Abstract
Guillain Barré syndrome (GBS), which is triggered by autoantibodies produced in response to antigenic stimuli such as certain infections and vaccinations, is the most common cause of acute flaccid paralysis worldwide. Campylobacter, the most common bacterial enteric infection in the USA, is reported to be the most commonly diagnosed antecedent of GBS, yet little information is available about the risk of post-Campylobacter GBS. Data collected through active, population-based surveillance in the Emerging Infections Program during the 2009–2010 novel Influenza A (H1N1) vaccination campaign allowed us to compare confirmed and probable GBS cases to non-cases to determine whether antecedent Campylobacter infection (or a diarrhoeal illness consistent with campylobacteriosis) was more common among cases and to assess the risk of GBS following Campylobacter infection. We estimate that 8–12% of GBS cases in the USA are attributable to Campylobacter infection (or a diarrhoeal illness consistent with campylobacteriosis), with 434–650 cases of post-diarrhoeal GBS annually and about 49 cases of GBS per 100 000 Campylobacter infections. These results provide updated estimates for post-Campylobacter GBS incidence in the USA and highlight an important benefit of effective measures to prevent Campylobacter infections.
Key words: Campylobacter, Guillain-Barre syndrome, incidence, surveillance
Introduction
Guillain Barré syndrome (GBS) is an autoimmune disorder of the peripheral nervous system triggered by autoantibodies formed in response to antigenic stimuli [1]. Antecedent exposures can include certain vaccinations (e.g. influenza) and viral or bacterial (especially Campylobacter) infections [2–6]. GBS is the most common cause of acute flaccid paralysis worldwide [1]; studies in Europe and North America report estimates of GBS incidence of 0.6 to 3.0 cases per 100 000 person-years [1, 7]. GBS is associated with severe morbidity, with patients frequently requiring extended ICU stays and up to 67% experiencing at least one major complication [8, 9]. The economic cost is estimated to be $1.7 billion annually in the USA [10].
Campylobacter causes an estimated 1.3 million enteric illnesses annually in the USA, making it the most common bacterial cause of gastroenteritis [11]. Campylobacter jejuni accounts for most Campylobacter infections and has been estimated in various settings and countries to precede 20%–31% of GBS cases with incidence estimated at 20–65 GBS cases per 100 000 Campylobacter infections [2, 12–20]. However, recent estimates for US populations are not available [7, 20].
Determining the risk of post-Campylobacter GBS is challenging for several reasons. Campylobacter infection is often undetectable by the time GBS symptoms begin because Campylobacter is typically shed for less than 2 weeks after onset of diarrhoea, whereas GBS symptoms typically present between 1 and 3 weeks after diarrhoea onset [2, 16, 20, 21]. In addition, due to mild symptoms or asymptomatic infection, many Campylobacter infections go undiagnosed, with an estimated 30 undiagnosed infections occurring for each laboratory-confirmed infection [11]. Diarrhoea can be mild [22], so infected persons may not seek care. Even if a stool sample is submitted, Campylobacter can be difficult to detect [23]. In the USA, surveillance for Campylobacter infection is conducted by the Centers for Disease Control and Prevention's (CDC) Foodborne Diseases Active Surveillance Network (FoodNet), the foodborne disease component of the Emerging Infections Program (EIP) [24]; however, no routine surveillance for GBS exists [7].
An increased risk of GBS following vaccination with a specific formulation of the vaccine targeted at an H1N1 influenza virus was identified in 1976 [25, 26], though no significant increased risk was observed with subsequent seasonal influenza vaccines formulations [27–29]. However, when a novel influenza A (H1N1) virus similar to the type identified in 1976 emerged in 2009 [30–32], concerns about post-vaccination GBS arose and CDC initiated a special EIP surveillance activity. This surveillance activity, conducted during the 2009–2010 novel influenza A vaccination campaign to assess the risk of post-vaccination GBS found no additional excess risk beyond typical that of seasonal influenza vaccines [33] and offered the opportunity for secondary analysis focused on post-Campylobacter GBS. This included extensive data collection on persons who were determined to not have GBS, providing a unique, well-characterized comparison group. Here, we report an analysis of the association of GBS with laboratory-confirmed Campylobacter infection (the most specific measure for campylobacteriosis) and with diarrhoeal illness (the most sensitive, available measure) to estimate the fraction of GBS attributable to laboratory-confirmed Campylobacter infection or diarrhoeal illness. We also present estimated rates of post-Campylobacter GBS.
