Abstract
Purpose
This review article focuses on the prevention of vertical transmission of hepatitis B virus (HBV) among pregnant women living in sub-Saharan Africa (SSA), where disease is endemic and maternal HBV seroprevalence is estimated to be >8%. Available interventions that have been studied in low-income and middle-income countries (LMIC) are compared in terms of efficacy and real-world effectiveness. Global disease elimination targets, barriers to HBV prevention efforts, and critical research gaps are discussed.
Methods
A PUBMED literature search in February 2018 identified relevant studies of interventions to reduce or prevent the transmission of hepatitis B virus during pregnancy or the peripartum period. Studies were included if they focused on interventions that are currently available or could be made available in SSA. Trials conducted in SSA and other low-income countries were prioritized although interventions in middle and high-income countries were included.
Findings
Among 127 studies and reports included in the review, 60 included data from SSA. The most cost-effective intervention to reduce HBV infection rates in SSA is timely birth dose vaccination followed by completion of the 3-dose infant vaccine series. The identification and treatment of pregnant women with elevated HBV viral load to further reduce the risk of vertical transmission in SSA shows promise but efficacy and safety trials in Africa are lacking.
Implications
Scale up of currently available tools is required to reach HBV disease elimination goals in SSA. Many countries in SSA are in the process of rolling out national birth dose vaccination campaigns; this provides an opportunity to evaluate and improve processes in order to expand coverage. Early antenatal care, promotion of facility deliveries and increased awareness of HBV prevention are also key components of prevention success. Future studies in SSA should identity an “HBV prevention package” that is effective, safe and feasible and can be administered in antenatal clinic and tailored to vertical transmission risk.
Keywords: hepatitis B virus (HBV) infection, hepatitis B antiviral therapy, sub-Saharan Africa (SSA), vertical HBV transmission, hepatitis B in pregnancy
Introduction
There are 257 million people living with chronic hepatitis B virus (HBV) infection worldwide and 88% of them reside in sub-Saharan Africa (SSA).1–7 Viral hepatitis was the 7th leading cause of global mortality in 2015 and deaths caused by viral hepatitis surpassed the number of deaths caused by HIV, tuberculosis and malaria infection. 7 If current trends continue, an estimated 63 million new cases of HBV will occur between 2015-2030, and the World Health Organization (WHO) has set a goal of disease elimination. Reaching this ambitious goal by 2030 will require a significant scale up of prevention and treatment efforts in SSA with a focus on efforts to prevent transmission of HBV during pregnancy and the peripartum period.8–12 Vertical transmission is a key factor driving endemic HBV infection rates in SSA.13
Chronic active HBV infection is usually asymptomatic and only 9% of infected persons worldwide are aware of their infection.14 Routine screening in antenatal clinic consists of testing for the presence of HBV surface antigen (HBsAg) but screening is not consistently performed among pregnant women in SSA.15 Similar to other viral infections in pregnancy, the risk of vertical transmission of HBV is directly correlated with the maternal viral load titer. Studies have documented that women with HBV DNA levels >200,000 IU/mL (10⁶ copies/mL) have higher risk of vertical transmission.16 Infectiousness is also predicted by positive maternal HBV e antigen (HBeAg) serostatus which correlates with elevated HBV DNA levels (ranging from 2,000 IU/mL to >200,000 IU/mL).5,17
Vertical transmission of HBV is mucosal and caused by perinatal exposure to infected maternal blood and body fluids at the time of delivery. Transplacental transmission and transmission via breastfeeding are rare. Routine cesarean delivery is not recommended for the sole purpose of reducing the risk of vertical transmission to HBV-exposed infants, but data are limited. 18–20 A potential association between HBV infection and preterm delivery requires further validation.21
The likelihood of developing chronic HBV infection is inversely proportional to age at the time of HBV exposure. 7,16,20,22–28 Ninety percent of HBV-exposed neonates will develop chronic infection, compared to 5-10% of HBV-exposed adults. Interventions to prevent HBV vertical transmission are highly cost-effective since they reduce both short-term adverse outcomes and long-term morbidity and mortality.29,30 The long term outcomes of chronic HBV infection include cirrhosis, end stage liver disease (ESLD) and hepatocellular carcinoma (HCC). 31 The risk of HCC after perinatal HBV infection is 5% per decade – 100 times higher than the risk of HCC following horizontal transmission later in life.32 Medical and surgical management options for ESLD and HCC in SSA are limited; this strengthens the rationale to allocate resources to disease prevention efforts. 33,34 HIV/HBV coinfection is important as well since an estimated 18 million women in SSA are living with HIV and most are of childbearing age. 35,36 Adults with HIV have elevated risk of acquiring HBV and HIV/HBV co-infected patients have more rapid progression of liver disease to fibrosis and cirrhosis. 37 Most pregnancy outcomes in women treated for HIV/HBV coinfection are reassuring to date, and coinfection does not appear to increase the risk of vertical HIV transmission.38,39
Fewer than 1% of pregnant women worldwide and very few women in sub-Saharan Africa are offered targeted antiviral therapy for HBV infection during pregnancy. 40,41 Several first-line antiretroviral therapy regimens used in SSA have dual activity against HIV and HBV viruses (including tenofovir, lamivudine and emtricitabine). 42Although antiretroviral medications may be available free of charge through international and national HIV programs in SSA (such as the US Presidents Emergency Plan for AIDS Relief or PEPFAR), there is no equivalent program to cover the expense of antiviral therapy for HBV.
Fortunately, highly effective tools to prevent the vertical transmission of HBV with a long track record of safe administration in pregnant women and neonates are available. Low awareness about HBV prevalence and prevention interventions among providers and the general public in SSA limits uptake.43,44 This review will focus on the HBV prevention options that are available in SSA – namely, HBV vaccination and HBV-targeted antiviral therapy.45–50 Gaps in knowledge and research priorities necessary to reach vertical transmission HBV elimination goals in SSA will also be discussed.
Methods
A full PUBMED search on February 19, 2018 was conducted to identify relevant human studies of interventions to reduce or prevent the transmission of hepatitis B virus during pregnancy or the peripartum period. The terms or keywords used in the search were “hepatitis B antiviral pregnancy”, “prevention of vertical transmission of hepatitis B”, “mother to child hepatitis B prevention”, “hepatitis B birth dose vaccination”, “hepatitis B infant vaccination”, “hepatitis B immunoglobulin” or “HBIG”, or “HBV therapy in pregnancy”. Studies conducted in Africa or other low-income countries were prioritized for inclusion although studies of HBV prevention interventions in middle and high-income countries were included when relevant. Only articles written in English were reviewed. Studies of interventions to prevent horizontal transmission or the prevention of hepatitis B in adults were excluded. Articles were reviewed to address thematic areas of interest.
Results
Overall the search yielded a total of 4543 reports of which 127 were relevant and reviewed for this publication. Sixty reports focused on or included data from countries in SSA. We address ten thematic questions in the sections below.
