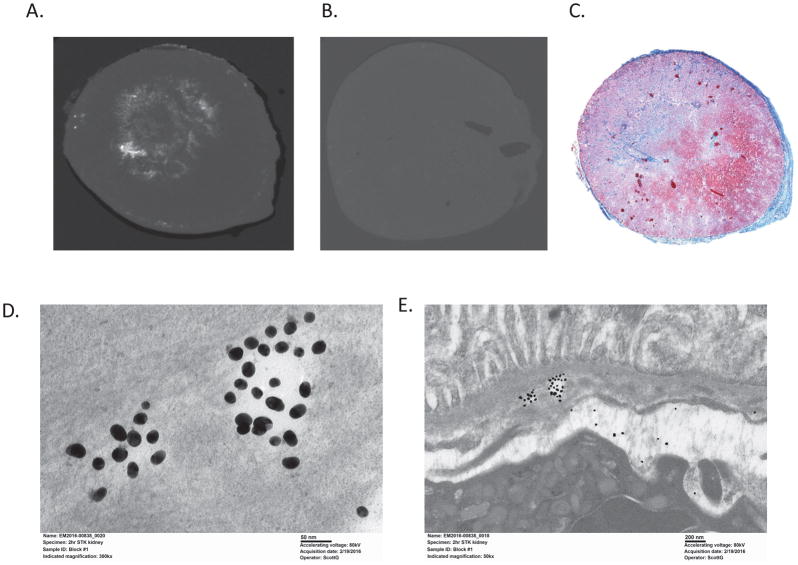
In vivo, MDCT images acquired 2 hrs after Co-I-AuNPs injection showed the stenotic kidney to be smaller and brighter than the contralateral kidney (59.3±5.1 vs. 45.1±1.7 HU, p=0.05) (Figure 3C), while the stenotic kidney receiving unconjugated AuNPs had similar intensity compared to the contralateral kidney (48.4±5.9 vs. 45.9±5.8 HU) (Figure 3D). Ex vivo, micro-CT detected gold signals in stenotic kidneys of Co-I-AuNPs-injected mice (white) at locations similar to collagen-positive stained (trichrome) regions in histological sections processed from the same kidney (Figures 4A–C). Furthermore, electron microscopy images confirmed AuNPs retention within the stenotic kidney (Figures 4D and E).
Figure 4.
Micro-CT images showed that gold signals in stenotic kidneys of anti-Co-I-AuNPs injected mice (A) were located in regions similar to collagen staining (C, trichrome) in histological slice subsequently processed from the same kidney, while unconjugated AuNPs were undetectable by micro-CT (B). C and D. Electron microscopy images showing AuNPs retention in the stenotic kidney (300kx and 50kx magnifications, respectively).
There were no significant changes in mouse hematology (red blood cells, hemoglobin, hematocrit, white blood cells, and platelets), electrolyte levels (Na+, k+, TCO2, Cl−, and Ca++), liver function (ALT, AST, bilirubin, albumin), and renal function (BUN and creatinine) after AuNPs injection in mice after 24 hours (Table 1), indicating relative short-term safety of AuNPs at the current dose. Interestingly, alkaline phosphatase (ALP) levels were lower in mice injected with AuNPs, but remained within the normal range for rodents. (http://cal.vet.upenn.edu/projects/ssclinic/refdesk/rodentrr.htm).
Table 1.
Blood biochemistry results of mice 24h after sham (saline) or collagen I antibody conjugated AuNP injection.
| Sham | AuNPs | |
|---|---|---|
| Red blood cells (1012/l) | 9.9±0.2 | 10.1±0.2 |
| Hemoglobin (g/dl) | 13.9±0.2 | 14.4±0.0 |
| Hematocrit (%) | 41.9±0.3 | 43.3±1.0 |
| White blood cells (109/l) | 10.1±0.8 | 9.6±0.4 |
| Platelets (109/l) | 656.0±34.0 | 687±15.0 |
| Na+ (mmol/l) | 143.0±1.0 | 132.5±8.5 |
| K+ (mmol/l) | 6.5±1.6 | 4.75±0.2 |
| TCO2 (mmol/l) | 21.5±1.5 | 22±1.0 |
| Cl− (mmol/l) | 106.0±0.0 | 99.5±6.5 |
| Ca++ (mg/dl) | 9.2±0.1 | 8.8±0.7 |
| BUN (mg/dl) | 39.5±11.5 | 24±3.0 |
| Creatinine (mg/dl) | 0.4±0.2 | 0.2±0.0 |
| ALP (U/L) | 49.0±3.0 | 26±4.0* |
| ALT (U/L) | 33.5±3.5 | 24.5±3.5 |
| AST (U/L) | 37.0±1.0 | 41±1.0 |
| Bilirubin (mg/dl) | 0.3±0.0 | 0.3±0.0 |
| Albumin (g/dl) | 2.0±0.1 | 1.6±0.1 |
| Total protein (g/dl) | 5.0±0.2 | 4.6±0.4 |
p<0.05 vs Sham, BUN blood urea nitrogen, ALT alanine transaminase, AST aspartate aminotransferase, ALP alkaline phosphatase,
Discussion
This study showed that the collagen-I-AuNPs that were produced specifically bind to collagen-I in both a collagen-coated plate in vitro as well as fibrotic murine kidneys ex vivo. Furthermore, the stenotic kidney showed increased Co I-AuNPs retention, which correlated with its collagen levels, and micro CT images demonstrated gold signals in situ in the collagen-I-AuNPs injected kidney colocalizing with subsequently trichrome-stained kidneys. Thus, we developed collagen-I-AuNPs which are able to visualize kidney fibrosis in vitro and in vivo.