Methods
EIP GBS special surveillance activity
We used data from the EIP GBS surveillance activity conducted during the 2009–2010 novel influenza A vaccination campaign to analyse the association of GBS with diarrhoeal illness or laboratory-confirmed Campylobacter infection and to calculate the fraction of GBS attributable to diarrhoeal illness or laboratory-confirmed Campylobacter infection.
EIP GBS special surveillance activity population
The EIP includes ten sites and a population that is approximately representative of the US population with respect to demographic and other health indicators, such as poverty (http://www.cdc.gov/ncezid/dpei/eip/). The catchment area for the GBS surveillance activity included 44.9 million persons. Data were collected between 1 October 2009 and 31 May 2010, yielding 22.9 million person-years under surveillance [33]. Possible GBS cases were identified by exhaustive, active, population-based case-finding to identify every resident of the catchment area presenting with symptoms possibly consistent with GBS. This case-finding was conducted through several avenues, including a network of clinicians (e.g. neurologists, clinical pharmacists, other providers), review of hospital admission and discharge data for the International Classification of Diseases-9-Clinical Modification code for GBS (357.0; acute infective polyneuritis) and monitoring of the Vaccine Adverse Events Reporting System. For additional details, see Wise et al. [33].
GBS and non-GBS diagnoses
Data were collected by review of inpatient and outpatient medical records for all possible GBS cases identified with onset of symptoms during the surveillance period [33]. After data collection and review, all possible GBS cases were classified using the Brighton Collaboration criteria for GBS, a classification of diagnostic certainty [34]. Cases were classified as confirmed (meeting Brighton level 1 or 2 criteria) or probable (Brighton level 3 criteria) based on clinical, cerebrospinal fluid and electrophysiologic criteria. We considered cases that did not meet the Brighton criteria for levels 1, 2, or 3 or cases in which an alternative diagnosis was reported as non-GBS controls.
Antecedent illness
Information about signs, symptoms and infections experienced in the 42 days before presentation, including diarrhoea, influenza-like illness (ILI), upper respiratory tract infection (URI) and laboratory-confirmed Campylobacter infection, was collected for all of the reported possible GBS cases, including persons ultimately determined to not have GBS. GBS is known to be strongly associated with Campylobacter infection but less with other common causes of diarrhoeal illness, so we examined the association of antecedent illness with GBS diagnosis using five definitions that ranged from highly specific and less sensitive to highly sensitive and less specific for Campylobacter infection. The most specific, least sensitive definition was laboratory-confirmed Campylobacter infection. The most sensitive, least specific was any diarrhoeal illness, which, as described above, was used because Campylobacter infection is usually not laboratory-confirmed. Three additional definitions of intermediate specificity and sensitivity included diarrhoea without ILI, diarrhoea without URI and diarrhoea without either ILI or URI. These were used because ILI and URI can also precede GBS and can sometimes include diarrhoea [12].
FoodNet
FoodNet, the foodborne diseases component of the EIP, is a collaboration among CDC, ten state health departments, the US Department of Agriculture's Food Safety and Inspection Service (USDA-FSIS) and the Food and Drug Administration (FDA). It conducts active, laboratory-based surveillance for selected pathogens transmitted commonly by food, including Campylobacter and publishes annual estimates of incidence. The FoodNet population is similar though not completely identical to the 2009–2010 GBS surveillance population. Based on 2010 US Census data, about 18% of the FoodNet surveillance population resided in areas not included in the EIP catchment and about 15% of the EIP GBS special surveillance population was not included in the FoodNet catchment. We used FoodNet data on laboratory-confirmed Campylobacter infections reported from 15 September 2009 to 14 September 2010. Since the EIP GBS surveillance activity did not cover a full year, we used FoodNet data from 2009 to 2010 on the timing of laboratory-confirmed Campylobacter infection in our extrapolation from 8 to 12-month estimates. Thus, we calculated the proportion of laboratory-confirmed Campylobacter infections reported to FoodNet that occurred during 15 September 2009–15 May 2010, a period shifted 2 weeks earlier than the GBS surveillance activity, to account for an average 2-week lag between onset of Campylobacter-related diarrhoea and onset of GBS.