HBV Testing Strategies in Pregnant Women and Infants in SSA
When women are screened for hepatitis B infection during pregnancy in SSA, serologic testing for HBsAg is performed. However, since few pregnant women (0-20%) are routinely tested for hepatitis B in SSA, cross-sectional studies are used to document regional HBsAg prevalence. 51–53 Given the expense and expertise required for quantitative molecular diagnostic testing, HBeAg is the only available measure of infectiousness for most HBV-positive pregnant women in SSA. The feasibility of rapid antenatal HBV testing is under investigation.54
HBV Vaccine Products for Neonates and Infants
The first commercially available HBV vaccine was a plasma-derived product that was approved in 1981. Within 10 years, this formulation was replaced by a yeast-derived, recombinant DNA HBV vaccine product that remains in use today.55,56 There are several HBV vaccine products currently available for pediatric populations in SSA; all contain 5-10 micrograms of HBV surface antigen (HBsAg) in a 0.5 mL standard volume dose. Any licensed and approved vaccine for HBV can be used interchangeably in national vaccination programs. In SSA, administration of a monovalent form of the HBV vaccine is recommended at birth for all infants (birth dose vaccine), followed by HBV vaccination as part of a pentavalent combination vaccine (HBV/DTP/Hib) at 6, 10 and 14 weeks of age. Three or four vaccines are required to complete the HBV series (four if the birth dose is followed by the 3-dose pentavalent series). Although most HBV vaccines have a long shelf life (up to 4 years), current cold-chain requirements mandate transportation and storage of vaccine at 2-8° C. This can be a challenge in areas of SSA with a lack of consistent electrical supply. Specific vaccine storage details are available in the product package insert and a recent WHO report focused on HBV prevention includes practical details for national HBV vaccination programs in low and middle-income countries (LMIC).14
HBV Vaccine Immunogenicity, Duration of Protection and Safety
Fortunately, pediatric HBV vaccination is highly immunogenic and vaccine series completion alone prevents 80-95% of vertical HBV transmission. Most healthy infants (>96%) have evidence of protective immunity upon completion of the primary series.57,58 Protection is more limited without vaccine series completion. The standard definition of protective immunity is detectable antibody (HBsAb) levels >10 mIU/mL at 9 months of age (1-2 months after the last dose). According to the WHO, preterm, low birthweight infants (<2000 grams) are recommended to receive birth dose vaccination followed by the 3 dose pentavalent series, and serologic response rates are excellent.59 Although US recommendations for low birthweight infants are similar for HBV-exposed infants (or if maternal status is unknown), CDC recommends HBV vaccination starting at 4 weeks of age among infants who are not HBV-exposed.60
Infants with HIV-infection appear to develop lower antibody levels in response to HBV vaccine but vaccination recommendations are unchanged.61 Studies show that the duration of the protective response after completion of the primary HBV vaccine series is long lasting (>20-30 years), in areas of both high and low endemnicity.13,14,62–65 A booster dose of HBV is not recommended, but since 5% of infants do not respond to vaccine, a search for underlying immunologic or genetic differences in this group is ongoing.66 HBV vaccination is safe for use in infants and children with serious adverse events (anaphylaxis) occurring in fewer than 1.1 per million vaccinations.67
HBV Vaccine Timing
The ideal timing of HBV vaccination to prevent vertical transmission is at birth.13,68 Receipt of the initial pentavalent vaccine at 6 weeks of age leaves an HBV-exposed neonate with inadequate protection for weeks and many infants in SSA have delayed initiation of the series, which prolongs the risk period.47 Although many studies have shown efficacy of the monovalent birth dose HBV vaccine, only one controlled, non-randomized, vaccine effectiveness trial compared infection rates among infants who did and did not receive birth dose vaccination.69 In Cote d’Ivoire, Ekra and colleagues compared HBV infection rates among 4600 infants vaccinated at 0, 6, and 14 weeks of age to those vaccinated at 6, 10, and 14 weeks of age.70 Infection rates at 9 months of age were 0.5% in both groups, but in the subgroup of 41 infants born to HBeAg+ mothers, the infection rate was 38% in the birth dose group and 59% in the group with series initiation at 6 weeks. (p=−.18) It is not clear why the infection rate was elevated in both groups despite vaccination and study findings have not been replicated.
The initiation of HBV vaccination at birth vs. 6 weeks has been the subject of controversy for national programs in SSA. Although studies in Uganda (where birth dose HBV vaccine is not available) have shown efficacy of vaccine series initiation at 6 weeks, large scale HBV elimination in SSA depends on increasing access to birth dose vaccine. 71–74 In one model, 50% of new chronic cases of HBV worldwide in 2030 will have been acquired by vertical transmission.75 A population-based, cross-sectional study of children in the Amazon region documented the impact of a birth dose vaccine program established in 2001.76 The rate of chronic HBV infection decreased to 0.5% and receipt of the birth dose decreased the risk of HBV infection by 95%. A similar rate of chronic HBV infection (0.4%) was noted in Israeli children following nationwide adoption of the birth dose vaccine in 1992.77 In Indonesia, low coverage of the birth dose vaccine was cited as one explanation for persistent pediatric HBV infection rates (seroprevalence as high as 6%) despite adoption of universal HBV vaccination starting at birth in 1997.78 Randomized or other well-designed studies comparing HBV transmission rates with vaccine initiation at birth vs. 6 weeks are lacking.
HBV Vaccine Coverage, Availability and Cost-effectiveness in SSA
The WHO has recommended universal HBV birth dose vaccination for all infants since 2009. 13(Figure 1) HBV vaccination within 24 hours of birth is also recommended as a key performance indicator for national immunization programs.14 HBV birth dose vaccination has been supported by Gavi, the Vaccine Alliance, since 2000. Gavi recognizes HBV birth dose as a high-impact vaccination that should be included in SSA vaccination platforms. Despite these longstanding recommendations, coverage of the HBV birth dose vaccine in 2015 was only 38% worldwide and 10% in SSA.50 Fewer than 100 (97) countries have adopted a policy of birth dose vaccination, and only 11 of 54 countries in SSA, although many are working towards adoption.15,79 Rates of completion for the three dose pentavalent HBV-containing vaccine series are 87% worldwide and 76% in SSA.15,47 Infant HBV vaccination rates in SSA are correlated with higher maternal age and education, urban residence and access to health care. 47,80
Figure 1.