The major components of fibrotic tissue belong to the collagen family of extracellular matrix proteins. Molecular imaging techniques that target collagen fibers, therefore, constitute a useful approach for fibrosis evaluation. Current widely used molecular imaging techniques include optical imaging, which uses fluorescence and bioluminescence probes to detect target molecules(14), but does not provide tomographic anatomic images and is limited to small animals. Single-photon emission computed tomography (SPECT) and positron emission tomography (PET) can be used for molecular imaging of scar tissue(15) with better dose sensitivity, and hybrid systems can complement the sensitivity of PET/SPECT with the anatomy and spatial resolution of CT or MRI, but the requirement for radioactive reagents limit their applications. Importantly, surface functionalization of AuNPs for active targeting, like the PEG coating and collagen antibody conjugation in our study, could enable a transformational shift in CT from an anatomic imaging modality to a combined high-resolution anatomic and molecular imaging modality.
Gold exhibits a relatively high X-ray attenuation coefficient and a longer vascular retention time compared with iodine; it absorbs more photons than iodine (3× at 100 keV), enabling higher contrast at lower X-ray dose and higher resolution(16). Furthermore, AuNPs provide greater X-ray attenuation compared with iodine at low (40–60 kVp) and high (100–140 kVp) tube potentials(17), which are clinically relevant ranges for diagnostic CT. Another significant advantage of AuNPs compared with other nanoparticles is their facile molecular surface functionalization to promote colloidal stability and enable active targeting(18). PEGylation increases the blood half-life of AuNPs in vivo(19), which enable longer circulation time in the bloodstream and accumulation at the site of interest. Thiol ligands have been most widely used due to their strong covalent bonding with gold, and strong sites for bispecific peptide bonding(20). These features enable production of collagen I antibody conjugated AuNPs for fibrosis imaging using CT.
In general, AuNPs are considered to be inert(21), but their size may determine their toxicity and applications. AuNPs under 2 nm in size are more likely to induce toxicity due to the ability to irreversibly bind to biomolecules, including DNA(22), whereas AuNPs >3 nm in size are relatively safe. In our acute study, we did not observe any changes in liver and renal function in mice after arterial AuNPs injection, suggesting relative safety. Our data show that 20 nm AuNPs exhibit a broad range of biodistribution, resulting in more organs being exposed to AuNPs, especially in the liver and spleen. Long-term toxicity may be dependent on the accumulation of AuNPs in specific organs and need longer evaluation. A previous study(18) demonstrated that 20 and 40 nm AuNPs were stable for >48 h in physiologic media, while 80 nm AuNPs aggregated by 24 h. Considering a 100–200 nm diameter of transvascular pores and fenestrations(23), AuNPs under 100nm in size may be preferable for molecular CT imaging. The human dose converted from mouse dose used in our study is around 15mg/kg(24), which should be viable for human studies, although the cost might be prohibitive.
One of the main limitations of CT compared with other imaging modalities is the relatively high mass concentration of contrast agents necessary for contrast-enhanced imaging at the site of interest(25), and the required high dose of AuNPs could result in toxicity or other adverse side effects. Furthermore, previous cell-labeling studies(26, 27) demonstrated that the mass concentration of nontargeted AuNPs internalized by cells increased with the initial mass concentration or dose of AuNPs, but a saturation point was reached. For cost effectiveness and decreased off-target accumulation, arterial injections of AuNPs are superior, but increase the invasiveness of this approach. Furthermore, this approach needs to be tested in larger animal models, human subjects, and in targeting other matrix components. A newer high resolution mode is now available and is expected to improve the quality of in vivo MDCT imaging. Our studies did not include K-edge imaging with enhanced AuNP contrast. Photon-counting system could be optimized and may be combined in the future with image post-processing to estimate tissue AuNPs concentration (28) and bio-distributions (29) in vivo, and to differentiate gold and iodine in the tissues(30). Nevertheless, our study demonstrates that surface modified AuNPs could be used as molecular imaging probe in CT imaging, combining high-resolution anatomic and molecular images. Further developments to refine this approach might render it useful for future applications.
Acknowledgments
This research was partly supported by NIH grants numbers DK73608, DK102325, DK104273, HL123160, and DK10081.