Statistical analyses
Confirmed and probable GBS cases (Brighton 1–3) were compared with non-cases to determine whether antecedent illness, as determined using the five definitions detailed above, was more common in cases. We calculated odds ratios (OR) to evaluate the association between each definition of antecedent illness and GBS and we used these OR to estimate the attributable risk (AR) [35].
The AR estimates, in turn, were used to estimate the number of post-Campylobacter and post-diarrhoeal GBS cases that occurred in the EIP GBS surveillance activity population during the surveillance period. Next, incorporating the national estimate of Campylobacter incidence data, we estimated national rates of post-Campylobacter (post-diarrhoeal) GBS in the USA using each of the five definitions of antecedent illness. All analyses were performed in SAS 9.3 (Cary, NC), Microsoft Excel, or the R Package, epiR.
For sensitivity analysis, we also used a more specific definition of GBS limited to confirmed GBS (Brighton levels 1 and 2). We also repeated analyses excluding the 11% of patients referred for possible GBS who had a previous history of GBS.
Results
EIP GBS special surveillance activity
GBS and non-GBS diagnoses
The GBS surveillance activity identified 638 persons with possible GBS, of whom 398 were determined to have confirmed (Brighton levels 1 or 2, n = 349) or probable (Brighton level 3, n = 62) GBS. The other 227 patients were classified as not cases of GBS and served as controls. These included persons whose illness did not meet the criteria for Brighton levels 1–3 and persons who received another diagnosis. These other diagnoses were not collected systematically but included cancer or cancer-related treatment (N = 8), cardiac-related conditions (7), conversion disorder/seizures (6), radiculopathy (6), drug or alcohol abuse (4), chronic obstructive pulmonary disease (3), diabetes-related conditions (3), stroke (3), multiple sclerosis (2), renal failure (2) and other conditions.
Antecedent illness
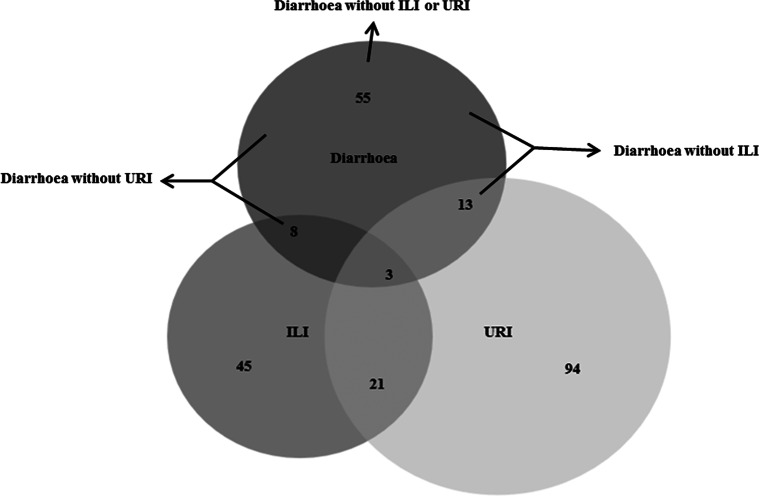
Complete antecedent illness reports were available for all 638 patients (Fig. 1, Table 1). From most sensitive to most specific for Campylobacter infection, antecedent illnesses in the 42 days before onset of symptoms of possible GBS included, 79 (12%) with diarrhoea, 68 (11%) with diarrhoea without ILI, 63 (10%) with diarrhoea without URI, 55 (9%) with diarrhoea without ILI or URI and 6 (1%) with laboratory-confirmed Campylobacter infection. The number of laboratory-confirmed Campylobacter infections was, as expected, substantially smaller than for the other antecedent illness definitions, though generally consistent with the other definitions (Table 1). Therefore, we focus on the other, more sensitive, antecedent illness definitions. Estimates of association with GBS ranged from OR = 3.2–4.2. Attributable risk percent ranged from 8.2% to 12.3%, indicating that 33.7 to 50.5 of the 411 GBS cases diagnosed in the EIP GBS surveillance were attributable to Campylobacter infection, as measured by the various antecedent illness definitions (Table 1).