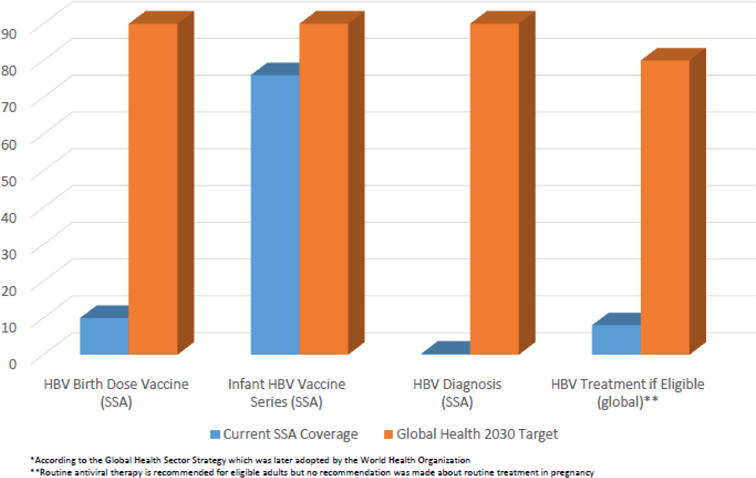
Current Rates and 2030 Targets for HBV Elimination in sub-Saharan Africa *
The monovalent vaccine is quite affordable at 20 cents per dose, although country procurement costs and patient charges may be significantly higher. Gavi does not currently provide financial support for the birth dose vaccine as it does for the pentavalent vaccine. The cost-effectiveness of HBV prevention with vaccination is favorable, whether analyses include short-term outcomes of infection prevention in children or long-term outcomes of HBV-associated morbidity and mortality.29,81,82 At an estimated cost of $15.5 million USD, scale up of HBV birth dose vaccination in South Africa was shown to be the most cost-effective intervention compared to several other intervention efforts modeled to reduce national viral hepatitis rates.83
Methods to improve HBV vaccine coverage in SSA
There are four critical barriers to wider implementation of the birth dose vaccine in SSA: 1) limited awareness of HBV prevalence and prevention interventions, 2) vaccine availability, 3) out-of-facility deliveries, and 4) cold-chain storage requirements.84,85
Training and supervision of healthcare workers to increase awareness about the importance of HBV birth dose, ensuring consistent vaccine supply and developing standing orders for birth dose vaccination in facilities significantly improved vaccine coverage in the Western Pacific. 86 Similarly, in China, birth dose coverage improved when facility delivery rates increased from 58% to 93% and access to vaccine was ensured. 87 In the Philippines, timely birth dose coverage was 40% in 2011 but private facilities had lower coverage rates compared to government facilities.88 Since many health officials and antenatal providers in SSA have worked to promote facility deliveries in order to improve a variety of maternal and infant health incomes, facility delivery rates in most countries now approach 80%.89,90 However, in regions where out-of-facility delivery rates remain high, innovative strategies to offer HBV birth dose vaccination have been developed.91 One effective project in rural Asia provided regional and local health workers with mobile phones to track home deliveries; birth dose coverage in intervention districts was 57% compared to 20% in control districts.92
HBV monovalent vaccine is “relatively heat-stable” according to in-vivo and in-vitro studies. 14,93–95 This provides some indication that HBV vaccine may retain its potency in the absence of continuous cold-chain transport and storage. There are no thermostable HBV vaccine products available at present but ongoing studies are promising given the relevance of this challenge in SSA.96–99
Data from HBV birth dose national vaccination campaigns from outside SSA provide useful information for national programs that are in the process of adopting or rolling out their own birth dose vaccination programs (including Benin, Cameroon, Republic of the Congo, Cote d’Ivoire, Ethiopia, Ghana and Sierra Leone). 10,47 Published findings highlight the need to ensure consistent vaccine access, engage relevant clinical and public health partners in training opportunities, and conduct public awareness campaigns about the importance of HBV prevention efforts.
HBIG Efficacy and Availability in SSA
HBIG contains high levels of purified antibodies from plasma donors that are specific to the hepatitis B surface antigen (HBsAg). HBIG provides short-term protection for the HBV-exposed neonate for 3-6 months after delivery when provided along with birth dose vaccination. From an ethical perspective, HBIG should be available to HBV-exposed neonates in every country, but unfortunately, it is not widely available in SSA. Even if a local supply is identified, HBIG is rarely affordable at a cost up to several hundred dollars per dose. Safety concerns further limit the feasibility of HBIG administration in SSA.14 Current evidence shows that HBIG provides protection for HBV-exposed infants that is additive to the protection afforded by the birth dose vaccine, particularly among women with an elevated HBV viral load.68,69,100 However, studies also show minimal benefit of HBIG for HBV-exposed infants who are born to HBeAg negative women; additional study will be helpful in defining the precise role for HBIG in LMIC. 101–103 According to the WHO, the option of HBIG immunoprophylaxis in SSA has limited utility until cost, further efficacy and safety concerns are addressed. 13
HBV Antiviral Treatment in Pregnancy to Reduce Vertical Transmission
The main causes of prophylaxis failure for vertical transmission are high maternal viral load or HBeAg positivity, in utero infection, escape mutants and the maternal immune status.104 Of these, high maternal serum viral load (HBV DNA level > 200,000 IU/mL) appears to be the major cause of prophylaxis failure, with up to 3-9% of perinatal transmissions despite both active and passive immunization. 105
Antiviral drugs are safe and effective in the third trimester to prevent intrauterine transmission of hepatitis B virus and are generally recommended for HBV infected pregnant women with high viral load, followed by neonatal HBV vaccination. 106,107 Most major international liver society guidelines recommend antiviral therapy for women at higher risk of vertical transmission of HBV with initiation during the 3rd trimester (28-32 weeks gestational age) and cessation during the postpartum period for women who do not meet criteria for continuation of therapy.43 Antiviral therapies which have been used to decrease HBV DNA levels during late pregnancy include nucleotide/nucleoside analogue polymerase inhibitors: lamivudine, telbivudine, tenofovir and entecavir. Although lamivudine and tenofovir may be available at no cost to treat patients with HIV or HIV/HBV coinfection in SSA, no similar program covers the expense of these medications for adults with HBV monoinfection. Since antiviral therapy is necessary to reach elimination targets, increased global funding is needed to expand access to these medications.10,83
Lamivudine was the first antiviral drug used in HBV-infected mothers to lower vertical transmission rates. A nucleoside analogue and reverse transcriptase inhibitor, it can significantly reduce the HBV viral load. In 2014, 45 women in Ireland met criteria for lamivudine treatment, and no cases of perinatal transmission occurred in infants born to mothers who received treatment. 108 The study authors concluded that lamivudine therapy in highly viremic, HBV-infected pregnant women could help reduce the rate of vertical transmission. In 2011, a meta-analysis of randomized controlled trials including 1,693 HBV-infected mothers showed that lamivudine initiated at 28 weeks substantially reduced vertical HBV transmission compared to immunoprophylaxis with HBIG alone. 109
Telbivudine has anti-HBV activity with no known fetal toxic effects. Wu and colleagues performed a prospective study of 450 HBeAg positive pregnant woman with 279 woman receiving telbivudine and 171 women participating as controls 110. None of the infants whose mothers were given telbivudine tested positive for HBsAg at 6 months of age, compared to 14.7% of infants in the control group. The authors concluded that telbivudine was safe and significantly reduced vertical transmission of HBV.
One major drawback that complicates lamivudine and telbivudine use is HBV antiviral resistance. In contrast, tenofovir disoproxil fumarate (TDF), a nucleotide reverse transcriptase inhibitor, is a potent medication with minimal resistance and a favorable pregnancy safety profile. Tenofovir is the antiviral therapy of choice for HBV in pregnancy according to the American Association for the Study of Liver Diseases (AASLD).111 Data are mixed about the efficacy of tenofovir in reducing vertical HBV transmission. A retrospective review of 48 women treated with tenofovir throughout pregnancy reported a vertical transmission rate of 0% with a spontaneous abortion rate of 6% in the first trimester 112. Two large randomized controlled trials have studied the impact of tenofovir on vertical transmission of HBV. In the first trial in China involving HBeAg-positive women with an HBV DNA level > 200,000 IU/mL during the third trimester, the rate of vertical transmission was 5% among those who received tenofovir therapy compared to 18% among who received usual care without antiviral therapy.113 In contrast, in the second, multicenter, double-blind clinical trial performed in Thailand, maternal tenofovir therapy in 331 HBEAg+ pregnant women did not result in a significantly lower rate of HBV transmission compared to women who did not receive antiviral therapy (0% vs 2%, p=0.12) when provided in conjunction with HBIG immunoprophylaxis and HBV vaccination.114 Findings from this study may not generalize to SSA where immunoprophylaxis is not consistently available. There is significant interest in the potential role of tenofovir alafenamide fumarate (TAF) therapy during pregnancy (a prodrug of tenofovir disoproxil fumarate with improved bone and renal safety profiles in adults) but studies have not yet been reported.