References
- 1.McGaraughty S, Davis-Taber RA, Zhu CZ, et al. Targeting Anti-TGF-beta Therapy to Fibrotic Kidneys with a Dual Specificity Antibody Approach. J Am Soc Nephrol. 2017;28:3616–26. doi: 10.1681/ASN.2017010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu XY, Urbieta-Caceres V, Krier JD, et al. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31:117–25. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu XY, Chade AR, Rodriguez-Porcel M, et al. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–9. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Wang ZJ, Liu M, et al. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol. 2014;69:1117–22. doi: 10.1016/j.crad.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Martin DR, Semelka RC, Chapman A, et al. Nephrogenic systemic fibrosis versus contrast-induced nephropathy: risks and benefits of contrast-enhanced MR and CT in renally impaired patients. J Magn Reson Imaging. 2009;30:1350–6. doi: 10.1002/jmri.21968. [DOI] [PubMed] [Google Scholar]
- 6.Silvestri A, Zambelli V, Ferretti AM, et al. Design of functionalized gold nanoparticle probes for computed tomography imaging. Contrast Media Mol Imaging. 2016;11:405–14. doi: 10.1002/cmmi.1704. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Xiong Z, Zhu J, et al. PEGylated polyethylenimine-entrapped gold nanoparticles loaded with gadolinium for dual-mode CT/MR imaging applications. Nanomedicine. 2016;11:1639–52. doi: 10.2217/nnm-2016-0093. [DOI] [PubMed] [Google Scholar]
- 8.Popovtzer R, Agrawal A, Kotov NA, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8:4593–6. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trojanowska M, LeRoy EC, Eckes B, Krieg T. Pathogenesis of fibrosis: type 1 collagen and the skin. J Mol Med (Berl) 1998;76:266–74. doi: 10.1007/s001090050216. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimi B, Crane JA, Knudsen BE, et al. Evolution of cardiac and renal impairment detected by high-field cardiovascular magnetic resonance in mice with renal artery stenosis. J Cardiovasc Magn Reson. 2013;15:98. doi: 10.1186/1532-429X-15-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danila D, Johnson E, Kee P. CT imaging of myocardial scars with collagen-targeting gold nanoparticles. Nanomedicine. 2013;9:1067–76. doi: 10.1016/j.nano.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Reuveni T, Motiei M, Romman Z, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int J Nanomedicine. 2011;6:2859–64. doi: 10.2147/IJN.S25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Z, Leng S, Jorgensen SM, et al. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. Phys Med Biol. 2016;61:1572–95. doi: 10.1088/0031-9155/61/4/1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Shao C, Wang R, et al. Optical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyes. J Urol. 2013;189:702–10. doi: 10.1016/j.juro.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathi M, Agarwal KK, Mukherjee A, et al. 99mTc-DMSA planar imaging versus dual-detector SPECT for the detection of renal cortical scars in patients with CKD-3. Nucl Med Commun. 2016;37:911–6. doi: 10.1097/MNM.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 16.Galper MW, Saung MT, Fuster V, et al. Effect of computed tomography scanning parameters on gold nanoparticle and iodine contrast. Invest Radiol. 2012;47:475–81. doi: 10.1097/RLI.0b013e3182562ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson PA, Rahman WN, Wong CJ, et al. Potential dependent superiority of gold nanoparticles in comparison to iodinated contrast agents. Eur J Radiol. 2010;75:104–9. doi: 10.1016/j.ejrad.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Yang Z, Lu W, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30:1928–36. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niidome T, Yamagata M, Okamoto Y, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–7. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Love JC, Estroff LA, Kriebel JK, et al. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem Rev. 2005;105:1103–69. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 21.Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. 2010;12:2313–33. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsoli M, Kuhn H, Brandau W, et al. Cellular uptake and toxicity of Au55 clusters. Small. 2005;1:841–4. doi: 10.1002/smll.200500104. [DOI] [PubMed] [Google Scholar]
- 23.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 24.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee N, Choi SH, Hyeon T. Nano-sized CT contrast agents. Adv Mater. 2013;25:2641–60. doi: 10.1002/adma.201300081. [DOI] [PubMed] [Google Scholar]
- 26.Xu C, Tung GA, Sun S. Size and Concentration Effect of Gold Nanoparticles on X-ray Attenuation As Measured on Computed Tomography. Chem Mater. 2008;20:4167–9. doi: 10.1021/cm8008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 28.Alivov Y, Baturin P, Le HQ, et al. Optimization of K-edge imaging for vulnerable plaques using gold nanoparticles and energy resolved photon counting detectors: a simulation study. Phys Med Biol. 2014;59:135–52. doi: 10.1088/0031-9155/59/1/135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Si-Mohamed S, Cormode DP, Bar-Ness D, et al. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution in vivo. Nanoscale. 2017;9:18246–57. doi: 10.1039/c7nr01153a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cormode DP, Si-Mohamed S, Bar-Ness D, et al. Multicolor spectral photon-counting computed tomography: in vivo dual contrast imaging with a high count rate scanner. Sci Rep. 2017;7:4784. doi: 10.1038/s41598-017-04659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]