Fig. 1.
Diarrhoea, influenza-like illness (ILI) and upper respiratory illness (URI) during the 42 days before onset of symptoms of possible Guillain Barré syndrome (GBS), Emerging Infections Program GBS surveillance, October 2009–May 2010.
Table 1.
Association of antecedent illness, based on definitions ranging from highly sensitive to highly specific for Campylobacter infection and association with Guillain Barré Syndrome (GBS) (Brighton Criteria 1–3)
| EIP GBS special Surveillance catchment | US Population | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antecedent Illness definition (within 42 days before GBS onset) | Expected sensitivity/specificity of antecedent illness definition | Patients with antecedent illness (N) | GBS cases with antecedent illness n (%) | Non-cases with antecedent illness n (%) | Odds ratio (95% CI) | Fraction of GBS attributable to antecedent illness (AR%) | Post-antecedent illness-GBS cases n (95% CI) | Annual post-antecedent illness-GBS cases n (95% CI)a | Annual post-antecedent illness-GBS cases n (95% CI)a | Post-antecedent illness-GBS cases, per 100 000 person-years (95% CI)a | Rate GBS, per 100 000 Campylobacter infections (95% CI)b,c |
| Diarrhoea reportedc | Most sensitive least specific | 79 | 68 (86) | 11 (14) | 3.9 (2.0, 7.5) | 12.3 | 50.5 (31.3, 68.8) | 94.6 (58.5, 128.8) | 650.4 (402.3, 886.0) | 0.2 (0.1, 0.3) | 49.2 (30.4, 67.0) |
| Diarrhoea, without ILI reported | Intermediate | 68 | 57 (84) | 11 (16) | 3.2 (1.6, 6.2) | 9.5 | 39.0 (20.4, 56.6) | 73.0 (38.2, 106.0) | 501.6 (262.9, 729.0) | 0.2 (0.1, 0.2) | 37.9 (19.9, 55.1) |
| Diarrhoea, without URI reported | Intermediate | 63 | 55 (87) | 8 (13) | 4.2 (2.0, 9.0) | 10.2 | 42.0 (24.9, 58.4) | 78.6 (46.5, 109.3) | 540.5 (319.9, 751.4) | 0.2 (0.1, 0.2) | 40.9 (24.2, 56.8) |
| Diarrhoea without ILI or URI reported | Intermediate | 55 | 47 (85) | 8 (15) | 3.5 (1.6, 7.6) | 8.2 | 33.7 (17.2, 49.5) | 63.1 (32.3, 92.6) | 433.8 (221.8, 636.9) | 0.1 (0.1, 0.2) | 32.8 (16.8, 48.2) |
| Laboratory-confirmed Campylobacter infectionc | Most specific least sensitive | 6 | 5 (83) | 1 (17) | 2.8 (0, 2.1) | 0.8 | 3.2 (0, 8.8) | 6.0 (4.6, 16.5) | 41.2 (0, 113.1) | 0.01 (0, 0.04) | 3.1 (0, 8.6) |
FoodNet
From 15 September 2009 through 15 May 2010, 3394 cases of Campylobacter infection were reported in FoodNet, representing 53% of all Campylobacter cases reported to FoodNet during the 1-year period from 15 September 2009 to 14 September 2010 (N = 6353). Applying this proportion to the estimate for each antecedent illness definition shows that, for the more sensitive case definitions (i.e. definitions based on symptomatology rather than laboratory-confirmation) an estimated 63.1 to 94.6 attributable GBS cases occurred in the EIP catchment population during the 1-year period from 1 October 2009 to 30 September 2010 (Table 1). Extrapolating from the EIP population, an estimated 433.8 to 650.4 post-Campylobacter GBS cases occurred in the USA during this 1-year period, yielding a rate of 0.1 to 0.2 cases per 100 000 person-years. Using our 1-year estimates of post-antecedent illness GBS and the 1-year estimate of Campylobacter infections (1 322 137infections) [11], approximately 32.8 to 49.2 cases of GBS occurred for every 100 000 Campylobacter infections in the USA. Table 1 also shows the lower estimates obtained using the highly specific definition of laboratory-confirmed Campylobacter infection; they are in the expected range, given the known underreporting of Campylobacter infection.