Entecavir, another nucleoside analog that inhibits reverse transcription and DNA replication has an excellent resistance profile and comparable efficacy and safety with tenofovir for the treatment of HBV. However, data is limited about its efficacy in pregnancy to reduce vertical transmission.115
Combination Interventions to Reduce HBV Prevalence in SSA
Several models have been created to identify high impact interventions using current tools (single or in combination) to reach global HBV elimination goals. In a recent model by Nayagam and colleagues, worldwide scale up of birth dose vaccination prevented 18.7 million new cases of HBV by 2030, while scale up of pentavalent vaccine coverage without birth dose vaccine prevented 4.3 million new infections.75 In another model, a package of interventions (population wide test and treat, peripartum antiviral therapy for HBeAg+ women, universal birth dose vaccination and series completion), reduced new chronic HBV infections worldwide by 90% and mortality by 65%. A response of this scale would be required in order to reach Global Health Sector Strategy targets for elimination goals.10 (Figure 1) The study also enumerates the high cost of this intervention package ($5.5 billion/year) and some of the challenges of scale up in SSA where disease prevalence is high and public health resources are limited.
Studies have also compared the cost-effectiveness of various antenatal HBV prevention strategies: universal birth dose vaccination, universal infant vaccination starting at 6 weeks or maternal HBsAg screening with targeted birth dose vaccination for exposed infants. In Cameroon, universal HBV vaccination with birth dose may be the most effective strategy in terms of reducing pediatric HBV infection by age 10 at a willingness to pay threshold of $150. 46 Similarly, universal HBV vaccination with birth dose was the least costly HBV prevention option in a population in Thailand with maternal seroprevalence of 7%. 116 Provision of HBIG for infants born to HBV infected women in Thailand was cost-effective at a willingness-to-pay threshold of $1200.
An optimal package to prevent vertical HBV transmission in SSA includes the identification of pregnant women who are highly infectious since these women may transmit HBV vertically despite birth dose vaccination and HBIG. This risk averages 8.5% but can be as high as 30% among women with elevated HBV viral load. 16,24,117,118 Identification of these women during early antenatal care would allow time for providers to discuss the risks and benefits of maternal antiviral therapy during pregnancy.
Research Gaps in the Prevention of HBV Vertical Transmission in SSA
Research innovation is needed on several fronts simultaneously to launch new efforts to reach global HBV vertical elimination targets in SSA: diagnosis, vaccination and treatment119 For diagnosis, universal screening for HBsAg in all pregnant women would increase the awareness of infection status in this key population. The development of rapid and affordable point-of-care diagnostic testing with excellent performance characteristics would also advance the field. Point-of-care HBeAg testing could be a useful strategy in SSA to determine treatment eligibility in ANC clinic. Another pragmatic goal would involve the incorporation of HBV testing into a single testing platform to facilitate the diagnosis of multiple infections at the time of the initial antenatal visit in SSA (HIV/HBV/Syphilis).
For vaccination, innovative implementation studies in SSA are needed to optimize facility delivery rates and maximize access to monovalent birth dose in any birth setting compared to initiation at 4-6 weeks. Additional studies to develop an effective and safe heat-stable HBV vaccine product are critical. National campaigns working on birth dose vaccination should reduce patient cost constraints as much as possible.
In terms of HBV therapy, there are many new exciting pharmacologic developments in the pipeline, including combination therapies and new life cycle targets. Each new antiviral therapy or strategy will require well-designed, prospective studies to determine drug safety and efficacy in pregnant women and infants exposed to antiviral medication in-utero.41,120 In the meantime, studies in SSA documenting the efficacy and safety of tenofovir use in HBV-infected pregnant women are needed. Relevant questions for the use of tenofovir therapy during pregnancy include: participant selection (in the absence of routine virologic testing), duration of therapy, timing of cessation of therapy (to reduce postpartum disease flares), mode of delivery and breastfeeding safety.121–123 Treatment outcomes for the newest formulation of tenofovir (tenofovir alafenamide or TAF) should also be investigated since, in non-pregnant adults, TAF has lower rates of bone and nephrotoxicity compared to tenofovir disopoxil. 43 Additional studies of cost effectiveness are needed to help prioritize prevention options. Since effective antiviral therapy is already available in much of SSA (but limited to those with HIV), pregnant women with HBV will need better access to affordable therapy if HBV “treatment as prevention” becomes standard of care. 124–126 Examples of successful HBV treatment programs in SSA already exist but expansion will be necessary if certain pregnant women become eligible for routine antiviral therapy in the future. 127
Conclusions
HBV infection is endemic in SSA and a major cause of morbidity and mortality. New and ambitious elimination targets provide an ideal opportunity to focus resources on optimizing the prevention of HBV vertical transmission. Current prevention efforts in SSA require universal access to timely HBV vaccination at birth. Public health officials and providers in SSA must continue to work to develop effective national HBV elimination strategies that are well-resourced, sustainable, supported by the community and linked to other antenatal infection prevention efforts (such as HIV prevention). Models with SSA-specific data should be used to prioritize cost-effective intervention combinations and advocate for appropriate allocation of resources. The optimal antenatal HBV prevention package in SSA is yet to be defined but future research will define the efficacy, safety and feasibility of a package that may include universal antenatal testing, targeted antiviral therapy during pregnancy and provision of HBV vaccine starting with the birth dose for all infants.
Acknowledgments
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23 HD090993 to JDO)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the authors have a conflict of interest to declare.
References
- 1.Alam N, Hajizadeh M, Dumont A, Fournier P. Inequalities in maternal health care utilization in sub-Saharan African countries: a multiyear and multi-country analysis. PLoS One. 2015;10(4):e0120922. doi: 10.1371/journal.pone.0120922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasim GI, Murad IA, Adam I. Hepatitis B and C virus infections among pregnant women in Arab and African countries. Journal of infection in developing countries. 2013;7(8):566–578. doi: 10.3855/jidc.3243. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann CJ, Mashabela F, Cohn S, et al. Maternal hepatitis B and infant infection among pregnant women living with HIV in South Africa. Journal of the International AIDS Society. 2014;17:18871. doi: 10.7448/IAS.17.1.18871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howell J, Lemoine M, Thursz M. Prevention of materno-foetal transmission of hepatitis B in sub-Saharan Africa: the evidence, current practice and future challenges. Journal of viral hepatitis. 2014;21(6):381–396. doi: 10.1111/jvh.12263. [DOI] [PubMed] [Google Scholar]
- 5.Ott JJ, Stevens GA, Wiersma ST. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC infectious diseases. 2012;12:131. doi: 10.1186/1471-2334-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet (London, England) 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 7.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet (London, England) 2016;388(10049):1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nations U. Sustainable Development Goals. 2015 [Google Scholar]
- 9.Organization WH. Combating Hepatitis B and C to Reach Elimination by 2030. http://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/
- 10.Spearman CW, Afihene M, Ally R, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. The lancet Gastroenterology & hepatology. 2017;2(12):900–909. doi: 10.1016/S2468-1253(17)30295-9. [DOI] [PubMed] [Google Scholar]
- 11.Ward JW. Building the evidence base to eliminate hepatitis B and C as public health threats. The Lancet Infectious Diseases. 16(12):1314–1316. doi: 10.1016/S1473-3099(16)30272-9. [DOI] [PubMed] [Google Scholar]
- 12.Wong GL-H, Wong VW-S. Eliminating hepatitis B virus as a global health threat. The Lancet Infectious Diseases. 16(12):1313–1314. doi: 10.1016/S1473-3099(16)30214-6. [DOI] [PubMed] [Google Scholar]
- 13.World Health O. Hepatitis B vaccines: WHO position paper, July 2017 - Recommendations. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 14.Hepatitis B vaccines: WHO position paper - July 2017. Releve epidemiologique hebdomadaire. 2017;92(27):369–392. [PubMed] [Google Scholar]
- 15.Breakwell L, Tevi-Benissan C, Childs L, Mihigo R, Tohme R. The status of hepatitis B control in the African region. The Pan African medical journal. 2017;27(Suppl 3):17. doi: 10.11604/pamj.supp.2017.27.3.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JS, Pan CQ. Viral factors for HBV mother-to-child transmission. Hepatology international. 2017;11(6):476–480. doi: 10.1007/s12072-017-9825-y. [DOI] [PubMed] [Google Scholar]
- 17.Tran TT, Gordon SC, Fung S, et al. Hepatitis B e Antigen Status and Hepatitis B DNA Levels in Women of Childbearing Age with Chronic Hepatitis B Infection Screening for Clinical Trials. PLOS ONE. 2015;10(3):e0121632. doi: 10.1371/journal.pone.0121632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan CQ, Zou HB, Chen Y, et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11(10):1349–1355. doi: 10.1016/j.cgh.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Qin Q, Fang Q, Jiang L, Nie S. Cesarean section to prevent mother-to-child transmission of hepatitis B virus in China: A meta-analysis. BMC pregnancy and childbirth. 2017;17(1):303. doi: 10.1186/s12884-017-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dionne-Odom J, Tita AT, Silverman NS. #38: Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. American journal of obstetrics and gynecology. 2016;214(1):6–14. doi: 10.1016/j.ajog.2015.09.100. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Zhang S, Liu M, Wang Q, Shen H, Zhang Y. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. The Lancet Global Health. 5(6):e624–e632. doi: 10.1016/S2214-109X(17)30142-0. [DOI] [PubMed] [Google Scholar]
- 22.Mavilia MG, Wu GY. Mechanisms and Prevention of Vertical Transmission in Chronic Viral Hepatitis. Journal of clinical and translational hepatology. 2017;5(2):119–129. doi: 10.14218/JCTH.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keane E, Funk AL, Shimakawa Y. Systematic review with meta-analysis: the risk of mother-to-child transmission of hepatitis B virus infection in sub-Saharan Africa. Alimentary pharmacology & therapeutics. 2016;44(10):1005–1017. doi: 10.1111/apt.13795. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Chang MS, Tran TT, Nguyen MH. Management of Chronic Hepatitis B in Pregnancy. Journal of clinical gastroenterology. 2017;51(9):789–795. doi: 10.1097/MCG.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 25.Nelson NP, Jamieson DJ, Murphy TV. Prevention of Perinatal Hepatitis B Virus Transmission. Journal of the Pediatric Infectious Diseases Society. 2014;3(Suppl 1):S7–s12. doi: 10.1093/jpids/piu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obi SN, Onah HE, Ezugwu FO. Risk factors for hepatitis B infection during pregnancy in a Nigerian obstetric population. Journal of obstetrics and gynaecology: the journal of the Institute of Obstetrics and Gynaecology. 2006;26(8):770–772. doi: 10.1080/01443610600963986. [DOI] [PubMed] [Google Scholar]
- 27.Wen WH, Chang MH, Zhao LL, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. Journal of hepatology. 2013;59(1):24–30. doi: 10.1016/j.jhep.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus–a systematic review. Virology journal. 2008;5:100. doi: 10.1186/1743-422X-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu SQ, McGhee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine. 2013;31(14):1864–1869. doi: 10.1016/j.vaccine.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Bray F, Jemal A, Torre LA, Forman D, Vineis P. Long-term Realism and Cost-effectiveness: Primary Prevention in Combatting Cancer and Associated Inequalities Worldwide. Journal of the National Cancer Institute. 2015;107(12):djv273. doi: 10.1093/jnci/djv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaka H, Mshana SE, Rambau PF, Masalu N, Chalya PL, Kalluvya SE. Hepatocellular carcinoma: clinicopathological profile and challenges of management in a resource-limited setting. World journal of surgical oncology. 2014;12:246. doi: 10.1186/1477-7819-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemoine M, Thursz MR. Battlefield against hepatitis B infection and HCC in Africa. Journal of hepatology. 2017;66(3):645–654. doi: 10.1016/j.jhep.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Beasley RP. Rocks along the road to the control of HBV and HCC. Annals of epidemiology. 2009;19(4):231–234. doi: 10.1016/j.annepidem.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 34.O’Hara GA, McNaughton AL, Maponga T, et al. Hepatitis B virus infection as a neglected tropical disease. PLoS neglected tropical diseases. 2017;11(10):e0005842. doi: 10.1371/journal.pntd.0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown K, Williams DB, Kinchen S, et al. Status of HIV Epidemic Control Among Adolescent Girls and Young Women Aged 15-24 Years - Seven African Countries, 2015-2017. MMWR Morbidity and mortality weekly report. 2018;67(1):29–32. doi: 10.15585/mmwr.mm6701a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection–a global challenge. The New England journal of medicine. 2012;366(19):1749–1752. doi: 10.1056/NEJMp1201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinikoor MJ, Sinkala E, Chilengi R, et al. Impact of Antiretroviral Therapy on Liver Fibrosis Among Human Immunodeficiency Virus-Infected Adults With and Without HBV Coinfection in Zambia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;64(10):1343–1349. doi: 10.1093/cid/cix122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benhammou V, Tubiana R, Matheron S, et al. HBV or HCV Coinfection in HIV-1-Infected Pregnant Women in France: Prevalence and Pregnancy Outcomes. Journal of acquired immune deficiency syndromes (1999) 2018;77(5):439–450. doi: 10.1097/QAI.0000000000001618. [DOI] [PubMed] [Google Scholar]