Assessment of more or less specific definitions
Analyses repeated using the more specific GBS case definition (confirmed cases only) and excluding persons with a previous history of GBS yielded similar results (data not shown).
Discussion
High quality, comprehensive, population-based, active surveillance data are rarely available for GBS, which, though uncommon, is responsible for high morbidity and economic burden [8–10]. We conducted a secondary analysis of GBS surveillance data collected during the 2009–2010 novel influenza A vaccination campaign to generate contemporaneous estimates of the burden of GBS attributable to Campylobacter infection in the USA; the primary analysis demonstrated that the risk of GBS following novel H1N1 vaccination was extremely low and not greater than what is typically observed for seasonal influenza vaccines. We estimate that 8.2–12.3% of GBS is attributable to antecedent Campylobacter infection, with 433–650 cases of GBS occurring annually in the USA (32.8–49.2 per 100 000 Campylobacter infections) that are attributable to antecedent Campylobacter infection. Although attributable risk estimates are at the lower end of the range of previous estimates for the USA and other developed countries, the incidence estimates are in the mid- to upper- range [17–20].
The EIP GBS surveillance activity provided a unique opportunity to investigate the association between Campylobacter and GBS. Strengths of the analysis include detailed health history collected through intensive, active, population-based surveillance not only from individuals who met the GBS case definitions but also from a comparison group. Given that Campylobacter infection is usually not laboratory-confirmed (only six laboratory-confirmed cases were reported) and diarrhoea often resolves before GBS symptom onset [2, 16, 20, 21], the collection of signs and symptoms in the 42 days prior allowed exploration of multiple definitions of varying sensitivity and specificity to represent antecedent Campylobacter illness. Of note, although the catchment areas of the EIP GBS surveillance activity and FoodNet did not perfectly overlap and Campylobacter incidence estimates were geographically contingent, the low proportion of mismatch in the catchment areas would not be expected to lead to a large difference in our results.
Campylobacter is the most common bacterial cause of domestically-acquired acute gastroenteritis in the USA [11, 36]. With rare exceptions [3, 37], the other top three acute gastroenteritis pathogens (norovirus, Salmonella and Clostridium perfringens) have not been consistently associated with GBS. However, the less specific but more sensitive definitions of antecedent Campylobacter illness based on diarrhoeal symptoms may have misclassified other diarrhoeal infections that are rare antecedents of GBS. The impact of these biases is hard to predict. On one hand, using diarrhoea as a proxy for campylobacteriosis should overestimate antecedent illness in both cases and controls, leading to underestimation of the association between Campylobacter infection and GBS. On the other hand, to the extent that other diarrhoeal syndromes are truly associated with GBS, attributing them to Campylobacter would lead to an overestimate of the post-Campylobacter association.
A limitation is that some patients with Campylobacter infection may not have reported diarrhoea. For example, one patient with culture-confirmed Campylobacter infection did not report diarrhoea and therefore was not captured by the diarrhoeal definition of antecedent illness. However, GBS diagnosis (case vs. non-case) would not have influenced testing for Campylobacter or report of diarrhoea in the previous 42 days because these occurred before the onset of the symptoms that led to reporting of possible GBS. In addition, identification of Campylobacter infection was limited to reported symptoms and clinical culture; serological testing was not performed. This may have led to underreporting of Campylobacter infection, thus underestimating the reported association.
Campylobacteriosis was not nationally notifiable at the time of the EIP GBS surveillance project. Therefore, a major strength of using FoodNet special surveillance data for the annual incidence of Campylobacter infection in the USA is that the data were collected through active laboratory-based surveillance, which estimates infections and incidence rates more accurately than passive surveillance. The estimated annual incidence of campylobacteriosis was generated using 2006 data, while the EIP GBS special surveillance covered an 8-month period during 2009–2010. This is unlikely to have substantially affected our results, as the incidence of Campylobacter infection remained relatively stable between 2006 and 2010 [38].