- 39.Mave V, Kadam D, Kinikar A, et al. Impact of maternal hepatitis B virus coinfection on mother-to-child transmission of HIV. HIV medicine. 2014;15(6):347–354. doi: 10.1111/hiv.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016 a modelling study. The lancet Gastroenterology & hepatology. 2018 doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 41.Wilson P, Parr JB, Jhaveri R, Meshnick SR. Call to Action: Prevention of Mother-to-Child Transmission of Hepatitis B in Africa. The Journal of infectious diseases. 2018;217(8):1180–1183. doi: 10.1093/infdis/jiy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellier PO, Schnepf N, Amarsy R, et al. Short article: Hepatitis B virus status in children born to HIV/HBV coinfected women in a French hospital: a cross-sectional study. European journal of gastroenterology & hepatology. 2016;28(3):328–332. doi: 10.1097/MEG.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 43.Chen HL, Wen WH, Chang MH. Management of Pregnant Women and Children: Focusing on Preventing Mother-to-Infant Transmission. The Journal of infectious diseases. 2017;216(suppl_8):S785–s791. doi: 10.1093/infdis/jix429. [DOI] [PubMed] [Google Scholar]
- 44.Paul T, Marie TP, Bechem E. Knowledge, attitude and practice of staff of 4 hospitals in Yaounde on the prevention of vertical transmission of hepatitis B. The Pan African medical journal. 2017;28:174. doi: 10.11604/pamj.2017.28.174.10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dionne-Odom J, Mbah R, Rembert NJ, et al. Hepatitis B, HIV, and Syphilis Seroprevalence in Pregnant Women and Blood Donors in Cameroon. Infectious diseases in obstetrics and gynecology. 2016;2016:8. doi: 10.1155/2016/4359401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson S, Harper LM, Dionne-Odom J, Halle-Ekane G, Tita ATN. A decision analytic model for prevention of hepatitis B virus infection in Sub-Saharan Africa using birth-dose vaccination. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2018 doi: 10.1002/ijgo.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dionne-Odom J, Westfall AO, Nzuobontane D, et al. Predictors of Infant Hepatitis B Immunization in Cameroon: Data to Inform Implementation of a Hepatitis B Birth Dose. The Pediatric infectious disease journal. 2018;37(1):103–107. doi: 10.1097/INF.0000000000001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gueye SB, Diop-Ndiaye H, Lo G, et al. HBV carriage in children born from HIV-seropositive mothers in Senegal: The need of birth-dose HBV vaccination. Journal of medical virology. 2016;88(5):815–819. doi: 10.1002/jmv.24409. [DOI] [PubMed] [Google Scholar]
- 49.Spearman CW, Sonderup MW. Preventing hepatitis B and hepatocellular carcinoma in South Africa: The case for a birth-dose vaccine. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2014;104(9):610–612. doi: 10.7196/samj.8607. [DOI] [PubMed] [Google Scholar]
- 50.WHO- UNICEF. Vaccine Coverage Estimates. 2016 [Google Scholar]
- 51.Adeyemi AB, Enabor OO, Ugwu IA, Bello FA, Olayemi OO. Knowledge of hepatitis B virus infection, access to screening and vaccination among pregnant women in Ibadan, Nigeria. Journal of obstetrics and gynaecology: the journal of the Institute of Obstetrics and Gynaecology. 2013;33(2):155–159. doi: 10.3109/01443615.2012.711389. [DOI] [PubMed] [Google Scholar]
- 52.Chernet A, Yesuf A, Alagaw A. Seroprevalence of Hepatitis B virus surface antigen and factors associated among pregnant women in Dawuro zone, SNNPR, Southwest Ethiopia: a cross sectional study. BMC research notes. 2017;10(1):418. doi: 10.1186/s13104-017-2702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metaferia Y, Dessie W, Ali I, Amsalu A. Seroprevalence and associated risk factors of hepatitis B virus among pregnant women in southern Ethiopia: a hospital-based cross-sectional study. Epidemiology and health. 2016;38:e2016027. doi: 10.4178/epih.e2016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chotun N, Preiser W, van Rensburg CJ, et al. Point-of-care screening for hepatitis B virus infection in pregnant women at an antenatal clinic: A South African experience. PLoS One. 2017;12(7):e0181267. doi: 10.1371/journal.pone.0181267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schillie SVC, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports. 2018;67(RR-1):1–31. doi: 10.15585/mmwr.rr6701a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu FC, Sun KX, Pan HX, et al. The immunogenicity in healthy infants and efficiency to prevent mother to child transmission of Hepatitis B virus of a 10mug recombinant yeast-derived Hepatitis B vaccine (Hep-KSC) Vaccine. 2016;34(24):2656–2662. doi: 10.1016/j.vaccine.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 57.van den Ende C, Marano C, van Ahee A, Bunge EM, De Moerlooze L. The immunogenicity of GSK’s recombinant hepatitis B vaccine in children: a systematic review of 30 years of experience. Expert review of vaccines. 2017;16(8):789–809. doi: 10.1080/14760584.2017.1338569. [DOI] [PubMed] [Google Scholar]
- 58.Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine. 2013;31(21):2506–2516. doi: 10.1016/j.vaccine.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Tan CX, Chan SM, Lee LY, et al. Serologic Responses After Hepatitis B Vaccination in Preterm Infants Born to Hepatitis B Surface Antigen-Positive Mothers: Singapore Experience. The Pediatric infectious disease journal. 2017;36(8):e208–e210. doi: 10.1097/INF.0000000000001578. [DOI] [PubMed] [Google Scholar]
- 60.Robinson CL, Romero JR, Kempe A, Pellegrini C. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):134–135. doi: 10.15585/mmwr.mm6605e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falconer O, Newell ML, Jones CE. The Effect of Human Immunodeficiency Virus and Cytomegalovirus Infection on Infant Responses to Vaccines: A Review. Frontiers in immunology. 2018;9:328. doi: 10.3389/fimmu.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jezek J, Chen D, Watson L, et al. A heat-stable hepatitis B vaccine formulation. Human vaccines. 2009;5(8):529–535. doi: 10.4161/hv.5.8.8600. [DOI] [PubMed] [Google Scholar]
- 63.Peto TJ, Mendy ME, Lowe Y, Webb EL, Whittle HC, Hall AJ. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986-90) and in the nationwide immunisation program. BMC infectious diseases. 2014;14:7. doi: 10.1186/1471-2334-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Middleman AB, Baker CJ, Kozinetz CA, et al. Duration of protection after infant hepatitis B vaccination series. Pediatrics. 2014;133(6):e1500–1507. doi: 10.1542/peds.2013-2940. [DOI] [PubMed] [Google Scholar]
- 65.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Human vaccines & immunotherapeutics. 2013;9(8):1679–1684. doi: 10.4161/hv.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hennig BJ, Hall AJ. Host genetic factors in hepatitis B infection, liver cancer and vaccination response: a review with a focus on Africa. The Science of the total environment. 2012;423:202–209. doi: 10.1016/j.scitotenv.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 67.Bohlke K, Davis RL, Marcy SM, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112(4):815–820. doi: 10.1542/peds.112.4.815. [DOI] [PubMed] [Google Scholar]