This analysis provides updated estimates related to GBS cases following Campylobacter infection in the USA. Post-Campylobacter GBS tends to be more severe than GBS following other antecedent events, with worse outcomes and slower recovery [14]. Campylobacter infections in the USA have an estimated economic burden of ($1.9 billion), over half which is attributed to GBS-related morbidity and mortality [39]. Efforts to decrease Campylobacter infections, a priority of the Food Safety Modernization Act, would likely contribute to a decrease in GBS, specifically the most severe GBS cases, thereby substantially mitigating morbidity and mortality associated with Campylobacter infection.
Acknowledgements
We gratefully acknowledge Patricia M. Griffin for the initial idea for the analysis and for helpful suggestions on the manuscript. We also thank the many Emerging Infections Program staff for their contributions to the active, population-based surveillance during the 2009–2010 novel Influenza A (H1N1) vaccination campaign. This work was not supported by grant funding from any agency, commercial or non-for-profit organization.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of interest
None.
References
- 1.Sejvar JJ et al. (2011) Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology 36, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadden RD et al. (2001) Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology 56, 758–765. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes KM and Tattersfield AE (1982) Guillain-Barre syndrome associated with Campylobacter infection. British Medical Journal (Clin Res Ed) 285, 173–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowling PC and Cook SD (1981) Role of infection in Guillain-Barré syndrome: laboratory confirmation of herpesviruses in 41 cases. Annals of Neurology 9(suppl.), 44–55. [DOI] [PubMed] [Google Scholar]
- 5.Ju YY et al. (2004) Haemophilus influenzae as a possible cause of guillain-barre syndrome. Journal of Neuroimmunology 149, 160–166. [DOI] [PubMed] [Google Scholar]
- 6.Sencer DJ and Millar JD (2006) Reflections on the 1976 swine flu vaccination program. Emerging Infectious Diseases 12, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrogan A et al. (2009) The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology 32, 150–163. [DOI] [PubMed] [Google Scholar]
- 8.Henderson RD et al. (2003) The morbidity of Guillain-Barré syndrome admitted to the intensive care unit. Neurology 60, 17–21. [DOI] [PubMed] [Google Scholar]
- 9.Dhar R, Stitt L and Hahn AF (2008) The morbidity and outcome of patients with Guillain–Barré syndrome admitted to the intensive care unit. Journal of the Neurological Sciences 264, 121–128. [DOI] [PubMed] [Google Scholar]
- 10.Frenzen PD (2008) Economic cost of Guillain-Barré syndrome in the United States. Neurology 71, 21–27. [DOI] [PubMed] [Google Scholar]
- 11.Scallan E et al. (2011) Foodborne illness acquired in the United States–major pathogens. Emerging Infectious Diseases 17, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam CC et al. (2007) Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS ONE 2, e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poropatich KO, Walker CL and Black RE (2010) Quantifying the association between Campylobacter infection and Guillain-Barré syndrome: a systematic review. Journal of Health, Population and Nutrition 28, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rees JH et al. (1995) Campylobacter jejuni infection and Guillain-Barré syndrome. New England Journal of Medicine 333, 1374–1379. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RA and Rees JH (1997) Clinical and epidemiologic features of Guillain-Barré syndrome. Journal of Infectious Diseases 176(suppl. 2), S92–S98. [DOI] [PubMed] [Google Scholar]
- 16.Nachamkin I and Blaser MJ (2000) Campylobacter, 2nd Edn. Washington, DC: ASM Press, xxiii, 545 p. [Google Scholar]
- 17.McCarthy N and Giesecke J (2001) Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni. American Journal of Epidemiology 153, 610–614. [DOI] [PubMed] [Google Scholar]