- 68.Chen ZX, Zhuang X, Zhu XH, et al. Comparative Effectiveness of Prophylactic Strategies for Perinatal Transmission of Hepatitis B Virus: A Network Meta-analysis of Randomized Controlled Trials. Open forum infectious diseases. 2017;4(4):ofx225. doi: 10.1093/ofid/ofx225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. The Cochrane database of systematic reviews. 2006;(2):Cd004790. doi: 10.1002/14651858.CD004790.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Ekra D, Herbinger KH, Konate S, et al. A non-randomized vaccine effectiveness trial of accelerated infant hepatitis B immunization schedules with a first dose at birth or age 6 weeks in Cote d’Ivoire. Vaccine. 2008;26(22):2753–2761. doi: 10.1016/j.vaccine.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Seremba E, Van Geertruyden JP, Ssenyonga R, et al. Early childhood transmission of hepatitis B prior to the first hepatitis B vaccine dose is rare among babies born to HIV-infected and non-HIV infected mothers in Gulu, Uganda. Vaccine. 2017;35(22):2937–2942. doi: 10.1016/j.vaccine.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teshale EH, Kamili S, Drobeniuc J, Denniston M, Bakamutamaho B, Downing R. Hepatitis B virus infection in northern Uganda: Impact of pentavalent hepatitis B vaccination. Vaccine. 2015;33(46):6161–6163. doi: 10.1016/j.vaccine.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 73.Tamandjou CR, Maponga TG, Chotun N, Preiser W, Andersson MI. Is hepatitis B birth dose vaccine needed in Africa? The Pan African medical journal. 2017;27(Suppl 3):18. doi: 10.11604/pamj.supp.2017.27.3.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clinics in liver disease. 2016;20(4):607–628. doi: 10.1016/j.cld.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nayagam S, Thursz M, Sicuri E, et al. Requirements for global elimination of hepatitis B: a modelling study. The Lancet Infectious Diseases. 16(12):1399–1408. doi: 10.1016/S1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 76.Garcia D, Porras A, Rico Mendoza A, et al. Hepatitis B infection control in Colombian Amazon after 15years of hepatitis B vaccination. Effectiveness of birth dose and current prevalence. Vaccine. 2018 doi: 10.1016/j.vaccine.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Klinger G, Chodick G, Levy I. Long-term immunity to hepatitis B following vaccination in infancy: Real-world data analysis. Vaccine. 2018;36(17):2288–2292. doi: 10.1016/j.vaccine.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Purwono PB, Juniastuti Amin M, et al. Hepatitis B Virus Infection in Indonesia 15 Years After Adoption of a Universal Infant Vaccination Program: Possible Impacts of Low Birth Dose Coverage and a Vaccine-Escape Mutant. The American journal of tropical medicine and hygiene. 2016;95(3):674–679. doi: 10.4269/ajtmh.15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bodo B, Malande OO. Delayed introduction of the birth dose of Hepatitis B vaccine in EPI programs in East Africa: a missed opportunity for combating vertical transmission of Hepatitis B. The Pan African medical journal. 2017;27(Suppl 3):19. doi: 10.11604/pamj.supp.2017.27.3.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyahara R, Jasseh M, Gomez P, et al. Barriers to timely administration of birth dose vaccines in The Gambia, West Africa. Vaccine. 2016;34(29):3335–3341. doi: 10.1016/j.vaccine.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiesen E, Diorditsa S, Li X. Progress towards hepatitis B prevention through vaccination in the Western Pacific, 1990-2014. Vaccine. 2016;34(25):2855–2862. doi: 10.1016/j.vaccine.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 82.Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. Jama. 1995;274(15):1201–1208. [PubMed] [Google Scholar]
- 83.Hecht R, Hiebert L, Spearman WC, et al. The investment case for hepatitis B and C in South Africa: adaptation and innovation in policy analysis for disease program scale-up. Health policy and planning. 2018;33(4):528–538. doi: 10.1093/heapol/czy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiesen E, Lagani W, Sui G, et al. Assessment of the hepatitis B birth dose vaccination program, Papua New Guinea, 2014. Vaccine. 2016;34(3):367–372. doi: 10.1016/j.vaccine.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 85.Adjei CA, Asamoah R, Atibila F, Ti-Enkawol GN, Ansah-Nyarko M. Mother-to-child transmission of hepatitis B: extent of knowledge of physicians and midwives in Eastern region of Ghana. BMC public health. 2016;16:537. doi: 10.1186/s12889-016-3215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia Region, 1992-2015. Vaccine. 2018;36(1):6–14. doi: 10.1016/j.vaccine.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui F, Luo H, Wang F, et al. Evaluation of policies and practices to prevent mother to child transmission of hepatitis B virus in China: results from China GAVI project final evaluation. Vaccine. 2013;31(Suppl 9):J36–42. doi: 10.1016/j.vaccine.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 88.Patel MK, Capeding RZ, Ducusin JU, de Quiroz Castro M, Garcia LC, Hennessey K. Findings from a hepatitis B birth dose assessment in health facilities in the Philippines: opportunities to engage the private sector. Vaccine. 2014;32(39):5140–5144. doi: 10.1016/j.vaccine.2013.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellizzi S, Sobel HL, Mathai M, Temmerman M. Does place and attendance at birth improve early neonatal mortality? Secondary analysis of nine Demographic and Health Surveys. BJOG: an international journal of obstetrics and gynaecology. 2016 doi: 10.1111/1471-0528.14422. [DOI] [PubMed] [Google Scholar]
- 90.Diamond-Smith N, Sudhinaraset M. Drivers of facility deliveries in Africa and Asia: regional analyses using the demographic and health surveys. Reproductive health. 2015;12:6. doi: 10.1186/1742-4755-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Heffelfinger J, Wiesen E, et al. Improving hepatitis B birth dose coverage through village health volunteer training and pregnant women education. Vaccine. 2017;35(34):4396–4401. doi: 10.1016/j.vaccine.2017.06.056. [DOI] [PubMed] [Google Scholar]
- 92.Xeuatvongsa A, Datta SS, Moturi E, et al. Improving hepatitis B birth dose in rural Lao People’s Democratic Republic through the use of mobile phones to facilitate communication. Vaccine. 2016;34(47):5777–5784. doi: 10.1016/j.vaccine.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Braun LJ, Jezek J, Peterson S, et al. Characterization of a thermostable hepatitis B vaccine formulation. Vaccine. 2009;27(34):4609–4614. doi: 10.1016/j.vaccine.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 94.Hipgrave DB, Tran TN, Huong VM, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. The American journal of tropical medicine and hygiene. 2006;74(2):255–260. [PubMed] [Google Scholar]
- 95.Van Damme P, Cramm M, Safary A, Vandepapeliere P, Meheus A. Heat stability of a recombinant DNA hepatitis B vaccine. Vaccine. 1992;10(6):366–367. doi: 10.1016/0264-410x(92)90064-q. [DOI] [PubMed] [Google Scholar]
- 96.Kolwaite AR, Xeuatvongsa A, Ramirez-Gonzalez A, et al. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR. Vaccine. 2016;34(28):3324–3330. doi: 10.1016/j.vaccine.2016.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hipgrave DB, Maynard JE, Biggs BA. Improving birth dose coverage of hepatitis B vaccine. Bulletin of the World Health Organization. 2006;84(1):65–71. doi: 10.2471/blt.04.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel MK, Kahn A-L. Game changing: hepatitis B vaccine in a controlled temperature chain. The Lancet Global Health. 2018;6(6):e596–e597. doi: 10.1016/S2214-109X(18)30233-X. [DOI] [PubMed] [Google Scholar]
- 99.Scott N, Palmer A, Morgan C, et al. Cost-effectiveness of the controlled temperature chain for the hepatitis B virus birth dose vaccine in various global settings: a modelling study. The Lancet Global Health. 2018;6(6):e659–e667. doi: 10.1016/S2214-109X(18)30219-5. [DOI] [PubMed] [Google Scholar]
- 100.Wei KP, Zhu FC, Liu JX, et al. The efficacy of two different dosages of hepatitis B immunoglobulin combined with hepatitis B vaccine in preventing mother-to-child transmission of hepatitis B virus: A prospective cohort study. Vaccine. 2018;36(2):256–263. doi: 10.1016/j.vaccine.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 101.Milne A, West DJ, Chinh DV, Moyes CD, Poerschke G. Field evaluation of the efficacy and immunogenicity of recombinant hepatitis B vaccine without HBIG in newborn Vietnamese infants. Journal of medical virology. 2002;67(3):327–333. doi: 10.1002/jmv.10071. [DOI] [PubMed] [Google Scholar]
- 102.Lu Y, Liang XF, Wang FZ, et al. Hepatitis B vaccine alone may be enough for preventing hepatitis B virus transmission in neonates of HBsAg (+)/HBeAg (−) mothers. Vaccine. 2017;35(1):40–45. doi: 10.1016/j.vaccine.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 103.Machaira M, Papaevangelou V, Vouloumanou EK, Tansarli GS, Falagas ME. Hepatitis B vaccine alone or with hepatitis B immunoglobulin in neonates of HBsAg+/HBeAg-mothers: a systematic review and meta-analysis. The Journal of antimicrobial chemotherapy. 2015;70(2):396–404. doi: 10.1093/jac/dku404. [DOI] [PubMed] [Google Scholar]
- 104.Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology (Baltimore, Md) 2014 doi: 10.1002/hep.27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan CQ, Han GR, Jiang HX, et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(5):520–526. doi: 10.1016/j.cgh.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 106.Yu MM, Jiang Q, Ji Y, et al. Comparison of telbivudine versus lamivudine in interrupting perinatal transmission of hepatitis B virus. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2014;61(1):55–60. doi: 10.1016/j.jcv.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 107.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of hepatology. 2012;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 108.Jackson V, Ferguson W, Kelleher TB, et al. Lamivudine treatment and outcome in pregnant women with high hepatitis B viral loads. Eur J Clin Microbiol Infect Dis. 2015;34(3):619–623. doi: 10.1007/s10096-014-2270-0. [DOI] [PubMed] [Google Scholar]
- 109.Han GR, Cao MK, Zhao W, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55(6):1215–1221. doi: 10.1016/j.jhep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 110.Wu Q, Huang H, Sun X, et al. Telbivudine Prevents Vertical Transmission of Hepatitis B Virus from Women High Viral Loads: A Prospective Long-term Study. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 111.TN A, LA SF, MB J, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology (Baltimore, Md) 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pan CQ, Mi LJ, Bunchorntavakul C, et al. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: a case series. Digestive diseases and sciences. 2012;57(9):2423–2429. doi: 10.1007/s10620-012-2187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pan CQ, Duan Z, Dai E, et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. The New England journal of medicine. 2016;374(24):2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 114.Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus Placebo to Prevent Perinatal Transmission of Hepatitis B. The New England journal of medicine. 2018;378(10):911–923. doi: 10.1056/NEJMoa1708131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Njei B, Gupta N, Ewelukwa O, Ditah I, Foma M, Lim JK. Comparative efficacy of antiviral therapy in preventing vertical transmission of hepatitis B: a network meta-analysis. Liver international: official journal of the International Association for the Study of the Liver. 2016;36(5):634–641. doi: 10.1111/liv.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Devine A, Harvey R, Min AM, et al. Strategies for the prevention of perinatal hepatitis B transmission in a marginalized population on the Thailand-Myanmar border: a cost-effectiveness analysis. BMC infectious diseases. 2017;17(1):552. doi: 10.1186/s12879-017-2660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sellier P, Maylin S, Amarsy R, et al. Untreated highly viraemic pregnant women from Asia or sub-Saharan Africa often transmit hepatitis B virus despite serovaccination to newborns. Liver international: official journal of the International Association for the Study of the Liver. 2015;35(2):409–416. doi: 10.1111/liv.12561. [DOI] [PubMed] [Google Scholar]
- 118.Liu CP, Zeng YL, Zhou M, et al. Factors associated with mother-to-child transmission of hepatitis B virus despite immunoprophylaxis. Internal medicine (Tokyo, Japan) 2015;54(7):711–716. doi: 10.2169/internalmedicine.54.3514. [DOI] [PubMed] [Google Scholar]
- 119.Thio CL, Guo N, Xie C, Nelson KE, Ehrhardt S. Global elimination of mother-to-child transmission of hepatitis B: revisiting the current strategy. The Lancet Infectious diseases. 2015;15(8):981–985. doi: 10.1016/S1473-3099(15)00158-9. [DOI] [PubMed] [Google Scholar]
- 120.Wilson EM, Tang L, Kottilil S. Eradication Strategies for Chronic Hepatitis B Infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;62(Suppl 4):S318–325. doi: 10.1093/cid/ciw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J, Wang J, Qi C, et al. Baseline Hepatitis B Virus Titer Predicts Initial Postpartum Hepatic Flare: A Multicenter Prospective Study. Journal of clinical gastroenterology. 2017 doi: 10.1097/MCG.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 122.Chang CY, Aziz N, Poongkunran M, et al. Serum Aminotransferase Flares in Pregnant and Postpartum Women With Current or Prior Treatment for Chronic Hepatitis B. Journal of clinical gastroenterology. 2018;52(3):255–261. doi: 10.1097/MCG.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 123.Chang CY, Aziz N, Poongkunran M, et al. Serum Alanine Aminotransferase and Hepatitis B DNA Flares in Pregnant and Postpartum Women with Chronic Hepatitis B. The American journal of gastroenterology. 2016;111(10):1410–1415. doi: 10.1038/ajg.2016.296. [DOI] [PubMed] [Google Scholar]
- 124.Shan S, Cui F, Jia J. How to control highly endemic hepatitis B in Asia. Liver international: official journal of the International Association for the Study of the Liver. 2018;38(Suppl 1):122–125. doi: 10.1111/liv.13625. [DOI] [PubMed] [Google Scholar]
- 125.Debarry J, Cornberg M, Manns MP. Challenges in warranting access to prophylaxis and therapy for hepatitis B virus infection. Liver international: official journal of the International Association for the Study of the Liver. 2017;37(Suppl 1):67–72. doi: 10.1111/liv.13320. [DOI] [PubMed] [Google Scholar]
- 126.Fan L, Owusu-Edusei K, Jr, Schillie SF, Murphy TV. Cost-effectiveness of active-passive prophylaxis and antiviral prophylaxis during pregnancy to prevent perinatal hepatitis B virus infection. Hepatology (Baltimore, Md) 2016;63(5):1471–1480. doi: 10.1002/hep.28310. [DOI] [PubMed] [Google Scholar]
- 127.Aberra H, Desalegn H, Berhe N, et al. Early experiences from one of the first treatment programs for chronic hepatitis B in sub-Saharan Africa. BMC infectious diseases. 2017;17(1):438. doi: 10.1186/s12879-017-2549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