- 18.Tam CC et al. (2006) Incidence of Guillain-Barré syndrome among patients with Campylobacter infection: a general practice research database study. Journal of Infectious Diseases 194, 95–97. [DOI] [PubMed] [Google Scholar]
- 19.Orlikowski D et al. (2011) Guillain-Barré syndrome following primary cytomegalovirus infection: a prospective cohort study. Clinical Infectious Diseases 52, 837–844. [DOI] [PubMed] [Google Scholar]
- 20.Mishu B and Blaser MJ (1993) Role of infection due to Campylobacter jejuni in the initiation of Guillain-Barré syndrome. Clinical Infectious Diseases 17, 104–108. [DOI] [PubMed] [Google Scholar]
- 21.Svedhem A and Kaijser B (1980) Campylobacter fetus subspecies jejuni: a common cause of diarrhea in Sweden. Journal of Infectious Diseases 142, 353–359. [DOI] [PubMed] [Google Scholar]
- 22.Blaser MJ (1997) Epidemiologic and clinical features of Campylobacter jejuni infections. Journal of Infectious Diseases 176(suppl. 2), S103–S105. [DOI] [PubMed] [Google Scholar]
- 23.Voetsch AC et al. (2004) Laboratory practices for stool-specimen culture for bacterial pathogens, including Escherichia coli O157:H7, in the FoodNet sites, 1995–2000. Clinical Infectious Diseases 38(suppl 3), S190–S197. [DOI] [PubMed] [Google Scholar]
- 24.Henao OL et al. (2015) Foodborne diseases active surveillance network—2 decades of achievements, 1996–2015. Emerging Infectious Diseases 21, 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schonberger LB et al. (1979) Guillain-Barré syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. American Journal of Epidemiology 110, 105–123. [DOI] [PubMed] [Google Scholar]
- 26.Langmuir AD et al. (1984) An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccines. American Journal of Epidemiology 119, 841–879. [DOI] [PubMed] [Google Scholar]
- 27.Lasky T et al. (1998) The Guillain–Barré syndrome and the 1992–1993 and 1993–1994 influenza vaccines. New England Journal of Medicine 339, 1797–1802. [DOI] [PubMed] [Google Scholar]
- 28.Roscelli JD, Bass JW and Pang L (1991) Guillain-Barré syndrome and influenza vaccination in the US Army, 1980–1988. American Journal of Epidemiology 133, 952–955. [DOI] [PubMed] [Google Scholar]
- 29.Haber P et al. (2009) Vaccines and Guillain-Barré syndrome. Drug Safety 32, 309–323. [DOI] [PubMed] [Google Scholar]
- 30.Louie JK et al. (2009) Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA: The Journal of the American Medical Association 302, 1896–1902. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Padilla R et al. (2009) Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. New England Journal of Medicine 361, 680–689. [DOI] [PubMed] [Google Scholar]
- 32.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team (2009) Emergence of a novel swine-origin influenza A (H1N1) virus in humans. New England Journal of Medicine 360, 2605–2615. [DOI] [PubMed] [Google Scholar]
- 33.Wise ME et al. (2012) Guillain-Barré syndrome during the 2009–2010 H1N1 influenza vaccination campaign: population-based surveillance among 45 million Americans. American Journal of Epidemiology 175, 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sejvar JJ et al. (2011) Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 29, 599–612. [DOI] [PubMed] [Google Scholar]
- 35.Jewell NP(2004) Statistics for Epidemiology. Boca Raton: Chapman & Hall/CRC, xiv, 333 p. [Google Scholar]
- 36.Centers for Disease Control and Prevention (2013) Surveillance for foodborne disease outbreaks–United States, 2009–2010. MMWR Morbidity and Mortality Weekly Report 62, 41–47. [PMC free article] [PubMed] [Google Scholar]
- 37.Eltayeb KG and Crowley P (2012) Guillain–Barré syndrome associated with norovirus infection. BMJ Case Reports 2012, 1–2. doi: 10.1136/bcr.02.2012.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crim SM et al. (2014) Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 US sites, 2006–2013. MMWR Morbidity and Mortality Weekly Report 63, 328–332. [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann S, Maculloch B and Batz M (2015) Economic burden of major foodborne illnesses acquired in the United States. Current Politics and Economics of the United States, Canada and Mexico 17.4, 543–616. [Google Scholar]